 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Damage to body tissue either deeply or superficially causes pain sensation in response to which Inflammation occurs which is a normal protective natural response of the body to a variety of hostile agents. Different treatment options, including conventional and herbal therapies, are available for pain and inflammation. This study was designed to evaluate the polyherbal n-hexane and methanolic extracts and their hydrogel formulations prepared from commonly available medicinal plants for their analgesic and anti-inflammatory potential. Cinnamomum verum J. (bark), Moringa oleifera L. (leaves), Nigella sativa L. (seed), and Trigonella foenum graecum L. (seed) were collected and purchased from the vicinity and local market of Multan Punjab, Pakistan. After drying, extraction was carried out by maceration separately with two different solvents (methanol and n-hexane). An equal quantity of all methanolic and n-hexane extracts was used to formulate polyherbal methanolic and n-hexane extracts separately. Then 1%, 3%, and 5% pH-based hydrogel formulations of methanolic and n-hexane polyherbal extract were prepared. All prepared six formulations were evaluated for pH, rheological study, spreadability, extrudability, stability study, texture, color, and for toxicological study. Then analgesic and anti-inflammatory potential of methanol and n-Hexane polyherbal extracts at (200, 400 and 800.0 mg/kg) in comparison with diclofenac sodium (100 mg/kg) and prepared gel formulations (1%, 3%, and 5%) were also evaluated in comparison to diclofenac sodium 1% gel (standard drug). Both polyherbal extracts and their gel formulations have significant analgesic and anti-inflammatory potential but methanolic extract and its hydrogel have slightly more potential as compared to n-hexane extracts and its prepared hydrogel. Results also revealed that their gel formulations have more significant analgesic and anti-inflammatory potentials as compared to extracts. All pH-based gels have good texture, spreadability, extrudability, stability, and are safe to use. It is concluded that methanolic polyherbal extract and hydrogel prepared from methanolic extract have more analgesic and anti-inflammatory potential as compare to n-Hexane extract and hydrogel prepare from n-Hexane extract.
Introduction
Topical drug delivery system is the most preferred route for local delivery of therapeutic agents especially in pain and inflammation management, hormonal therapy, treatment of diseases of the cardiovascular and central nervous systems. Because of its convenience and affordability, it also prevents drug loss due to first-pass metabolism and maximizes the therapeutic effect without interference of pH, enzyme, and intestinal bacteria.[Citation1] Hydrogel is one of the semi-solid topical dosage forms, which includes cross-linked three-dimensional networks of hydrophilic polymer chains; therefore, it can entrap a larger amount of water. Hydro-gels have the advantages of increased biocompatibility, porous structure, tunable biodegradability, and proper mechanical strength as compare to other type of topical drugs.[Citation2]
Pain can be described as a somatic sensation, a symptom of a few bodily damage or sickness, or maybe emotional misery. Pain is a fundamental issue of the frame’s safety mechanisms, and it is a segment of a rapid caution relay training the motor neurons of the important frightened machine to lower physical damage.[Citation3] Pain is directly related with inflammation. Inflammation is an alliance of different routes including generations of interleukin, adhesive proteins, prostaglandins, and factor for activation of platelets. These are agonizing the chemo-taxis. Its mechanism comprises a series of occasions wherein the metabolism of arachidonic acid performs a vital function.[Citation4]
Medicinal plants are supposed to be an important source of chemical substances with therapeutic effects in the body and considered to treat various diseases with least side effects. Mostly non-steroidal anti-inflammatory drugs are taken for reducing the inflammation and pain, but these drugs show many side effects like bleeding, ulceration, skin irritation, and failure of organ function. So, development and evaluation of new drug moiety from natural sources with multifarious activity is needed to reduce the unwanted effects with low price and least side effects. Therefore, these commonly available local medicinal plants, i.e. Nigella sativa, Cinnamomum verum, Trigonella foenum-graecum, and Moringa oleifera, were selected for this research project.
Nigella sativa L
Nigella sativa L. family (Ranunculaceae) is widely known as black cumin or black seeds.[Citation5] The phytochemical analysis of the seed has shown a mixture of 8 fatty acids and 32 volatile terpenes. The major terpenes, thymoquinone (TQ), dithymoquinone (DTQ), transanethole, p-cymene, limonene, and carvone have been identified. Black cumin seeds are used as antioxidant, antibacterial, antifungal, antiparasitic, antiviral, antiinflammatory, anticancer, antidiabetic, hepatoprotective, immunomodulatory.[Citation6] Studies have shown that thymoquinone and its para-benzoquinone analogues have both analgesic and anti-inflammatory potential.[Citation7]
Cinnamomum verum J
Cinnamon belongs to family (Lauraceae); its botanical name is Cinnamomum verum (J.). Cinnamon contains resinous compounds such as cinnamaldehyde, cinnamate, cinnamic acid, and numerous essential oils (transcinnamaldehyde, cinnamyl acetate, eugenol, L-borneol, caryophyllene oxide, b-caryophyllene, L-bornyl acetate, E-nerolidol, α-cubebene, α-terpineol, terpinolene, and α-thujene). Bark of cinnamon is used as analgesic, antiinflammatory, antiallergic, antibacterial, antiviral, anticancer, cardioprotective and antidiabetic agents.[Citation8] Studies have shown that Cinnamaldehyde and eugenol are responsible for analgesic and anti-inflammatory potential.[Citation9]
Trigonella foenum-graecum L
Fenugreek belongs to the family Fabaceae; its botanical name is Trigonella foenum-graecum L. Its seed contains significant quantities of alkaloids (trigonelline, trigo-coumarin, nicotinic acid, trimethyl coumarin), flavonoids (quercetin, luteolin, vitexin, and 7, 4-dimethoxy flavanones), amino acids (aspartic acid, glutamic acid, leucine, tyrosine and phenylalanine), and also saponin (fenugrin B).[Citation10] These alkaloid and flavonoid contents are responsible for antinociception and antiinflammatory effects.[Citation11] Seeds are used as antibacterial, gastric stimulant, antidiabetic, galactogogue, hepatoprotective, hypocholesterolemic, and anticancer agents.[Citation12]
Moringa oleifera L
Moringa oleifera L. belongs to the family Moringaceae. Major chemical constituents present in the Moringa plant are 1,2,3triolein, 1,3dilinoleoyl-2-olein,1,3dioleoyl-2linolein, Linoleic acid, Benzyl isothiocyanates, Niazimicin, Moringyne, Glycine and alanine, Aspartic acid and Valine and tryptophan.[Citation13] It is are used as antimicrobial, antidiabetic, antifungal, anticancer, anthelmintic, and hepatoprotective.[Citation14] kaempferol-3-glucoside in the polar extract and fatty acids like chlorogenic acid in nonpolar extract have analgesic antiinflammatory potential.[Citation15]
Although these herbs are commonly used for their analgesic and anti-inflammatory potential, their combined effect has not yet been studied and also their combined dosage form has not been prepared. Hence, the current research work was designed to find out the combined analgesic and anti-inflammatory potential of polyherbal extracts (PNE, PME) and their hydrogel formulations.
Materials and methods
Chemicals
Black cumin seeds, cinnamon bark, fenugreek seeds, moringa leaf Carbopol 940 (Duksan pure chemicals co., ltd. South korea), Carrageenan (Duksan pure chemicals co., ltd. South korea), Diclofenac sodium (Novartis Pharma, (Pakistan) ltd), Methanol (Duksan pure chemicals co., ltd. South korea), n-Hexane (Liaoning Yufeng Chemical Co.,Ltd. china), Propylene glycol (Duksan pure chemicals co., ltd. South korea) and Tri-ethanolamine (Duksan pure chemicals co., ltd. South korea) were purchased from local market.
Identification of plant materials
Black cumin seeds, cinnamon bark, fenugreek seeds and moringa leaf were identified by Dr. Zafar Ullah Zafar (Associate Professor), Institute of Pure and Applied Biology, Bahauddin Zakariya University Multan. Identification number of black cumin is (kew -2,698,428), cinnamon (kew -2,721,292), fenugreek (kew -2,721,378) and of moringa (kew -2,721,576) and specimen were submitted to the herbarium of Faculty of Pharmacy, Bahauddin Zakariya University Multan, for further reference.
Preparation of extracts
Black cumin seeds, cinnamon bark, and fenugreek seeds 400.0 g each were taken in pre-dried form. 600.0 g of moringa leaves were washed and cleaned properly, then cut into small pieces and dried completely under shade for several days. 400.0 g of each plant material was soaked in 1000 ml n-hexane in a closed glass bottle with occasional shaking for 7 days and then filtered. Filtrate was evaporated by the rotary evaporator R-200 (Buchi, Switzerland) to concentrate the extract at 40–60°C under reduced pressure (4–6 mm of Hg). This procedure was repeated three times.
After that residue of each plant material was dried, weighed, and soaked in 1000.0 ml methanol separately. This maceration was repeated three times. Extracts were collected and stored in the glass bottle. Their extractive percentage has been shown in .
Table 1. Formulation of Hydrogels with and without Polyherbal extract.
Preparation of polyherbal n-hexane and methanolic extracts:
Polyherbal n-hexane extract and polyherbal methanolic extract were prepared by mixing 20.0 g each n-hexane extract and mixed them to form polyherbal n-hexane extract (PNE). Similarly, polyherbal methanolic extracts were prepared by mixing 20.0 g of methanolic extracts (PME).
Preparation of hydrogel
Distilled water (94.0 g) C-1: Avoid sentence starts with number was taken in a beaker and placed on the magnetic stirrer. Then 1.0 g carbopol 940 was added slowly in the beaker with continuous stirring until all carbopol was dissolved. After that 5.0 g propylene glycol was added and mixed with it. Then 3 to 4 drops of tri-ethanolamine were added to it to form homogeneous hydrogel.[Citation16] Tri-ethanolamine is a common and safe stabilizer, fatty acid neutralizer, and pH adjuster in skincare products.[Citation17]
Preparation of poly herbal n-hexane and methanolic extracts hydrogel
The hydrogel based on Carbopol 940 were prepared to incorporate two herbal extracts, namely polyherbal n-hexane extract (PNE) and polyherbal methanolic extract (PME). Present hydrogels were prepared by adding a fixed amount of Carbopol 940, i.e., 1%. Propylene glycol and tri-ethanolamine were used as wetting agent and pH modifier or neutralizer, respectively. First Carbopol 940 was added in distilled water by gentle stirring. Later on, three different concentrations (1.0, 3.0, and 5.0 g) of both polyherbal n-hexane and methanolic extract were separately mixed with Propylene glycol (5.0 g). Finally, Carbopol 940 solution was mixed with solution containing herbal extracts and 3–4 drops of tri-ethanolamine were added with continuous stirring till the homogeneous formation of hydrogel.[Citation16] Various compositions of hydrogels formulation containing herbal extracts are presented in .
Table 2. Percentage yield of n-hexane and methanol extract.
Characterization of hydrogels
pH: pH of all hydrogel’s formulations was determined by using digital pH meter. 1.0 g of all hydrogels was dissolved in distilled water separately and stored for 2 hours at room temperature. Then pH was noted in triplicates and their average was calculated.[Citation18] These values were tabulated in .
Table 3. pH of hydrogel formulations with and without Polyherbal extract.
Homogeneity and appearance: The appearance of all hydrogel formulations was examined by visual inspection. While homogeneity was determined by taking the small amounts of hydrogel formulations between the thumb and index finger separately and observed for the presence of any coarse particle. Similarly a small amount of hydrogel formulations was applied on the back side of the hand and rubbed separately. The same procedure was repeated 3 times for each formulation[Citation18] and their values are tabulated in .
Table 4. Appearance, homogeneity, spreadability, and extrudability of hydrogel formulations.
Spreadability test: Spreadability was calculated by measuring the spreading area of 1.0 g hydrogel between two glass slides having diameter of both glass slides 19 cm x 19 cm. The standard weight about 100.0 g was placed on the upper slide for 60 sec to check the spread ability. The diameter of the dispersed gel was measured in cm and the result was calculated by using the following formula[Citation18] and their values are tabulated in .
Here, M = weight (g) placed on the upper glass slide, L = length in cm moved on the glass slide and T = time in seconds
Extrudability study: Aluminum collapsible tubes were used for this test. Six aluminum tubes were taken and filled with 10 g gel in each and holding them between two fingers. Aluminum tubes were compressed by applying finger pressure. Extrudability percentage for each formulation was calculated by measuring the weight of extruded gel.[Citation18] The results are shown in .
Rheological study
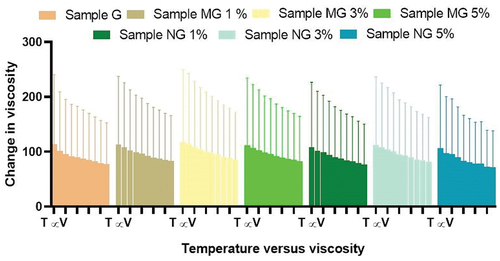
Rheological study of all gels was performed by using Brookfield viscometer. The Brookfield digital viscometer was filled with gel and inserted into the flow jacket of viscometer. Sample adapter rotated at a speed of 20 rpm was used to determine the viscosity of gel formulations. The temperature was maintained 24.8°C by rotating the water on the thermostated water jacket. Sample was settled before 6 minutes to take readings. The viscosity of all hydrogels can be measured by increasing the value of the share rate.[Citation19] Values are represented in .
Figure 1. Rheological studies of hydrogel formulations with and without polyherbal extract (PNE, PME). Sample G is hydro-gel formulation without polyherbal extract, Sample MG 1% is hydrogel formulation containing 1.0 g of polyherbal methanolic extract, Sample MG 3% is hydrogel formulation containing 3.0 g of polyherbal methanolic extract and sample MG 5% is hydrogel formulation containing 5.0 g of polyherbal methanolic extract, Sample NG 1% is hydrogel formulation containing 1.0 g of polyherbal n-hexane extract, Sample NG 3% is hydrogel formulation containing 3.0 g of polyherbal n-hexane extract and sample NG 5% is hydrogel formulation containing 5.0 g of polyherbal n-hexane extract
Stability test
Freshly prepared hydro-gel formulations were observed for 60 days by examining their general appearance like color and odor while storing them at different conditions of temperature and humidity.[Citation20] Results are shown in .
Table 5. Stability study of hydrogel formulations with and without different extracts.
Skin irritation study
Skin irritation study was performed on three rabbit the room temperature was maintained at 22 ± 3°C with 30–70% relative humidity. The hair on the back of each rabbit was removed 24 hours before the experiment. Exposing cleaned shaved areas of skin. All polyherbal gels were applied carefully on the skin and observed for 7 days for any irritation, redness, or lesions.[Citation16] Results have been shown in .
Table 6. Skin irritation test of hydrogel formulations with and without polyherbal extract.
Preparation of Experimental Animal
Wistar albino rats both male and female of 150–250 grams were used in the experiment. These animals were purchased from animal house of Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The experiment was approved by a bio-ethical committee of Bahauddin Zakariya University Multan vide letter No BZ-237-PH-10/1. These animals had free access to standard pellet diet and water.
Analgesic activity of polyherbal extracts and their hydrogel formulations
Tail flick method
Analgesic activity of polyherbal extracts and their hydrogel formulations were evaluated by tail flick method. For this purpose, 45 animals were weighed and divided into 15 groups, each group containing three animals. Group 1 was the control group to whom normal saline was given orally via oral gavage. Group 2 was the standard group for polyherbal extracts to whom diclofenac sodium 100.0 mg/kg was given orally. Solution of polyherbal n-hexane extract 200.0 mg/kg, 400.0 mg/kg, and 800.0 mg/kg were given orally to groups 3, 4, and 5. Likewise, solution of polyherbal methanolic extract 200.0 mg/kg, 400.0 mg/kg, and 800.0 mg/kg were given orally to groups 6, 7, and 8. Group 9 was the standard group for hydrogel formulations to whom diclofenac sodium 1% gel was applied topically on hind paws. Hydrogel formulations with polyherbal n-hexane extract (1% NG gel, 3% NG gel and 5% NG) were applied to groups 10, 11, and 12. Hydrogel formulations with polyherbal methanolic extract (1% MG gel, 3% MG gel, and 5% MG) were applied on hind paws to groups 13, 14, and 15 respectively. In this method tail of the rat, about 3–5 cm was dipped into hot water bath and the temperature of bath was maintained at about 50–52°C. Avoiding the cutoff time was 30 sec. After the oral administration of dosage, observations were first taken at zero time, then at 60 min, 90 min, 120 min, and 180 min with the help of a stopwatch[Citation5] and values are represented in . Whereas in the case of hydrogel formulations observations were made first at zero time then at 30 min, 60 min, 90 min, and 120 min.[Citation21] Results have been shown in .
Figure 2. Analgesic activity of polyherbal n-hexane extracts (PNE) by tail flick method.
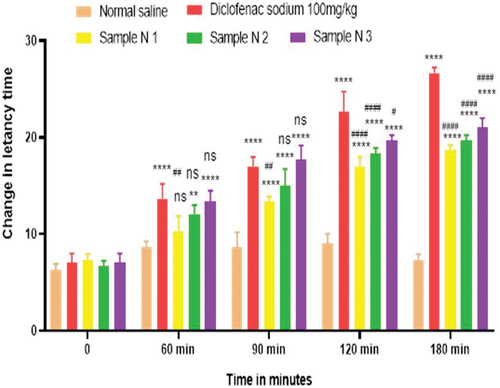
Figure 3. Analgesic activity of polyherbal methanolic extracts (PME) by tail flick method.
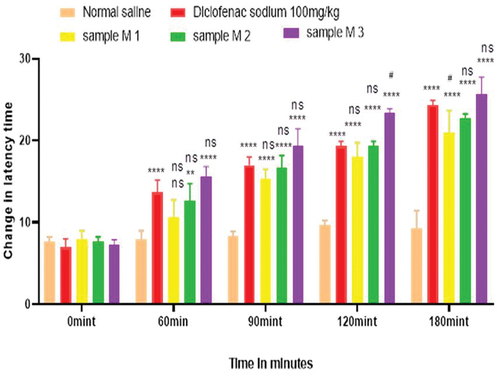
Figure 4. Analgesic activity of hydrogel formulations (NG) with polyherbal n-hexane extracts by tail flick method.
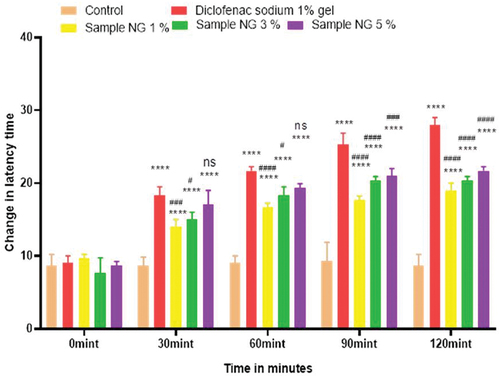
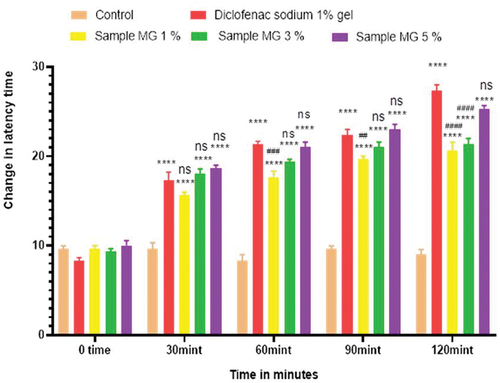
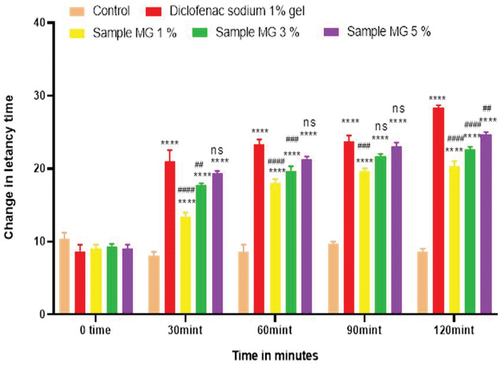
Figure 5. Analgesic activity of hydrogel formulations (MG) with polyherbal methanolic extracts by tail flick method.
Hot plate method
Hot plate method was used to evaluate the analgesic activity of polyherbal extracts and their hydrogel formulations. For this purpose, 45 animals were weighed and divided into 15 groups, each group containing three rats. Group 1 was the control group to whom normal saline was given orally via oral gavage. Group 2 was the standard group for polyherbal extracts to whom diclofenac sodium 100.0 mg/kg was given orally. Solution of polyherbal n-hexane extract 200.0 mg/kg, 400.0 mg/kg, and 800.0 mg/kg were given orally to groups 3, 4, and 5. Likewise solution of polyherbal methanolic extract 200.0 mg/kg, 400.0 mg/kg and 800.0 mg/kg were given orally to groups 6, 7, and 8. Group 9 was standard group for hydrogels to whom diclofenac sodium 1% gel was applied topically on hind paws. Hydrogel formulations with polyherbal n-hexane extract (1% NG gel, 3% NG gel, and 5% NG) were applied to groups 10, 11, and 12. Hydrogel formulations with polyherbal methanolic extract (1% MG gel, 3% MG gel, and 5% MG) were applied on hind paws of groups 13, 4, and 15 respectively. In this procedure rats were placed on the hot plate and the temperature of the plate was maintained at about 55 ± 1 °C. Reaction time was noted with the help of the stop watch till the rat starts licking paws or jumping. Reaction time was taken first at zero time, then at 60 min, 90 min, 120 min, and 180 min for polyherbal extracts[Citation5] and results have been shown in whereas for hydrogel formulations readings were taken at zero time, 30 min, 60 min, 90 min, and 120 min.[Citation21] Results have been shown in .
Figure 6. Analgesic activity of polyherbal n-hexane extracts (PNE) by hot plate method.
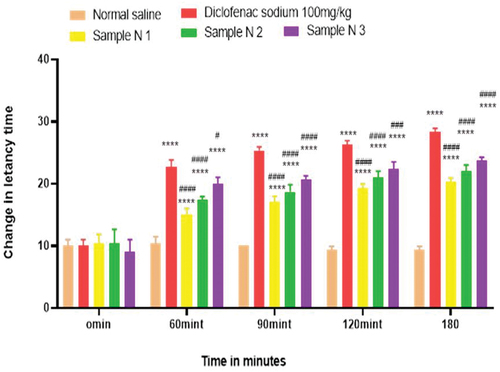
Figure 7. Analgesic activity of polyherbal methanolic extracts (PME) by hot plate method.
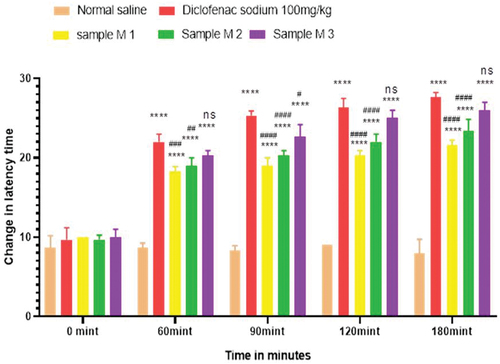
Figure 8. Analgesic activity of hydrogel formulations (NG) with polyherbal n-hexane extracts by hot plate method.
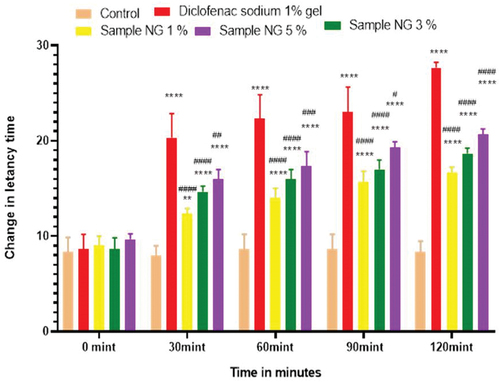
Figure 9. Analgesic activity of hydrogel formulations (MG) with polyherbal methanolic extracts by hot plate method.
Anti-inflammatory activity of the polyherbal extract and its hydrogel formulations
All animals (45) were weighed and divided into 15 groups each containing three rats. Group 1 was the considered as control group to whom only normal saline was given orally via oral gavage. Group 2 was named as standard group for polyherbal extracts to whom diclofenac sodium 100.0 mg/kg was given orally. Polyherbal n-hexane extract 200.0, 400.0, and 800.0 mg/kg were given orally to groups 3, 4, and 5 respectively. Likewise, solution of polyherbal methanolic extract 200.0, 400.0, and 800.0 mg/kg were given orally to groups 6, 7, and 8. Group 9 was the standard group for the evaluation of hydrogels to whom Diclofenac sodium 1% gel was applied topically on hind paws. Hydrogel formulations with polyherbal n-hexane extract (1%, 3%, and 5% NG) were applied to groups 10, 11, and 12. Hydrogel formulations with polyherbal methanolic extract (1%, 3%, and 5% MG) were applied on hind paws of group 13, 14 and 15 respectively. Before one hour to start the experiment, 0.1 ml of 1% carrageenan was administered intra-dermally on plantar surface of the left hind paw of each rat with the help of a 27-gauge ½ inch needle except group 1. Values are expressed in for poly herbal extracts and in for polyherbal gel formulations. Observations were noted after administration of test solutions and gel formulations first at time zero minute then, at 30 min, 60 min, and 120 min by using the plethysmo meter.[Citation5] Percentage inhibition of rat paw edema was calculated by using the formula
Figure 10. Anti-inflammatory activity of polyherbal n-hexane extracts (PNE).
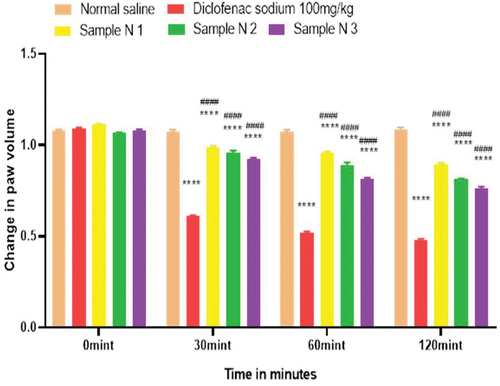
Figure 11. %inhibition of anti-inflammatory activity of polyherbaln-hexane extracts (PNE).
Figure 12. Anti-inflammatory activity of polyherbal methanolic extracts (PME).
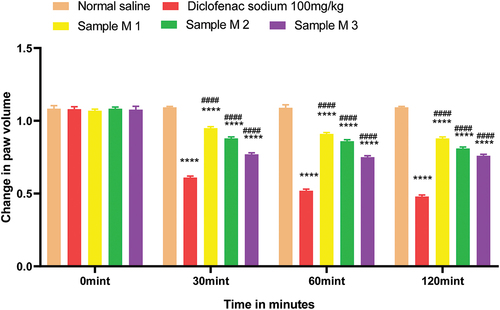
Figure 13. %inhibition of anti-inflammatory activity of polyherbal methanolic extracts (PME).
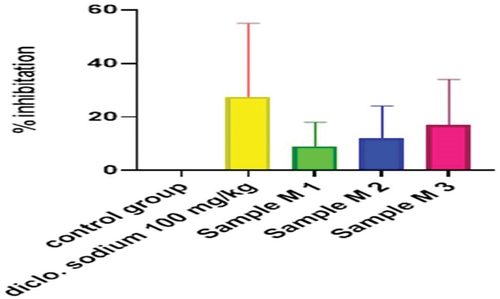
Figure 14. Anti-inflammatory activity of hydrogel formulations (NG) with polyherbal n-hexane extracts.
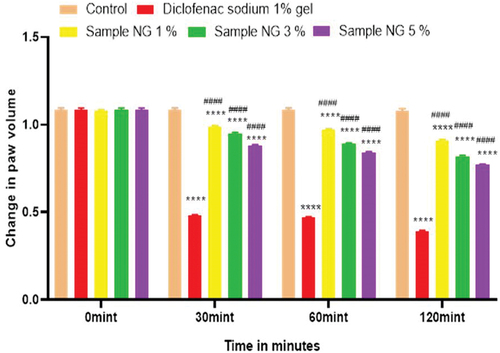
Figure 15. % inhibition of anti-inflammatory activity of hydrogel formulations (NG) with polyherbal n-hexane extracts.
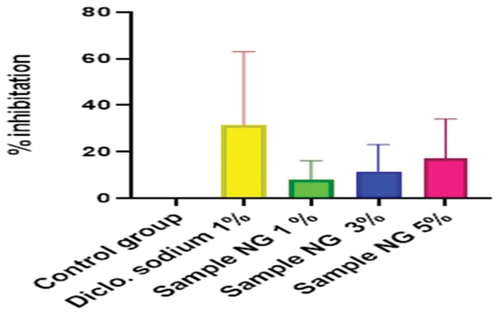
Figure 16. Anti-inflammatory activity of hydrogel formulations (MG) with polyherbal methanolic extracts.
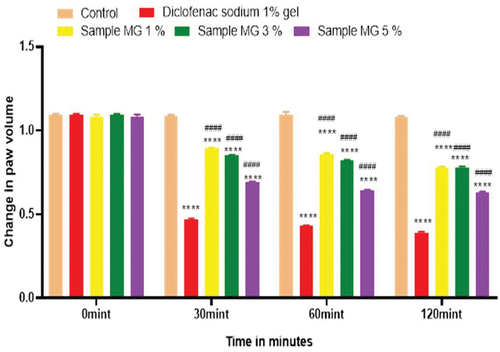
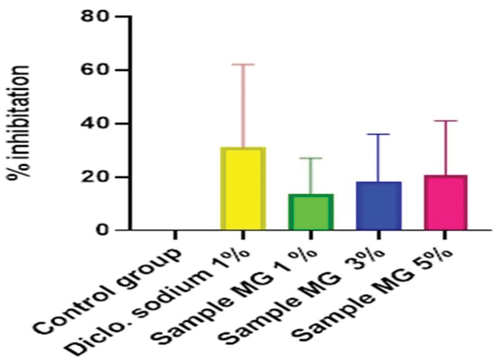
Figure 17. %inhibition of anti-inflammatory activity of hydrogel formulations(MG) with polyherbal methanolic extracts.
Where PI = percentage inhibition, Cv = paw volume of the control group and Tv = paw volume of the test group
Discussion
Herbal medicines have always been an important part of the public healthcare system all over the world. From the last decade, herbal medicines are getting fame because they possess fewer side effects as compared to conventional dosage form and are economical.[Citation18] The extraction technique is the first step to separate the desired active constituents from the raw materials by using suitable solvent. Solvent-based extraction is a method to separate compounds on the basis of their relative solubility.[Citation22] In the current study solvent-based extraction was performed by using n-hexane and methanol. n-hexane is a nonpolar substance and is the solvent of choice for nonpolar compounds extractions while methanol is a polar organic solvent due to the presence of hydroxyl group therefore methanol is more likely to dissolve polar constituents.[Citation23] The percentage yield of extraction was based on the mass of extract divided by the mass of the dry material and multiplied with 100, which indicates the potential of the solvent to extract different constituents from the sample raw material. In the current study the percentage yield of n-hexane extract is greater than that of methanolic extract. This indicates that the amount of nonpolar constituents is larger than polar constituents in these parts of plants.
Homogeneity and color were observed for all polyherbal gel formulations. It was noted that all gels show uniformity and clarity. Color was light green of all gel formulation with polyherbal extracts while gel formulation without polyherbal extract was white of color as shown in . Therapeutic property of any gel formulations is related to its spreadability value. It also shows extent to which gel readily spreads while applied to the skin. Spreadability test of all gel formulations indicate the better spread ability[Citation24] and results are shown in . The extrudability test is used to check the force required to extrude the gel from the tubes. More than 90% gel when extruded out from tubes indicate great extrudability, more than 80% extruded out considered better extrudability and more than 70% extruded out considered good extrudability.[Citation18] All gel formulations in this study possess good and better extrudability as shown in . Rheological study indicate the effect of different range of temperature and shear stress on viscosity.[Citation25] In the current research work, it was observed with the increase in the temperature the viscosity of the gel formulations decreases which represent the inversely proportional correlation between temperature and viscosity and presented in . Quality of a drug product varies with time under the influence of the different environmental factors, such as temperature and humidity.[Citation26] In the current study, the results of stability test over the 2 months indicate that all formulations are homogeneous and there was no change in general appearance like color and odor represented in . All gel formulations for evaluated for irritancy or allergen for seven days by skin irritation test, which indicates that all formulations are free of any irritant material and results are presented in .
Anti-nociceptors reduce the propagation of pain reflex either acting on peripheral or on central receptors. In the current study two different solvent extracts (n-Hexane and methanol) of four different plants parts like black cumin seeds, cinnamon bark, fenugreek seeds, and moringa leaf were used to evaluate their combined effect at (200.0 mg/kg, 400.0 mg/kg and 800.0 mg/kg) by tail flick and hot plate method for analgesic activity. Diclofenac sodium (100 mg/kg) was used as a standard drug. Results have been shown by change in latency time in . Similarly analgesic activity of all hydrogel formulations including G, MG 1%, MG 3%, MG 5%, NG 1%, NG 3% and NG 5% were evaluated by tail flick and hot plate method. Diclofenac sodium 1% was used as standard and change in latency time was observed as shown in . Maximum analgesic potential has been observed with the hydrogel formulation containing 5 g of polyherbal extract PNE and PME (Sample NG 5%, Sample MG 5%). The hydrogel formulations with polyherbal methanolic extracts have more analgesic potential as compared to those of n-hexane polyherbal hydrogels. These effects may be due to the thymoquinone of black cumin seeds (inhibits the propagation of pain through peripheral analgesic activity),[Citation27] cinnamaldehyde of cinnamon (both peripheral and central anti-nociceptive potential),[Citation28] trigonelline of fenugreek (inhibits the pain reflexes by acting on both peripheral and central receptors of pain),[Citation29] kaempferol-3-glucoside fatty acids like chlorogenic acid of moringa leaves (both peripheral and central potential central anti-nociceptive).[Citation30] Hence, polyherbal n- hexane and methanolic extracts of these herbs have significant peripheral and central analgesic potential. It was also observed that polyherbal methanolic extract and its hydrogel has slightly more analgesic potential as compare to n-hexane polyherbal extract and hydrogel prepared from this extract.
Inflammation is a process by which your body’s white blood cells and other defense cells protect you from infection. But prolonged untreated inflammation can cause many disorders therefore research on safe and effective anti-inflammatory drugs is necessary.[Citation31] In this study, anti-inflammatory activity was performed on both polyherbal n-hexane and methanolic extract via carrageenan induced paw edema method. Bi-phasic edema induced by carrageenan injection on the sub-plantar surface. Early phase mediates by the release of histamine, bradykinin, serotonin and prostaglandins while the delayed phase mediates the neutrophil-related free radicals and continues prostaglandins secretions.[Citation32] Different concentrations of both extracts (PNE, PME) were used, i.e 200.0 mg/kg, 400.0 mg/kg, and 800.0 mg/kg. Diclofenac sodium 100 mg/kg was used as a standard. Results have been shown by change in paw edema in , and both polyherbal extracts (PNE and PME) have active anti-inflammatory constituents like Thymoquinone of Black cumin seeds (inhibits the second phase inflammation by acting on prostaglandin alley).[Citation27] Cinnamaldehyde of cinnamon (inhibits the second phase of inflammation),[Citation33] Polyphenol compounds such as flavonoids and phenolic groups of fenugreek (effective during delayed phase of inflammation),[Citation34] isothiocyanate and phenolic derivatives of Moringa (have anti-inflammatory potential during both phase of inflammation).[Citation35] Results of % inhibition of paw edema have shown that polyherbal n-hexane and methanolic extracts both have significant anti-inflammatory potential in a dose-dependent manner which means with an increasing concentration of polyherbal extract the anti-inflammatory potential also increased. Therefore, maximum anti-inflammatory potential has been observed with 800 mg/kg (Sample M 3, Sample N 3) of polyherbal extract (PNE and PME). Results have also shown that polyherbal methanolic extract has higher anti-inflammatory potential than polyherbal n-hexane extract. Results have shown the percentage inhibition of paw edema in .
Anti-inflammatory activity of all hydrogel formulations including G, MG 1%, MG 3%, MG 5%, NG 1%, NG 3%, and NG 5% were performed by carrageenan-induced edema method. 1% carrageenan was used to induce inflammation which is bi-phasic mechanism. Diclofenac sodium 1% was used as standard and the change in paw was observed as shown in and . It has been observed that combined effect of active anti-inflammatory constituent in hydrogel formulations with polyherbal n-hexane and methanolic extract has a significant anti-inflammatory potential in a dose-dependent manner, which means with increasing concentration of polyherbal extracts the anti-inflammatory potential also increased. Therefore, the maximum anti-inflammatory potential has been observed with the hydrogel formulation containing 5 g of polyherbal extract PNE and PME (Sample NG 5%, Sample MG 5%). The hydrogel formulations with polyherbal methanolic extracts have more anti-inflammatory potential as compared to those of n-hexane hydrogels. Results have shown the percentage inhibition of paw edema in .
Conclusion
It is hereby concluded from this study polyherbal methanolic extract and gel formulations prepared from methanolic extract have analgesic that is more significant and anti-inflammatory potential.
Abbreviations
G; hydro-gel formulation without polyherbal extract, MG 1%; hydro-gel formulation with 1 g of polyherbal methanolic extract, MG 3%; hydro-gel formulation with 3 g of polyherbal methanolic extract, MG 5%; hydro-gel formulation with 5 g of polyherbal methanolic extract, MNTC; Moringa oleifera, Nigella sativa, Trigonella foenum graecum and Cinnamomum verum, NG 1%; hydro-gel formulation with 1 g of polyherbal n-hexane extract, NG 3%; hydro-gel formulation with 3 g of polyherbal n-hexane extract and NG 5%; hydro-gel formulation with 5 g of polyherbal n-hexane extract, PME; polyherbal methanolic extract, PNE; polyherbal n-hexane extract, Sample M 1; polyherbal methanolic extract 200 mg/kg, Sample M 2; polyherbal methanolic extract 400 mg/kg, Sample M 3; polyherbal methanolic extract 800 mg/kg, Sample N 1; polyherbal n-hexane extract 200 mg/kg, Sample N 2; polyherbal n-hexane extract 400 mg/kg, Sample N 3; polyherbal n-hexane extract 800 mg/kg
Ethical approval and consent to participate
According to national research council guidelines, all procedures and techniques of this study were performed. Laboratory animals were approved by the bio-ethical committee of Baha Uddin Zakariya University Multan vide letter No BZ-237-PH-10/1 were also used in their accordance.
Acknowledgments
The authors are thankful to the Dean of Faculty of Pharmacy, Baha Uddin Zakariya University Multan, for providing necessary facilities to carry out this research work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jeong, W. Y.; Kwon, M.; Choi, H. E.; Kim, K. S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25(1), 1–15. DOI: 10.1186/s40824-021-00226-6.
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms Behind Them. Gels. 2017, 3(1), 1–6. DOI: 10.3390/gels3010006.
- Kumar, M.; Shek, A.; Akbar, Z. A Review on Analgesic: From Natural Sources. Int. J. Pharm. Biol. Arch. 2010, 1, 95–100.
- Rajeswari, G.; Murugan Mohan, V. Anti Inflammatory Activity of Leaf and Bark of Hugonia Mystax Linn. J. Harmonized Res. Pharm. 2013, 2(2), 80–83.
- Gurung, T. M.; Kaundinnyayanna, A.; Parajuli, K. Evaluation of Antioxidant, Anti-Inflammatory and Analgesic Activities of Traditional Polyherbal Combination Used in Western Nepal. World J. Pharm. Pharm. Sci. 2019, 8(3), 757–775.
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytotherapy Res. 2000, 14(5), 323–328. DOI: 10.1002/1099-1573(200008)14:5<323:AID-PTR621>3.0.CO;2-Q.
- Amin, B.; Hosseinzadeh, H. Black Cumin (Nigella Sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-Inflammatory Effects. Planta. med. 2016, 82(1/02), 8–16. DOI: 10.1055/s-0035-1557838.
- Rao, P. V.; Gan, S. H. Cinnamon: A Multifaceted Medicinal Plant. Evidence-Based Complementary and Alternative Medicine. Evid. Based Complement. Altern. Med. 2014, 2014(1), 1–12. DOI: 10.1155/2014/642942.
- Ranasinghe, P.; Pigera, S.; Premakumara, G. S.; Galappaththy, P.; Constantine, G. R.; Katulanda, P. Medicinal Properties of ‘True’cinnamon (Cinnamomum Zeylanicum): A Systematic Review. BMC Complement. Altern. Med. 2013, 13(1), 275. DOI: 10.1186/1472-6882-13-275.
- Syed, Q. A.; Rashid, Z.; Ahmad, M. H.; Shukat, R.; Ishaq, A.; Muhammad, N.; Rahman, H. U. U. Nutritional and Therapeutic Properties of Fenugreek (Trigonella Foenum-Graecum): A Review. Int. J. Food Prop. 2020, 23(1), 1777–1791. DOI: 10.1080/10942912.2020.1825482.
- Mandegary, A.; Pournamdari, M.; Sharififar, F.; Pournourmohammadi, S.; Fardiar, R.; Shooli, S. Alkaloid and Flavonoid Rich Fractions of Fenugreek Seeds (Trigonella Foenum-Graecum L.) with Antinociceptive and Anti-Inflammatory Effects. Food Chem. Toxicol. 2012, 50(7), 2503–2507. DOI: 10.1016/j.fct.2012.04.020.
- Wani, S. A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. Sci. 2018, 17(2), 97–106. DOI: 10.1016/j.jssas.2016.01.007.
- Mishra, G.; Singh, P.; Verma, R.; Kumar, S.; Srivastav, S.; Jha, K. K.; Khosa, R. L. Traditional Uses, Phytochemistry and Pharmacological Properties of Moringa Oleifera Plant: An Overview. Der. Pharmacia. Lettre. 2011, 3(2), 141–164.
- Rastogi, T.; Bhutda, V.; Moon, K.; Aswar, P. B.; Khadabadi, S. S. Comparative Studies on Anthelmintic Activity of Moringa Oleifera and Vitex Negundo. Asian J. Res. Chem. 2009, 2(2), 181–182.
- Martínez-González, C. L.; Martínez, L.; Martínez-Ortiz, E. J.; González-Trujano, M. E.; Déciga-Campos, M.; Ventura-Martínez, R.; Díaz-Reval, I. Moringa Oleifera, a Species with Potential Analgesic and Anti-Inflammatory Activities. Biomed. Pharmacother. 2017, 87(1), 482–488.10.1016/j.biopha.2016.12.107
- Aiyalu, R.; Govindarjan, A.; Ramasamy, A. Formulation and Evaluation of Topical Herbal Gel for the Treatment of Arthritis in Animal Model. Braz. J. Pharm. Sci. 2016, 52(3), 493–507. DOI: 10.1590/s1984-82502016000300015.
- Meza-Valle, K. Z.; Saucedo-Acuña, R. A.; Tovar-Carrillo, K. L.; Cuevas-González, J. C.; Zaragoza-Contreras, E. A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe Vera Hydrogel on Wound-Healing Process. Polymers. 2021, 13(22), 39–58. DOI: 10.3390/polym13223958.
- Gupta, R.; Gupta, G. D. Formulation Development and Evaluation of Anti-Inflammatory Potential of Cordia Obliqua Topical Gel on Animal Model. Pharmacogn. J. 2017, 9(6), 93–98. DOI: 10.5530/pj.2017.6s.163.
- Rupal, J.; Kaushal, J.; Mallikarjuna, S. C.; Kaushal, J. Preparation and Evaluation of Topical Gel of Valdecoxib. Int. J. Pharm. Sci. Drug Res. 2010, 1(1), 51–54. DOI: 10.25004/IJPSDR.2009.010108.
- Bhinge, S. D.; RANDIVE, D. S.; WADKAR, G. H.; KAMBLE, S. Y.; BHINGE, S. Formulation and Evaluation of Polyherbal Gel Containing Extracts of Azadirachta Indica, Adhatoda Vasica, Piper Betle. Ocimum tenuiflorum and Pongamia pinnata. Marmara Pharm J. 2019, 23(1), 44–54. DOI: 10.12991/jrp.2018.107.
- Kulkarni, P. A.; Kewatkar, S.; Lande, M. D.; Phanse, M. A.; Chaudhari, P. D. Topical Anti-Inflammatory Activity of Herbal Gel Formulation. J. Med. Chem. Pharm. Chem. Pharm. Sci. Comput. Chem. 2010, 2(3), 338–342.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin.Med. 2018, 13(1), 1–26. DOI: 10.1186/s13020-018-0177-x.
- Fraterrigo Garofalo, S.; Tommasi, T.; Fino, D. A Short Review of Green Extraction Technologies for Rice Bran Oil. Biomass Conv. Bioref. 2021, 11(2), 569–587. DOI: 10.1007/s13399-020-00846-3.
- Dantas, M. G. B.; Reis, S. A. G. B.; Damasceno, C. M. D.; Rolim, L. A.; Rolim-Neto, P. J.; Carvalho, F. O.; Quintans-Junior, L. J.; Almeida, J. R. G. D. S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 1–4. DOI: 10.1155/2016/7394685.
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers. 2019, 11(2), 1–11. DOI: 10.3390/polym11020259.
- Ramadon, D.; Pramesti, S. S.; Anwar, E. Formulation, Stability Test and in vitro Penetration Study of Transethosomal Gel Containing Green Tea (Camellia Sinensis L. Kuntze) Leaves Extract. Int. J. App. Pharm. 2017, 9(5), 91–96. DOI: 10.22159/ijap.2017v9i5.20073.
- Shaheen, N.; Azam, A.; Ganguly, A.; Anwar, S.; Parvez, M. S. A.; Punyamurtula, U.; Hasan, M. K. Anti-Inflammatory and Analgesic Activities of Black Cumin (BC, Nigella Sativa L.) Extracts in in vivo Model Systems. Bull. Natl. Res. Cent. 2022, 46(1), 1–8. DOI: 10.1186/s42269-022-00708-0.
- Churihar, R.; Solanki, P.; Vyas, S.; Tanwani, H.; Atal, S. Analgesic Activity of Cinnamaldehyde per Se and It’s Interaction with Diclofenac Sodium and Pentazocine in Swiss Albino Mice. Int. J. Phamacognosy. 2016, 3(1), 97–102.
- Vyas, S.; Agrawal, R. P.; Solanki, P.; Trivedi, P. Analgesic and Anti-Inflammatory Activities of Trigonella Foenum-Graecum (Seed) Extract. Acta Poloniae. Pharm. 2008, 65(4), 473–486.
- Tamrat, Y.; Nedi, T.; Assefa, S.; Teklehaymanot, T.; Shibeshi, W. Anti-Inflammatory and Analgesic Activities of Solvent Fractions of the Leaves of Moringa Stenopetala Bak.(moringaceae) in Mice Models. BMC Complement. Altern. Med. 2017, 17(1), 1–10. DOI: 10.1186/s12906-017-1982-y.
- Dinarello, C. A. Anti-Inflammatory Agents: Present and Future. Cell. 2010, 140(6), 935–950.
- Mansouri, M. T.; Mansouri, M.; Hemmati, A.; Naghizadeh, B.; Mard, S.; Rezaie, A. A Study of the Mechanisms Underlying the Anti-Inflammatory Effect of Ellagic Acid in Carrageenan-Induced Paw Edema in Rats. Indian J. Pharmacol. 2015, 47(3), 292–306. DOI: 10.4103/0253-7613.157127.
- Rathi, B.; Bodhankar, S.; Mohan, V.; Thakurdesai, P. Ameliorative Effects of a Polyphenolic Fraction of Cinnamomum Zeylanicum L. Bark in Animal Models of Inflammation and Arthritis. Sci. Pharm. 2013, 81(2), 567–589.
- Subhashini, N.; Nagarajan, G.; Kavimani, S. Anti-Inflammatory and in vitro Antioxidant Property of Trigonella Foenum Graecum Seeds. J. Pharmacol. Toxicol. 2011, 6(4), 371–380. DOI: 10.3923/jpt.2011.371.380.
- Rao, K. V.; Gopalakrishnan, V.; Loganathan, V.; Nathan, S. S. Anti Inflammatory Activity of Moringa Oliefera. Lam. Ancient Sci. Life. 1999, 18(3–4), 195–206.
