ABSTRACT
The target delivery of sensitive components to get the intended benefits is a challenge for the global food industry. One of the principal strategies used to boost individualized absorptivity, nutrient stability, and enhanced food quality is the application of liposomal systems in the food industry, which allows controlled release of bioactive compounds. Lipid-oriented encapsulation strategies such as liposomes are superior for encapsulating sensitive components, increasing product solubility and bioavailability, and accurately targeting encapsulated content in food and nutraceutical production. In this review, the nature, composition, and different methodologies for the preparation of liposomes, such as the Bangham Method, ethanol injection method, microfluidic channel method, and freeze-drying method summarized. Moreover, the crucial role of liposomes in delivering sensitive bioactive compounds to cure different health maladies has been emphasized.
Introduction
Liposomal nanomedicines are based on lipids such as phospholipids. They are employed to protect medications from degradation in vivo, control drug release, and target drug delivery to the disease site. Liposomes are sphere-shaped vesicles made up of one or more than one phospholipid bilayers. The word “Liposome” comes from two Greek words including “lipo and soma” which means “fat and body” respectively.[Citation1] Liposomes have been used as a microencapsulation technology for medications to cure ailments since 1971.[Citation2] Liposomes improve drug delivery by enhancing oral bioavailability and protecting from GI tract mechanisms by increasing the retention period and cellular interaction of drugs. Liposomes have been used in various therapeutic techniques and clinical studies, including the delivery of gene medicines, anti-inflammatory pharmaceuticals, and antibiotics as well as anesthetics, anti-cancer, anti-fungal, and anti-bacterial drugs.[Citation3] Liposomes can capture both hydrophobic and hydrophilic molecules, thus, preventing the entrapped mixtures from corrosion and release entrapped molecules at specific locations.[Citation4,Citation5] Liposomes have advanced from being a delivery mechanism for therapeutics, pharmaceuticals and personal care products to becoming an eventual target for functional food ingredient investigation. Adopting a liposomal system in the food industry, which enables the targeted release of bioactive molecules, has been hailed as one of the most effective ways to improve nutrient stability, customized absorption, and food quality.[Citation2]
Most microencapsulation methods today employ biopolymer matrices comprised of sugar, starch, gum, protein, synthetic, dextrin, and alginates. On the other hand, liposomes, which are tiny spheres of fat, have gained popularity in the applications of the food industry.[Citation6] Suitable dietary supplemental carriers are required to transport the chemicals for targeted delivery, which must also preserve bioactive compounds and enhance their accessibility. Liposomes are appropriate encapsulated materials that have nutraceutical carrier and delivery capabilities.[Citation7]
Encapsulation of liposomes has shown to be the most effective method of encapsulating nutraceuticals, particularly when used in cancer treatment. Various studies have demonstrated that liposomal encapsulation is a promising approach for improving nutraceutical stability, targeted distribution, and bioavailability; however, the targeted drug delivery or distribution of liposomes achieved by the stabilization of phospholipids into fatty acid bilayer with antibody derivatives.[Citation8,Citation9] Studies have demonstrated the potential of liposomes to be used in various food applications such as to increase taste (i.e., flavor encapsulation), texture, stability, preserve delicate active components such as nisin and vitamin C, improve ripening solubility, and promote antimicrobial and antioxidant effects of food.[Citation10,Citation11] To properly employ liposomes in these sectors, various challenges must be addressed, including chemical corrosion and stability in the form of oxidation, hydrolysis, aggregation, and fusion.[Citation12] The current review presents information on the composition and various preparation methods of liposomes. Moreover, considering liposomes as a potential carrier, applications of liposomes in food industry and nutraceuticals, as well as their therapeutic potential have been discussed.
Nature and composition of liposomes
Liposomes are primarily synthesized from phospholipids with various head groups and a small number of additional additives, all of which are combined into an aqueous core and an outside lipid bilayer.[Citation13] Phospholipids possess both hydrophilic (head) and hydrophobic (tail) parts, making them amphiphilic molecules.[Citation14] Liposomes comprise a hydrophobic head and a hydrophilic tail, makeup glycerol-phospholipids. Different head group substitutions result in different glycerol-phospholipids, including phosphatidylethanolamine, phosphatidylserine, phosphatidylcholine, cardiolipin, phosphatidylinositol, phosphatidic acid and phosphatidyl glycerol.[Citation15]
The composition of liposomes is based on the number of phospholipid bilayers, liposomes are classified as unilamellar (ULV), multilamellar (MLV), oligolamellar (OLV), or multivesicular vesicles (MVV).[Citation16] ULVs comprise a single lipid bilayer with a diameter of 20–250 nm that encloses a huge aqueous core and is excellent for encapsulating hydrophilic medicine. MLVs, on the other hand, are composed of two or more concentric 1–5 μm diameter lipid bilayers that selectively entangle lipid soluble components.[Citation17] Lipids can control the surface charge, cellular uptake, penetration, release, and elimination of different liposome compositions.[Citation18]
However, in reaction to acidic pH, pH-sensitive liposomes undergo structural and chemical change, heat – sensitive liposomes maintain their encapsulated material at body temperature but release it upon local heating.[Citation19] The presence of PEG (polyethylene glycol) on the surface of liposome increases circulation, protects the trapped medication from deactivation or metabolic breakdown and also promotes intracellular intake.[Citation20]
Preparation methods of liposomes
Various liposomes were developed as part of the advancement of liposome technology that includes targeted liposomes, stealth liposomes, immune liposomes, and stimuli-sensitive liposomes. The stealth method relies on the ability to cover the exterior surface of liposome with biocompatible polymer conjugates that are hydrophilic in nature, such as PEG (poly-ethylene glycol), chitosan and various other types of liposomes, which lowers immunogenicity and macrophage absorption. Stealth liposome technology allows liposomes to evade phagocytosis, minimize toxicity caused by charged lipids, and extend the half-life of blood circulation.[Citation21,Citation22]
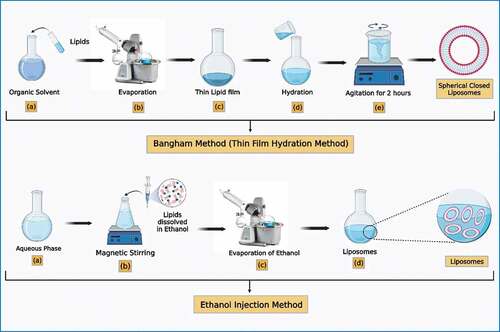
Traditional techniques for liposome production include the Bangham method which is also known as the thin film hydration method. Along with this foremost method, there are various methods for the preparation of liposomes that includes ether/ethanol injection, detergent depletion, reverse phase evaporation, heating, membrane extrusion method, microfluidic channel method, homogenization, and sonication method. For a decade, new approaches for liposomal-based drug delivery have been developed by various methods such as, freeze dried method, supercritical fluid method and dual asymmetric centrifugation method.[Citation23] Various methods for the preparation of liposomes have been discussed below:
Bangham method (thin film hydration method)
The Bangham technique was the first widely utilized liposome preparation process.[Citation24] This process dissolves lipids in an organic solvent (dichloromethane, chloroform, ethanol, and a chloroform – methanol combination); the organic solvent is then removed by evaporation under vacuum at 45–60°C to produce a thin lipid film as shown in . After hydration in an aqueous medium for 2 hours at 60–70°C, where it swells to generate spherical closed liposomes.[Citation25]
The Bangham technique is the most primary method for liposome production since the process is simple and useful for any lipid combination. On the other hand, this approach has certain disadvantages, such as low encapsulation effectiveness for water-soluble medicines, difficulties scaling up, problematic degradation of metabolic solvent and huge vesicles with no control on particle size.[Citation18]
Ethanol injection methods
In 1973, Batzri and Korniniti first reported this technique, Ethanol injection is a simple and fast way to make unilamellar liposomes. Several criteria affect the outcome of this approach, and changes were made to maximize circumstances for producing small homogenous liposomes.[Citation26] Organic solvents such as ether-methanol, diethyl ether, and their combination or ethanol are used to dissolve lipids. A needle is used to rapidly inject an ethanolic solution of lipids into an aqueous media, spreading the phospholipids throughout the medium and encouraging the formation of vesicles . Furthermore, because ether is incompatible with aqueous media, the produced liposomes must be heated to evaporate the organic solvent.[Citation27]
Ethanol injection is a straightforward, quick, repeatable, and easy to utilize approach. Concentrated liposome with a higher entrapment properties can be produced via this method. Inappropriate mixing, however, can result in heterogeneous liposomes. It is difficult to remove ethanol as it forms an azeotrope with water. Even small concentrations of ethanol render a variety of physiologically active macromolecules inert.[Citation18]
Heating method
Mortazavi and Mozafari invented the heating method for producing liposomes.[Citation28] The phospholipids are hydrated using glycerol/alcohol, and continuous stirring at 60° or 120°C for 1 hour. The liquid must be centrifuged at 4000 rpm for 15 minutes after cooling to get the liposomes. This approach produces liposomes with no phospholipid degradation, and sterilization is not required because the procedure is carried out at a high temperature (120°C), however, the high temperature increases the acyl chain length of liposomes and liposomes become more stable with the increase in temperature.[Citation22,Citation29] Furthermore, the Mozafari approach is an improved heating process conducted in an aqueous medium before being heated without organic solvents; this improves stability and is employed for the delivery of flavorsome.[Citation30 The procedure is simple and quick, requiring no contamination (e.g., organic solvent) or sterilization. However, it has limited encapsulation effectiveness, needs a high temperature, and may cause phospholipid and drug degradation. [Citation18]
Microfluidic channel method
Jahn developed a microfluidic approach for regulated liposome production.[Citation31] Isopropyl alcohol is usually used to dissolve lipids which subsequently travels through tubes carrying an aqueous medium in this process. The lipids in the isopropyl alcohol stream are mixed together to produce liposomes. Liposome size and size distribution is properly regulated via laminar flow. This is an efficient process to get self-assembled liposomes by directly encapsulating the medication.[Citation32] This is a low-cost approach that is easy and has high particle size control. However, organic solvent cleanup may be difficult, making it unsuitable for mass manufacturing.[Citation18]
Freeze drying method
A lyophilization monophasic solution procedure has been introduced to synthesize liposomes. The lipid and solvents are dissolved in alcohol at 450°C, while the lyo-protectant is dissolved in water at the same temperature. Both resulting solutions are combined to prepare a third identical monophasic solution, which is filtered and freeze-dried to make pro-liposomes. Freeze drying requires two steps: first, the sample is frozen at 40°C, then dried at room temperature via the air drying, after hydration resulting in liposomes of very small size from 100–300 nm.[Citation33] It is a single-step approach for commercial-scale manufacture that extends the shelf life of liposomes and avoids physical deterioration. Although it is energy-intensive procedure, it may cause physical changes, such as changes in vesicle size and loss of enclosed content.[Citation18]
High shear homogenization method
For the lamellarity and size reduction of liposomes, homogenization is frequently utilized. High homogenization forces are usually applied on liposome suspension, which collides with a stainless-steel wall to form liposomes that are smaller in size, when high pressure is applied. This technique allows for good particle size control and large-scale production. Contrarily, it also has certain disadvantages, such as the fact that this approach employs extremely high pressure, and the liposome size distribution is quite broad and changeable.[Citation18]
Encapsulation applications of liposomes
Encapsulation systems are a great way to preserve bioactive substances and use them as food additives. It allows for the controlled release of hydrophobic or hydrophilic substances at precise locations to a food lipophilic/lipophobic and hydrophilic compound.[Citation34] The most common nanocarrier systems are lipid or phospholipid-based innovative encapsulating methods that have helped a significant contribution to the burgeoning field of food and nutraceuticals and nanotechnology applications.[Citation35] Lipid nano-emulsions, liposomes, nano-liposomes, nano-cochleates, archaeosomes, solid lipid nanoparticles, and nanostructured lipids are among the promising delivery systems.[Citation36] In the food sector, liposome and nano-liposome methods have been widely employed in supplementing and enriching nutrients. It improves the quality and stability of the food product while also acting as a substitute for microencapsulation.[Citation37,Citation38]
By changing the capsule characteristics, limiting interactions with other undesired molecules, and concealing unpleasant odors and flavors, liposome encapsulation optimizes the bioavailability of functional substances.[Citation39] These lipid and water-oriented systems provide distinct advantages in food processing, such as increased surface area, which improves product solubility.[Citation40] There are just a few food-grade liposomes combined with the coating of polymers such as chitosan and gelatin that are GRAS (generally regarded as safe).[Citation41,Citation42]
Liposomes have been studied as a possible medicine delivery system because of their biomedical applications, delivery mechanisms and the capacity to include both hydrophilic and hydrophobic materials. It is expected that the use of liposomes in the food industry will expand dramatically in the next 5–10 years.
Applications of liposomes in food and nutrition
Consumers have chosen healthier lives, fueled the rise of novel functional food products.[Citation43] A recent research isolates classified liposomes based functional products, as well as identifies the impact of each constituent in the formulation and its stability, to better elaborate the functionality of liposomes under different processing conditions. The connection between bioactive substances, phospholipid type, cholesterol content, and various wall materials, as well as the stability of liposomal vesicles, should be considered first.[Citation44] Lecithin preparations including mixes of phosphatidylserines (PS), phosphatidylcholines (PC), phosphatidylethanolamines (PE) and phosphatidylinositol (PI), as well as additional compounds such as sterols and free fatty acids (FFA’s), are commercially known as food-derived phospholipids.[Citation45]
To increase the strength and functionality of food-grade liposomes, these are coated with layers of antioxidants and other bioactive components to increase the stability, to promote bioactive and antioxidant effects.[Citation46] Because of the lipid content of the membranes, the stability and quality of liposomes is diminished by environmental conditions such as, processing, storage factors and high temperatures, as well as harsh conditions of gastrointestinal tract such as acidity, as well as enzymes and bile salts.[Citation47]
In milk, liposomes are used for various purposes, the most prevalent of which is to improve antimicrobial peptide action.[Citation48] Utilizing liposomes in antimicrobial peptides might be a beneficial strategy for extending the shelf life of these products by protecting their stability and controlling food deterioration.[Citation49] Encapsulating two or more antimicrobials into liposomes in milk products produced synergistic benefits.[Citation50]
Liposomes were first utilized in the cheese industry in the early 1980s to speed up the ripening process and avoid taste flaws produced by the inclusion of proteinase.[Citation51] Encapsulating enzyme to facilitate cheese ripening is the most prevalent use of liposomes in cheese, and the cost of cheddar cheese ripening expedited by liposomes-encapsulated enzyme was around 100-fold lower than other frequently used techniques.[Citation2] Antimicrobials, such as nisin bacteriocin-like compound P34 and lemongrass oil, have been encapsulated in liposomes to boost their antibacterial action without affecting cheese’s physicochemical and sensory properties during Cheddar cheese ripening.[Citation52] Sour cherry extract, doum fruit extract, and fish oil have been encapsulated in liposomes.[Citation2 In , various studies have been listed regarding applications of Liposomes in functional foods.
Table 1. Liposomes in functional foods.
Nutraceuticals and liposomes in cancer therapy
Cancer is the biggest cause of death, claiming approximately 6 million lives each year, and the risk of mortality from cancer rises. Based on the current cancer prevalence rate, it is estimated that by 2050, cancer diagnoses impact 24 million people globally.[Citation42,Citation53] Surgery, chemotherapy, radiation therapy, hormone therapy, immunotherapy and stem cell transplant are some cancer treatments available today. Nutraceuticals are now being explored as a preventative and curative approach to combat cancer. Nutraceuticals have both nutritional and medicinal advantages; also, they are natural and biodegradable, and pose no risks.[Citation54] Liposomes are treated with polyethylene glycol (PEG) pegylated liposomes, which reduce liposome breakdown by macrophages in the reticulo-endothelial system and improve medication retention.[Citation55] Liposomes are commonly used for this type of drug administration because they prolong the buffer time of encapsulated medicines and extend their circulatory time and to modify their pharmacokinetic profile.[Citation56]
Drug delivery methods based on liposomes have the potential to improve the therapeutic index of anti-cancer drugs. By employing targeting tactics, they take advantage of the improved permeability and retention effect phenomenon. Liposomes can lower medication exposure in healthy tissues while increasing drug concentration in malignant cells.[Citation57]
A study evaluated the potential of employing liposomes to deliver epigallocatechin gallate (EGCG) and its derivative to tumors. The findings imply that injecting EGCG-containing liposomes into BCCs with a mild modification effectively increase EGCG deposition.[Citation58] In another study, chitosan compounds that are cationic and hydrophobic were produced and studied. Effective coating with these compounds allowed the stabilization of curcumin-containing liposomes. The cell membrane is easily penetrated by such coated liposomes, in which curcumin released in a controlled manner.[Citation59] Stable vesicles for effective curcumin encapsulation, transport, and controlled release have been achieved by encapsulating liposomes with a thin layer of freshly synthesized chitosan derivatives.[Citation5]
In another study by Khan et al.[Citation60] a tuftsin-tagged liposome was developed for the cytosolic transport of the 2,6-di-isopropylphenol-linolenic acid combination in a liver cancer cell. This nanoformulation therapy dramatically inhibited the development of liver cancer cells in diethylnitrosamine-induced mice by suppressing cyclooxygenase-2 (COX-2), b-cell lymphoma 2 (Bcl-2), and activating Bcl-2-associated x (Bax). A positive recovery of the application framework and the violent apoptosis of cancer cells at the site of the tumor were also demonstrated by histopathological investigation. Cancer is the most severe diseases in the world, claims many lives. Encapsulation of nutraceuticals is thought to be a successful therapeutic, acting as both a preventative and a curative strategy for cancer, despite the major side effects of chemotherapy. had showed the utilization of liposmesin treatment of different cancers
Table 2. Adjacent effect of Liposomes and Nutraceuticals in cancer treatment.
Breast cancer
In 2020, 685 000 women worldwide died as a result of breast cancer, although affected 2.3 million women. The most common disease in the globe as of the end of 2020 was breast cancer, which had been diagnosed in 7.8 million women in the previous five years.[Citation61] One of the best treatments for patients with breast cancer is anthracyclines. Their use has been constrained by the hazardous side effects they are known to have, particularly cardiotoxicity but also bone marrow suppression, alopecia, diarrhea, and nausea.[Citation62] Drug-resistant cancer stem cells (CSCs) are linked to tumor progression. For the therapy and prevention of tumor recurrence, targeted berberine liposomes were designed in a study. The treatments had a significant effect in mice, indicating that targeting berberine liposomes can be a promising therapeutic for the treatment and prevention of breast cancer recurrence caused by CSCs.[Citation63]
The exceedingly low water solubility, poor absorption, and pharmacokinetics of genistein restrict its clinical utility against cancer. Liposomal vehicle compositions were developed and adjusted for optimum integration of genistein’s flavonoid composition based on structural similarities with steroidal substances. Model standard and stealth genistein liposomes (GenLip), which include unsaturated phospholipids and cholesterol, have shown improved drug solubilization, longer release profiles, and shelf-life durability. The results demonstrated that the loading capacity and physicochemical features of the integrated medication boosted cellular transport and particular pro-apoptotic efficiency against diverse malignancies.[Citation64] Although, further clinical studies are needed to demonstrate the potential role of liposomes encapsulated in nutraceuticals for breast cancer treatment.
Pancreatic cancer
Pancreatic cancer remains one of the most difficult human malignancies to treat. Pancreatic cancer has been recorded in 458,918 new cases worldwide in 2018, and it is predicted that 355,317 more cases will develop before 2040. Despite improvements in its identification and treatment, only 9% is still the 5-year survival rate for pancreatic cancer.[Citation65] Developments in liposomal delivery systems have facilitated the targeting of specific agents for cancer treatment. Such systems can be developed as platforms for future multi-functional nano-devices adapted for the combined detection of early cancer and functional drug delivery. For the treatment of pancreatic cancer, liposome-based drug-delivery systems can offer superior pharmacokinetics, fewer side effects, and possibly higher tumor uptake.[Citation66] A study investigated encapsulated curcumin in a liposomal delivery system to evaluate its impact on pancreatic cancer. The researchers used human pancreatic cancer cells to investigate the in vitro and in vivo effects of this drug on proliferation, apoptosis, signaling, and angiogenesis Curcumin decreased tumor angiogenesis and lowered pancreatic cancer development in vivo.[Citation67]
Low bioavailability, poor aqueous solubility, and quick metabolism are characteristics of curcumin. It is mostly enclosed in liposomes to suppress these restrictions. A protective layer made of chitosan oligosaccharides lactate was used to coat the bilayer of diarachidonyl phosphatidyl choline liposomes (DAPC) in recent work to encapsulate curcumin for the first time (DAPC-Cur-COL NCs). The findings indicated that DAPC liposomes, when compared to other liposomes, provided an encapsulation effectiveness of 90%. Therefore, the inclusion of chitosan improved curcumin’s 99.40% encapsulation efficiency.[Citation68]
Prostate cancer
Prostate cancer is the most prevalent form of cancer and the leading cause of cancer-related mortality in males.[Citation69] Targeted drug delivery with liposomal drug delivery systems is a promising method for enhancing the efficacy of anticancer drugs, minimizing adverse effects, and as a result increasing the therapeutic action. In the majority of preclinical prostate cancer trials, passive liposomal targeting of anticancer drugs (caused by enhanced permeability and retention of the therapeutic molecule) leads to increase antitumor activity and lower side effects compared to non-targeted therapy.[Citation70]
Curcumin, an anticancer drug, was incorporated into liposomes coated with prostate membrane to target specific antigens by specific antibodies as part of a study to improve the targeted delivery of this medication for prostate cancer therapy.[Citation71] A recent study used the sol-gel method to prepare curcumin-loaded liposomes with varied lipid contents, with an average size of 100–150 nm.[Citation72] Using an antibody-based method, liposomes can actively target tumor tissues. This can be accomplished by incorporating particular antibodies, known as immune liposomes (ILP), onto the liposomal surface targeted against cancer cells or the endothelial cells of the tumor vasculature.[Citation73]
According to a study, a liposomal delivery system with two distinct functions was created. Its surface was changed with transferrin (Tf) for receptor-mediated transcytosis and a cell-penetrating peptide-penetratin (Pen) for improved cell penetration. By using the post-insertion approach, Transferrin-Penetratin (Tf-Pen) liposomes were created. Tf-Pen liposomes also showed remarkable biocompatibility for delivery in vivo. Tf-Pen liposomes showed outstanding anticancer activity by regressing 90% of tumors in mice brains with considerable improvement in median survival time (36 days) and no harm. Therefore, it has been determined that this study will have a significant influence on the way to treat cancer patients.[Citation74]
Colorectal cancer
The third most prevalent cancer worldwide and the fourth most lethal malignancy is colorectal cancer (CRC).[Citation65] The quality of life and results specific to cancer for CRC patients are greatly impacted by the toxicity of chemotherapy and radiotherapy to healthy tissues and antibiotic resistance. Numerous attempts have been made to develop medication delivery systems based on nanomaterials, liposomes are one of these nano-carriers that are most effective at delivering focused oncological treatment, enhancing the safety profile, and enhancing the therapeutic efficacy of encapsulated pharmaceuticals.[Citation75]
Curcumin has been linked to treating various ailments, including cancer, in traditional medicine. The main obstacles to its acceptance as a medicinal agent are limited solubility in water and low in vivo bioavailability. Liposomes are regarded as efficient medication carriers because of their propensity to solubilize hydrophobic substances and modify their pharmacokinetic characteristics.[Citation76]
A study developed lyophilized liposomal curcumin that was fully characterized in terms of physical characteristics, as well as tested it in vitro cytotoxicity against colorectal cancer cell lines in a short-term and long-term assay.[Citation77] Curcumin can be absorbed into egg-phosphatidylcholine (EPC) liposomes at a 1:14 drug-to-lipid molar ratio, resulting in an incorporation effectiveness of about 85%. In vitro investigations on colorectal cancer cell lines have revealed that liposomes boost curcumin activity, particularly in the long-term test, and the liposomal formulation is more powerful against HCT116 and HCT15, cell lines that have the MDR phenotype.[Citation78,Citation79]
Lung cancer
Lung cancer is the leading cause of cancer-related mortality worldwide, as its incidence rates are among the highest cancers.[Citation80] It is usually considered that angiogenesis plays a crucial role in cancer development and metastasis. Anti-angiogenesis therapy has progressed to show promising outcomes; however, despite their widespread use, anti-angiogenic medications have yet to achieve sufficient effectiveness.[Citation81] Various studies have demonstrated the anticancer potential of liposomes by reducing vasculogenic mimicry (VM) channel development, tumor angiogenesis, migration, and invasion.[Citation82] The liposomes likewise downregulated vasculogenic mimicry (VM) and angiogenesis-related proteins in vitro.[Citation83] Fisetin (3,3′,4′,7-tetrahydroxyflavone) is a natural flavonoid incorporating antiangiogenic and anticancer effects.[Citation84] Fisetin has a low water solubility. Therefore a liposomal formulation was developed and tested in vitro and in Lewis lung cancer (LLC)-bearing mice. The results of the study demonstrate that a liposomal formulation improved Fisetin’s digestibility and antitumor effects in mice. Fisetin liposomes significantly increased Fisetin bioavailability and anticancer activity in mice, suggesting that this formulation can make it easier to administer this flavonoid in the treatment.[Citation85]
Liposomes as effective therapeutics
Natural compound delivery through liposomes has been regarded as a viable technique to circumvent the constraints of in vitro treatment. Natural compounds have promising nutritional and therapeutic potential but have limited biological activity due to environmental variables, low dispersion, uncontrolled release, and gastrointestinal breakdown.[Citation86] Liposomes are intriguing and efficient nano-carriers for chemicals and medicines, enhancing their bioavailability and therapeutic efficacy.[Citation87] Encapsulation is a powerful tool to increase the bioactivity of natural materials. Liposomes are a colloidal encapsulation system that can hold both lipophilic and hydrophilic chemicals, as they have polar, nonpolar, and amphiphilic parts therefore, all compounds are encapsulated within a phospholipid bilayer compartment.[Citation88] The application of liposomes in nutraceuticals and drug delivery systems might be useful to prevent chronic diseases therapeutically. Moreover, the neuroprotective, anti-oxidant, anti-hyperlipidemic, and anti-inflammatory activity of drugs can be increased by utilizing liposomes as carriers.
Neuroprotective activity
Cerebrovascular diseases are a leading cause of long-term disease worldwide and are associated with high death rates.[Citation89] Ischemic stroke accounts for approximately 50–60% of the overall diseases and the remaining comprising of cerebral hemorrhage and subarachnoid hemorrhage.[Citation90] A therapy for ischemic stroke was presented in a treatment using a liposomal drug delivery system. In this study, the liposomal medicines were administered in rats, as a result, this drug reduced the adverse effect of plasminogen activator (t-PA) and increased the therapeutic time window (TTW).[Citation91]
The prevalence of Alzheimer’s disease is increasing due to a number of variables, such as blood-brain barrier, however, the progress in treatment effectiveness is not remarkable. Recent research has shown the importance of oxidative stress in the pathophysiology of illness.[Citation92] According to a study, quercetin liposomes administered orally can prevent neurodegeneration in Alzheimer’s disease due to its strong antioxidant effects and the burden of the blood-brain barrier.[Citation93]
It has been demonstrated that capsaicin-containing liposomes function as a potentially effective therapeutic agent in lowering the oxidative stress on the liver brought on by various stressors.[Citation94] Additionally, curcumin-entrapped nano-liposomes showed an immunomodulatory effect on dimethylhydrazine-experimental rats by modifying the neurotransmitters in the brain’s hypothalamus (which decreased glutamate and increased -amino butyric acid) and striatum (which decreased dopamine) and 5-hydroxytryptamine.[Citation95] A study used four spices to treat depression, including Annona muricata, Trichilia catigua, Hyssopus officinalis, and Cereus grandiflorus, as well as their mixtures. The spices were screened as monoamine oxidase inhibitors (MAO-A), and evaluated their synergistic, antagonistic, and additive interactions.[Citation96] However, more studies involving human participants are needed to elaborate on the crucial role of liposomes-based drug delivery systems in treatment of neurological disorders.
Anti-oxidant activity
Antioxidant compounds, such as resveratrol, have gained great interest in the pharmaceutical research area due to their therapeutic properties. Because of its broad range of therapeutic effects its safety profile, Generally Recognized as Safe (GRAS) status, and natural origin, resveratrol is an appealing prospect for the development of innovative pharmaceutical drugs.[Citation97] Furthermore, the development and optimization of liposomes modified with polyethylene glycol (PEG) and Quercetin encapsulated with chitosan or lecithin polymeric nanoparticles have resveratrol properties.[Citation98]
Another study suggested that Eudragit coated liposomes are efficient vesicle systems for quercetin integration, retention, and transport. Adding the Eudragit coating to liposomes boosted their physical stability under simulated gastrointestinal conditions (acidic pH and high ionic strength).[Citation99] Furthermore, the in vitro results in human intestinal cells reveal the antioxidant activity of the quercetin Eudragit-coated liposomes, extending the action of quercetin inside the cells for up to 6 hours. As a result, the suggested formulation can control inflammatory and oxidative circumstances that can lead to DNA damage and cell alterations.[Citation100]
A simple electrostatic deposition method was developed to encapsulate quercetin within chitosan/lecithin polymeric nano-capsules, preventing it from degrading and increasing its biocompatibility. Size, shape, encapsulation efficiency, antioxidant effect, storage stability, and antiproliferative effects of quercetin-encumbered polymeric nano-capsules (Q-NPs) were studied. When compared to native quercetin, the shelf life and free radical scavenging activity was enhanced.[Citation101] More research is needed to assess the efficacy of liposomes coated with chitosan.
A study indicated that the generated polyethylene glycol (PEG) modified liposomes which are a suitable carrier system for bioactive resveratrol because it has long-term stability and biocompatibility.[Citation64] The suggested technique has the potential for therapeutic applications against oxidative stress, providing promising prospects for using resveratrol PEG-modified liposomes in vivo, with a particular emphasis on increasing the shelf life in blood circulation.[Citation98] Although, more literature is needed to elaborate on the antioxidant potential of liposomes-based drug delivery systems in the treatment of chronic disorders.
Anti-hyperlipidemic activity
According to the World Health Organization (WHO), cardiovascular disorders are the most common diseases in the world the leading cause of death in the developing world.[Citation102] Functional foods loaded with critical micronutrients such as polyunsaturated fatty acids (PUFAs) are one of the promising methods of preventing such disorders.[Citation103] A study determined the appropriate amount of omega- 3 fatty acids concentrated in a liposomal as a seal fat in fortified bread. In this study, an experimental model of an atherogenic diet generated by adding cholesterol to the diet of laboratory animals revealed that PUFAs concentrate in a liposomal form has a significant hypocholesterolemic, hypolipidemic, and antioxidant impact.[Citation104]
An enhanced perillaldehyde-loaded liposomal nano-formulation (PAH-LNF) was successfully employed to boost the pharmacological effects of perillaldehyde in poloxamer 407-induced hyperlipidemia (PAH). Compared to free PAH, oral administration of PAH-LNF (240 mg/kg per body weight) enhanced the absorption and efficacy of the drug (250–270%) in experimental rats. In order of decreasing affinity, a tissue distribution investigation found PAH buildup in different organs (heart, liver, spleen, lungs, brain, and kidney). As a result, a substantial reduction in triglyceride level, cholesterol level, and low-density lipoprotein level in blood was found.[Citation105]
Additionally, PAH-LNF decreased malondialdehyde (MDA) while increasing the activities of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), indicating that PAH has antioxidant and hepato-protective properties. As a result, liposomal nano-formulation is a promising medication delivery strategy for PAH development.[Citation106]
Anti-inflammatory activity
Liposomal entrapped curcumin formulation significantly increased antioxidant activity (increased DPPH scavenging activity) and anti-inflammatory activity by reducing lipopolysaccharide-induced nitric oxide, interleukin 1, and tumor necrosis factor production in RAW 264.7 cells when compared to unentrapped curcumin.[Citation107]
Curcumin can inhibit the expression of cyclooxygenase inhibitor enzymes and the production of prostanoids in human cells.[Citation108] Therefore, it has anti-inflammatory properties, although it is seldom employed for medical purposes due to its less water absorption and vulnerability to the UV action of sunlight.[Citation109] As carriers for skin distribution, novel lipid vesicles have been produced. demonstrated that propylene glycol liposomes (PGL) were applied as a skin medication because curcumin is water-insoluble and unstable to the UV.[Citation110] A recent study assessed the enoxaparin (ENX) loaded propylene glycol (PG) liposome’s anticoagulant potential. PG-Liposome may significantly increase ENX’s skin penetration. Enoxaparin is a medication that can be applied topically to stop thrombosis.[Citation111]
Propylene glycol-embodying liposomes, often known as PG-liposomes, are a unique class of lipid vesicles made of phospholipid, propylene glycol, and water. High entrapment efficiency and stability have been displayed by PG-liposomes during storage for at least one month. The PG-liposomes appear promising delivery systems for drugs when applied topically to the skin. Studies have demonstrated that PG-liposomes outperformed traditional liposomes, deformable liposomes, and etho-somes are effective.[Citation112]
Conclusion
Different types of liposomal synthetic drugs are now authorized in clinical trials and commercially accessible. There are just a few studies focusing on improving the biological activities of natural substances and nutraceuticals via liposomal delivery. It should be noted that most of the studies employed stealth liposomes, stimuli-responsive liposomes, or ligand-targeted liposomes in cell-type models, while only a few used animal models and clinical studies. To assess the therapeutic activity of natural substances, further biological research and clinical investigations using stealth-stimuli-sensitive, stealth-ligand-targeted, or theragnostic liposomes are needed to better understand digestive behavior, target mechanisms, and cellular absorption.
Credit authorship contribution statement
Noor Akram, Farhan Saeed, and Muhammad Afzaal designed the study and wrote the manuscript. Mohd Asif Shah, Gulzar Nayik, Sajad Wani and Amin Mousavi participated in drafting the article. Farhan Saeed, Huda Ateeq and Muhammad Afzaal helped in developing the whole concept and editing. Yasir Abbas Shah, Zargham Faisal Huda Ateeq and Aasma Asghar helped in preparing Figures and Tables and the overall quality of the manuscript. Noor Akram, Muzzamal Hussain, Farhan Saeed, and Muhammad Afzaal wrote, edited, and revised the manuscript critically. Mohd Asif Shah, Gulzar Nayik, Sajad Wani and Amin Mousavi Khaneghah revised the final written paper. The final version of the manuscript has been read and approved by all listed authors
Acknowledgments
The authors are thankful to Government College University Faisalabad for providing literature collection facilities.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Even though adequate data has been given in the form of tables and figures, however, all authors declare that if more data is required then the data will be provided on a request basis.
Additional information
Funding
References
- Cruz, C. H.; Marzuoli, I.; Fraternali, F. Virus-Inspired Designs of Antimicrobial Nanocapsules. Faraday Dis. 2021, 232, 448–462.
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189.
- Sathe, K. P.; Bangar, V. B. LIPOSOMES: AN OVERVIEW. World J Pharm. Res. 2021, 10, 935–954.
- Sharma, V. K.; Agrawal, M. K. A Historical Perspective of Liposomes-A Bio Nanomaterial, Mater. Today Proc. 2021, 45, 2963–2966. DOI: 10.1016/j.matpr.2020.11.952.
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. DOI: 10.3389/fbioe.2020.00437.
- Patel, A. K.; Mutta, S. K.; Yadav, R. P. Liposomes–A Overview. Asian J. Pharm. Res. Dev. 2021, 9(3), 82–88. DOI: 10.22270/ajprd.v9i3.934.
- Hasan, M.; Messaoud, G. B.; Michaux, F.; Tamayol, A.; Kahn, C. J.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-Coated Liposomes Encapsulating Curcumin: Study of Lipid–Polysaccharide Interactions and Nanovesicle Behavior. R.S.C. Adv. 2016, 6(51), 45290–45304. DOI: 10.1039/C6RA05574E.
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X. D. Combination Therapy Using Co-Encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016, 6(1), 1–11. DOI: 10.1038/srep22390.
- Dutta, S.; Moses, J. A.; Anandharamakrishnan, C. Encapsulation of Nutraceutical Ingredients in Liposomes and Their Potential for Cancer Treatment. Nutr. Cancer. 2018, 70(8), 1184–1198. DOI: 10.1080/01635581.2018.1557212.
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S. M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid Interface Sci. 2020, 278, 102140. DOI: 10.1016/j.cis.2020.102140.
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S. H.; Valizadeh, H.; Hesari, J. Liposomes as Carrier Vehicles for Functional Compounds in Food Sector. J. Exp. Nanosci. 2016, 11, 737–759.
- Mirafzali, Z.; Thompson, C. S.; Tallua, K. Application of Liposomes in the Food Industry, Microencapsul. Food Ind. Elsevier. 2014, 195–207.
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 2015, 10(2), 81–98. DOI: 10.1016/j.ajps.2014.09.004.
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics. 2020, 12(12), 1235.
- Large, D. E.; Abdelmessih, R. G.; Fink, E. A.; Auguste, D. T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. DOI: 10.1016/j.addr.2021.113851.
- Eskandari, V.; Sadeghi, M.; Hadi, A. Physical and Chemical Properties of Nano-Liposome, Application in Nano Medicine. J. Comput. Appl. Mech. 2021, 52, 751–767.
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975. DOI: 10.2147/IJN.S68861.
- Andra, V. V. S. N. L.; Bhatraju, L. V. K. P.; Ruddaraju, L. K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanosci. 2022, 12(1), 274–291.
- Rehman, A. U.; Omran, Z.; Anton, H.; Mély, Y.; Akram, S.; Vandamme, T. F.; Anton, N. Development of Doxorubicin Hydrochloride Loaded pH-Sensitive Liposomes: Investigation on the Impact of Chemical Nature of Lipids and Liposome Composition on pH-Sensitivity. Eur J. Pharm. And Biopharm. 2018, 133, 331–338.
- Nunes, S. S.; Fernandes, R. S.; Cavalcante, C. H.; da Costa César, I.; Leite, E. A.; Lopes, S. C. A.; Ferretti, A.; Rubello, D.; Townsend, D. M.; de Oliveira, M. C. Influence of PEG Coating on the Biodistribution and Tumor Accumulation of pH-Sensitive Liposomes, Drug Deliv. Transl. Res. 2019, 9, 123–130.
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon. 2022, 8(5), e09394. DOI: 10.1016/j.heliyon.2022.e09394.
- Nkanga, C. I.; Bapolisi, A. M.; Okafor, N. I.; Krause, R. W. M. General Perception of Liposomes: Formation, Manufacturing and Applications. Liposo. adv. Perspec. 2019.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutic. 2017, 9(4), 12. DOI: 10.3390/pharmaceutics9020012.
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Process. 2020, 8(9), 1022.
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Liposomes. Springer. 2017, 17–22.
- Gouda, A.; Sakr, O. S.; Nasr, M.; Sammour, O. Ethanol Injection Technique for Liposomes Formulation: An Insight into Development, Influencing Factors, Challenges and Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. DOI: 10.1016/j.jddst.2020.102174.
- Guimarães, D.; Noro, J.; Loureiro, A.; Lager, F.; Renault, G.; Cavaco-Paulo, A.; Nogueira, E. Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomed. 2020, 8(12), 630. DOI: 10.3390/biomedicines8120630.
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyo. 2021, 7(12), e08632.
- Liu, G.; Hou, S.; Tong, P.; Li, J. Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry. Crit. Rev. Anal. Chem. 2022, 52(2), 392–412. DOI: 10.1080/10408347.2020.1805293.
- Vafabakhsh, Z.; Khosravi, D. K.; Mortazavi, S.; Khajeh, K.; Jahadi, M.; Mohammadi, J.; Bahramian, G.; Komeili, F. R. Liposomal Encapsulation of Flavourzyme and Its Impact on Kinetic Properties. 2015, 427–439.
- Has, C.; Sunthar, P. A Comprehensive Review on Recent Preparation Techniques of Liposomes. J. Liposome Res. 2020, 30(4), 336–365. DOI: 10.1080/08982104.2019.1668010.
- Kotouček, J.; Hubatka, F.; Mašek, J.; Kulich, P.; Velínská, K.; Bezděková, J.; Fojtíková, M.; Bartheldyová, E.; Tomečková, A.; Stráská, J. Preparation of Nanoliposomes by Microfluidic Mixing in Herring-Bone Channel and the Role of Membrane Fluidity in Liposomes Formation. Sci. Rep. 2020, 10(1), 1–11. DOI: 10.1038/s41598-020-62500-2.
- Yen, T. T.; Le Dan, N.; Duc, L. H.; Tung, B. T.; Hue, P. T. M. Preparation and Characterization of Freeze-Dried Liposomes Loaded with Amphotericin B. Curr. Drug Ther. 2019, 14, 65–73.
- Toniazzo, T.; Berbel, I. F.; Cho, S.; Fávaro-Trindade, C. S.; Moraes, I. C. Pinho, β-Carotene-Loaded Liposome Dispersions Stabilized with Xanthan and Guar Gums: Physico-Chemical Stability and Feasibility of Application in Yogurt. LWT-Food Sci. Technol. 2014, 59, 1265–1273.
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome Technology for the Food and Nutraceutical Industries, Trends Food Sci. Technol. 2018, 79, 106–115.
- Shukla, S.; Haldorai, Y.; Hwang, S. K.; Bajpai, V. K.; Huh, Y. S.; Han, Y. K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. DOI: 10.3389/fmicb.2017.02398.
- Hosseini, H.; Tajiani, Z.; Jafari, S. M. Improving the Shelf-Life of Food Products by Nano/micro-Encapsulated Ingredients. Food Qual. Shelf Life Elsevier. 2019, 159–200.
- Fattahian, A.; Fazlara, A.; Maktabi, S.; Pourmahdi, M.; Bavarsad, N. The Effects of Chitosan Containing Nano-Capsulated Cuminum Cyminum Essential Oil on the Shelf-Life of Veal in Modified Atmosphere Packaging. J. Food Meas. Charact. 2022, 16, 920–933.
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59(19), 3129–3151. DOI: 10.1080/10408398.2018.1484687.
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57(10), 3545–3555. DOI: 10.1007/s13197-020-04360-2.
- Timilsena, Y. P.; Haque, M. A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11(6), 481. DOI: 10.4236/fns.2020.116035.
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods. 2022, 11(19), 3017. DOI: 10.3390/foods11193017.
- Saini, A.; Panesar, P. S.; Bera, M. B. Valorization of Fruits and Vegetables Waste Through Green Extraction of Bioactive Compounds and Their Nanoemulsions-Based Delivery System. Bioresour. Bioprocess. 2019, 6(1), 1–12. DOI: 10.1186/s40643-019-0261-9.
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; González-Cavieres, L.; Lemus-Mondaca, R. An Overview Focusing on Food Liposomes and Their Stability to Electric Fields. Food Eng. Rev. 2022, 14(2), 292–306.
- Hirose, M.; Ueno, T.; Nagumo, H.; Sato, Y.; Sakai-Kato, K. Enhancing the Endocytosis of Phosphatidylserine-Containing Liposomes Through Tim4 by Modulation of Membrane Fluidity. Mol Pharm. 2021, 19, 91–99.
- Feng, S.; Sun, Y.; Wang, P.; Sun, P.; Ritzoulis, C.; Shao, P. Co‐Encapsulation of Resveratrol and Epigallocatechin Gallate in Low Methoxyl Pectin‐Coated Liposomes with Great Stability in Orange Juice. Int. J. Food Sci. Technol. 2020, 55, 1872–1880.
- Tian, M.; Han, J.; Ye, A.; Liu, W.; Xu, X.; Yao, Y.; Li, K.; Kong, Y.; Wei, F.; Zhou, W. Structural Characterization and Biological Fate of Lactoferrin‐Loaded Liposomes During Simulated Infant Digestion. J. Sci. Food Agri. 2019, 99(6), 2677–2684. DOI: 10.1002/jsfa.9435.
- Marsanasco, M.; Calabró, V.; Piotrkowski, B.; Chiaramoni, N. S.; Alonso, S. V. Fortification of Chocolate Milk with Omega‐3, Omega‐6, and Vitamins E and C by Using Liposomes. Eur. J. lipid sci. technol. 2016, 118, 1271–1281.
- Al-Moghazy, M.; El-Sayed, H. S.; Salama, H. H.; Nada, A. A. Edible Packaging Coating of Encapsulated Thyme Essential Oil in Liposomal Chitosan Emulsions to Improve the Shelf Life of Karish Cheese. Food Biosci. 2021, 43, 101230. DOI: 10.1016/j.fbio.2021.101230.
- Pinilla, C. M. B.; Brandelli, A. Antimicrobial Activity of Nanoliposomes Co-Encapsulating Nisin and Garlic Extract Against Gram-Positive and Gram-Negative Bacteria in Milk. Innov. Ood Sci. Emerg. Technol. 2016, 36, 287–293.
- Bhosale, S.; Fulpagare, Y.; Desale, R. Nanoliposomes: Applications in Food and Dairy Industry. Int. J. Adv. Res. Biol. Sci. 2019, 6, 79–84.
- Malheiros, P. S.; Cuccovia, I. M.; Franco, B. D. Inhibition of Listeria Monocytogenes in vitro and in Goat Milk by Liposomal Nanovesicles Containing Bacteriocins Produced by Lactobacillus Sakei Subsp. Sakei 2a. Food Contr. 2016, 63, 158–164.
- Pilleron, S.; Soto‐Perez‐de‐Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated Global Cancer Incidence in the Oldest Adults in 2018 and Projections to 2050. Int. J. Cancer. 2021, 148(3), 601–608.
- Cicero, A. F.; Allkanjari, O.; Busetto, G. M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Della Cuna, F. S. R.; Russo, G. I.; Stamatiou, K., Trinchieri, A. Nutraceutical Treatment and Prevention of Benign Prostatic Hyperplasia and Prostate Cancer. Arch. Ital. Urol. Androl. 2019, 91(3).
- Vandchali, N. R.; Moadab, F.; Taghizadeh, E.; Tajbakhsh, A.; Gheibihayat, S. M. CD47 Functionalization of Nanoparticles as a Poly (Ethylene Glycol) Alternative: A Novel Approach to Improve Drug Delivery. Curr. Drug Targets. 2021, 22(15), 1750–1759. DOI: 10.2174/1389450122666210204203514.
- Signorell, R. D.; Luciani, P.; Brambilla, D.; Leroux, J. C. Pharmacokinetics of Lipid-Drug Conjugates Loaded into Liposomes. Eur. J. Pharm. Biopharm. 2018, 28, 188–199.
- Ingram, N.; McVeigh, L. E.; Abou-Saleh, R. H.; Maynard, J.; Peyman, S. A.; McLaughlan, J. R.; Fairclough, M.; Marston, G.; Valleley, E. M.; Jimenez-Macias, J. L., Charalambous, A. Ultrasound-Triggered Therapeutic Microbubbles Enhance the Efficacy of Cytotoxic Drugs by Increasing Circulation and Tumor Drug Accumulation and Limiting Bioavailability and Toxicity in Normal Tissues. Theranostic. 2020, 10(24), 10973.
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 Modified Liposome Encapsulating Epigallocatechin-3-Gallate in the Presence of Magnesium: Characterization and Protective Effect Against Oxidative Damage. Int. J. Pharm. 2018, 552(1–2), 225–234. DOI: 10.1016/j.ijpharm.2018.10.004.
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecul. 2018, 23(4), 739.
- Khan, A. A.; Alanazi, A. M.; Jabeen, M.; Hassan, I.; Bhat, M. A. Targeted Nano-Delivery of Novel Omega-3 Conjugate Against Hepatocellular Carcinoma: Regulating COX-2/bcl-2 Expression in an Animal Model. Biomed. Pharm. 2016, 81, 394–401.
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global Patterns of Breast Cancer Incidence and Mortality: A Population‐Based Cancer Registry Data Analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194.
- Arciniegas Calle, M. C.; Sandhu, N. P.; Xia, H.; Cha, S. S.; Pellikka, P. A.; Ye, Z.; Herrmann, J.; Villarraga, H. R. Two-Dimensional Speckle Tracking Echocardiography Predicts Early Subclinical Cardiotoxicity Associated with Anthracycline-Trastuzumab Chemotherapy in Patients with Breast Cancer. BMC Cancer. 2018, 18(1), 1–8. DOI: 10.1186/s12885-018-4935-z.
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer Efficiency of Doxorubicin and Berberine-Loaded PCL Nanofibers in Preventing Local Breast Cancer Recurrence. J Drug Delivery Sci Technol. 2022, 67, 102984. 10.1016/j.jddst.2021.102984
- Chopra, H.; Bibi, S.; Goyal, R.; Gautam, R. K.; Trivedi, R.; Upadhyay, T. K.; Mujahid, M. H.; Shah, M. A.; Haris, M.; Khot, K. B., Gopan, G. Chemopreventive Potential of Dietary Nanonutraceuticals for Prostate Cancer. An Extens Review, Front. Oncol. 2022, 12, 2941.
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Gastroenterol Review/Prz. Gastroenterol. 2019, 14, 89–103.
- Singh, S.; Sharma, N.; Grover, R.; Grewal, I. K. Liposome-Based Drug Delivery System for Cancer Chemotherapeutics. Plant Arch. 2020, 20, 3305–3315.
- Ramakrishnan, P.; Loh, W. M.; Gopinath, S. C.; Bonam, S. R.; Fareez, I. M.; Mac Guad, R.; Sim, M. S.; Wu, Y. S. Selective Phytochemicals Targeting Pancreatic Stellate Cells as New Anti-Fibrotic Agents for Chronic Pancreatitis and Pancreatic Cancer. Acta Pharm. Sin. B. 2020, 10(3), 399–413.
- Kurdi, R. E.; Mesmar, J.; Estephan, M.; Badran, A.; Baydoun, E.; Patra, D. Anticancer Activity of Diarachidonyl Phosphatidyl Choline Liposomal Curcumin Coated with Chitosan Against Breast and Pancreatic Cancer Cells. Bionanosci. 2022, 12(4), 1158–1165.
- Kimura, T.; Egawa, S. Epidemiology of Prostate Cancer in Asian Countries. Int. J. Urol. 2018, 25(6), 524–531. DOI: 10.1111/iju.13593.
- Edis, Z.; Wang, J.; Waqas, M. K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. IJN. 2021, 16, 1313. 10.2147/IJN.S289443
- Al-Rabia, M. W.; Alhakamy, N. A.; Rizg, W. Y.; Alghaith, A. F.; Ahmed, O. A.; Fahmy, U. A. Boosting Curcumin Activity Against Human Prostatic Cancer PC3 Cells by Utilizing Scorpion Venom Conjugated Phytosomes as Promising Functionalized Nanovesicles. Drug. Deliv. 2022, 29(1), 807–820.
- Zarrabi, A.; Zarepour, A.; Khosravi, A.; Alimohammadi, Z.; Thakur, V. K. Synthesis of Curcumin Loaded Smart PH-Responsive Stealth Liposome as a Novel Nanocarrier for Cancer Treatment. Fibers. 2021, 9(3), 19.
- Olusanya, T. O.; Haj Ahmad, R. R.; Ibegbu, D. M.; Smith, J. R.; Elkordy, A. A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecul. 2018, 23(4), 907. DOI: 10.3390/molecules23040907.
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual Functionalized Liposomes for Efficient Co-Delivery of Anti-Cancer Chemotherapeutics for the Treatment of Glioblastoma. J. Control Release. 2019, 307, 247–260. DOI: 10.1016/j.jconrel.2019.06.033.
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome Technologies Towards Colorectal Cancer Therapeutics. Acta. Biomater. 2021, 127, 24–40. DOI: 10.1016/j.actbio.2021.03.055.
- Weng, W.; Goel, A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine, Semin. Cancer Biol. Elsevier. 2020, 80, 73–86.
- Schmitt, C.; Lechanteur, A.; Cossais, F.; Bellefroid, C.; Arnold, P.; Lucius, R.; Held-Feindt, J.; Piel, G.; Hattermann, K. Liposomal Encapsulated Curcumin Effectively Attenuates Neuroinflammatory and Reactive Astrogliosis Reactions in Glia Cells and Organotypic Brain Slices. Int. J. Nanomed. 2020, 15, 3649. DOI: 10.2147/IJN.S245300.
- Pandelidou, M.; Dimas, K.; Georgopoulos, A.; Hatziantoniou, S.; Demetzos, C. Preparation and Characterization of Lyophilised Egg PC Liposomes Incorporating Curcumin and Evaluation of Its Activity Against Colorectal Cancer Cell Lines. J. Nanosci. Nanotechnol. 2011, 11(2), 1259–1266. DOI: 10.1166/jnn.2011.3093.
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21(7), 2364. DOI: 10.3390/ijms21072364.
- Gantenbein, N.; Bernhart, E.; Anders, I.; Golob-Schwarzl, N.; Krassnig, S.; Wodlej, C.; Brcic, L.; Lindenmann, J.; Fink-Neuboeck, N.; Gollowitsch, F. Influence of Eukaryotic Translation Initiation Factor 6 on Non–Small Cell Lung Cancer Development and Progression. Eur. J. Cancer. 2018, 101, 165–180. DOI: 10.1016/j.ejca.2018.07.001.
- Han, L.; Wang, J. N.; Cao, X. Q.; Sun, C. X.; Du, X. An-Te-Xiao Capsule Inhibits Tumor Growth in Non-Small Cell Lung Cancer by Targeting Angiogenesis. Biomed. Pharmacotherap. 2018, 108, 941–951.
- Chavoshi, H.; Poormolae, N.; Vahedian, V.; Kazemzadeh, H.; Mir, A.; Nejabati, H. R.; Behroozi, J.; Isazadeh, A.; Hajezimian, S.; Nouri, M. Vascular Mimicry: A Potential Therapeutic Target in Breast Cancer, Pathol. Res. Pract. 2022, 33(7), 2919–2930.
- Kong, L.; Cai, F. Y.; Yao, X. M.; Jing, M.; Fu, M.; Liu, J. J.; He, S. Y.; Zhang, L.; Liu, X. Z.; Ju, R. J., Li X. T. RPV‐Modified Epirubicin and Dioscin Co‐Delivery Liposomes Suppress Non‐Small Cell Lung Cancer Growth by Limiting Nutrition Supply. Cancer Sci. 2020, 111, 621–636.
- Velazhahan, V.; Glaza, P.; Herrera, A. I.; Prakash, O.; Zolkiewski, M.; Geisbrecht, B. V.; Schrick, K.; Maki, C. G. Dietary Flavonoid Fisetin Binds Human SUMO1 and Blocks Sumoylation of p53. PLoS One. 2020, 15(6), e0234468. DOI: 10.1371/journal.pone.0234468.
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y. M.; Romano, M. R.; Touil, Y. S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G. G. Liposomal Encapsulation of the Natural Flavonoid Fisetin Improves Bioavailability and Antitumor Efficacy. Int. J. Pharm. 2013, 444(1–2), 146–154. DOI: 10.1016/j.ijpharm.2013.01.050.
- Jakaria, M.; Haque, M. E.; Kim, J.; Cho, D. Y.; Kim, I. S.; Choi, D. K. Active Ginseng Components in Cognitive Impairment: Therapeutic Potential and Prospects for Delivery and Clinical Study. Oncotarget. 2018, 9, 33601.
- Shishir, M. R. I.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal Delivery of Natural Product: A Promising Approach in Health Research. Trends Food Sci. Technol. 2019, 85, 177–200. DOI: 10.1016/j.tifs.2019.01.013.
- Shishir, M. R. I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60.
- Lopez, A. D.; Adair, T. Is the Long-Term Decline in Cardiovascular-Disease Mortality in High-Income Countries Over? Evidence from National Vital Statistics. Int. J. Epidemiol. 2019, 48(6), 1815–1823. DOI: 10.1093/ije/dyz143.
- Briasoulis, A.; Ueyama, H.; Kuno, T.; Asleh, R.; Alvarez, P.; Malik, A. H. Trends and Outcomes of Device-Related 30-Day Readmissions After Left Ventricular Assist Device Implantation. Eur. J. Int. Med. 2021, 84, 56–62. DOI: 10.1016/j.ejim.2020.10.004.
- Fukuta, T.; Ishii, T.; Asai, T.; Oku, N. Applications of Liposomal Drug Delivery Systems to Develop Neuroprotective Agents for the Treatment of Ischemic Stroke. Biol. Pharm. Bull. 2019, 42, 319–326.
- Oltra-Cucarella, J.; Ferrer-Cascales, R.; Alegret, M.; Gasparini, R.; Díaz-Ortiz, L. M.; Rios, R.; Martínez-Nogueras, Á. L.; Onandia, I.; Perez-Vicente, J. A.; Cabello-Rodriguez, L., Sánchez-SanSegundo, M. Risk of Progression to Alzheimer’s Disease for Different Neuropsychological Mild Cognitive Impairment Subtypes: A Hierarchical Meta-Analysis of Longitudinal Studies. Psychol. Aging. 2018, 33, 1007.
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of Quercetin in Neurological Disorders: From Nutrition to Nanomedicine. Rev. Neurosci. 2019, 30(5), 555–572. DOI: 10.1515/revneuro-2018-0080.
- Shaif, M.; Kushwaha, P.; Usmani, S.; Pandey, S. Exploring the Potential of Nanocarriers in Antipsoriatic Therapeutics. J. Dermatol. Treat. 2022, 33(7), 2919–2930.
- Giri, T. K.; Mukherjee, P.; Barman, T. K.; Maity, S. Nano-Encapsulation of Capsaicin on Lipid Vesicle and Evaluation of Their Hepatocellular Protective Effect. Int. J. Bio. Macromol. 2016, 88, 236–243. DOI: 10.1016/j.ijbiomac.2016.03.056.
- Grosso, C.; Valentão, P.; Andrade, C.; Andrade, P. B. HPLC–DAD Analysis and in vitro Enzyme Inhibition: An Integrated Approach to Predict Herbal Binary Mixture Behaviour Employing Median Effect Equation. Microchem. J. 2015, 119, 176–182.
- Intagliata, S.; Modica, M. N.; Santagati, L. M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxid. 2019, 8(8), 244. DOI: 10.3390/antiox8080244.
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A. M.; Manconi, M. Stability, Biocompatibility and Antioxidant Activity of PEG-Modified Liposomes Containing Resveratrol. Int. J. Pharm. 2018, 538, 40–47.
- Elmotasem, H.; Awad, G. E. A Stepwise Optimization Strategy to Formulate in situ Gelling Formulations Comprising Fluconazole-Hydroxypropyl-Beta-Cyclodextrin Complex Loaded Niosomal Vesicles and Eudragit Nanoparticles for Enhanced Antifungal Activity and Prolonged Ocular Delivery. Asian J. Pharm. Sci. 2020, 15, 617–636.
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A. M.; Pucci, L.; Manconi, M. Antioxidant Activity of Quercetin in Eudragit-Coated Liposomes for Intestinal Delivery. Int. J. Pharm. 2019, 565, 64–69. DOI: 10.1016/j.ijpharm.2019.05.007.
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the Flavonoid Quercetin with Chitosan-Coated Nano-Liposomes, LWT-Food Sci. Technol. 2017, 85, 37–44.
- Nowbar, A. N.; Gitto, M.; Howard, J. P.; Francis, D. P.; Al-Lamee, R. Mortality from Ischemic Heart Disease: Analysis of Data from the World Health Organization and Coronary Artery Disease Risk Factors from NCD Risk Factor Collaboration, Circ.: Cardiovasc. Qual. Outcomes. 2019, 12, e005375.
- Wang, C.; Sun, C.; Lu, W.; Gul, K.; Mata, A.; Fang, Y. Emulsion Structure Design for Improving the Oxidative Stability of Polyunsaturated Fatty Acids, Compr. Rev. Food Sci. Food Saf. 2020, 19, 2955–2971.
- Kozlova, T.; Syngeeva, E.; Zhamsaranova, S.; Lamazhapova, G. The Use of Unsaturated Fatty Acids in Liposomal Form to Get Bread Enriched with ω-3 Fatty Acids. Curr. Nutr. Food Sci. 2018, 14(1), 54–60. DOI: 10.2174/1573401313666170316114252.
- de Oliveira, G. Z. D. S.; Ramalho, T. C.; de Almeida, V. P. A.; Moura, V.; Rolim, H. M. L. The Major Scientific Advances of Liposomal Pharmaceutical Preparations with Neuroprotective and Antioxidant Effects. J. Glob. Innov. 2020, 2, 2184–7738.
- Omari-Siaw, E.; Wang, Q.; Sun, C.; Gu, Z.; Zhu, Y.; Cao, X.; Firempong, C. K.; Agyare, R.; Xu, X.; Yu, J. Tissue Distribution and Enhanced in vivo Anti-Hyperlipidemic-Antioxidant Effects of Perillaldehyde-Loaded Liposomal Nanoformulation Against Poloxamer 407-Induced Hyperlipidemia. Int. J. Pharm. 2016, 513(1–2), 68–77. DOI: 10.1016/j.ijpharm.2016.08.042.
- Basnet, P.; Hussain, H.; Tho, I.; Skalko-Basnet, N. Liposomal Delivery System Enhances Anti-Inflammatory Properties of Curcumin. J. Pharm. Sci. 2012, 101(2), 598–609. DOI: 10.1002/jps.22785.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018, 10, 1618.
- Mert, T.; Sahin, M.; Sahin, E.; Yaman, S. Anti-Inflammatory Properties of Liposome-Encapsulated Clodronate or Anti-Ly6G Can Be Modulated by Peripheral or Central Inflammatory Markers in Carrageenan-Induced Inflammation Model. Inflammopharmacol. 2019, 27, 603–612.
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Klarić, D. A.; Bogdanov, A.; Raffai, T.; Virok, D. P. Azithromycin-Liposomes as a Novel Approach for Localized Therapy of Cervicovaginal Bacterial Infections. Int. J. Nanomed. 2019, 14, 5957. DOI: 10.2147/IJN.S211691.
- Jain, S.; Prajapati, S. K.; Jain, S.; Jain, S.; Jain, A. Propylene Glycol-Liposome for Anticoagulant Drug Delivery Through Skin. J. Bionanosci. 2018, 12, 721–727.
- Madhavi, N.; Sudhakar, B.; Reddy, K. Ethosome: A Potential Tool for Drug Delivery Through the Skin, Smart Nanomater. Biomed. App. Springer. 2021, 487–506.