ABSTRACT
Quercetin, a flavonoid, is a dietary component that has attracted the attention of dietitians and medicinal chemists due to its anticancer potential. It is an antioxidant that has a significant role in reducing different human cancers such as breast, pancreatic, prostate, colon, blood, and oral. Being as an additional anti-cancer agent, it can stop cancer cell invasion and metastasis, induce apoptosis and autophagy, decrease cancer cell growth and proliferation. Multiple mechanisms, including suppression of pathways involved in cell survival, angiogenesis, and inflammation, are thought to be responsible for these anticancer effects. Quercetin has anti-inflammatory properties in both in vitro and in vivo tests, which is important as chronic inflammation is a major contributor to cancer. Preventing the synthesis of cytokines and chemokines that encourage inflammation can support to lessen inflammation and stop the spread of cancer. In cancer cells, quercetin can cause programmed cell death, which can stop the cells from rapidly multiplying and replicating. It provides protection against degradation of DNA induced by radiation and other carcinogens. The PI3K/Akt/mTOR pathway, the NF-κB system, and the Wnt/β-catenin pathway is few of the signaling pathways that quercetin modulates. It can activate the process of autophagy, which breaks the growth and range of cancer by having cells terminate and recover damaged or unwanted cellular mechanisms. The best treatment plans for various cancer kinds will need to be determined through additional research, which will also help confirm its safety and effectiveness in humans.
Introduction
Many fruits and veggies have a lot of flavonols. There has been a lot of study done on the flavonols kaempferol, quercetin, myricetin, and fisetin. Flavonols can be found in high amounts in apples, berries, kale, onions, lettuce, and tomatoes, among other fruits and veggies. Flavonols can be found in foods like fruits and veggies, and they can also be found in beverages like red wine and tea.[Citation1] Quercetin (3, 3′, 4′, 5, 7-pentahydroxy flavone), flavone that may be found in red and green peppers as well as apples, cranberries, blueberries, cherries, grapes, tea, wine, and strawberries, among other fruits and veggies. Quercetin is a chemical that have anti-cancer, anti-proliferative, and apoptosis-stimulating qualities. Major food sources of quercetin are shown in .
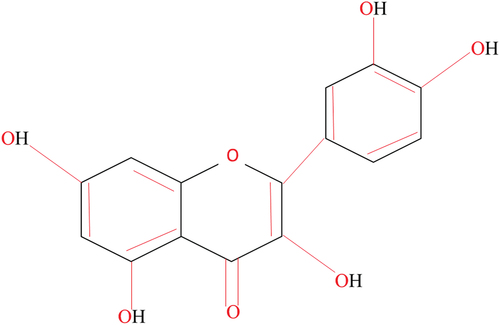
If look at the structure of quercetin (); the two aromatic rings that make up the structure of quercetin are connected by a third ring that contains an oxygen atom. Quercetin’s antioxidant capabilities are due to the five hydroxyl groups (OH) that are joined to the rings. Because the hydroxyl groups can interact with other molecules through hydrogen bonds hence, quercetin is a versatile substance that is capable of interacting with a range of biological systems.[Citation2]
In old Chinese medication, quercetin was thought to be the greatest important plant constituents.[Citation3] It is often used to treat cancer, asthma, and high blood pressure. Quercetin is good for the health of blood vessels because it lowers blood pressure, reduces inflammation, and works as antioxidant/anti-inflammatory mediator.[Citation4]
Also, quercetin controls the function of cell carriers like plasma ion channels. This is a trait that is linked to keeping the right amount of ions in cells, which is called ion homeostasis. Quercetin stops lipid degradation in addition to being a strong antioxidant and stopping ROS from forming. This keeps atherosclerosis from getting worse and helps protect the nervous system.[Citation5]
Flavonoids-rich foods offer a range of brain-protective properties, including the potential to guard against brain damage and recover memory, learning, and cognitive function. It’s often found in plants which are used to make food. It is known as one of the most powerful vitamins that plants can make.[Citation6] Quercetin has been shown to protect the brain and nervous system from a number of problems, such as epilepsy, Parkinson’s infection, Alzheimer’s syndrome, and head injuries.[Citation7] Animal studies have shown that quercetin can stop seizures by reducing oxidative damage, inflammation.[Citation8] It is important to know that quercetin can pass through the (BBB) blood-brain barrier. This makes it a possible handling for a wide range of neurological diseases, such as epilepsy. Anticancer effects of quercetin were shown in .
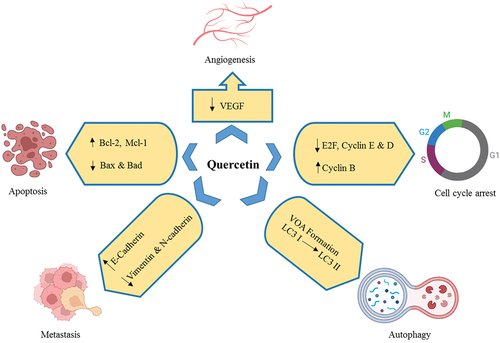
Cancer is a big deal on a global scale because it is the second top reason of mortality around the globe.[Citation9] Cancer cells can change the way their metabolism works to help their uncontrolled growth. This is done by growing their biomass even if it means using more energy. This shows how important metabolic reprogramming is as a key biological method for accepting how a standard cell turns into a cancer originator. Quercetin is the most important flavonol in the flavonoid family of flavonols.[Citation10] Quercetin is one of the flavonols that people in the western countries consume the most since it is found in practically all fruits and vegetables. Quercetin has been shown to fight cancer in a number of ways. Apoptosis and autophagy are the key factors that lead to the death of cells. Natural substances like quercetin have been found to be important in both treating and preventing cancer. They have been shown to have reliable results, a high therapeutic potential, and low toxicity. A number of experts looked at the effects of a single quercetin molecule on different types of cancer cell lines. There is little doubt that quercetin has stopped the progression of a number of diseases, especially breast cancer, gastric tumor, oral cancer, (CRC) colorectal cancer, prostate tumor, liver cancer, leukemia, thyroid cancer, lung cancer and pancreatic cancer. It comes from natural sources, quercetin could be a very useful food ingredient for treating, managing, and preventing cancer. When compared to manmade cancer drugs, the price of quercetin as a supplement or food supplement is much lower.[Citation11] This is a benefit of the drug that needs to be thought about. Mechanisms of action of quercetin against cancers is shown in .
Antioxidant status of quercetin
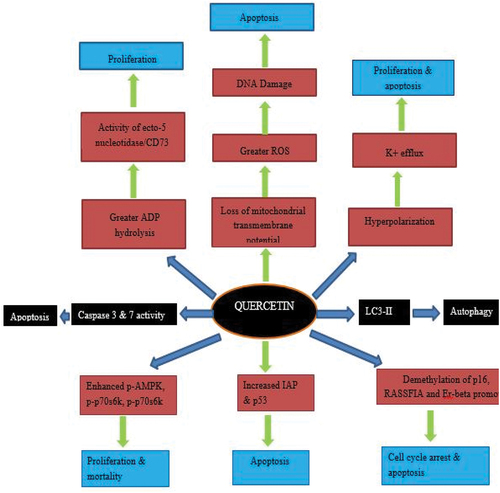
A flavonoid recognized for its antioxidant effects is quercetin. In the body, free radicals and other reactive oxygen species (ROS) that can result into oxidative stress and tissue damage are neutralized by molecules known as antioxidants. The effectiveness of quercetin as an antioxidant has been shown in several in vivo and in vitro analyses. There are numerous ways in which quercetin works as an antioxidant. By directly neutralizing free radicals, it can lessen their harmful effects on biological components like proteins, DNA and lipids. In addition to providing further protection against oxidative damage to cells, quercetin has the ability to upregulate antioxidant enzymes including superoxide dismutase (SOD) and catalase.[Citation12] Chelating metal ions is one method quercetin may defend against oxidative damage. ROS-producing reactions can involve metal ions like iron and copper, and quercetin can bind to these ions to stop them from catalyzing these processes. In diseased animal models, quercetin has also been discovered to guard against oxidative stress. For instance, quercetin administration decreased oxidative stress indicators and enhanced perceptive purpose in a rat model of Alzheimer’s syndrome.[Citation13] Quercetin enhanced motor performance and shielded dopaminergic neurons from oxidative damage in a rat model of Parkinson’s disorder.[Citation14] In addition to its antioxidant capabilities, quercetin has been exposed to have anti-inflammatory benefits.[Citation15] Oxidative stress and the emergence of numerous illnesses, including as cancer, cardiovascular disease, and neurological disorders, are linked to chronic inflammation. It has been demonstrated that quercetin inhibits the activation of inflammatory signaling trails such MAPKs and NF-κB, as well as the generation of proinflammatory cytokines and chemokines. Quercetin has been discovered to provide a extensive range of health advantages in addition to its anti-inflammatory and antioxidant characteristics.[Citation16] By lowering blood pressure, enhancing lipid profiles, and lowering the risk of atherosclerosis, it has been demonstrated to promote cardiovascular health. The effectiveness of quercetin as an antioxidant is greatly influenced by its bioavailability. Since just a small portion of quercetin consumed is absorbed into the bloodstream, quercetin has a limited bioavailability.[Citation17] The concentration of quercetin in the body can be increased and its antioxidant effects can be strengthened by using quercetin supplements, meals high in quercetin, and quercetin derivatives with greater bioavailability, according to research.[Citation18] In conclusion, quercetin is a strong antioxidant with a variety of health advantages. It is a better option for the prevention and management of many ailments, including cancer, cardiovascular disease, and neurological disorders because of its capacity to neutralize free radicals, upregulate antioxidant enzymes, chelate metal ions, and decrease inflammation. For quercetin to completely realize its promise as an antioxidant and therapeutic agent, however, its bioavailability is a restriction that needs to be addressed.[Citation19]
Pharmacokinetic study of quercetin
Quercetins potential for health benefits, especially its anticancer effects, have been well researched. The pharmacokinetic study of quercetin provides information on its absorption, distribution, metabolism, and excretion cancer patients. The capacity of a patient to absorb quercetin can vary dependent on the type of tumor, the phase of the disease, and the course of treatment.[Citation20] The bioavailability of quercetin from dietary sources typically ranges from 1% to 10%, however it can reach up to 50% when taken as supplements.[Citation21] The addition of other flavonoids and dietary lipids improves the absorption of quercetin. However, gastrointestinal damage brought on by chemotherapy, which can hinder the absorption of nutrients and medications, may have an impact on quercetin absorption in cancer patients. After being ingested, quercetin is carried in the bloodstream by plasma proteins. The distribution of quercetin in cancer patients can vary dependent on the variety of tumor, the disease’s stage, and the type of therapy used.[Citation22] For instance, quercetin has been demonstrated to accumulate in breast cancer tissue in patients with mammary cancer, indicating that it could have a targeted anticancer impact.[Citation23] In the liver and intestine, quercetin goes through a thorough metabolic process. Sulphates, Glucuronides and methylated derivatives are the three main types of quercetin’s metabolites. UDP-glucuronosyltransferases (UGTs), Catechol-O-methyltransferase (COMT), and sulfotransferases (SULTs) are a few of the enzymes that help quercetin be metabolized. Chemotherapy-induced hepatic toxicity might disrupt the performance of hepatic enzymes involved in drug metabolism in cancer patients, which may have an impact on the metabolism of quercetin in those patients. Mostly through the urine and feces, quercetin and its metabolites are excreted from the body. Quercetin has an approximately 2- to 3-hour half-life in the body.[Citation24] Chemotherapy-induced renal toxicity, which can decrease how well medicines and their metabolites are eliminated through the kidneys, can impact cancer patients’ ability to eliminate quercetin.[Citation25] Numerous studies have looked into the pharmacokinetic characteristics of quercetin as well as its possible anticancer effects in both preclinical and clinical studies.[Citation26] These investigations have revealed that quercetin may possess a number of anticancer mechanisms, including anti-inflammatory and antioxidant properties, apoptosis induction, angiogenesis suppression, and cell signaling pathway regulation.[Citation27] Quercetin’s pharmacokinetics in males with prostate tumor were examined in one study. According to the study, quercetin and its metabolites were significantly more concentrated in the plasma when quercetin was taken orally in a supplement form at a dosage of 1 g/day for three weeks. The investigation also discovered that quercetin, a prostate cancer progression indicator known as prostate-specific antigen (PSA).[Citation28] In a different study, quercetin’s pharmacokinetics in people with breast cancer were examined. According to the study, taking quercetin orally at a dose of 1 g per day for four weeks caused a noticeably higher plasma concentration of quercetin and its metabolites. Quercetin was also shown to be well tolerated and to have no discernible negative impacts on the markers of breast cancer progression, according to the study.[Citation29]
Anticancer perspectives
Given that it has been found to have anticancer properties, it might be employed as a therapeutic agent to fight tumor. It has been demonstrated that quercetin hinders the development of carcinomas from the breast, prostate, colon, and lung. It accomplishes this by producing apoptosis and cell cycle arrest, both of which finally result in the death of tumor cells.[Citation30] The occurrence and spread of cancer are significantly influenced by chronic inflammation. Through the inhibition of the synthesis of pro-inflammatory molecules like prostaglandins and cytokines, quercetin has been shown to reduce inflammation.Tumors develop new blood vessels through the process of angiogenesis in order to get oxygen and nutrients. It has been demonstrated that quercetin prevents the creation of new blood vessels by preventing the generation of vascular endothelial growth factor (VEGF) that is significant for angiogenesis.[Citation31] It has been investigated that quercetin elevates the susceptibility of tumor cells to chemotherapy medicines, improving the effectiveness of chemotherapy.[Citation32] By preventing harm to healthy cells, it also lessens the negative effects of chemotherapy.[Citation33] Cancer suppressor genes like p53 and p21, which are crucial in preventing the growth of cancer, have been found to be expressed in a manner that is controlled by quercetin.[Citation34]
Finally, quercetin exhibits promising anticancer properties, making it a probable beneficial agent for the prevention and handling of tumor. To completely comprehend its modes of action and possible clinical applications, more study is necessary.[Citation35] Cancer cells from the initial tumor can migrate to other areas of the body through a process known as metastasis. By lowering the activity of enzymes necessary for cancer cells to invade and spread, quercetin has been demonstrated to limit metastasis.[Citation36] The PI3K/Akt, MAPK/ERK, and NF-κB pathways are just a few of the signaling pathways that quercetin has been demonstrated to affect.[Citation37] These pathways are all involved in the growth and spread of cancer. Quercetin can hinder cancer cells’ ability to survive and develop via altering these pathways. The detection and treatment of cancer are both significantly aided by the insusceptible system. Both of these variables are thought to prompt the fundamental incendiary condition, through the unevenness of favorable to provocative as well as mitigating go-between. Natural killer cells (NK), T cells and macrophages are just a few of the immunity cells that quercetin has been discovered to activate to improve immune function.[Citation38] Degradation of damaged proteins and organelles is a step in the cellular process of autophagy. By preserving cellular homeostasis, it helps to hinder the growth of tumor. The anticancer properties of quercetin may be attributed to the fact that it has been proven to stimulate autophagy. Additionally, curcumin, resveratrol, and green tea catechins, quercetin has been discovered to work in concert with other anticancer substances. This cooperative action can lessen the toxicity of these substances while boosting their overall anticancer efficacy.[Citation39] Quercetin is a better option for the management and prevention of tumor since it has various modes of action. It is a desirable polyphenol for more research and development because of its low toxicity and accessibility in numerous food sources.[Citation40]
Breast cancer
On a variety of breast tumor cell shapes, MDA-MB-231, MCF7, BT549, T47D, and 4T1, quercetin exhibits anticancer activity through a number of mechanisms, including preventing cancer progression, thwarting tumor cell propagation, relocation, and colony development, and exhibiting matrix metalloproteinase signaling and inducing cell death pathways.[Citation41] The intracellular mitochondrial respiration and glycolytic activity were significantly decreased by quercetin, which restricted the synthesis of large amounts of ATP. Reactive oxygen species (ROS) were then increased, antioxidant levels were decreased, and anti-metastatic and cell apoptosis were induced. The main ingredient of the Xiaoyao Kangai Jieyu Formula was determined to be quercetin.[Citation42] Through the downregulation of card domain and PYD containing (ASC)NLR family pyrin domain comprising 1, and caspase-3 (NLRP3) quercetin significantly reduced BCRD-induced neuron pyroptosis and was able to restore the inhibition of neuron viability caused by BCRD.[Citation42] Quercetin was able to restore the concentrations of 5-hydroxytryptamine (5-HT), dopamine (DA), and neutrophil elastase (NE) in mouse and dramatically reduced the symptoms of BCRD in those animals. In addition, quercetin may enhance immunological reactions in xenograft rats by increasing interleukin-2 (IL-2) and IL-10 levels.[Citation43] Activation of the overexpressed insulin-like growth factor-1 receptor (IGF1R) and its downstream kinases Akt and Erk1/2 were reduced by quercetin in MDA-MB-23 1 cells in a dose-dependent way. The expression of the EMT transcription features Snail and Slug was retarded, which significantly reduced the (EMT) epithelial-mesenchymal transition and metastatic phenotype of MDA-MB-231 cells. As a result, quercetin disrupted the paracrine or autocrine loop of IGF1 signaling. Quercetin also enhanced the excretion of IGF-binding protein-3 while decreasing the production of IGF1 in the conditioned media of MDA-MB-23, 1 cells. In xenograft rat models, quercetin treatment inhibited the development of MDA-MB-231 lung metastasis and MDA-MB-231 tumor xenografts. IGF1R was inactivated along with this, and the expression of Slug, Snail and the mesenchymal markers vimentin and fibronectin were all downregulated.[Citation44] 5 Molar quercetin induced apoptosis in MCF-7, MCF-10AT, and MDA-MB-231 cells in a concentration-and time-dependant mode. Along with enhancing the control of T cells, it also enhanced the protein levels of IFN-R, p-STAT1 and p-JAK2 while reducing the protein levels of PD-L1.[Citation45] Breast cancer T47D cells and CD44+/CD24- cells experienced a decreased degree of apoptosis when quercetin and doxorubicin were used alone or in combination to suppress cell proliferation. Doxorubicin’s ability to cause apoptosis in both cell types and inflict cytotoxicity were greatly enhanced by quercetin. To a lesser extent, isolated cancer cell lines were also affected by the amalgamation of quercetin and doxorubicin, which caused G2/M seizure in T47D cells.[Citation46]
Pancreatic cancer
Quercetin has pharmacological effects that can be either stimulating or inhibiting in pancreatic cancer (PC), including increased susceptibility to chemotherapeutic drugs and autophagy, cell growth inhibition or decrease, EMT, oxidative stress, and induction of apoptosis.[Citation47] In particular, quercetin triggers gemcitabine (GEM)-resistant pancreatic cancer cells while also controlling cell apoptosis and increasing medication influence through receptor for advanced glycation end products (RAGE) decrease. By hindering the AKT/PI3K/mTOR axis in MIA Paca-2 GEMR and (GEM-resistant cells) MIA Paca-2 cells, recent findings demonstrated that decreasing RAGE expression by RAGE-specific siRNA transfection greatly accelerated apoptosis by autophagy, apoptosis, and GEM-activated cytotoxicity.[Citation48] Notably, quercetin demonstrated an impressive effect that was comparable to RAGE silencing and successfully reduced RAGE expression to promote cell cycle apoptosis, autophagy, cell cycle seizure and GEM chemo sensitivity in MIA Paca-2 GEMR cells.[Citation49] Different resveratrol and quercetin concentrations (5, 10, 25, 50, and 100 M) were applied to PANC-1 cells that were CD133-and CD133+. The experiment was used to assess cytotoxicity and cell growth. Using antibodies against IL-1, N-cadherin, ACTA-2, vimentin, TNF-α, and immunocytochemistry was used to analyze the anti-metastatic and anticancer impacts of quercetin and resveratrol.[Citation50] Immunostaining on CD133+ cells was more intense than on CD133- cells. In quercetin-treated CD133+ cells, the immunoreactivities of IL-1, ACTA-2, and N-cadherin were dramatically reduced, whereas the immunoreactivities of TNF-α and vimentin were significantly elevated.[Citation51] Furthermore, in CD133+ cells treated with resveratrol, TNF and N-cadherin immunoreactivities expressively reduced.[Citation52]
Gemcitabine and quercetin have a synergistic effect on preventing pancreatic cancer cells from migrating, according to research.[Citation53] As a result, in this study, quercetin and gemcitabine were loaded into decomposable nanoparticles (NPs) made of poly (lactic-co-glycolic acid) that were superficially draped with hyaluronic acid (HA; viz., PPHA NPs). HA is important for drug targeting to tumors because it has the capacity to interact precisely with the CD44 response, which is dominated in many cancers. In comparison to both the simple medications and the medications loaded in nanoparticles that do not uncover hyaluronic acid on the exterior side, the produced HA-decorated nanoparticles loaded with quercetin and gemcitabineshown better and cytotoxicity and cellular uptake toward two cell lines of pancreatic ductal adenocarcinoma, namely PANC-1 and Mia-PaCa-2. Additionally, HA-decorated NPs were capable of enhancingQCT’s anti-inflammatory characteristics, which caused in a reduction in the stages of interleukin in both cancer cell lines that had previously been stimulated with lipopolysaccharides.[Citation54] Quercetin has demonstrated effects on pancreatic cancer cells’ apoptosis, proliferation, migration and invasion as well as metastasis and cancer growth in pancreatic ductal adenocarcinoma (PDA) xenograft animal models.[Citation55] By reducing the expression of c-Myc, quercetin drastically reduced pancreatic cancerous cells proliferation. Additionally, quercetin inhibited pancreatic cancerous cells migration and invasion by lowering the amount of TGF-1, which in turn hindered (EMT). Additionally, quercetin prompted pancreatic cancerous cells apoptosis via the death receptor and mitochondrial pathways. When quercetin was administered to nude mice models, dopamine (PDA) metastasis and growth were slowed down.[Citation56] In terms of mechanics, quercetin treats dopamine (PDA) by reducing SHH activity. Gli2, but not Gli1, is an interesting factor in the quercetin-induced inactivation of SHH[Citation57] PCC proliferation, migration, and invasion were inhibited by quercetin but were eliminated when SHH movement was elevated by recombinant Shh protein. Shh also induced the expression of Snail1 and Zeb2, which ultimately lead to a partial difficulty of quercetin-mediated suppression of pancreatic cancerous cells invasion and migration. This activation of TGF-1/Smad2/3 signaling and promotion of EMT were accomplished through these two mechanisms.[Citation58]
Prostate cancer
Quercetin has also been found to be effective against prostate cancers. In a study it was demonstrated that increasing the concentration of quercetin nanoparticles caused a progressive decline in cell viability.[Citation59] Nearly 50% of the viable cells were still present after 48 hours following the 40 mM quercetin treatment. In the LNCaP cells, quercetin nanoparticles alter the expression of the Su (Fu) and gli mRNAs.[Citation60] Resveratrol and Quercetin (600 and 60 mg/kg, correspondingly, in diet) have an anticancer impact on prostate tumor in the Transgenic Adenocarcinoma of Mice Prostate-model (TRAMP) with notable inhibition in proliferation, tumor survival and oxidative stress markers, as well as enhanced apoptotic markers.[Citation61] Both substances alter the activity of genes in relation to androgen response, PTEN and PI3K/AKT signaling, cell cycle apoptosis, transcription factors, promoter methylation. IGF1 and BCL2 were found to be key actors in two gene networks by Ingenuity Pathway Analysis (IPA).[Citation62] Inhibiting cell proliferation/viability, vasculogenesis, hyperplasia, and angiogenesis with two-fold therapy was anticipated by functional annotation to increase apoptosis. Also predicted by IPA was the upstream hinderance of key PCa signaling (Ca2+, VEGF, PI3K, PTH, CSF2). Based on the results of a PCR array, it was they discovered elevated levels of NKX3.1 and IGFBP7 and lower levels of EGR3, EGFR, and IL6—all of which support quercetin-resveratrol’s anti-PCa properties.[Citation62] Doxorubicin and quercetin therapy dramatically increased the rate of doxorubicin-induced apoptosis in prostate tumor (PC)3 cell line (PC3/R) cells by activating the mitochondria and reactive oxygen species pathway.[Citation63] Tyrosine-protein kinase-met (c-met), which is knowingly upregulated in R/PC3 cells was found to be compared to normal PC3 cells. The downstream PI3K/AKT pathway and c-met expression were both suppressed in response to quercetin administration in R/PC3 cells.[Citation64] In R/PC3 cells preserved with doxorubicin, quercetin-promoted apoptosis was also prevented by enhanced expression of c-met.[Citation65]
Inhibiting cell migration, drastically inducing endoplasmic reticulum (ER) stress, and increasing ROS production were all effects of the quercetin and paclitaxel treatment regimen.[Citation66] It also greatly reduced cell propagation, elevated apoptosis, stopped the cell cycle at the G2/M stage, and significantly decreased apoptosis. Quercetin enhanced paclitaxel’s ability to kill cancer cells in a PC-3 cancer-bearing mouse model, and this combination treatment had the fewest negative side effects when compared to the solo paclitaxel treatment group.[Citation67] By inhibiting TGF-induced expression of vimentin and N-cadherin and increasing the expression of E-cadherin in PC-3 cells, quercetin has an anticancer effect on TGF-inducedEpithelial-Mesenchymal Transition (EMT) in prostate tumor (PC-3) cell line. This prevents TGF-induced EMT. Quercetin dramatically reduced TGF-induced Twist, Slug and Snail expression, as demonstrated by the relative expression of Twist, Slug and Snail. In the current investigation, it was discovered that the expression of mesenchymal markers was downregulated in the simple phase and upregulated in the induced phase, in contrast to the expression of epithelial markers, which was shown to be elevated in the simple phase and downregulated in the activated phase.[Citation68] The expression of CXCR and CXCR7 chemokine receptors, 4, 5, and 1 integrin subunits, VEGF, and Ki-67 proliferation markers was considerably decreased in DU145 and PC3 prostate tumor cell lines when quercetin (75 M) and vitamin C (100 M) were combined.[Citation69] In a dose-dependent manner, quercetin hindered the growth of PC-3 cells. Additionally, it prevented the growth of new blood vessels and tumors, even as quercetin concentration was markedly raised by the expression of the thrombospondin-1 (TSP-1) protein and mRNA.[Citation70,Citation71]
Liver cancer
Quercetin triggers apoptosis and cell cycle arrest in both in vivo and in vitro experiments, which reduces the development of hepatocellular carcinoma cells (HCC). LM3 cell cycle distribution and cell’s proliferation may be disrupted by quercetin, which could lead to the death of the cells.[Citation72] Quercetin simultaneously aided HCC autophagy and decreased LM3 cells’ ability to migrate and invade. Quercetin’s ability to reduce JAK2 and STAT3 activation was at least partially responsible for these outcomes.[Citation73] In vitro, quercetin could markedly reduce the proliferative, migratory, and invading traits of HCC cells and increase cell death in a concentration-dependant way. Tumor volume and weight both markedly decreased in vivo after quercetin administration. The levels of G-CSF, GM-CSF and PD-L1 were all lowered by quercetin, as well as the levels of granulocyte colony-inducing factor and granulocyte-macrophage (G-CSF and GM-CSF, respectively).[Citation74] It is possible that quercetin causes macrophages to become more M1 polarized because the ratio of CD86+ cells elevated while the ratio of CD206+ cells dropped. In contrast to this, p62 expression was down-regulated whereas LC3 II/I expression was elevated. TNF-α, IL-6, IL-17A, and NF-κB signaling were all reduced in a quercetin concentration-dependant way, as were other pro-inflammatory markers.[Citation74] The in vitro and in vivo findings described here indicate that quercetin dramatically decreased hepatocellular carcinoma (HCC) growth, caused cell cycle arrest, and triggered necrosis and apoptosis whether used alone or in combination with sorafenib. Additional molecular results presented in this article suggest that quercetin alone or in amalgamation with sorafenib reserved the expression of important genes linked to inflammation, angiogenesis and proliferation (TNF-α, VEGF, P53, and NF-κB). Significant antioxidant and anti-tumor benefits were seen in combined sorafenib/quercetin treatment, which also significantly enhanced the morphology of the caused liver impairment.[Citation75] In a dose-dependant manner, quercetin dramatically reduced the movement of HuH7 cells triggered by both HGF and TGF. Myricetin, another flavonol, also showed a considerable reduction of cell migration. Quercetin and myricetin had no effect on the auto phosphorylation of any receptors that was activated by transforming growth factor (TGF) or hepatocyte growth factor (HGF). The phosphorylation of p38 MAPK brought on by HGF or TGF was not inhibited by quercetin. On the other hand, quercetin and myricetin prevented the phosphorylation of AKT brought on by growth factors.[Citation76] Seventy-two male Sprague-Dawley mouse were treated with selenium (Se) in the form of nanoparticles (SeNPs) and combined with quercetin (QCT) to prevent the development of hepatocellular carcinoma (HCC) as a result of thioacetamide (TAA)-induced HCC. This was accomplished through a variety of mechanisms, including increased levels of reduced glutathione (GSH).[Citation77,Citation78] Notch1 and Gli2 mRNA and protein expression were downregulated by quercetin, which also inhibited CK2. Caspase-3 but not Caspase-8 expression was likewise reduced by quercetin. Cyclin D1 and Ki-67, two indicators of cell proliferation and cycle were downregulated whereas p53 expression was increased by quercetin.
Albumin, bilirubin, alanine and aspartate aminotransferase (ALT, AST), alkaline phosphatase (ALP), liver γ-glutamyl transferase (GGT), and other indicators of hepatic cellular integrity were all dramatically improved.[Citation79] According to an in vitro investigation, quercetin showed a negligible cytotoxic effect on healthy hepatocytes while inhibiting the propagation of human HCC cells in a time- and dose-dependent way. Auto phagosomes and autolysosomes were also increased. By blocking the mTOR/AKT pathway and initiating the MAPK pathways, it promoted autophagy in part. Autophagy inhibitors were used in further functional experiments to show that quercetin stimulated autophagy in part to cause apoptosis in HCC cells. According to the in vivo research, quercetin considerably reduced the growth of tumors linked to the stimulation of apoptosis and the stimulation of autophagy, and the dominance of autophagy significantly lessened the influence of quercetin on the reduction of tumor development and the initiation of apoptosis.[Citation80,Citation81] HIF-1’s expression was downregulated and p53, the regulator of apoptosis, was expressed at higher levels as a result of the anti-cancer medication and quercetin combination. The efflux activity of MDR1 was also found to be inhibited by quercetin, which was another finding.[Citation82]
Gastric cancer
In human gastric tumor AGS cells, quercetin generated morphological alterations in the cells and decreased overall viability via inducing apoptotic cell death.[Citation83] It led to an elevation in the formation of reactive oxygen species (ROS), a decrease in the mitochondrial membrane strength (m), and an elevation in the proportion of apoptotic cells in AGS cells. Western blotting results revealed that quercetin boosted pro-apoptotic protein of Bax, Bad, and Bid while decreasing anti-apoptotic protein of Bcl-2, Bcl-x, and Mcl-1.[Citation84] Additionally, quercetin decreased the expression of VEGFB, (cyclin-dependent kinase 10) CDK10, and (KDEL [Lys-Asp-Glu-Leu] KDELC2, which are linked to cell death means, while increasing the expression of TP53INP1, TNFRSF10D, TP53INP1, and (jun B proto-oncogene) JUNB.[Citation83,Citation85] Quercetin’s effects on gastric cancerous cells were examined by researchers. They subjected human gastric cancerous cells (NCI-N87) to a 48-hour treatment with 15 M quercetin and a dimethyl sulfoxide control. Comparing (DEGs) differentially conveyed genes between clusters was done using the HiSeq 2500 DNA sequence data. The network of protein-protein interactions (PPI) was evaluated by a sophisticated technique. Cytoscape was used to clarify the directing network of (TFs-DEGs) transcription factors. The DEGS discovered included the aryl hydrocarbon response (AHR, degree = 12), (JUN, degree = 11) Jun proto-oncogene, the Fos proto-oncogene (FOS, degree = 12), and the cytochrome P450 family 1 subfamily A member (CYP1A1, degree = 11). These DEGS were knowingly related with other proteins in the PPI system with higher points. In contrast to AHR, which was downregulated, the five TF-DEGs had greater levels of (EGR1) Early Growth Response 1, (FOSL1) FOS like 1, JUN, FOS, additionally, the FOSL1, WNT7B and JUN, family members were supplemented in the Wnt signaling pathway. CYP1A1 and AHR were closely related in the PPI network. Because of this, it is possible that quercetin affected the GC enzymes FOS, JUN, AHR, EGR1, CYP1A1, FOSL1, and WNT7B.[Citation86]
Another study examined the ability of quercetin to trigger apoptosis, cause the death of human GC cells, and change gene expression in in vitro experiments. Their findings showed that quercetin could alter gene expression and trigger GC cell death. According to flow cytometry, quercetin enhanced the production of (ROS) reactive oxygen species, reduced the levels of a few specific proteins that protect the mitochondrial membrane, and ultimately caused death in AGS cells. According to the results of the Western blotting, quercetin elevated the levels of pro-apoptotic proteins such as Bid, Bax, and Bad while decreasing the levels of anti-apoptotic proteins such as Bcl-2, Bcl-x, and Mcl-1. Different changes in gene expression were brought about by quercetin. For example, quercetin reduced the expression of (KDEL [Lys-Asp-Glu-Leu] comprising 2) KDELC2F, (vascular endothelial growth factor B) VEGF-B, and (cyclin-dependent kinase 10) CDK10 but elevated the expression of (tumor protein p53 inducible nuclear protein 1) TP53INP1, (tumor necrosis factor receptor superfamily 10D, decoy with truncated death domain) TNFRSF10D, TP53INP1 and (jun B proto-oncogene) JUN-B. In the end, their research provided information on the molecular processes, gene expression, and signaling pathways that quercetin uses to cause cell death in human gastric cancer cells.[Citation83]
When mice were injected with EBV (-) human gastric cancer cells (MKN74) cells, isoliquiritigenin and quercetin both demonstrated anti-tumor effects that were comparable. Interesting, cancer tissues from mouse injected with EBV (+) human gastric cancer cells showed decreased expression of EBV viral proteins, including LMP-2 and EBNA-1 proteins, when quercetin was present. In EBV (+) human gastric cancer, quercetin more potently caused p53-dependent cell death compared to isoliquiritigenin, and this initiation was associated with elevated expression of the cleaved forms of caspase-9, −3, and Parp. Quercetin and isoliquiritigenin both similarly improved the expression of p53, Puma, and Bax, as well as the cleaved forms of caspase-9 and − 3 and Parp in EBV (-) human gastric cancer (MKN74).[Citation87]
As evidenced by the downregulation of Bcl-2, the initiation of caspase-9 and − 3, the decrease in mitochondrial membrane strength, the upregulation of Bax and (Cyt-c) cytochrome c, and the overexpression of caspase-3 and − 9, quercetin promoted cell death in gastric CSCs (GCSCs) in a way that was mitochondrial-dependent. In addition, a significant reduction in Akt phosphorylation intensities was seen after quercetin management, whereas fumonisin B1 (FB1, an Akt activator) pre-treatment expressively mitigated quercetin’s inhibitory impacts on cell development and its stimulating effects on mitochondrial-dependent cell death. Particularly, FB1 reversed the decline in mitochondrial membrane potential caused by quercetin and increased the production of Bcl-2, which was suppressed by quercetin. However, the adding of FB1 to the GCSCs also attenuated the rise in caspase, Cyt-c and Bax, and levels brought on by quercetin. As a result, findings show that quercetin inhibits mitochondrial development in GCSCs through inhibiting (PI3K) phosphoinositide 3-kinase -Akt signaling, suggesting a potential objective for the therapy of gastric tumor.[Citation88]
In both in vivo and in vitro experiments, the combination of quercetin with SN-38 (irinotecan (CPT-11)) resulted in reduced protein levels of -catenin up-regulation in the AGS human (GC) gastric cancer cell line. The combination of quercetin and low-dose irinotecan was more effective than high-dose irinotecan only in reducing the gene expression of cyclooxygenase-2 and markers related to the epithelial-mesenchymal transition, such as Twist1 and ITG-6, in the AGS xenograft mice model. In addition, mutual treatments with irinotecan and quercetin dramatically decreased the levels of angiogenesis-related factors (vascular endothelial growth factor (VEGF)-A and VEGF-receptor 2) and the proportion of Tie2-expressing monocytes.[Citation89] In vitro study, (SGC-7901) human gastric tumor cell line, quercetin presented reticence in cell growth, initiation of apoptosis, improved the quantity of apoptotic cells, stimulated caspase-8, which sliced Bid into tBid; instantaneously, Bax transported from cytosol into mitochondria to decrease MMP; inevitably, cytochrome c out from mitochondria started caspase-3, and then caspase-3 triggered caspase-9, which implemented the apoptosis in amount dependent way.[Citation90]
Cysteine-rich angiogenic activator 61 (CYR61) is a protein connected to the extracellular matrix that has a role in drug resistance, cancer, and survival. Due to their extraordinary resistance to 5-fluorouracil (5-FU), Adriamycin (ADR), tamoxifen (TAM), paclitaxel (PAC), and docetaxel (DOC), CYR61-overexpressing human gastric (AGS-cyr61) adenocarcinoma AGS cells were the target of the study on the effects of flavones. Quercetin significantly decreased the viability of AGS-cyr61 cells compared to AGS cells and had the lowermost 50% inhibitory dose (IC50) of any of the tested flavones. Quercetin reversed multidrug resistance (MDR); decreased levels of nuclear factor (NF)-kappa B p65 subunit and multidrug resistance-associated protein 1; reserved induced caspase-dependent cell death and colony formation; and downregulated levels of proteins related to the epithelial-mesenchymal transition in AGS-cyr61. Additionally, quercetin and 5-FU or ADR showed significant synergism in AGS-cyr61 cells managed with applications of quercetin close to the IC50 and instantaneously treated with these two drugs in the sub-lethal range.[Citation91]
It has been demonstrated that mycotherapy can lessen some of the negative effects of chemotherapy while also increasing the overall response rate during cancer treatment. A common mushroom used for therapeutic purposes is called Ganoderma lucidum. Several cancers may be resistant to the antitumor effects of G. lucidum extracts (GLE). GLE was shown to have tumor-inhibiting properties that were ascribed to its reduction of metastasis and cancer cell growth. EBV-associated gastric cancer, also known as EBVaGC, is considered by the monoclonal propagation of carcinoma cells with latent EBV inflammation. It is unclear whether GLE has any inhibitory effects on EBVaGC. The purpose of this review was to observe GLE as strong anticancer agents and a rival to quercetin for the cotreatment in preventing the development of EBVaGC. In order to determine whether GLE could inhibit the growth of tumors, this study performed antitumor experiments using a mouse model that had EBVaGC xenografts. Due to the modest concentration of quercetin treatment in the xenograft mice, these inhibitory effects were noticeably increased. Apoptosis produced by quercetin and EBV lytic reactivation were strengthened by the addition of GLE in low concentrations. The polysaccharide and triterpene ganoderic acid is one among those found in GLE. It’s interesting to note that adding ganoderic acid A (GAA) can have bioactive effects that are comparable to GLE’s in quercetin-mediated anticancer activity. Low concentrations of GAA added together strengthened the lytic reactivation of the EBV and the apoptosis induced by quercetin. As effective as GLE, GAA was also sufficient. Therefore, findings suggested that GLE with a quercetin supplement can be a useful food auxiliary in preventing the development of EBVaGC.[Citation92]
Oral cancer
Quercetin (5 M) efficiently stopped cell development, decreased glucose uptake, and hindered cellular insensitivity in two HSC-3-derived erlotinib-resistant cell lines, ERL-R10 and ERL-R5, used in xenograft mice models. Furthermore, quercetin-like effects from siRNA-mediated PKM2 knockdown re-sensitized ERL-R cells to erlotinib and mimicked the impacts of quercetin. Quercetin was also added, and via promoting apoptosis, it prevented the emergence of erlotinib-mediated resistance.[Citation93] In the CAL27 oral squamous cell carcinogenic line, quercetin inhibited glycolysis, glucose uptake, cell growth, and YWHAZ/G3BP1 signaling in a dose-dependent manner. G3BP1 overexpression reversed these effects. It appears that quercetin works through blocking the YWHAZ/G3BP1 axis to reduce glycolysis and cell propagation in oral squamous cell cancer.[Citation94] In YD10B and YD38 OSCC cells, quercetin reduced cell viability and brought on a G1 cell cycle arrest. In addition, quercetin markedly raised the expression of a CDK inhibitor and lowered the production of proteins that upregulate the cell cycle. In both varieties of OSCC cells, quercetin also markedly boosted the quantity of annexin-V-positive cells in a dose-dependent manner. Quercetin’s apoptotic potential caused PARP to be broken down, which then caused the p38 MAPK signaling pathway to be activated.[Citation95] On the basis of the outcomes of a cell feasibility assess, elevated Annexin PI/V staining, elevated production of reactive oxygen species (ROS) and Ca2+, reduced levels of mitochondrial membrane strength (m), improved proportion of apoptotic cells, and improved levels of apoptosis-associated protein expression in SAS cells, the rate of cell apoptosis elevated with the duration of quercetin therapy.[Citation96] Western blotting outcomes displayed that quercetin raised the levels of Fas-Ligand, Fas, fas-associated protein with death domain, and caspase-8, all of which were linked to the cell surface death receptor. In addition, quercetin boosted the amount of stimulating transcription elements ATF-6β, (ATF)-6α, and gastrin-releasing peptide-78 that showed a rise in endoplasm reticulum pressure, raised levels of the pre cell death protein BH3 conversing-domain death opponent, and reduced amounts of (Bcl) 2 anti-apoptotic proteins B-cell lymphoma and Bcl-extra-large that might have caused to the reduces of ΔΨm. Apoptosis-inducing element, endonuclease G, and cytochrome c concentrations were also able to be elevated by quercetin. These molecules are linked to apoptotic pathways.[Citation96] In oral cancer cells, quercetin decreased metalloproteinase-9 (MMP-9) and MMP-2 abundances as well as cell survival, movement, and invasion. The addition of quercetin reduced the level of microRNA-16 (miR-16) and reversed it. Furthermore, miR-16 overexpression reduced MMP-9 and MMP-2 abundances, cell migration, invasion, and viability in oral carcinomas. (HOXA10) Homeobox A10 was also under attack by miR-16, and its reestablishment reduced the impact that miR-16 had in the growth of oral cancer cells. Additionally, quercetin’s effect on oral cancer advancement was prevented by miR-16 knockdown. By controlling HOXA10 and miR-16 in oral carcinomas, quercetin decreased cell feasibility, migration, and incursion.[Citation97]
In (OSCC) oral squamous cell carcinoma, quercetin can control miR-22 and its downstream pathway WNT1/-catenin to exert its anti-tumor effects. In OSCC, quercetin therapy and miR-22 domination lead to a decrease in cell feasibility and an elevation in cell death rate, according to the results of (CCK-8) Cell Counting Kit-8 and flow cytometry studies. Researchers used bioinformatics, RNA immunoprecipitation (RIP) tests and luciferase reporter, and to demonstrate that WNT1 was a miR-22 target. Quercetin amplified the expression of miR-22 and reduced the expression of WNT1/-catenin in OSCC cells, according to a test using real-time quantitative PCR. An inhibitor of miR-22 reversed this effect. Additionally, miR-22 deletion reduced the potency of quercetin’s ability to suppress viability and enhance apoptosis in OSCC cells. By upregulating miR-22 expression and downregulating the WNT1/-catenin pathway in vivo, quercetin reduced the development of OSCC xenograft tumors.[Citation98]
As well as down-regulating CD36 and up-regulating miR-1254, quercetin dramatically reduced the proliferativeness and incursion of CAL-27 cells in a dosage-dependent way. CAL-27 cell invasion and viability were significantly inhibited by quercetin, and CD36’s expression was similarly markedly downregulated by the overexpression of miR-1254. Agonizing the inhibitory effects of quercetin, the suppression of miR-1254 dramatically increased CD36 expression.[Citation99] By causing cell cycle arrest during the G2/M stage, quercetin reduced OSCC cells’ survival. Quercetin did not, however, have an impact on the ability of human keratinocytes such (immortal keratinocyte) HaCaT and (primary normal human oral keratinocyte) nHOK cells to maintain their vitality. In addition, quercetin reduces TGF-1-induced EMT in HaCaT cells and inhibits cell migration in OSCC cells through inhibiting EMT and (MMP) matrix metalloproteinase.[Citation100,Citation101]
Cervical cancer
In vitro and in vivo tumor-suppressive miRNAs exist in quercetin, which is effective against cervical cancer. The tumor-suppressive miR-126, miRNAs miR-26b, and miR-320a have them in vivo and in vitro expression upregulated by quercetin. Through the upregulation of miR-320a in HeLa cells, quercetin decreased the amount of WNT1/-catenin, which is programmed by (CTNNB1) catenin beta 1. The expression of the progenitors of the mir-126, mir-26b, and mir-320a genes was also elevated in HeLa cells by quercetin.[Citation102] In order to boost quercetin loading ability, dissociation, transport, and therefore bioavailability in cervical tumor cells, (SBE-CD) chitosan/sulfonyl-ether-cyclodextrin conjugated delivery systems have been investigated in this study. Using two kinds of chitosan with multiple molecular weights, SBE-CD/quercetin insertion complexes and SBE-CD/chitosan/quercetin-conjugated delivery systems were both investigated. Regarding categorization studies, HMW SBE-CD/chitosan/quercetin preparations showed the top outcomes, with nanoparticle sizes of 272.07 2.87 nm, a (PdI) polydispersity index of 0.287 0.011, a zeta potential of + 38.0 1.34 mV, and an encapsulation capability of roughly 99.9%. Studies on the discharge of quercetin in vitro for formulations of 5 kDa chitosan showed that at pHs of 7.4 and 5.8, respectively, the release of quercetin was 9.6% and 57.53%. HMW SBE-CD/chitosan/quercetin delivery systems’ IC50 values on HeLa cells demonstrated an improved cytotoxic effect (43.55 M), indicating a notable improvement in quercetin bioavailability.[Citation103]
Cyclin D1 exhibited a propensity to decline gradually, whereas Bax and Bcl-2 levels in the quercetin intervention group showed a propensity to gradually elevate when compared to the blank control group. In comparison to the control group that received no quercetin, the expression levels of Caspase-3, glycoprotein 78 (GRP78), and (CHOP) enhancer-binding protein homologous protein significantly increased (P 0.05). Comparing the quercetin intervention group to the standard group without quercetin, the levels of IRE1, p-Perk, and c-ATF6 revealed a tendency to rise gradually.[Citation104] SiHa and HeLa cell assault was reduced by quercetin in a time- and dose-dependent fashion. The CI values for quercetin with paclitaxel, cisplatin, doxorubicin, and 5-fluorouracil were, respectively, 1, >1, >1, and > 1. Quercetin and cisplatin together had a more potent impact on cell growth than either substance alone. In comparison to a group receiving a single drug, the co-treatment group could reduce further cell invasion and migration. In addition, compared to the single-drug group, the cisplatin and quercetin combination group increased the amount of cell death. Western blotting results revealed that the expression levels of Ezrin, MMP2, METTL3 and P-Gp in the co-treatment group were, correspondingly, lesser than those in the cisplatin group.[Citation105] The propagation of cervical tumor cells was markedly reduced by quercetin. Combining the studies of the GEO database with the RNA-seq results yielded 74 (DEGs) differentially expressed genes, and the functional enrichment experiment of the DEGs revealed 32 cellular components, 861 biological processes, 56 KEGG pathways and 50 molecular functions. A total of five therapeutic candidate genes, including JUN, EGFR, CD44, AR, and MUC1, were chosen. Ten miRNAs, 71 circRNAs and one lncRNA, were found to be upstream of these genes. A network of regulating lncRNAs, circRNAs, miRNAs, and mRNAs was created in the end.[Citation106] When compared to its impact on HaCaT cells, the anticancer effects of quercetin on HeLa cells are demonstrated. Using MTT and deoxy uridine triphosphate nick-end labeling tests that are catalyzed by terminal deoxy nucleotidyl transferase, cell viability and mortality were quantified. By using immunoprecipitation and succinylated wheat germ agglutinin pulldown, the O-GlcNAcylation of (AMPK) AMP-activated protein kinase was investigated. O-linked N-acetyl glucosamine transferase (OGT) and sterol regulatory element binding protein 1 (SREBP-1) were stained with immunofluorescence to identify their immunoreactivity. Its effects on HaCaT cells were less than those on HeLa cells, but quercetin nevertheless reduced cell development and caused cell apoptosis in both types of cells. HeLa cells had higher levels of O-GlcNAcylation than HaCaT cells did. The O-GlcNAcylation of AMPK is lowered by quercetin, which also decreases the expression of global O-GlcNAcylation and increases AMPK initiation. Treatment with 6-diazo-5-oxo-L-norleucine confirmed that the reduced O-GlcNAcylation of AMPK caused its activation.[Citation107]
Quercetin slows colony growth, inhibits the G2-M cell cycle, causes DNA impairment, and promotes cell death. It also diminishes cell viability and colony formation. The antioxidant quercetin causes apoptosis by triggering both apoptotic pathways, with the extrinsic pathway having a higher impact due to the combined strength of FASL, TRAIL, and TNF as well as the up-regulation of caspases and pro-apoptotic genes. By studying how proteins bind, quercetin may be able to suppress anti-apoptotic proteins. The MAPK, PI3K, and WNT pathways are also blocked by quercetin.[Citation108] In addition to promoting cell cycle assault in the G2/M stage and cell death, quercetin also decreased the viability of cervical cancer cells while preventing their migration and invasion. Quercetin significantly activated the two kinases at the EGFR Tyr1068 phosphorylation site and the associated ERK target, both of which were studied. Afatinib, an EGFR inhibitor, and U0126, an ERK inhibitor, also prevented the phosphorylation of the EGFR and ERK proteins from rising, which markedly increased apoptosis and cell cycle arrest.[Citation109] Quercetin nanoparticles (NQ) were given to cells as part of this investigation to look into the fundamental mechanisms controlling cervical cancer. In order to conduct in vivo and in vitro investigations, JAK2-inhibited cervical cancer cell lines were utilized. The main signaling pathway controlled by JAK2 for cervical cancer development was identified using Western blotting, quantitative (qRT-PCR) RT-PCR, ELISA, flow-cytometric analysis and Immunohistochemistry. Additionally, the process led to the identification of the function of quercetin nanoparticles. The information presented here showed that, in contrast to healthy cervical cells, cancer cell lines did indeed exhibit high levels of JAK2. Additionally, JAK2-inhibited cancer cells were found to undergo apoptosis and autophagy by triggering Caspase-3, inhibiting mTOR and Cyclin-D1, which were controlled by the PI3K/AKT and STAT 3/5 signaling pathways, autophagy and apoptosis were also observed in JAK2-inhibited tumor cells. It stopped the multiplication of cervical cancer cells. Additionally, JAK2 inhibition was demonstrated in vivo to reduce tumor size and weight. Notably, treatment of quercetin nanoparticles had a comparable role with JAK2 inhibition, which could prevent cervical tumor cells from proliferating, migrating, or invading. Apoptosis and autophagy were also stimulated, encouraging the death of cervical cancer cells.[Citation72]
Uterine cancer
In leiomyoma and myometrial cells, quercetin significantly reduced the levels of collagen 1A1 mRNA. In myometrial cells and leiomyoma cells, quercetin decreased the amounts of fibronectin protein and mRNA. In leiomyoma and myometrial cells, I3C lowered the levels of collagen 1A1 mRNA at higher doses. Even while mRNA levels in leiomyoma cells were unaffected, the protein levels of fibronectin were also decreased in leiomyoma and myometrial cells with the highest dose of I3C. The levels of vertical mRNA in both cell types were unaffected by quercetin or I3C treatment. In response to quercetin and I3C therapy, it was shown that both myometrial and leiomyoma cells’ migration was significantly reduced. In a considerable way, quercetin and I3C both slowed the growth of myometrial cells.[Citation110] It caused cell death, reduced EC cell proliferation and migration, and had an impact on the cell cycle. Additionally, the induction of ferroptosis in the EC cells was linked to the anti-tumor effect of quercetin.[Citation111]
Lung cancer
Over the course of five weeks, quercetin was capable to significantly reduce the propagation of the cells exposed to as. Additionally, the lung cancer cells treated with as also experienced quercetin-induced reactive oxygen species-mediated DNA double-strand breaks. It was also demonstrated that reactive oxygen species production generated by quercetin initiated caspase-3 to a level adequate to cause DNA impairment but inappropriate to cause cell apoptosis in lung cancer cells managed with aspirin. In addition, cells treated with quercetin and aspen were found to temporarily activate the caspase-2 enzyme. According to the flow cytometry-based cell cycle study, the anti-proliferative effect of quercetin was facilitated by S-phase cell cycle seizure, which was connected to an increase in the Ataxia Telangiectasia-mutated (ATM), but not to RAD3 and ATM related proteins.[Citation112] Both H1299 and A549 cells were subjected to a dose-dependentreticence of cell viability and activation of mitochondria-dependent cell death by quercetin. In addition, quercetin reduced the expression of p62 and increased that of LC3-II, beclin 1, and other molecules. By using quercetin, it was possible to increase the expression of LC3-II, beclin, Atg5, Atg12, and Atg7. Quercetin-induced apoptosis might be successfully countered by 3-methyladenine-induced autophagy suppression. The SIRT1 protein level and the AMPK-pAMPK ratio were both elevated by quercetin in a dose-dependentway. EX527, a SirT1 knockdown and SIRT1 inhibitor via (siRNA) small interfering RNA both decreased the autophagy that quercetin elicited.[Citation113]
For non-small-cell lung cancer (NSCLC) patients with activating EGFR alterations, (TKIs) tyrosine kinase inhibitors of the epidermal growth factor receptor (EGFR) are approved therapies. One of the recognized mutations that has developed acquired avoidance to the most recent third-generation TKIs is the EGFR C797S alteration. Treatment preferences for patients who develop resistance to third-generation tyrosine kinase inhibitors are currently unclear. A receptor tyrosine kinase AXL from the (TYRO3-AXL-MER) TAM family, was shown to express itself more frequently after acquiring the EGFR C797S alteration, and the expression of AXL can be suppressed to slow the development of NSCLC cells that had the EGFR C797S mutation. Due to quercetin’s lately discovered ability to suppress AXL, it may be useful for treating NSCLC cells that have the EGFR C797S mutation. In this study, TKI-resistant NSCLC cells with the EGFR C797S mutation were used to study the cytotoxic impacts of quercetin and its capacity to prevent tumor development. Through the inhibition of AXL and induction of apoptosis, they showed that quercetin had strong cytotoxic impacts on NSCLC cells that had the EGFR C797S alteration. When combined with brigatinib, quercetin appeared to work synergistically to reduce the development of cancers in vivo. This was demonstrated in xenografted NSCLC cells that had the EGFR C797S alteration. To sum up, they have shown that quercetin is a useful inhibitor for treating non-small-cell lung cancer that has the EGFR C797S mutation in this article.[Citation114] In radiation-resistant cells, WEE1 expression increased whereas miR-16-5p expression decreased. According to dose and duration, quercetin increased the radio-sensitivity of NSCLC cells. In addition, miR-16-5p expression was up whereas WEE1 expression was lowered after quercetin treatment. MiR-16-5p transfection or inhibitors of plasmids that overexpress WEE1 were able to reverse the effects of quercetin.[Citation115,Citation116]
Following that, a lung cancer xenograft model in mice was used to confirm quercetin’s in vivo antitumor activity. The mice’s body weight was unaffected by 200 g/mL quercetin’s considerable reduction in tumor volume. Moreover, quercetin-treated tumor tissues showed evidence of the compound’s ability to induce apoptosis. In tumor tissue, quercetin raised the expression of numerous cell death-related genes, such as p53, Bax, and Fas, as well as the ratio of Bcl-2/Bax. Results indicated quercetin’s important inhibitory impact on (NSCLC) non-small cell lung cancer by modulating the Bax/Bcl-2 ratio as the key active component of the YYQFT.[Citation117,Citation118] In comparison to Erlotinib with Carnosic Acid/Fisetin/Luteolin, they found that the combination of Erlotinib and Quercetin is more simultaneous against NCI H460 and A549 cells. By adopting the heat homogenization process with Chitosan-MA-TPGS polymer, Quercetin and Erlotinib loaded solid lipid nanoparticles were created to significantly increase efficacy and get beyond the limitations of free therapies. The drug-loaded (NPs) nanoparticles shown a high degree of encapsulation (71.4% Quercetin and 77% Ertb), a tiny particle magnitude of 87.3 0.78 nm, and a +ve zeta potential of + 13.4 1.12 mV. Quercetin and Ertb were discharged at their greatest concentrations at pH 5.5. They discovered that EQNPs reduced the expression of (nEGFR) nuclear epidermal growth factor receptor and P-glycoprotein (P-gp). In Ertb-resistant A549/ER cells, EQNPs boosted the absorption of Ertb and Quercetin and the triggering of apoptosis. Additionally, in vivo testing of the EQNPs formulation revealed increased nanoparticle uptake in the lung tissue and noticeably decreased nEGFR expression. As a result, EQNPs may be created as a targeted drug with few side effects for the treatment of NSCLC to increase the survival and quality of life of NSCLC patients.[Citation119] At 24 and 48 hours after treatment, quercetin inhibits cell viability in the lung cancer cell line (A549) in a dose-dependent (10–80 M) manner. While TIMP-2 expression elevated after quercetin management, the expression of the EMT marker proteins Akt/MAPK/-catenin and was significantly reduced. A-549 cells’ invasion and migration are suppressed by quercetin. Additionally, the results of the immunocytochemistry demonstrated that quercetin can decrease the nuclear Trans localization of -catenin in A549 cells.[Citation120] When compared to free quercetin powder (Q) in vitro, novel quercetin-loaded micro particles (QM) performed at a much faster release rate. Additionally, compared to quercetin, QM induced more G0/G1 phase cell cycle arrest and more potent inhibitory effects on A549 cell growth, resulting in decreased cell viability, decreased cell migration, and reduced cell migration.[Citation121]
Programmable cell death is classified as ferroptosis, which triggers oxidative cell death in a way that depends on lipid peroxides and iron. An innovative method of treating cancer is by targeting ferroptosis. The most common type of cancer that results in mortality worldwide is lung cancer. In the realm of cancer research, circular RNAs (circRNAs), a type of noncoding RNA having a particular closed circular sequence, are becoming more and more important. However, it is still difficult to pinpoint the circRNAs’ regulatory mechanisms in ferroptosis during the growth of lung cancer. In this study, they clarify how the circular RNA circFOXP1 plays a critical role in the ferroptosis of lung cancer as well as its potential predictive usefulness. When compared to tissues nearby, they discovered that clinical lung sample tissues had a noticeably higher expression of circFOXP1. It was found that patients with lung cancer had a low overall survival rate when their circFOXP1 levels were up-regulated. Cell viability in lung cancer cells was reduced by circFOXP1 knockdown. Depletion of circFOXP1 also reduced the number of lung cancer cells that form colonies. Silencing circFOXP1 resulted in a reduction of the Edu-positive lung cancer cells. CircFOXP1 short hairpin RNA (shRNA) inhibited the invasion and migration of lung cancer cells. By knocking down circFOXP1, E-cadherin expression was increased whereas vimentin expression was decreased. The lung cancer cells’ ability to survive was further suppressed by the use of the ferroptosis activator erastin or RSL3, and the overexpression of circFOXP1 restored the phenotype. Silencing of circFOXP1 in lung cancer cells induced by erastin and RSL3 increased the levels of iron, lipid reactive oxygen species (ROS), and malondialdehyde (MDA). Through direct miR-520a-5p sponging in lung cancer cells, circFOXP1 mechanically increased SLC7A11 expression. CircFOXP1 shRNA-induced ferroptosis phenotypes in lung cancer cells were rescued by miR-520a-5p inhibitor or SLC7A11 overexpression. SLC7A11 was improved in vivo by circFOXP1, which was significant in its contribution to the development of lung tumors.[Citation122]
In comparison to normal cells, the expression levels of the microRNA miR-34a-5p were lower and the long non-coding RNA (lncRNA) small nucleolar RNA host gene 7 (SNHG7) was expressed at higher levels in NSCLC cells. Quercetin lowered SNHG7 and increased miR-34a-5p levels in NSCLC cells, according to another research. Target-binding sequences between SNHG7 and miR-34a-5p were discovered by transfection investigations, luciferase reporter gene assays, and RNA-binding protein immunoprecipitation assays. Inhibition of miR-34a-5p and dominance of SNHG7 amplified the growth and spread of cancer cells and endorsed the proliferation of NSCLC cells. Co-transfection of an inhibitor of miR-34a-5p or an SNHG7 mimic blocked the therapeutic effect of quercetin on NSCLC cells. In addition to enhancing apoptosis, quercetin prevented NSCLC cells from surviving, proliferating, migrating, and invading. Quercetin has been demonstrated to stop tumor growth in the mouse model.[Citation123]
Brain cancer
By promoting cell apoptosis in vitro, quercetin administration reduced GBM cell proliferation. In vivo, quercetin enhanced survival rates without causing damage to nearby organs, reduced the growth of GBM, and restored spontaneous locomotion. The glycolytic metabolism in tumor tissue was also reduced by quercetin.[Citation124,Citation125] While maintaining 85% of healthy astrocytes alive, quercetin treatment significantly decreased cell viability in the two GBM cell lines U87MG and U373MG. Further western blot studies revealed that quercetin promotes apoptosis but has no effect on Akt or mitogen-activated protein kinases, which are components involved in cell proliferation. IL-6 production and STAT3 phosphorylation in GBM cells were both reduced by quercetin. Quercetin also inhibited the half-life of the produced Axl protein as well as the expression of genes, proteins, and proteins. ScientistsTheywere able to establish that Axl’s function was essential for the apoptotic effect of quercetin on GBM cells by using shRNA to knock down Axl. By enhancing the malignant microenvironment of GBM through the Axl/IL-6/STAT3 pathway, it was concluded by recommending quercetin as a possible anticancer drug.[Citation126] Dietary polyphenols may offer protection from a number of malignancies, according to a growing body of research. Their involvement in brain tumors, however, is still largely unknown. In this study, they examined how the human glioblastoma A172 and LBC3 cell lines responded to the dietary flavonoid quercetin (QCT). In both of the cell lines that were examined, they researchers showed that quercetinelicited cytotoxic effects. Microscopic findings, Annexin V-FITC/PI staining, and increased expression and activity of caspase 3/7 demonstrated that quercetin primarily led to apoptotic death of A172 cells. Additional investigations supported the activation of oxidative stress and ER stress following quercetin exposure, as evidenced by increased ROS generation, deregulated expression of SOD1 and SOD2, depleted ATP levels, and an overexpression of CHOP. Last but not least, it was found that caspase 9 expression and activity were enhanced, which is a sign that apoptosis is occurring via a mitochondrial mechanism. In contrast, the pro-apoptotic effect in LBC3 cells was not seen until 24 hours following treatment with quercetin, and a change toward necrotic cell death was not seen until 48 hours. According to all of the data, quercetin exposure caused A172 cells to undergo intrinsic apoptosis, which is a form of cell death.[Citation127]
In this investigation, (lactic-co-glycolic acid) quercetin-loaded poly nanoparticles (Qu2NP, Qu1NP, and Qu3NP) of various sizes and encapsulated qualities were created, characterized, and tested for their effects on C6 glioma cells in vitro. The single emulsion solvent evaporation process was used to create nanoparticles. Then, it was determined what the nanoparticles’ zeta potential particle size, encapsulation effectiveness and polydispersity index were. The particle sizes of Qu2NP, Qu1NP, and Qu3NPs were found to be 282.3 7.9, 215.2 6.2, and 584.5 15.2 nm, correspondingly. All nanoparticle formulations were effective at reducing the rate of cell division in C6 glioma cells. In comparison to other nanoparticles and Quercetin, Qu1NPs demonstrated the lowest IC50 value in 48 hours at 29.9 g/ml and had a greater level of cellular absorption. Qu1NPs therapy for 48 hours also resulted in a significant reduction in MDA levels in C6 glioma cells (14.90 nmol/g protein), which is linked to lower oxidative pressure in cells. The results of this research showed that in C6 glioma cells, small-sized Qu1NPs enhance quercetin’s cellular absorption and anti-oxidant activities.[Citation128] In both in vitro and in vivo experiments, quercetin greatly reduced the viability of glioblastoma cells, as well as their ability to migrate and invade. In addition, quercetin inverted the EMT-alike mesenchymal phenotype and decreased the expression of EMT-related indicators. In glioblastoma, quercetin was also observed to inhibit GSK-3/-catenin/ZEB1 signaling.[Citation129]
Inhibiting migration and inducing apoptosis were two of the anticancer effects of the quercetin-loaded polymeric mixed micelles (Qu-PMMs) on the glioma cell lines C6 and U87MG.[Citation130] In rat C6 and human T98G glioblastoma cells, the quercetin (25 M) and sodium butyrate (NaB) (1 mM) combination increased apoptosis via inhibiting autophagy in the presence of nutritional deprivation. In the cells under nutrient starvation, both demonstrated a sharp decline in protective autophagy. Expression of Beclin-1 and LC3B II dropped along with autophagy, which was associated with membrane blebbing, nuclear fragmentation, and chromatin condensation, combination therapy exacerbated the morphological symptoms of apoptosis. By reducing pro-apoptotic Bax and raising pro-apoptotic Bcl-2, decreasing survivin, activating caspase-3, and degrading poly (ADP-ribose) polymerase (PARP), the combination of quercetin and sodium butyrate promoted apoptosis.[Citation131]
Blood cancer
Quercetin is a bioflavonoid obtained from fruits and vegetables that has anti-inflammatory properties in a variety of disorders. In human retinal pigment epithelial (ARPE-19) cells activated by tumor necrosis factor (TNF), earlier work found that quercetin may inhibit the production of matrix metalloprotease-9 (MMP-9) and intercellular adhesion molecule-1 (ICAM-1) to produce anti-inflammatory effects. The goal of the current investigation was to determine whether quercetin can reduce the amount of inflammatory cytokines and chemokines that ARPE-19 cells produce when interleukin-1 (IL-1) is present. ARPE-19 cells were pretreated with quercetin at a range of doses (2.5–20 M) before being stimulated by IL-1. The outcomes demonstrated that quercetin might, in a dose-dependent manner, lower ICAM-1, IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) protein and mRNA levels. Additionally, it reduced the adhesion of the human monocytic leukemia THP-1 cell line to ARPE-19 cells that had been stimulated by IL-1. The phosphorylation of mitogen-activated protein kinases (MAPKs), inhibitor of nuclear factor b kinase (IKK)/, c-Jun, cAMP response element-binding protein (CREB), activating transcription factor 2 (ATF2), and nuclear factor (NF)-B p65, as well as the blocking of NF-κB p65 translocation into the nucleus, were all shown to be affected by quercetin. Additionally, the expression of soluble ICAM-1 (sICAM-1), but not ICAM-1, was reduced by MAPK inhibitors such as an extracellular signal-regulated kinase (ERK) 1/2 inhibitor (U0126), a p38 inhibitor (SB202190), and a c-Jun N-terminal kinase (JNK) inhibitor (SP600125). SP600125 was unable to reduce the expression of IL-6, IL-8, or MCP-1, whereas U0126 and SB202190 could. Additionally, the expression of ICAM-1, sICAM-1, IL-6, IL-8, and MCP-1 was decreased by an NF-κB inhibitor (Bay 11–7082).
Together, these findings show that quercetin protects ARPE-19 cells by preventing the activation of the MAPK and NF-κB signaling pathways to reduce the inflammatory response and reduce the increase in ICAM-1, sICAM-1, IL-6, IL-8, and MCP-1 production that is induced by IL-1. In order to create nanoemulsions, theyutilized high-pressure homogenization; to measure cytostatic/cytotoxicity, used Trypan Blue was used; to detect apoptosis and DNA damage, they used an epifluorescence microscope and particular probes; to measure gene expression; to dock proteins using AutoDock Vina; and to analyze efflux, researchers used flow cytometry. According to,[Citation132] quercetin had an antiproliferative effect, caused cell necrosis, apoptosis, and DNA damage. The outcomes suggested that KG-1 cells could become more sensitive to TRAIL-induced apoptosis by being treated with quercetin. By downregulating the expression of the NF-κB subunit, antiapoptotic proteins, and death receptor messenger RNA, as well as upregulating the expression of death receptor genes, this result is attained.[Citation133] Acute myeloid leukemia (AML) is one of the most prevalent hematopoietic progenitor cell cancers in adults. Because of its anti-cancer efficacy and low toxicity, quercetin has become more well-known over time. In this article, scientists seek to understand the signaling pathway of quercetin in HL-60 leukemic cells as well as the anti-leukemia mechanism of quercetin. In this research, it was found that quercetin triggers apoptosis and autophagic cell death, both of which are crucial for decreasing the survival of leukemia cells. Primary AML cells, HL-60 cells, KG-1 cells, and THP-1 cells all express less phosphorylated AMPK (p-AMPK) protein than normal cells in peripheral blood. Following quercetin administration, p-AMPK expression is elevated while p-mTOR expression is, in a dose-dependent manner, decreased. In HL-60 cells exposed to quercetin, compound C, an AMPK phosphorylation inhibitor, increases the phosphorylation of mTOR and decreases autophagy and apoptosis, while CaMKK silencing prevents the quercetin-induced phosphorylation of AMPK, which leads to an increase in mTOR phosphorylation. Additionally, CaMKK silencing prevents HL-60 cells from undergoing autophagy. Findings show that quercetin inhibits cell viability while causing apoptosis and autophagy in leukemia cells, which is how it fights leukemia. By controlling the activity of AMPK, quercetin prevents mTOR from being phosphorylated, which in turn affects the regulation of autophagy and apoptosis. In HL-60 cells, quercetin induces autophagy via the AMPK/mTOR signaling pathway, which may have an upstream protein called CaMKK.[Citation134] AML is a blood cancer that is aggressive and is characterized by an increase of immature and defective myeloid cells that are highly proliferative. One form of flavonoid, quercetin (Qu), has been shown to have anti-cancer properties in a variety of solid tumor types. However, the effects of quercetin on acute myeloid leukemia have received less research attention, and the underlying processes are still completely unknown. This investigation sought to identify the precise mechanism and target of quercetin-induced cell death in AML. First, it was discovered that quercetin causes caspase-dependent apoptosis, a sort of cell death, which is induced by the compound. Second, it was discovered that declines in mitochondrial membrane potential (MMP) and Bcl-2 proteins are necessary for quercetin-induced apoptosis. They observed the downregulation of PI3K/Akt and VEGFR2 signaling in cells treated with quercetin using quantitative chemical proteomics. The VEGFR2 and PI3K/Akt signaling pathways are implicated in the impact of quercetin on mitochondria and Bcl-2 proteins, according to cell experiments that have repeatedly shown this. It is possible to reverse the decrease in MMP and cell death when PI3K/Akt signaling is triggered, indicating that VEGFR2 and PI3K/Akt operate as upstream regulators for quercetin’s effect on inducing apoptosis in AML cells. Finally, findings from this study provide strong evidence that quercetin causes cell death in AML cells by suppressing the VEGF/Akt signaling pathways and mitochondria-mediated apoptosis.[Citation135]
In K562 cells, the quercetin and curcumin combination exhibited anti-proliferative and apoptotic effects. On genes specifically linked to the p53, NF-κB, and TGF- pathways, the quercetin and curcumin combination was effective. A multi-targeted therapy for chronic myeloid leukemia cancer cells without harming healthy cells may be possible due to the impacts of down-regulatory (CDKN1B, AKT1, IFN-) and up-regulatory (BTG2, CDKN1A, FAS) effects on gene and related protein expressions.[Citation136] Pro-inflammatory NF-kB p65 levels were elevated in the nucleus and decreased in the cytoplasm in human leukemia NB4 cells after being exposed to 25 M quercetin for 24 hours. As well, quercetin decreased the expression of lipoxygenase. Nrf2 levels were lower in the nucleus after consuming quercetin than they were in the cytoplasm.[Citation137]
Colon cancer
A dose-dependent inhibition of colon cancer cell proliferation was seen after quercetin therapy in colon tumor cells. By controlling the expression of aging proteins like Sirtuin-6 and Klotho as well as by blocking telomerase activity to limit the telomere length, as shown by qPCR analysis, quercetin stopped the growth of colon cancer cells. By lowering proteasome 20S levels, quercetin demonstrated DNA damage prevention as well. The results of the miRNA expression profiling showed that distinct miRNAs were expressed in colon cancer cells, and that a substantially elevated miRNA was also implicated in the control of transcription, cell cycle, and proliferation.[Citation138,Citation139] By changing how hormones operate physiologically, quercetin protected colon HT-29 cells from being triggered by bisphenol A. Quercetin can control signaling trials via hormone receptors, which inhibits carcinomas. Treatments with Q+BPA and quercetin caused cell cycle seizure in the G0/G1 stage, while treatments with FEQ+BPA and FEQ lead to cell cycle arrest in the S phase. Quercetin positively regulated the ESR2 and GPR30 genes in comparison to other therapies. Q+BPA, quercetin, FEQ+BPA and FEQ, positively regulated genes included in apoptosis and cell cycle arrest and cell death using a gene microarray of the p53 pathway; bisphenol suppressed the expression of cell cycle repressor genes and pro-apoptotic.[Citation140]
Aspirin and quercetin may be effective against cancer, particularly colorectal cancer, according to mounting data. In this study, chitosan nanoparticles were loaded with polyethylene glycol 4000 and Pluronic F127 solid distribution for the transport of aspirin and quercetin to the colon. Solubility and complex formation were confirmed in a 1:1 stoichiometric polymer ratio. Ideal conditions led to the production of solid dispersion-loaded chitosan nanoparticles with dimensions of 244.45 8.5 nm, 34.1 3.3 mV on the surface, and 76.3 4.3% encapsulation efficiency. It has been observed that coating with Eudragit L100 occasionally resulted an elevation in particle size and a reduction in zeta potential. The coated formulation, taking into account the physicochemical characteristics of the actives, discharged them in a pH-dependent way. Surprisingly, IL-8 and PGE2 production in colonic tissues were significantly reduced by the coated formulation as comparison to the actives’ uncoated formulation and suspension, with the coated formulation having better anti-inflammatory activity (16.9 7.9 ng/g tissue and 134.9 10.1 pg/g tissue, correspondingly). Additionally, the majority of the colon’s histological changes brought on by dimethyl hydrazine were reversed. TNF expression in colonic tissues was likewise shown to be significantly reduced. Therefore, it is suggested that Eudragit L100-coated QT/AS-loaded chitosan NPs might offer a potential platform for colonic delivery of quercetin and aspirin.[Citation141]
The amount of TUNEL-positive cells, as well as the immunoreactivities for lamin B1, p16, and cyclin B1 in both Colo-741 and Colo-320 cells, increased after quercetin administration (25 g/ml for 48 hours) to both Colo-741 and Colo-320 cell lines.[Citation142] The administration of quercetin significantly decreased the quantity and size of colon tumors in the mouse model of colon cancer generated by azoxymethane/dextran sodium sulfate (AOM/DSS). In addition, by reducing the inflammation brought on by the AOM/DSS-induced colon tumor rat model, quercetin considerably increased the leukocyte numbers. Additionally, it was found that quercetin could reduce the expression of oxidative tension markers like (LPO), lipid peroxide, (NO) nitric oxide, (SOD) superoxide dismutase, (G6PD) glucose-6-phosphate, and (GSH) glutathione, indicating that the antioxidant effect of quercetin is what causes it to have an anti-inflammatory effect. Additionally, using serum metabolite profiling, potential biomarkers were found. Additionally, the mice treated with AOM/DSS had higher levels of 2-aminobutyrate, 2-hydroxybutyrate, and 2-oxobutyrate and lower levels of indole-3-methyl acetate, gentian violet, N-acetyl-5-hydroxytryptamine, indoxyl and indoxyl sulfate. However, 2-hydroxybutyrate, 2-aminobutyrate, 2-oxobutyrate, endocannabinoids, and sphinganine levels were successfully decreased by quercetin treatment, while gentian violet, N-acetyl-5-hydroxytryptamine, indoxyl sulfate, and indoxyl levels were successfully elevated.[Citation143]
Compared to the control and healthy groups, quercetin use ((10 mg/kg) decreased the cytological alterations in the colon cancer cells. Comparing the quercetin group to the control group, there was a significant rise in the expression of caspase 3 and a decrease in the expression of beta-catenin and Bcl-2 proteins.[Citation144] The expression of all five proteins of the -secretase network was considerably decreased in DLD-1 and HT-29 cells of human colon tumor xenografts in naked mouse when low dose radiation and quercetin were combined. The inhibitory effects of the combo therapy were also partially reversed by ectopic expression of the (NICD) Notch intracellular domain. As a result, these findings suggested that, by concentrating on colon tumor stem-like cells and blocking Notch-1 signaling, the arrangement of quercetin (20 M) and IR (5 Gy) would be a potential beneficial approach for colon tumor treatment.[Citation145] For three different periods (24, 48, and 72 hours), quercetin (40–200 M) was applied, and IC50 doses were calculated. The effective dose was established and used for various analyses, such as staining with BrdU to look into cell proliferation, terminal deoxynucleotidyl transferase dUTP nick and labeling (TUNEL) to look into apoptosis, and caspase-3 and Apoptosis Inducing Factor (AIF) to look into caspase-dependent or independent apoptotic pathways. In both 2D and 3D cultures of the SW480 cell lines, the quercetin treatment groups displayed increased cell mortality and less cell growth in comparison to the untreated control groups.[Citation146] These findings demonstrated that quercetin and 5-FU together had a stronger cytotoxic efficacy than 5-FU alone. In comparison to 5-FU only, co-administration of both medications increased apoptosis in HCT-116 and Caco-2 cells. It also significantly inhibited the expression of miR-27a, which resulted in an upregulation of released frizzled-related protein 1 and a inhibition of catenin/Wnt signaling, which was supported by an important reduction in cyclin D1 expression.[Citation147]
By using a proliferation and apoptosis experiment, the 33 M of quercetin dramatically increased the (Dox) doxorubicin cytotoxicity to P-gp-overexpressed Ad300/SW620 cells. Additional research on the mechanism revealed that Quercetin decreased P-gp’s ATP-driven transport activity, which in turn increased Dox accumulation within cells. According to metabolomics studies based on UPLC-MS/MS analysis, Quercetin could inhibit D-glutamine and D-glutamate metabolism to a significant extent, which could be used to reverse the MDR. The underlying mechanism is that Quercetin down-regulated the expression of the glutamine transporter solute carrier family 1, member 5 (SLC1A5) in SW620/Ad300 cells. In an in vivo examination of RKO cells, quercetin likewise successfully prevented migration and invasion, decreased levels of vimentin and N-cadherin, and elevated levels of E-cadherin.[Citation148]
A potent environmental toxin that specifically targets the colon is dimethyl hydrazine (DMH). The deregulation of the Wnt/β-catenin pathway caused by DMH is thought to be a key factor in the early stages of colon carcinogenesis, according to previous research. By concentrating on (APC) adenomatous polyposis coli and Wnt/β-catenin in Wistar mouse, study sought to learn how quercetin affected DMH-mediated colon cancer. Before being slaughtered after the 30th week, the animals were administered subcutaneously with DMH at a dosage of 20 mg/kg (bw) body weight up until the 15th week and orally with quercetin at dosage of either 25 or 50 mg/kg (bw) bodyweight. Reactive oxygen species are produced as a result of the administration of DMH, which leads to an inequality in membrane lipid peroxidation and redox homeostasis, which also partially caused by a decrease in the activity of tissue antioxidant machinery. In rats with colon cancer caused by DMH, elevated levels of inflammatory and proliferative proteins were found. The apoptotic pathway was likewise dysregulated after DMH therapy, with a lower Bax: Bcl-2 ratio. Pretreatment with quercetin reduces the effects of DMH on colonic tissue’s putative early indicators (goblet cell disintegration and mucin depletion), activities of detoxifying enzymes, and proliferation. Additionally, it significantly controls the expression of APC and -catenin and reduces the incidence and growth of tumors.[Citation149]
QuCaNP has a polydispersity index (PDI) of 0.340 0.027 and a mean diameter of 237.8 9.670 nm. Quercetin and CAPE both had encapsulation efficiencies of 74.28% and 65.24%, respectively. QuCaNP’s particle size and drug content were stable at − 20°C for 30 days. QuCaNP-treated HT-29 cells’ half-maximal inhibitory concentration (IC50) values were determined to be 11.2 g/mL (24 h) and 8.2 g/mL (48 h). In HT-29 cells, QuCaNP treatment boosted the mRNA levels of caspase-3 (2.38 fold) and caspase-9 (2-fold), as well as the expression of critical proteins in the intrinsic apoptosis pathway.[Citation150]
Recent research has indicated that probiotics like Lactobacillus gasseri (Lg) and Bifidobacterium bifidum (Bf) as well as flavonoids like quercetin may be useful in preventing the formation of colon cancer cells. The development of mouse (CRC) colorectal cancer was the subject of this study, which looked at the effects of quercetin and microencapsulated probiotics (Bf and Lg) on the diet. Mice with multiple intestinal neoplasia/adenomatous polyposis coli (ApcMin/+) were given a conventional diet, the same diet supplemented with probiotics (107 CFU/100 g food each of the Bf and Lg strains), or both probiotic strains plus microencapsulated quercetin (15 mg/100 g food), for 73 days. The weights of the body and its organs as well as the changes in colon tissue, intestinal flora, and energy metabolism were all measured. Additionally, in colon sample analysis, the expression of genes connected to the Wnt pathway was examined. As a result, ApcMin/+ mice who received dietary supplements of microencapsulated probiotics or microencapsulated probiotics along with quercetin saw less body weight loss and intestinal hemorrhage. After 8 weeks of treatment, but not after 10 weeks, there was a change in energy expenditure. When Bf, Lg, and quercetin were added to the diet, the number of aberrant crypt foci (ACF) and adenomas dropped by 57% and 80%, respectively, compared to a diet supplemented with microencapsulated Bf and Lg and 45% and 60%, respectively. Hindrance of the canonical catenin/Wnt signaling pathway in the colon of ApcMin/+ mice was possible using microencapsulated Lg and Bf in conjunction with quercetin. The formation of CRC in ApcMin/+ mouse is inhibited by the injection of microencapsulated Bf and Lg, either separately or in combination with quercetin.[Citation151] The prevention and treatment of colorectal cancer by polyphonic substances like quercetin have been shown in a growing body of recent research. In order to better understand how quercetin helps rats with colon cancer caused by the chemical 1,2-dimethylhydrazine (DMH), they evaluated the underlying mechanisms. In order to do this, male Wistar rats were divided into six groups, with group 1 receiving no treatment, group 2 receiving quercetin (50 mg/kg), group 3 receiving DMH (40 mg/kg), and groups 4–6 receiving both DMH and quercetin. All groups’ colon tissues underwent evaluation for DNA damage, DNA repair, antioxidant expression levels and activities, NRF2/Keap1 signaling, and enzymatic and non-enzymatic antioxidants. Research findings demonstrated a significant reduction in DNA damage and an increase of DNA repair in the DMH + Quercetin groups, especially during the entire period when compared to other groups (p .05). In the DMH + Quercetin groups, the expression levels and activity of both enzymatic and non-enzymic antioxidants were elevated. In groups that included DMH and quercetin, lipid and protein peroxidation was considerably reduced. Quercetin also altered the targets of NRF2/Keap1 signaling, which included the detoxifying enzymes in DMH + Quercetin groups.[Citation152]
Thyroid cancer
The thyroid tumor cells were also therapeutically treated with nonsteroidal and phytochemicals anti-inflammatory medications in a dosage-dependent way, which resulted in an increase in pro-NAG-1 expression. Particularly, quercetin strongly activates the influence of pro-NAG-1 but not mature NAG-1, with improved anticancer activity, involving cell cycle arrest and apoptosis induction. p53, C/EBP, or C/EBP played a key role in the NAG-1 impression that was stimulated by quercetin, according to analysis of the NAG-1 promoter activation.[Citation153] The endocrine system’s most prevalent malignancy is thyroid cancer. The most common course of treatment for patients is thyroidectomy, followed by radioiodine therapy. However, in some of the patients, a decrease in the sodium-iodide symporter (NIS) takes place, making radioiodine therapy ineffective. Additionally, epithelial-mesenchymal transition (EMT) may take place, resulting in more aggressive and invasive features. Here, researchers examined how the flavonoid quercetin affected the expression of EMT and NIS in the papillary thyroid carcinomas BCPAP. Cell feasibility, cell death, EMT markers, and NIS were assessed after BCPAP was treated for 24 hours with 100 M quercetin. By accelerating apoptosis, quercetin reduced cell viability. Aside from that, the flavonoid increased E-cadherin mRNA levels and decreased matrix metalloproteinase nine levels, which prevented BCPAP from adhering to surfaces and migrating. The expression and activity of NIS were also elevated by quercetin. Due to its ability to induce apoptosis, lessen invasion, and boost the effectiveness of radioiodine therapy, quercetin may be a valuable adjuvant in the handling of thyroid tumor.[Citation154]
For papillary thyroid carcinoma (PTC), quercetin, a flavonol with anti-cancer and anti-inflammatory effects, is anticipated to be a novel anticancer treatment mediator. However, it is still unclear which downstream signaling mechanisms are responsible for the anticancer effects of quercetin. Several bioinformatic tools, such as PharmMapper, the Gene Expression Omnibus database (GEO), molecular docking and the Protein-Protein Interaction network (PPI) were used in this study to screen potential targets of quercetin. In addition, colony formation tests, flow cytometric analysis, CCK-8 transwell, western blot, study of invasion and migration, and other procedures were carried out to look into the following procedure. By creating a PPI network with 23 common targets, they discovered four essential nodes (MMP9, JUN, SPP1, and HMOX1). By using functional enrichment analysis, they were able to demonstrate that the four target genes above are enriched in the inflammatory signaling pathways TNF, PI3K-AKT, and NF-κB. These pathways are involved in the milieu of inflammation and prevent the growth and progression of malignancies. Additionally, the results of molecular docking showed that quercetin has a strong affinity for the proteins that these 4 important proteins encode. Finally, in PTC cell lines, quercetin showed significant antitumor efficacy.[Citation155]
Inhibition of cell growth and an increase in the rate of apoptosis via activating caspase were the effects of quercetin therapy on human papillary thyroid carcinoma cells. It has also been shown that quercetin reduces Hsp90 levels, which causes cancer cells to undergo apoptosis. As a result, researchers have demonstrated that quercetin inhibits the production of Hsp90, which may be related to a decrease in the activity of the chymotrypsin-like proteasome, which, in turn, results in the suppression of development and the death of thyroid tumor cells.[Citation156] It has been discovered that quercetin can induce apoptosis in a number of cancer cell lines by blocking the formation of HSP in a dose-dependent manner, as well as exhibiting cell cycle arrest, when administered at various concentrations (between 10 and 75 mM), over 24 hours.[Citation157]
Bladder cancer
Apoptosis is promoted in BIU-87 cells by quercetin, which also inhibits the production of p-P70S6K and p-AMPK and decreases the growth of the human BC cell line. Quercetin also inhibits the growth of tumors by activating the AMPK/mTOR cascade and inhibits the colony-forming ability of BC cells in humans by inducing DNA damage. Researchers will gain a better understanding of the functional role of quercetin in BC prevention and treatment by studying this review paper.[Citation158,Citation159] Quercetin lowered cell survival and number in animal models of T24 bladder cancer cells at concentrations of 40 M and 60 M, respectively. It reduced membrane condensation, cell body, and cytoplasmic retraction. After quercetin treatment, the NMA showed an elevation in the percentage of nuclei indicative of cell death and senescent processes. In addition, cells showed dose-dependent biophysical abnormalities, such as increased roughness and aggregation of membrane proteins that were consistent with apoptosis, which is a kind of cell death. Using force curves to measure cellular elasticity, quercetin therapy resulted in increased stiffness.[Citation160,Citation161] When quercetin-zinc complex (Q-ZnCPX) at a concentration of 12.5 M was applied to human bladder cancer cell lines BFTC-905 in vitro, it was discovered that this significantly decreased the cells’ ability to migrate and invade as compared to untreated cells through the modulation of p-AKT and MT1-MMP. Furthermore, after a 24-hour treatment period, synthesized Q-ZnCPX with less than 75 M can even cause > 50% of cancer cell mortality.[Citation162]
Bone cancer
Quercetin inhibited JAK2 in osteosarcoma (OS) via JH2 domain in a non-covalent way, which prevented OS proliferating and immune escape through the JAK2-STAT3-PD-L1 signaling axis. What’s more, researchers encapsulated quercetin in a folic acid-modified liposome to overcome its drawbacks, such as limited water solubility and poor oral availability.[Citation163]
The cytotoxic effects of Cis on Saos-2 cells were enhanced by quercetin. Cis’ IC50 dropped from 6.12 M to 4.25 M. A down-regulation of RAD51 and an up-regulation of the miR-22 and DDR subunits P53, ATM, ATR, and H2AX were seen when quercetin and Cis were combined. Furthermore, compared to mono drugs, this combined regimen significantly increased the rate of apoptosis in Saos-2 cells.[Citation164]
The cytotoxicity of Cisplatin was markedly increased by quercetin (P 0.05). In MG-63 cells, Quercetin also markedly reduced the expression levels of NF-κB compared to mono-treatment. Additionally, Quercetinen courages the apoptosis that cisplatin causes in MG-63 cells.[Citation165]
Compared to other concentrations studied, quercetin (1 M, 48 h) significantly enhanced the number of healthy human osteoblasts (hFOB cells) and their vitality as measured by the MTS and trypan blue assays, respectively. Assays for cellular adhesion and wound healing also showed that 1 M of quercetin strongly increased both parameters in osteoblasts. By monitoring alkaline phosphatase activity, calcium deposition, and collagen levels, Quercetin was also found to stimulate osteoblast development in an osteogenic media. A Western blot study conducted with quercetin at a dose of 0.01 M revealed an increase in these differentiation indicators as well as an initiation of the GSK3/AKT/-catenin pathway. The location of -catenin in the plasma membrane increased following quercetin administration, according to investigations on immunocytochemistry and subcellular fractions. If not, after 48 hours, quercetin (20–100 M) reduced the number of cells and the feasibility in (ROS 17/2.8 cells) cancer osteoblasts. Additionally, the (Ser136) pro-apoptotic protein BAD and AKT (Ser473) were both phosphorylated less by quercetin (100 M). In addition, because pre-treatment with the MEK suppressor PD98059 had reversed the Quercetin effect, ERK1/2 phosphorylation elevated, resulting in osteosarcoma cell death.[Citation166] In this investigation, they exposed MG-63 cells to quercetin at doses of 50, 100, and 200 M for 24 h. Here, it was demonstrated that quercetin boosted autophagic flux in the MG-63 cells as demonstrated by the overexpression of LC3B-II/LC3B-I and the downregulation of P62/SQSTM1. Further evidence that quercetin caused autophagic cell death in MG-63 cells came from the reduction of quercetin-induced cell death by the autophagy suppressor Bafilomycin A1 or genetic hindrance of autophagy with ATG5 reduction. Particularly, quercetin enhanced NUPR1 expression and initiated NUPR1 reporter movement, which helped to express autophagy-linked genes and subsequently started autophagic apoptosis in osteosarcoma cells. It is significant to note that the increased expression of NUPR1 was closely correlated with the disruption of the homeostasis of reactive oxygen species (ROS), which could be avoided by reducing intracellular ROS using NAC. Last but not least, NAC reversed the in vivo autophagic cell death caused by quercetin.[Citation167] According to the MTT findings, Quercetin boosts MTX’s cytotoxicity toward Saos-2 cells. The measured IC50s for quercetin are 142.3 M and for MTX they are 13.7 ng/ml. In the presence of quercetin, a decrease in MTX IC50 value was seen, going from 13.7 ng/ml to 8.45 ng/ml. Furthermore, the combination therapy significantly up-regulates tumor suppressor genes like p53, CBX7, and CYLD while down-regulating anti-apoptotic genes like BCL-2 and miR-223, which can inhibit proliferation and induce apoptosis. This was revealed by the results of the mRNA expression analysis. In addition, after treatment with MTX and Que, the apoptotic rate in Saos-2 cells considerably increased, going from 6.03% in the control group to 38.35%.[Citation168] The cell viability of Saos-2 and U2OS cells was considerably decreased in a dose-dependent way after 48 hours of quercetin incubation at concentrations of 20, 40, 60, 80, or 100 M. At greater quercetin concentrations, cell viability was also significantly reduced along with significant decrease in cell adhesion, migration and invasion. Additionally, tissue suppressors of metalloproteinase (TIMP)-1 and − 2 mRNA expression levels were increased while matrix metalloproteinase (MMP)-2 and − 9 mRNA expression levels were lessened. Additionally, parathyroid hormone receptor 1 (PTHR1) mRNA expression levels were significantly reduced by quercetin treatment by 0.27, 0.55, and 0.19 folds at 80, and 0.41 folds at 100 M, respectively, compared to Saos-2 cells by 0.19 and 0.41 folds. At 80 and 100 M of quercetin, the expression of the PTHR1 protein was decreased in U2OS cells by 0.19 and 0.43 folds, respectively (P 0.05), but it was decreased by 0.17 and 0.35 folds in Saos-2 cells. After quercetin administration, immunofluorescence analysis showed that PTHR1 expression was decreased. At 80 and 100 M, PTHR1 expression in U2OS cells was decreased by 0.18 and 0.41 folds, respectively, but in Saos-2 cells it was reduced by 0.15 and 0.38 folds.[Citation169] In dogs, osteosarcoma is a mesenchymal malignant bone cancer that has a greater probability of lung metastasis and a short prognosis. Quercetin alters the mitochondrial depolarization, cell cycle, level of reactive oxygen species, and concentration of cytoplasmic calcium in the DSN and D-17 (canine osteosarcoma) cell lines. It also lowers proliferative characteristics and improved programmed cell death in these cell lines. In addition, it was discovered that quercetin increases the phosphorylation of ERK1 or 2, P38, P90RSK and c-Jun N-terminal kinase proteins in both cell lines while suppressing the phosphorylation of P70S6K, AKT, and S6 proteins in both cell lines.[Citation170]
Quercetin dramatically decreased cell invasion and migration in HOS and MG63 cells as compared to treatment with control media. In HOS cells treated with quercetin as opposed to HOS cells treated with controls, the expression levels of HIF-1, VEGF, MMP2, and MMP9 mRNA and proteins were also significantly downregulated.[Citation171] In a rat model of bone cancer pain, quercetin significantly lowers the expressions of osteocalcin, anti-tartaric acid phosphatase (TRAP), and serum CTX, as well as the percentage of TRAP-positive cells. The positive fraction of local bone tissue macrophages can be dramatically reduced by the medication in rats with painful bone cancer. It can also knowingly elevate the expressions of OPG and promote considerably impede the expressions of the TRPV1/PAR2 pathway-related molecules TRPV1, PAR2, PKA and PKC-, in DRG neurons. Additionally, it can expressively decrease the phases of major inciting mediators (trypsin), IL-1 and TNF-, in the TRPV1/PAR2 trail.[Citation172]
Ovarian cancer
The wurtzite (WZ) type of (ZnO) zinc oxide nanoparticles were made and then functionalized with quercetin (ZnO@Quercetin) in ovarian PA-1 cells. These nanoparticles demonstrated outstanding action by inducing intercellular oxidative tension and depolarizing mitochondrial membrane strength against human ovarian tumor cells. Through initiation of the intrinsic cell death signaling pathway in PA-1 cells, the dual-staining analyses demonstrated that ZnO@Quercetin induces late apoptosis.[Citation173,Citation174] By deactivating Akt/PI3k, Raf/Ras paths, and EGFR expression, quercetin treatment at concentrations of 50 mM and 75 mM reduces human metastatic ovarian tumor PA-1 cell proliferation and survival. N-cadherin expression in PA-1 cells is also changed by it. Additionally, quercetin reduces the expression of both MMP genes in metastatic ovarian cancer PA-1 cells as well as the release of the gelatinase enzyme, MMP-2/-9 proteolytic activity, and both MMPs. Further, quercetin prevents PA-1 cells from migrating. The tight junctional molecules Claudin-11 and Claudin-4 are also downregulated by quercetin therapy of PA-1 cells, whereas the expression of occludin is upregulated. By reducing the adhesion of PA-1 ovarian cancer cells in a cell adhesion assay, quercetin is further demonstrated to be effective.[Citation175]
The vitality of PA-1 cells is decreased in a dose-dependent way after receiving quercetin treatments (0–200 M) on human metastatic ovarian tumor PA-1 cell line for 24 hours. Based on the MTT experiment, 50 and 75 M were determined to be the efficacious doses. By using the AO/EtBr dual labeling, DNA fragmentation and DAPI staining test, researchers were able to confirm that quercetin causes apoptosis in metastatic ovarian carcinomas. Quercetin affected the molecules that are a part of the intrinsic apoptotic pathway. Intriguingly, quercetin treatment of PA-1 cells resulted in an increase in pro-apoptotic molecules such as caspase-9, caspase-3, Bid, Bax, Bad and cytochrome c and a decrease in antiapoptotic molecules like Bcl-xL and Bcl-2.[Citation176] Quercetin demonstrated better apoptosis induction and cell growth inhibitory effects in in vivo abdominal SKOV-3 ovarian cancer animal models. Anti-cancer mechanisms of quercetin are summarized in .
Table 1. Anti-cancer mechanisms of quercetin (summarized).
Conclusion
In this review, it was observed at the different ways that quercetin (Que) stops tumors from growing. These include the ways that lung, breast, ovarian, liver, blood, and prostate cancers start to grow. A big part of quercetin’s ability to promote apoptosis, stop spread, and affect other biological processes comes from its ability to control the cell cycle and the growth of blood vessels in tumors. The power of quercetin to change epigenetics, which can directly control the appearance of miRNA and how much DNA is methylated, can help fight cancer and make tumor cells more open to treatment. By changing epigenetics, quercetin can slow or stop the growth of cancers. Researchers found that nano-quercetin’s anti-proliferative effect caused MCF7 cells to die by apoptosis and stopped them from moving from the G1 phase to the S phase of the cell cycle. In another study, researchers looked at what happened to the disease when the epidermal growth factor receptor (EGFR)/VEGFR-2-mediated pathway was used to stop EMT, angiogenesis, and invasiveness in breast cancer. It has also been shown that quercetin used the p-STAT3/Bcl-2 pathway to make cells go through ER stress, apoptosis, and autophagy. Several studies have found that quercetin makes ovarian cancer cells that have spread to other organs go through a process called apoptosis, which makes them less likely to grow and spread. The amount of anti-apoptotic molecules, like Bcl-2 and Bcl-xL, and pro-apoptotic molecules, like caspase-3, caspase-9, Bid, Bax, and Bad, is also going up. Quercetin can also slow down cytochrome c. Because quercetin can cause mitochondrial-mediated death in cancer cells that have spread to other organs, it slows down the growth of ovarian cancer cells. Quercetin works on the angiogenic pathway, and it also stops the growth of P70S6K, which is a control factor for AKT/mTOR/ribosomal protein S6 kinase. VEGFR-2 is in charge of the angiogenic system. Several studies have shown that this slows or stops the growth of tumors in men with prostate cancer. Quercetin was able to stop lung cancer cells from spreading and invading by a lot. It was also able to lower the appearance of N-cadherin, vimentin, and ADAM9 while rising the appearance of E-cadherin and MMP-related proteins. More research is needed to learn more about how quercetin affects the body, as well as how much and how quickly it is absorbed. Quercetin’s metabolites should be used in studies because they are the most common types of the molecule found in human plasma. To get a good idea of how quercetin works in living organisms and how well it prevents diseases caused by oxidative damage, it is important to find and study the antioxidant action of Quercetin products and pathways of metabolic conversion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdu, S.; Juaid, N.; Amin, A.; Moulay, M.; Miled, N. Effects of Sorafenib and Quercetin Alone or in Combination in Treating Hepatocellular Carcinoma: In Vitro and In Vivo Approaches. Molecules. Nov 21, 2022, 27(22), DOI: 10.3390/molecules27228082. PMID: 36432184; PMCID: PMC9697794.
- Adami, B. S.; Diz, F. M.; Oliveira Gonçalves, G. P.; Reghelin, C. K.; Scherer, M.; Dutra, A. P.; Papaléo, R. M.; de Oliveira, J. R.; Morrone, F. B.; Wieck, A., et al. Morphological and mechanical changes induced by quercetin in human T24 bladder cancer cells. Micron. Dec, 2021, 151, 103152. DOI: 10.1016/j.micron.2021.103152. Epub 2021 Sep 23. PMID: 34607251.
- Ahmadi, M.; Valizadeh, A.; Bazavar, M.; Yousefi, B. Investigating the role of quercetin in increasing the rate of cisplatin-induced apoptosis via the NF-Κb pathway in MG-63 cancer cells. Drug Res. (Stuttg). Sep, 2022, 72(7), 385–389. DOI: 10.1055/a-1842-7424. Epub 2022 Jul 4. PMID: 35785813
- Alam, S.; Mohammad, T.; Padder, R. A.; Hassan, M. I.; Husain, M. Thymoquinone and quercetin induce enhanced apoptosis in non-small cell Lung cancer in combination through the Bax/Bcl2 cascade. J. Cell. Biochem. Feb, 2022, 123(2), 259–274. DOI: 10.1002/jcb.30162. Epub 2021 Oct 12. PMID: 34636440.
- Alban, L.; Monteiro, W. F.; Diz, F. M.; Miranda, G. M.; Scheid, C. M.; Zotti, E. R.; Morrone, F. B.; Ligabue, R. New quercetin-coated titanate nanotubes and their radiosensitization effect on human bladder cancer. Mater. Sci. Eng. C Mater. Biol. Appl. May, 2020, 110, 110662. DOI: 10.1016/j.msec.2020.110662. Epub 2020 Jan 15. PMID: 32204090.
- Ali, A.; Kim, M. J.; Kim, M. Y.; Lee, H. J.; Roh, G. S.; Kim, H. J.; Cho, G. J.; Choi, W. S. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat. Cell Biol. Dec, 2018, 51(4), 274–283. DOI: 10.5115/acb.2018.51.4.274. Epub 2018 Dec 29. PMID: 30637162; PMCID: PMC6318463
- Almeida, A. F.; Borge, G. I.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C. N. Bioavailability of quercetin in humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. May, 2018, 17(3), 714–731. DOI: 10.1111/1541-4337.12342.
- Amiri, A.; Abbasi, A.; Dehghani, M.; Ramezani, A.; Ramezani, F.; Zal, F.; Mostafavi-Pour, Z. New perspectives of quercetin and Vitamin C effects on fibronectin-binding integrins and chemokine receptors in prostate cancer cell lines. Bratisl. Lek. Listy. 2021, 122(7), 507–512. PMID: 34161119. DOI: 10.4149/BLL_2021_082.
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K. I.; Lampen, A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. Jan, 2018, 62(1), 1700447. DOI: 10.1002/mnfr.201700447.
- Ansari, M. A. Anticancer potential of quercetin: a comprehensive review. Phytother. Res. Sep, 2019, 33(9): 2065–2086.
- Asgharian, P.; Tazehkand, A. P.; Soofiyani, S. R.; Hosseini, K.; Martorell, M.; Tarhriz, V.; Ahangari, H.; Cruz-Martins, N.; Sharifi-Rad, J.; Almarhoon, Z. M., et al.Quercetin impact in pancreatic cancer: an overview on Its therapeutic effects. OXID. MED. CELL LONGEV. Nov 3, 2021, 4393266. DOI: 10.1155/2021/4393266. PMID: 34777687; PMCID: PMC8580629
- Asgharian, P.; Tazekand, A. P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S. M.; Tarhriz, V., et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer. Cell Inter. Aug 15, 2022, 22(1), 257. DOI: 10.1186/s12935-022-02677-w.
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: a global overview and recent trends. Europ. urol. Jan 1, 2017, 71(1), 96–108. DOI: 10.1016/j.eururo.2016.06.010.
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F. R.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym. Bull. Jan, 2023, 80(1), 241–262. DOI: 10.1007/s00289-022-04091-8.
- Azizi, E.; Fouladdel, S.; Komeili Movahhed, T.; Modaresi, F.; Barzegar, E.; Ghahremani, M. H.; Ostad, S. N.; Atashpour, S. Quercetin effects on cell cycle arrest and apoptosis and doxorubicin activity in T47D cancer stem cells. Asian Pac. J. Cancer Prev. Dec 1, 2022, 23(12), 4145–4154. DOI: 10.31557/APJCP.2022.23.12.4145. PMID: 36579996; PMCID: PMC9971456.
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: are flavonoids doing the trick? Biomed. Pharmacother. Jan 1, 2019, 109, 1488–1497. DOI: 10.1016/j.biopha.2018.10.086.
- Baruah, M. M.; Khandwekar, A. P.; Sharma, N. Quercetin modulates wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumour Biol. Oct, 2016, 37(10), 14025–14034. Doi: 10.1007/s13277-016-5277-6. Epub 2016 Aug 5. PMID: 27495232.
- Baghel, S. S.; Shrivastava, N.; Baghel, R. S.; Agrawal, P.; Rajput, S. A review of quercetin: antioxidant and anticancer properties. World J Pharm Pharmaceutical Sci. May 1, 2012, 1(1), 146–160.
- Benito, I.; Encío, I. J.; Milagro, F. I.; Alfaro, M.; Martínez-Peñuela, A.; Barajas, M.; Marzo, F. Microencapsulated bifidobacterium bifidum and lactobacillus gasseri in combination with quercetin inhibit colorectal cancer development in ApcMin/+ Mice. Int. J. Mol. Sci. May 5, 2021, 229, 4906. DOI: 10.3390/ijms22094906. PMID: 34063173; PMCID: PMC8124226.
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A. K.; Banerjee, A. A comprehensive study on the anti-cancer effects of quercetin and its epigenetic modifications in arresting progression of colon cancer cell proliferation. Arch Immunol Ther Exp (Warsz). Feb 20. 2023, 71(1), 6. DOI: 10.1007/s00005-023-00669-w. PMID: 36807774; PMCID: PMC9941246.
- Cao, Y.; Zhang, W.; Chen, X.; Chen, J.; Wang, X.Quercetin as a potential anticancer agent: from biology to clinical application.Cancer Lett. May 28. 2021, 505, 9–20.
- Çelik, H.; Arinç, E. Evaluation of the protective effects of quercetin, rutin, naringenin, resveratrol and trolox against idarubicin-induced DNA damage.
- Chai, R.; Xu, C.; Lu, L.; Liu, X.; Ma, Z. Quercetin inhibits proliferation of and induces apoptosis in non-small-cell lung carcinoma via the lncRna Snhg7/miR-34a-5p pathway. Immunopharmacol. Immunotoxicol. Dec, 2021, 43(6), 693–703. DOI: 10.1080/08923973.2021.1966032. Epub 2021 Aug 27. PMID: 34448661.
- Chan, C. Y.; Hong, S. C.; Chang, C. M.; Chen, Y. H.; Liao, P. C.; Huang, C. Y. Oral squamous cell carcinoma cells with acquired resistance to erlotinib are sensitive to anti-cancer effect of quercetin via pyruvate kinase M2 (PKM2). Cells. Jan 1. 2023, 12(1), 179. DOI: 10.3390/cells12010179. PMID: 36611972; PMCID: PMC9818869.
- Chaudhari, V. S.; Gawali, B.; Saha, P.; Naidu, V. G. M.; Murty, U. S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu Cells) with improved biodistribution profile. Eur. J. Pharmacol. Oct 15. 2021, 909, 174400. DOI: 10.1016/j.ejphar.2021.174400. Epub 2021 Jul 29. PMID: 34332920.
- Chen, B.; Li, X.; Wu, L.; Zhou, D.; Song, Y.; Zhang, L.; Wu, Q.; He, Q.; Wang, G.; Liu, X.et al.Quercetin suppresses human glioblastoma migration and invasion via GSK3β/β-Catenin/zeb1 signaling pathway.Front Pharmacol. Nov 1, 2022, 13, 963614. DOI: 10.3389/fphar.2022.963614. PMID: 36386155; PMCID: PMC9663482.
- Chen, L.; Xia, J. S.; Wu, J. H.; Chen, Y. G.; Qiu, C. J. Quercetin suppresses cell survival and invasion in oral squamous cell carcinoma via the MiR-1254/CD36 cascade in vitro. Hum. Exp. Toxicol. Sep, 2021, 40(9), 1413–1421. DOI: 10.1177/0960327121991912. Epub 2021 Mar 9. PMID: 33686878.
- Chen, W. J.; Tsai, J. H.; Hsu, L. S.; Lin, C. L.; Hong, H. M.; Pan, M. H. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/Igf1R-mediated EMT program. J. Food Drug Anal. Mar 15. 2021, 29(1), 98–112. DOI: 10.38212/2224-6614.3090. PMID: 35696220; PMCID: PMC9261845
- Chen, X.; Xu, P.; Zhang, H.; Su, X.; Guo, L.; Zhou, X.; Wang, J.; Huang, P.; Zhang, Q.; Sun, R. EGFR and ERK Activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro. Oncol. Lett. Nov, 2021, 22(5), 754. DOI: 10.3892/ol.2021.13015. Epub 2021 Aug 31. PMID: 34539858; PMCID: PMC8436358
- Cirmi, S.; Ferlazzo, N.; Lombardo, G. E., et al.Quercetin and cancer: biological implications and therapeutic potential.Front Pharmacol. Jul 3, 2020, 11, 1306.
- Colpan, R. D.; Erdemir, A. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. Sep, 2021, 38(6), 381–393. DOI: 10.1080/02652048.2021.1948623. Epub 2021 Jul 5. PMID: 34189998.
- Darband, S. G.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 Signaling pathway in rats with DMH induced colon carcinogenesis. Life. sci. Jul 15, 2020, 253, 117584. DOI: 10.1016/j.lfs.2020.117584. Epub 2020 Mar 24. PMID: 32220623.
- David, A. V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. Jul, 2016, 10(20), 84. DOI: 10.4103/0973-7847.194044.
- Deepika, M. P. Health benefits of quercetin in age-related diseases. Molecules. Apr 13, 2022, 27(8), 2498. DOI: 10.3390/molecules27082498.
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch. Biochem. Biophys Apr 15, 2021, 701, 108795. DOI: 10.1016/j.abb.2021.108795. Epub 2021 Feb 9. PMID: 33577840.
- Elmowafy, M.; Shalaby, K.; Elkomy, M. H.; Alsaidan, O. A.; Gomaa, H. A. M.; Hendawy, O. M.; Abdelgawad, M. A.; Ali, H. M.; Ahmed, Y. M.; El-Say, K. M. Exploring the potential of quercetin/aspirin-loaded chitosan nanoparticles coated with eudragit l100 in the treatment of induced-colorectal cancer in rats. Drug. Deliv. Transl. Res. Mar 31, 2023, DOI: 10.1007/s13346-023-01338-3. Epub ahead of print. PMID: 37000409.
- Elumalai, P.; Ezhilarasan, D.; Raghunandhakumar, S. Quercetin inhibits the epithelial to mesenchymal transition through suppressing akt mediated nuclear translocation of β-catenin in lung cancer cell line. Nutr. Cancer. 2022, 74(5), 1894–1906. DOI: 10.1080/01635581.2021.1957487. Epub 2021 Aug 2. PMID: 34338101.
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. Jul, 25(6), 757–766. 2020, DOI: 10.1080/10837450.2020.1740933. Epub 2020 Mar 20. PMID: 32192406.
- Ferreira, M.; Gomes, D.; Neto, M.; Passarinha, L. A.; Costa, D.; Sousa, Â. development and characterization of quercetin-loaded delivery systems for increasing its bioavailability in cervical cancer cells. Pharmaceutics Mar 14, 2023, 153, 936. DOI: 10.3390/pharmaceutics15030936. PMID: 36986797; PMCID: PMC10058887.
- Fulfager, A. D.; Yadav, K. S. Understanding the Implications of co-delivering therapeutic agents in a nanocarrier to combat multidrug resistance (MDR) in breast cancer. J. Drug Delivery Sci. Technol. Apr 1, 2021, 62, 102405. DOI: 10.1016/j.jddst.2021.102405.
- Ganthala, P. D.; Alavala, S.; Chella, N.; Andugulapati, S. B.; Bathini, N. B.; Sistla, R. Co-Encapsulated Nanoparticles of Erlotinib and Quercetin for Targeting Lung Cancer through Nuclear EGFR and PI3K/AKT Inhibition. Colloids Surf. B Biointerfaces. Mar, 2022, 211, 112305. DOI: 10.1016/j.colsurfb.2021.112305. Epub 2021 Dec 30. PMID: 34998178.
- Gao, R.; Zhang, R.; Chen, T.; Zheng, Y.; Wang, G. Quercetin Suppresses Cancer Stem Cell Properties in Neuroblastoma Via Downregulation of Wnt/β-Catenin Signaling. Oncol. Rep. Aug, 2021, 46(2), 83. DOI: 10.3892/or.2021.8179.
- García-Gutiérrez, N.; Luna-Bárcenas, G.; Herrera-Hernández, G.; Campos-Vega, R.; Lozano-Herrera, S. J.; Sánchez-Tusié, A. A.; García-Solis, P.; Vergara-Castañeda, H. A. Quercetin and Its Fermented Extract as a Potential Inhibitor of Bisphenol A-exposed HT-29 Colon Cancer Cells’ Viability. Int. J. Mol. Sci. Mar 2023, 15, 246, 5604. DOI: 10.3390/ijms24065604. PMID: 36982678; PMCID: PMC10052295.
- Gaikwad, S.; Srivastava, S. K. Role of Phytochemicals in Perturbation of Redox Homeostasis in Cancer. Antioxidants. Jan 9, 2021, 10(1), 83. DOI: 10.3390/antiox10010083.
- Golmohammadi, M.; Elmaghraby, D. A.; Ramírez-Coronel, A. A.; Rakhimov, N.; Mohammed, S. S.; Romero-Parra, R. M.; Jawad, M. A.; Zamanian, M. Y.; Soltani, A.; Taheri, N.et al. A Comprehensive View on the Quercetin impact on Bladder Cancer: Focusing on oxidative Stress, Cellular, And Molecular Mechanisms. Fundam. Clin. Pharmacol. Mar 24, 2023, DOI: 10.1111/fcp.12896. Epub ahead of print. PMID: 36960597
- Gonçalves, C. F. L.; Hecht, F.; Cazarin, J.; Fortunato, R. S.; Vaisman, M.; Carvalho, D. P.; Ferreira, A. C. F.The Flavonoid Quercetin Reduces Cell Migration And Increases Nis And E-cadherin mRNA In The Human Thyroid Cancer Cell Line BCPAP. Mol. Cell. Endocrinol. Jun 1, 529,111266. DOI: 10.1016/j.mce.2021.111266. Epub 2021 Apr 6. PMID: 33831503.
- Greco, S.; Islam, M. S.; Zannotti, A.; Delli Carpini, G.; Giannubilo, S. R.; Ciavattini, A.; Petraglia, F.; Ciarmela, P. Quercetin aNd Indole-3-carbinol Inhibit Extracellular Matrix Expression In Human Primary Uterine Leiomyoma Cells. Reprod Biomed Online. Apr, 2020, 40(4), 593–602. DOI: 10.1016/j.rbmo.2020.01.006. Epub 2020 Jan 23. PMID: 32276890.
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin Induces Pro-apoptotic Autophagy Via Sirt1/AMPK Signaling Pathway In Human Lung Cancer Cell Lines A549 And H1299 In Vitro. Thorac Cancer. May, 2021, 12(9), 1415–1422. DOI: 10.1111/1759-7714.13925. Epub 2021 Mar 11. PMID: 33709560; PMCID: PMC8088950.
- Guo, Y.; Tong, Y.; Zhu, H.; Xiao, Y.; Guo, H.; Shang, L.; Zheng, W.; Ma, S.; Liu, X.; Bai, Y. Quercetin Suppresses Pancreatic Ductal Adenocarcinoma Progression Via Inhibition Of Shh And Tgf-β/smad Signaling Pathways. Cell Biol. Toxicol. Jun, 2021, 37, 479–496. DOI: 10.1007/s10565-020-09562-0.
- Guo, Y.; Tong, Y.; Zhu, H.; Xiao, Y.; Guo, H.; Shang, L.; Zheng, W.; Ma, S.; Liu, X.; Bai, Y. Quercetin Suppresses Pancreatic Ductal Adenocarcinoma Progression via Inhibition of SHH and TGF-Β/smad Signaling Pathways. Cell Biol. Toxicol. Jun, 2021, 37(3), 479–496. DOI: 10.1007/s10565-020-09562-0. Epub 2020 Oct 17. PMID: 33070227.
- Hassan, S.; Peluso, J.; Chalhoub, S.; Idoux Gillet, Y.; Benkirane-Jessel, N.; Rochel, N.; Fuhrmann, G.; Ubeaud-Sequier, G.; Ulasov, I. Quercetin Potentializes the Respective Cytotoxic Activity of Gemcitabine or Doxorubicin on 3D Culture of AsPC-1 or HepG2 Cells, Through the Inhibition of HIF-1α and MDR1. PLoS One. Oct 14, 2020, 15(10), e0240676. DOI: 10.1371/journal.pone.0240676. PMID: 33052979; PMCID: PMC7556446.
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the Absorption, Metabolism, and Efficacy Exertion of Quercetin and Its Important Derivatives: Absorption, Metabolism and Function of Quercetin. Food Front. Dec, 2020, 1(4), 420–434. DOI: 10.1002/fft2.50.
- He, C.; Lu, X.; Li, J.; Shen, K.; Bai, Y.; Li, Y.; Luan, H.; Tuo, S. The Effect of Quercetin on Cervical Cancer Cells as Determined by Inducing Tumor Endoplasmic Reticulum Stress and Apoptosis and Its Mechanism of Action. Am. J. Transl. Res. May 15, 2021, 135, 5240–5247. PMID: 34150114; PMCID: PMC8205781
- Hoca, M.; Becer, E.; Kabadayi, H.; Yucecan, S.; Vatansever, H. S. The Effect of Resveratrol and Quercetin on Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem Cell. Nutr. Cancer. 2019, 1–12. DOI: 10.1080/01635581.2019.1670853.
- Hoca, M.; Becer, E.; Kabadayı, H.; Yücecan, S.; Vatansever, H. S. The Effect of Resveratrol and Quercetin on Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem Cell. Nutr. Cancer. Oct 2, 2020, 72(7), 1231–1242. DOI: 10.1080/01635581.2019.1670853.
- Hong, Y.; Lee, J.; Moon, H.; Ryu, C. H.; Seok, J.; Jung, Y. S.; Ryu, J.; Baek, S. J. Quercetin Induces Anticancer Activity by Upregulating Pro-NAG-1/GDF15 in Differentiated Thyroid Cancer Cells. Cancers (Basel). Jun 16. 2021, 13(12), 3022. DOI: 10.3390/cancers13123022. PMID: 34208730; PMCID: PMC8233818
- Hosseini, S. S.; Ebrahimi, S. O.; Haji Ghasem Kashani, M.; Reiisi, S. Study of Quercetin and Fisetin Synergistic Effect on Breast Cancer and Potentially Involved Signaling Pathways. Cell Biol. Int. Jan, 2023, 47(1), 98–109. DOI: 10.1002/cbin.11942. Epub 2022 Oct 23. PMID: 36273436.
- Hussain, Y.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Khan, H.; Daglia, M. Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance. Cancers. Mar 31, 2021, 13 7, 1602. DOI: 10.3390/cancers13071602.
- Hu, M.; Song, H. Y.; Chen, L. Quercetin Acts via the G3BP1/YWHAZ Axis to Inhibit Glycolysis and Proliferation in Oral Squamous Cell Carcinoma. Toxicol. Mech. Methods. Feb, 2023, 33(2), 141–150. DOI: 10.1080/15376516.2022.2103480. Epub 2022 Aug 9. PMID: 35945655.
- Huang, K. Y.; Wang, T. H.; Chen, C. C.; Leu, Y. L.; Li, H. J.; Jhong, C. L.; Chen, C. Y. Growth Suppression in Lung Cancer Cells Harboring EGFR-C797S Mutation by Quercetin. Biomolecules. Aug 25, 2021, 11(9), 1271. DOI: 10.3390/biom11091271. PMID: 34572484; PMCID: PMC8470952.
- Huang, R., et al.Quercetin-Loaded Nanocarriers for Cancer Treatment: A Review.J. Control Release. Oct 28, 2016, 238, 89–113.
- Huh, S.; Lee, S.; Choi, S. J.; Wu, Z.; Cho, J. H.; Kim, L.; Shin, Y. S.; Kang, B. W.; Kim, J. G.; Liu, K.et al. Quercetin Synergistically Inhibit EBV-Associated Gastric Carcinoma with Ganoderma Lucidum Extracts. Molecules. 2019 Oct 24, 2421, 3834. DOI: 10.3390/molecules24213834. PMID: 31653035; PMCID: PMC6864608.
- Hyun, H. B.; Moon, J. Y.; Cho, S. K. Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules. Jan 24, 2018, 23(2), 209. DOI: 10.3390/molecules23020209. PMID: 29364834; PMCID: PMC6017870
- Imran, M.; Salehi, B.; Sharifi-Rad, J., et al., Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules. Oct 7, 2019, 2419, 2277. DOI: 10.3390/molecules24122277
- Ji, Y.; Li, L.; Ma, Y. X.; Li, W. T.; Li, L.; Zhu, H. Z.; Wu, M. H.; Zhou, J. R. Quercetin Inhibits Growth of Hepatocellular Carcinoma by Apoptosis Induction in Part via Autophagy Stimulation in Mice. J. NUTR BIOCHEM. Jul, 2019, 69, 108–119. DOI: 10.1016/j.jnutbio.2019.03.018. Epub 2019 Apr 8. PMID: 31078904; PMCID: PMC9659433.
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Shao, Z.; Pu, F. Quercetin Encapsulated in Folic Acid-Modified Liposomes is Therapeutic Against Osteosarcoma by Non-Covalent Binding to the JH2 Domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. Aug, 2022, 182, 106287. DOI: 10.1016/j.phrs.2022.106287. Epub 2022 Jun 6. PMID: 35671921.
- Johnson, K. E.; Wilgus, T. A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Advances In Wound Care. Oct 1, 2014, 3(10), 647–661. DOI: 10.1089/wound.2013.0517.
- Kampa, R. P.; Gliździńska, A.; Szewczyk, A.; Bednarczyk, P.; Filipek, S. Flavonoid Quercetin Abolish Paxilline Inhibition of the Mitochondrial BKCa Channel. Mitochondrion. 2022, 65, 23–32. DOI: 10.1016/j.mito.2022.04.005.
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; HaQuercetin, S.; Hussain, A. Quercetin Modulates Signaling Pathways and Induces Apoptosis in Cervical Cancer Cells. Biosci. Rep. Aug 13, 2019, 398, BSR20190720. DOI: 10.1042/BSR20190720. PMID: 31366565; PMCID: PMC6692570.
- Kim, H. I.; Lee, S. J.; Choi, Y. J.; Kim, M. J.; Kim, T. Y.; Ko, S. G. Quercetin Induces Apoptosis in Glioblastoma Cells by Suppressing Axl/IL-6/STAT3 Signaling Pathway. Am. J. Chin. Med. 2021, 49(3), 767–784. DOI: 10.1142/S0192415X21500361. Epub 2021 Mar 3. PMID: 33657989.
- Kim, S. R.; Lee, E. Y.; Kim, D. J.; Kim, H. J.; Park, H. R. Quercetin Inhibits Cell Survival and Metastatic Ability via the EMT-Mediated Pathway in Oral Squamous Cell Carcinoma. Molecules. Feb 10, 2020, 25(3), 757. DOI: 10.3390/molecules25030757. PMID: 32050534; PMCID: PMC7037689.
- Kim, D. H.; Khan, H.; Ullah, H.; Hassan, S. T.; Šmejkal, K.; Efferth, T.; Mahomoodally, M. F.; Xu, S.; Habtemariam, S.; Filosa, R., et al. MicroRna Targeting by Quercetin in Cancer Treatment and Chemoprotection. Pharmacol. Res. Sep 1, 2019, 147, 104346. DOI: 10.1016/j.phrs.2019.104346.
- Korkina, L. G.; Mikhalchik, E. V. Suppression of Human Prostate Cancer Cell Metastatic Potential by Quercetin Treatment: A Prelude to Antioxidant Therapy. Acta Naturae. Oct-Dec, 2016, 8(4): 121–126.
- Kreczmer, B.; Dyba, B.; Barbasz, A.; Rudolphi-Szydło, E. Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells. Molecules. Aug 16, 2021, 26(16), 4945. DOI: 10.3390/molecules26164945. PMID: 34443533; PMCID: PMC8397999.
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients. Mar 5, 2021, 13(3), 845. DOI: 10.3390/nu13030845.
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. Jan 25, 2022, 233, 1345. DOI: 10.3390/ijms23031345. PMID: 35163269; PMCID: PMC8836052.
- Lan, C. Y.; Chen, S. Y.; Kuo, C. W.; Lu, C. C.; Yen, G. C. Quercetin Facilitates Cell Death and Chemosensitivity Through RAGE/PI3K/AKT/mTOR axis in Human Pancreatic Cancer Cells. J. Food Drug Anal. Oct, 2019, 27(4), 887–896. DOI: 10.1016/j.jfda.2019.07.001. Epub 2019 Aug 21. PMID: 31590760; PMCID: PMC9306979.
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43(2), 553–567. DOI: 10.1159/000480528. Epub 2017 Sep 21. PMID: 28965117.
- Lee, H. H.; Lee, S.; Shin, Y. S.; Cho, M.; Kang, H.; Cho, H. Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma. Molecules. Sep 26. 2016, 21(10), 1286. DO: 10.3390/molecules21101286. PMID: 27681719; PMCID: PMC6274130.
- Lee, J.; Han, S. I.; Yun, J. H.; Kim, J. H. Quercetin 3-O-Glucoside Suppresses Epidermal Growth Factor–Induced Migration by Inhibiting EGFR Signaling in Pancreatic Cancer Cells. Tumor Biol. Dec, 2015, 36(12), 9385–9393. DOI: 10.1007/s13277-015-3682-x.
- Lee, Y. H.; Tuyet, P. T. Synthesis and Biological Evaluation of Quercetin-Zinc (II) Complex for Anti-Cancer and Anti-Metastasis of Human Bladder Cancer Cells. Vitro Cell Dev Biol Anim. Jun, 2019, 55(6), 395–404. DOI: 10.1007/s11626-019-00363-2. Epub 2019 May 14. PMID: 31089950.
- Lei, C. S.; Hou, Y. C.; Pai, M. H.; Lin, M. T.; Yeh, S. L. Effects of Quercetin Combined with Anticancer Drugs on Metastasis-Associated Factors of Gastric Cancer Cells: In Vitro and In Vivo Studies. J. NUTR BIOCHEM. Jan, 2018, 51, 105–113. DOI: 10.1016/j.jnutbio.2017.09.011. Epub 2017 Sep 28. PMID: 29125991.
- Lezcano, V.; Morelli, S.; González-Pardo, V. Molecular and Cellular Outcomes of Quercetin Actions on Healthy and Tumor Osteoblasts. Biochimie. Aug, 2022, 199, 46–59, DOI: 10.1016/j.biochi.2022.04.003. Epub 2022 Apr 18. PMID: 35447220.
- Li, C.; Gao, L.; Zhang, Y.; Simpson, B. K. Preparation of Quercetin Loaded Microparticles and Their Antitumor Activity Against Human Lung Cancer Cells (A549) In Vitro. Curr. Pharm. Biotechnol. 2019, 20(11), 945–954. DOI: 10.2174/1573407215666190628145902. PMID: 31264544.
- Li, Z. Y.; Chen, S. Y.; Weng, M. H. Ursolic Acid Restores Sensitivity to Gemcitabine Through the RAGE/NF-κB/MDR1 Axis in Pancreatic Cancer Cells and in a Mouse Xenograft Model. Journal Of Food And Drug Analysis. 2021, 29(2), 262. DOI: 10.38212/2224-6614.3346.
- Li, H.; Tan, L.; Zhang, J. W.; Chen, H.; Liang, B.; Qiu, T.; Li, Q. S.; Cai, M.; Zhang, Q. H. Quercetin is the Active Component of Yang-Yin-Qing-Fei-Tang to Induce Apoptosis in Non-Small Cell Lung Cancer. Am. J. Chin. Med. 2019, 47(4), 879–893. DOI: 10.1142/S0192415X19500460. PMID: 31179723.
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin Suppresses the Proliferation and Metastasis of Metastatic Osteosarcoma Cells by Inhibiting Parathyroid Hormone Receptor 1. Biomed. Pharmacother. Jun, 2019, 114, 108839. DOI: 10.1016/j.biopha.2019.108839. Epub 2019 Apr 9. PMID: 30978523.
- Li, X.; Zhu, Q.; Ma, M.; Guo, H. Quercetin Inhibits the Progression of Endometrial HEC-1-A Cells by Regulating Ferroptosis-A Preliminary Study. Eur. J. Med. Res. Dec 15, 2022, 27(1), 292. DOI: 10.1186/s40001-022-00934-2. PMID: 36522794; PMCID: PMC9753389
- Li, Y.; Kou, J.; Wu, T.; Zheng, P.; Chao, X. Screening of Therapeutic Candidate Genes of Quercetin for Cervical Cancer and Analysis of Their Regulatory Network. Onco. Targets Ther. Feb 5, 2021, 14, 857–866. DOI: 10.2147/OTT.S287633. PMID: 33574679; PMCID: PMC7873026.
- Li, Y.; Wang, Z.; Jin, J.; Zhu, S. X.; He, G. Q.; Li, S. H.; Wang, J.; Cai, Y. Quercetin Pretreatment Enhances the Radiosensitivity of Colon Cancer Cells by Targeting Notch-1 Pathway. Biochem. Biophys. Res. Commun Mar 19, 2020, 523(4), 947–953. DOI: 10.1016/j.bbrc.2020.01.048. Epub 2020 Jan 18. PMID: 31964531.
- Li, Z.; Zhang, J.; Ren, X.; Liu, Q.; Yang, X. The Mechanism of Quercetin in Regulating Osteoclast Activation and the PAR2/TRPV1 Signaling Pathway in the Treatment of Bone Cancer Pain. Int. J. Clin. Exp. Pathol. Nov 1, 2018, 11(11), 5149–5156. PMID: 31949595; PMCID: PMC6963045.
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis Through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. May 21, 2020, 20(20), 1–10, DOI: 10.1155/2020/9242601. PMID: 32537472; PMCID: PMC7260625.
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M. H.; Baghbadorani, P. Z.; Eskandari, N. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Front. Immunol. Feb 28, 2023, 14, 1077531. DOI: 10.3389/fimmu.2023.1077531.
- Liu, X. H.; Pan, L. L.; Zhu, Y. Z. Active Chemical Compounds of Traditional Chinese Medicine Herba Leonuri: Implications for Cardiovascular Diseases. Clin. Exp. Pharmacol. Physiol. Mar, 2012, 39(3), 274–282. DOI: 10.1111/j.1440-1681.2011.05630.x.
- Liu, G. Pharmacokinetics and Safety of Quercetin in Healthy Volunteers and Prostate Cancer Patients. J. Pharm. Pharmacol. Nov, 2016, 68(11): 1439–1447.
- Luo, C. L.; Liu, Y. Q.; Wang, P.; Song, C. H.; Wang, K. J.; Dai, L. P.; Zhang, J. Y.; Ye, H. The Effect of Quercetin Nanoparticle on Cervical Cancer Progression by Inducing Apoptosis, Autophagy and Anti-Proliferation via JAK2 Suppression. Biomed. Pharmacother. Aug, 2016, 82, 595–605. DOI: 10.1016/j.biopha.2016.05.029. Epub 2016 Jun 9. PMID: 27470402.
- Ma, Y. S.; Yao, C. N.; Liu, H. C.; Yu, F. S.; Lin, J. J.; Lu, K. W.; Liao, C. L.; Chueh, F. S.; Chung, J. G. Quercetin Induced Apoptosis of Human Oral Cancer SAS Cells Through Mitochondria and Endoplasmic Reticulum Mediated Signaling Pathways. Oncol. Lett. Jun, 2018, 15(6), 9663–9672. DOI: 10.3892/ol.2018.8584. Epub 2018 Apr 26. PMID: 29928342; PMCID: PMC6004715.
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cellular & Molecular Biology Letters. Dec, 2022, 27(1), 1–26. DOI: 10.1186/s11658-021-00301-9.
- Malakoti, F.; Majidinia, M.; Ahmadi, Y.; Yousefi, B.; Shanebandi, D. Quercetin Augments Cisplatin-Induced Apoptosis, DNA Damage Response, and MiR-22 Expression While It Prevents DNA Repair in Osteosarcoma Cells. Drug Res. (Stuttg). Sep, 2022, 72(7), 378–384. DOI: 10.1055/a-1800-6030. Epub 2022 Jun 20. PMID: 35724673.
- Marques, M. B.; Machado, A. P.; Santos, P. A.; Carrett-Dias, M.; Araújo, G. S.; da Silva Alves, B.; de Oliveira, B. S.; da Silva Júnior, F. M. R.; Dora, C. L.; Cañedo, A. D., et al. Anti-MDR Effects of Quercetin and Its Nanoemulsion in Multidrug-Resistant Human Leukemia Cells. ACAMC. 2021, 21(14), 1911–1920. DOI: 10.2174/1871520621999210104200722. PMID: 33397267.
- Mebarek, S.; Skafi, N.; Brizuela, L. Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer. Cancers. May 12, 2023, 15(10), 2732. DOI: 10.3390/cancers15102732.
- Mohamed, A. A.; Zaghloul, R. A.; Abdelghany, A. M.; El Gayar, A. M. Selenium Nanoparticles and Quercetin Suppress Thioacetamide-Induced Hepatocellular Carcinoma in Rats: Attenuation of Inflammation Involvement. J. Biochem. Mol. Toxicol. Apr, 2022, 36(4), e22989. DOI: 10.1002/jbt.22989. Epub 2022 Feb 18. PMID: 35179263.
- Mohammadi, E.; Alemi, F.; Maleki, M.; Malakoti, F.; Farsad-Akhtar, N.; Yousefi, B. Quercetin and Methotrexate in Combination Have Anticancer Activity in Osteosarcoma Cells and Repress Oncogenic MicroRNA-223. Drug Res. (Stuttg). Apr, 2022, 72(4), 226–233. DOI: 10.1055/a-1709-0658. Epub 2022 Apr 6. PMID: 35385884.
- Mohammadpour, A. H., et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell. Mol. Biol. (Noisy-le-Grand). Oct 31, 2017, 6311. 121–126.
- Momchilova, A.; Nikolaev, G.; Pankov, S.; Vassileva, E.; Krastev, N.; Robev, B.; Krastev, D.; Pinkas, A.; Pankov, R. Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells. Int. J. Mol. Sci. Nov 11, 2022, 2322, 13916. DOI: 10.3390/ijms232213916. PMID: 36430423; PMCID: PMC9697772.
- Mousavi, N.; Rahimi, S.; Emami, H.; Kazemi, A. H.; Mohammad Taghi Kashi, R.; Heidarian, R. The Effect of Quercetin Nanosuspension on Prostate Cancer Cell Line LNCaP via Hedgehog Signaling Pathway. Rep Biochem Mol Biol. Apr, 2021, 10(1), 69–75. DOI: 10.52547/rbmb.10.1.69. PMID: 34277870; PMCID: PMC8279718.
- Murata, M.; Komatsu, S.; Miyamoto, E.; Oka, C.; Lin, I.; Kumazoe, M.; Yamashita, S.; Fujimura, Y.; Tachibana, H. Quercetin Up-Regulates the Expression of Tumor-Suppressive microRnas in Human Cervical Cancer. Biosci. Microbiota Food Health. 2023, 42(1), 87–93. DOI: 10.12938/bmfh.2022-056. Epub 2022 Oct 5. PMID: 36660602; PMCID: PMC9816044.
- Mutlu Altundağ, E.; Kasacı, T.; Yılmaz, A. M.; Karademir, B.; Koçtürk, S.; Taga, Y.; Yalçın, A. S. Quercetin-Induced Cell Death in Human Papillary Thyroid Cancer (B-CPAP) Cells. J Thyroid Res. 2016, 9843675. Epub 2016 Jan 20. PMID: 27057371; PMCID: PMC4745605. DOI: 10.1155/2016/9843675.
- Mutlu Altundag, E.; Mine Yilmaz, A.; Kasaci, T.; Corek, C.; Taga, Y.; Suha Yalçin, A. The Role of HSP90 in Quercetin-Induced Apoptosis in Human Papillary Thyroid (B-CPAP) Cancer Cells. Free Radic Biol Med. Oct, 2014, 75(Suppl 1), S43. DOI: 10.1016/j.freeradbiomed.2014.10.797. Epub 2014 Dec 10. PMID: 26461378.
- Mutlu Altundağ, E.; Yılmaz, A. M.; Serdar, B. S.; Jannuzzi, A. T.; Koçtürk, S.; Yalçın, A. S. Synergistic Induction of Apoptosis by Quercetin and Curcumin in Chronic Myeloid Leukemia (K562) Cells: II. Signal Transduction Pathways Involved. Nutr. Cancer. 2021, 73(4), 703–712. DOI: 10.1080/01635581.2020.1767167. Epub 2020 May 18. PMID: 32420759.
- Naimi, A.; Entezari, A.; Hagh, M. F.; Hassanzadeh, A.; Saraei, R.; Solali, S. Quercetin Sensitizes Human Myeloid Leukemia KG-1 Cells Against TRAIL-Induced Apoptosis. J. Cell. Physiol. 2019, 234(8), 13233–13241. DOI: 10.1002/jcp.27995.
- Nday, C. M.; Halevas, E.; Jackson, G. E.; Salifoglou, A. Quercetin Encapsulation in Modified Silica Nanoparticles: Potential Use Against Cu (II)-Induced Oxidative Stress in Neurodegeneration. J. Inorg. Biochem. Apr 1, 2015, 145, 51–64. DOI: 10.1016/j.jinorgbio.2015.01.001.
- Nakhaee, S.; Farrokhfall, K.; Miri-Moghaddam, E.; Foadoddini, M.; Askari, M.; Mehrpour, O. The Effects of Quercetin on Seizure, Inflammation Parameters and Oxidative Stress in Acute on Chronic Tramadol Intoxication. BMC Pharmacology And Toxicology. Dec, 2021, 22(1), 1–1. DOI: 10.1186/s40360-021-00532-8.
- Özgöçmen, M.; Bayram, D.; Armağan, İ.; Türel, G. Y.; Sevimli, M.; Şenol, N. Is Quercetin Beneficial for Colon Cancer? A Cell Culture Study, Using the Apoptosis Pathways. Anti Cancer Agents Med. Chem. 2022, 22(1), 193–200. DOI: 10.2174/1871520621666210624110547. PMID: 34170811.
- Özsoy, S.; Becer, E.; Kabadayı, H.; Vatansever, H. S.; Yücecan, S. Quercetin-Mediated Apoptosis and Cellular Senescence in Human Colon Cancer. Anti Cancer Agents Med. Chem. 2020, 20(11), 1387–1396. DOI: 10.2174/1871520620666200408082026. PMID: 32268873.
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, 47. DOI: 10.1017/jns.2016.41.
- Paranthaman, S.; Uthaiah, C. A.; Osmani, R. A. M.; Hani, U.; Ghazwani, M.; Alamri, A. H.; Fatease, A. A.; Madhunapantula, S. V.; Gowda, D. V. Anti-Proliferative Potential of Quercetin Loaded Polymeric Mixed Micelles on Rat C6 and Human U87MG Glioma Cells. Pharmaceutics. Aug 6. 2022, 14(8), 1643. DOI: 10.3390/pharmaceutics14081643. PMID: 36015268; PMCID: PMC9412540.
- Pradhan, A.; Kumari, A.; Srivastava, R.; Panda, D. Quercetin Encapsulated Biodegradable Plasmonic Nanoparticles for Photothermal Therapy of Hepatocellular Carcinoma Cells. ACS Appl Bio Mater. Dec 16. 2019, 2(12), 5727–5738. DOI: 10.1021/acsabm.9b00764. Epub 2019 Nov 22. PMID: 35021566.
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules. Oct 3, 2022, 27(19), 6545. DOI: 10.3390/molecules27196545.
- Qiu, D.; Yan, X.; Xiao, X.; Zhang, G.; Wang, Y.; Cao, J.; Ma, R.; Hong, S.; Ma, M. To Explore Immune Synergistic Function of Quercetin in Inhibiting Breast Cancer Cells. Cancer Cell Int. Nov 27, 2021, 21(1), 632. DOI: 10.1186/s12935-021-02345-5. PMID: 34838003; PMCID: PMC8626953.
- Ramalingam, V.; Muthukumar Sathya, P.; Srivalli, T.; Mohan, H. Synthesis of Quercetin Functionalized Wurtzite Type Zinc Oxide Nanoparticles and Their Potential to Regulate Intrinsic Apoptosis Signaling Pathway in Human Metastatic Ovarian Cancer. Life. sci. Nov 15, 2022, 309, 121022. DOI: 10.1016/j.lfs.2022.121022. Epub 2022 Oct 4. PMID: 36206836.
- Rubió, L.; Motilva, M. J.; Romero, M. P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Critical Reviews in Food Science and Nutrition. Crit. Rev. Food Sci. Nutr. Jan 1, 2013, 53(9), 943–953. DOI: 10.1080/10408398.2011.574802.
- Rubio, V.; García-Pérez, A. I.; Herráez, A.; Diez, J. C. Different Roles of Nrf2 and NFKB in the Antioxidant Imbalance Produced by Esculetin or Quercetin on NB4 Leukemia Cells. Chem. Biol. Interact. Oct 1, 2018, 294, 158–166. DOI: 10.1016/j.cbi.2018.08.015. Epub 2018 Aug 30. PMID: 30171828.
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G. L. The Flavonoid Quercetin in Disease Prevention and Therapy: Facts and Fancies. Biochem. Pharmacol. 2012 Jan 1, 83(1), 6–15. DOI: 10.1016/j.bcp.2011.08.010.
- Ryu, S.; Park, S.; Lim, W.; Song, G. Quercetin Augments Apoptosis of Canine Osteosarcoma Cells by Disrupting Mitochondria Membrane Potential and Regulating PKB and MAPK Signal Transduction. J. Cell. Biochem. Oct, 2019, 120(10), 17449–17458. DOI: 10.1002/jcb.29009. Epub 2019 May 26. PMID: 31131468.
- Salama, Y. A.; El-Karef, A.; El Gayyar, A. M.; Abdel-Rahman, N. Beyond Its Antioxidant Properties: Quercetin Targets Multiple Signalling Pathways in Hepatocellular Carcinoma in Rats. Life. sci. Nov 1, 2019, 236, 116933. DOI: 10.1016/j.lfs.2019.116933. Epub 2019 Oct 12. PMID: 31614146.
- Serri, C.; Quagliariello, V.; Iaffaioli, R. V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination Therapy for the Treatment of Pancreatic Cancer Through Hyaluronic Acid-Decorated Nanoparticles Loaded with Quercetin and Gemcitabine: A Preliminary In Vitro Study. J. Cell. Physiol. Apr, 2019, 234(4), 4959–4969. DOI: 10.1002/jcp.27297. Epub 2018 Oct 18. PMID: 30334571.
- Seyed, M. A.; Ghanadian, M.; Kamarul, T.; Fakurazi, S.; Bagheri, A. The Effect of Quercetin in the Treatment of Cancer: An Overview. Iran. J. Med. Sci. May, 2019, 44(3): 170–177.
- Shahidi, S.; Rostamizadeh, K.; Fathi, M.; Nedaei, K.; Ramazani, A. Combination of Quercetin Or/And siRna-Loaded DDAB-MPEG-PCL Hybrid Nanoparticles Reverse Resistance to Regorafenib in Colon Cancer Cells. BMC Complement. Med. Ther. 2022 Dec 27, 22(1), 340. DOI: 10.1186/s12906-022-03787-8. PMID: 36575448; PMCID: PMC9793538.
- Shang, H. S.; Lu, H. F.; Lee, C. H.; Chiang, H. S.; Chu, Y. L.; Chen, A.; Lin, Y. F.; Chung, J. G. Quercetin Induced Cell Apoptosis and Altered Gene Expression in AGS Human Gastric Cancer Cells. Environ. Toxicol. Nov, 2018, 33(11), 1168–1181. DOI: 10.1002/tox.22623. Epub 2018 Aug 27. PMID: 30152185.
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX Phosphorylation: A Marker for DNA Damage. Methods Mol. Biol. 2017, 1526, 125–133.
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive in situ Gel-A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics. Nov 20, 2022, 14(11), 2527. DOI: 10.3390/pharmaceutics14112527. PMID: 36432718; PMCID: PMC9695231.
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin Inhibits the Growth of Human Gastric Cancer Stem Cells by Inducing Mitochondrial-Dependent Apoptosis Through the Inhibition of PI3K/Akt Signaling. Int. J. Mol. Med. Aug, 2016, 38(2), 619–626. DOI: 10.3892/ijmm.2016.2625. Epub 2016 Jun 6. PMID: 27278820
- Shafabakhsh, R.; Asemi, Z. Quercetin: A Natural Compound for Ovarian Cancer Treatment. Journal Of Ovarian Research. Dec, 2019, 12(1), 1–9. DOI: 10.1186/s13048-019-0530-4.
- Shi, H.; Li, X. Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z. X.; Chen, P. H.; Dai, H. Q.; Feng, J.; Chatterjee, S., et al. Quercetin Induces Apoptosis via Downregulation of Vascular Endothelial Growth Factor/Akt Signaling Pathway in Acute Myeloid Leukemia Cells. Front Pharmacol. Dec, 2020, 10;11, DOI: 10.3389/fphar.2020.534171. Erratum in: Front Pharmacol. 2021 Feb 08;11:640750. PMID: 33362534; PMCID: PMC7758733, 534171.
- Shi, X.; Deepak, V.; Wang, L.; Ba, X.; Komori, T.; Zeng, X., et al. Thrombospondin-1 is a Putative Target Gene of Runx2 and Runx3. Int. J. Mol. Sci. 2013, 14(7), 14321–14332. DOI: 10.3390/ijms140714321.
- Shree, A.; Islam, J.; Sultana, S. Quercetin Ameliorates Reactive Oxygen Species Generation, Inflammation, Mucus Depletion, Goblet Disintegration, and Tumor Multiplicity in Colon Cancer: Probable Role of Adenomatous Polyposis Coli, β-Catenin. Phytother. Res. Apr, 2021, 35(4), 2171–2184. DOI: 10.1002/ptr.6969. Epub 2020 Dec 22. PMID: 33350534.
- Shu, Y.; Xie, B.; Liang, Z.; Chen, J. Quercetin Reverses the Doxorubicin Resistance of Prostate Cancer Cells by Downregulating the Expression of C-Met. Oncol. Lett. Feb, 2018, 15(2), 2252–2258. DOI: 10.3892/ol.2017.7561. Epub 2017 Dec 8. PMID: 29434932; PMCID: PMC5777119.
- Singla, R. K.; Sai, C. S.; Chopra, H.; Behzad, S.; Bansal, H.; Goyal, R.; Gautam, R. K.; Tsagkaris, C.; Joon, S.; Singla, S., et al. Natural Products for the Management of Castration-Resistant Prostate Cancer: Special Focus on Nanoparticles Based Studies. Front Cell Dev Biol. Nov 5, 2021, 9, 745177. DOI: 10.3389/fcell.2021.745177.
- Singh, C. K.; Chhabra, G.; Ndiaye, M. A.; Siddiqui, I. A.; Panackal, J. E.; Mintie, C. A.; Ahmad, N. Quercetin-Resveratrol Combination for Prostate Cancer Management in TRAMP Mice. Cancers (Basel). Aug 2. 2020, 12(8), 2141. DOI: 10.3390/cancers12082141. PMID: 32748838; PMCID: PMC7465013.Sobral, F., Calhelha, R. C., Barros, L., Dueñas, M., Tomás, A., Santos-Buelga, C. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules. 2017. 22, 22.
- Son, H. K.; Kim, D. Quercetin Induces Cell Cycle Arrest and Apoptosis in YD10B and YD38 Oral Squamous Cell Carcinoma Cells. Asian Pac. J. Cancer Prev. Jan 1, 2023, 24(1), 283–289. DOI: 10.31557/APJCP.2023.24.1.283. PMID: 36708578
- Suganthy, N.; Devi, K. P.; Nabavi, S. F.; Braidy, N.; Nabavi, S. M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. Dec 1, 2016, 84, 892–908. DOI: 10.1016/j.biopha.2016.10.011.
- Sun, Y.; Xie, W.; Kang, N.; Yi, J.; Ruan, X.; Hu, L.; Zhao, J.; Zheng, X.; Wei, S.; Gao, M. To Explore the Inhibitory Mechanism of Quercetin in Thyroid Papillary Carcinoma Through Network Pharmacology and Experiments. Dis. Markers. Dec 3, 2022, 9541080. DOI: 10.1155/2022/9541080. PMID: 36510497; PMCID: PMC9741536.
- Taylor, M. A.; Khathayer, F.; Ray, S. K. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. Jul, 2019, 44(7), 1715–1725. DOI: 10.1007/s11064-019-02802-8. Epub 2019 Apr 22. PMID: 31011879.
- Teekaraman, D.; Elayapillai, S. P.; Viswanathan, M. P.; Jagadeesan, A. Quercetin Inhibits Human Metastatic Ovarian Cancer Cell Growth and Modulates Components of the Intrinsic Apoptotic Pathway in PA-1 cell Line. Chem. Biol. Interact. Feb 25, 2019, 300, 91–100. DOI: 10.1016/j.cbi.2019.01.008. Epub 2019 Jan 9. PMID: 30639267.
- Terana, G. T.; Abd-Alhaseeb, M. M.; Omran, G. A.; Okda, T. M. Quercetin Potentiates 5-Fluorouracil Effects in Human Colon Cancer Cells Through Targeting the Wnt/β-Catenin Signalling Pathway: The Role of MiR-27a. Contemp Oncol (Pozn). 2022, 26(3), 229–238. DOI: 10.5114/wo.2022.120361. Epub 2022 Oct 24. PMID: 36381675; PMCID: PMC9641630.
- Tezerji, S.; Nazari Robati, F.; Abdolazimi, H.; Fallah, A.; Talaei, B. Quercetin’s Effects on Colon Cancer Cells Apoptosis and Proliferation in a Rat Model of Disease. Clin. Nutr. ESPEN. Apr, 2022, 48, 441–445. DOI: 10.1016/j.clnesp.2022.01.004. Epub 2022 Jan 10. PMID: 35331526.
- Tomas-Hernández, S.; Blanco, J.; Rojas, C.; Roca-Martínez, J.; Ojeda-Montes, M. J.; Beltrán-Debón, R.; Garcia-Vallvé, S.; Pujadas, G.; Arola, L.; Mulero, M. Resveratrol Potently Counteracts Quercetin Starvation-Induced Autophagy and Sensitizes HepG2 Cancer Cells to Apoptosis. Mol. Nutr. Food Res. Mar, 2018, 62(5), 1700610. DOI: 10.1002/mnfr.201700610.
- Trinh, N. T.; Nguyen, T. M. N.; Yook, J. I.; Ahn, S. G.; Kim, S. A. Quercetin and Quercitrin from Agrimonia Pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells Through the JNK Signaling Pathway. Pharm (Basel). Mar 17, 2022, 15(3), 364. DOI: 10.3390/ph15030364. PMID: 35337161; PMCID: PMC8951172.
- Ulusoy, H. G.; Sanlier, N. A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities. Crit. Rev. Food Sci. Nutr. Oct 27, 2020, 60(19), 3290–3303. DOI: 10.1080/10408398.2019.1683810.
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S., et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. PMID: 32175075; PMCID: PMC7063794. Mar 10 2020, 10, 32. DOI: 10.1186/s13578-020-00397-0.
- Wang, L.; Ji, S.; Liu, Z.; Zhao, J. Quercetin Inhibits Glioblastoma Growth and Prolongs Survival Rate Through Inhibiting Glycolytic Metabolism. Chemotherapy. 2022, 67(3), 132–141. DOI: 10.1159/000523905. Epub 2022 Mar 4. PMID: 35249013.
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin Radiosensitizes Non-Small Cell Lung Cancer Cells Through the Regulation of MiR-16-5p/WEE1 Axis. IUBMB Life. May, 2020, 72(5), 1012–1022. DOI: 10.1002/iub.2242. Epub 2020 Feb 6. PMID: 32027086.
- Wang, T.; Xiang, Z.; Zhang, X.; Cao, X.; Huang, G. Quercetin Enhances the Efficacy of Chemotherapy in Non-Small-Cell Lung Cancer Cells by Inhibiting Glucose Metabolism. Oncol. Lett. Sep, 2021, 223, 788. DOI: 10.3892/ol.2021.12866. Epub 2021 Jul 9
- Weng, C. J.; Yen, G. C. Flavonoids, a Ubiquitous Dietary Phenolic Subclass, Exert Extensive In Vitro Anti-Invasive and In Vivo Anti-Metastatic Activities. Cancer And Metastasis Reviews. Jun, 2012, 31(1–2), 323–351. DOI: 10.1007/s10555-012-9347-y.
- Wu, B.; Zeng, W.; Ouyang, W.; Xu, Q.; Chen, J.; Wang, B.; Zhang, X. Quercetin Induced NUPR1-Dependent Autophagic Cell Death by Disturbing Reactive Oxygen Species Homeostasis in Osteosarcoma Cells. J. Clin. Biochem. Nutr. Sep, 2020, 67(2), 137–145. DOI: 10.3164/jcbn.19-121. Epub 2020 Mar 6. PMID: 33041510; PMCID: PMC7533857.
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.et al. Quercetin Shows Anti-Tumor Effect in Hepatocellular Carcinoma LM3 Cells by Abrogating JAK2/STAT3 Signaling Pathway. Cancer Med. Aug, 2019, 810, 4806–4820. DOI: 10.1002/cam4.2388. Epub 2019 Jul 5. PMID: 31273958; PMCID: PMC6712453.
- Wu, R.; Zhou, T.; Xiong, J.; Zhang, Z.; Tian, S.; Wang, Y.; Chen, J.; Quercetin, T. X. The Ingredient of Xihuang Pills, Inhibits Hepatocellular Carcinoma by Regulating Autophagy and Macrophage Polarization. Front. Biosci. Dec 20, 2022, 27(12), 323. DOI: 10.31083/j.fbl2712323. PMID: 36624942.
- Wu, T., et al.Pharmacokinetics and Pharmacodynamics of Quercetin in Regulating Tumor Angiogenesis.Eur. J. Pharm. Sci. Aug 30, 2018, 121, 176–185.
- Xiao, J.; Zhang, B.; Yin, S.; Xie, S.; Huang, K.; Wang, J.; Yang, W.; Liu, H.; Zhang, G.; Liu, X., et al. Quercetin Induces Autophagy-Associated Death in HL-60 Cells Through CaMkkβ/AMPK/mTOR signal Pathway. Acta Biochim Biophys Sin (Shanghai). Sep 25, 2022, 54(9), 1244–1256. DOI: 10.3724/abbs.2022117. PMID: 36148953; PMCID: PMC9827794.
- Xu, G.; Li, B.; Wang, T.; Wan, J.; Zhang, Y.; Huang, J.; Shen, Y. Enhancing the Anti-Ovarian Cancer Activity of Quercetin Using a Self-Assembling Micelle and Thermosensitive Hydrogel Drug Delivery System. R.S.C. Adv. Jun, 2018, 11;8(38), 21229–21242. DOI: 10.1039/c8ra03274b. Erratum in: RSC Adv 2019 Oct 16;9(57):33193-33194. PMID: 35539921; PMCID: PMC9080896.
- Xu, W.; Xie, S.; Chen, X.; Pan, S.; Qian, H.; Zhu, X. Effects of Quercetin on the Efficacy of Various Chemotherapeutic Drugs in Cervical Cancer Cells. Drug Des. Devel. Ther. Feb 15, 2021, 15, 577–588. DOI: 10.2147/DDDT.S291865. PMID: 33623367; PMCID: PMC7894806.
- Xu, Y. X.; Wang, B.; Zhao, X. H. In Vitro Effects and the Related Molecular Mechanism of Galangin and Quercetin on Human Gastric Cancer Cell Line (SGC-7901). Pak. J. Pharm. Sci. Jul 2017, 30(4), 1279–1287. PMID: 29039326
- Yamada, N.; Matsushima-Nishiwaki, R.; Kozawa, O. Quercetin Suppresses the Migration of Hepatocellular Carcinoma Cells Stimulated by Hepatocyte Growth Factor or Transforming Growth Factor-α: Attenuation of AKT Signaling Pathway. Arch. Biochem. Biophys Mar 30. 2020, 682, 108296. DOI: 10.1016/j.abb.2020.108296. Epub 2020 Feb 4. PMID: 32032576.
- Yang, F.; Jiang, X.; Song, L.; Wang, H.; Mei, Z.; Xu, Z.; Xing, N. Quercetin Inhibits Angiogenesis Through Thrombospondin-1 Upregulation to Antagonize Human Prostate Cancer PC-3 Cell Growth In Vitro and In Vivo. Oncol. Rep. 2016, 35(3), 1602–1610. DOI: 10.3892/or.2015.4481.
- Yang, L.; Hu, Z.; Zhu, J.; Liang, Q.; Zhou, H.; Li, J.; Fan, X.; Zhao, Z.; Pan, H.; Fei, B. Systematic Elucidation of the Mechanism of Quercetin Against Gastric Cancer via Network Pharmacology Approach. Biomed Res. Int. 2020 Sep 3, PMID: 32964029; PMCID: PMC7486643. 2020, 1–11. DOI: 10.1155/2020/3860213.
- Yang, P.; Li, X.; Wen, Q.; Zhao, X. Quercetin Attenuates the Proliferation of Arsenic-Related Lung Cancer Cells via a Caspase-Dependent DNA Damage Signaling. Mol. Carcinog. Jul, 2022, 61(7), 655–663. DOI: 10.1002/mc.23408. Epub 2022 Apr 18. PMID: 35436022
- Zeng, Y.; Shen, Z.; Gu, W.; Wu, M. Bioinformatics Analysis to Identify Action Targets in NCI-N87 Gastric Cancer Cells Exposed to Quercetin. Pharm. Biol. 2018, 56(1), 393–398. DOI: 10.1080/13880209.2018.1493610.
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin Suppresses the Tumorigenesis of Oral Squamous Cell Carcinoma by Regulating microRNA-22/WNT1/β-Catenin Axis. J. Pharmacol. Sci. Jun, 2019, 140(2), 128–136. DOI: 10.1016/j.jphs.2019.03.005. Epub 2019 May 4. PMID: 31257059.
- Zhang, L.; Cheng, X.; Gao, Y., et al. Quercetin: A Promising Antioxidant for Cancer Prevention and Treatment. Biomed. Pharmacother. Mar, 2020, 121, 109604. DOI: 10.1016/j.biopha.2019.109604.
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. Onco. Targets Ther. Jan 16, 2020, 13, 513–523. DOI: 10.2147/OTT.S228453. PMID: 32021294; PMCID: PMC6970612.
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin Inhibits Cell Viability, Migration and Invasion by Regulating MiR-16/HOXA10 Axis in Oral Cancer. Eur. J. Pharmacol. Mar 15, 2019, 847, 11–18. DOI: 10.1016/j.ejphar.2019.01.006. Epub 2019 Jan 9. PMID: 30639311.
- Zhaorigetu, F. I.; Belal, A.; Badawi, M. H. A.; Abdelhady, A. A.; Galala, F. M. A. A.; El-Sharkawy, A.; El-Dahshan, A. A.; Mehany, A. B. M.; Mehany, A. B. M. Antiproliferative, Apoptotic Effects and Suppression of Oxidative Stress of Quercetin Against Induced Toxicity in Lung Cancer Cells of Rats: In Vitro and In Vivo Study. J. Cancer Jun, 26, 2021, 1217, 5249–5259. DOI: 10.7150/jca.52088. PMID: 34335941; PMCID: PMC8317526.
- Zhu, Q.; Yang, L.; Yang, H.; Han, Y.; Chen, Y.; He, Y. Quercetin Alleviates the Progression of Breast Cancer-Related Depression via Inhibiting the Pyroptosis and Promoting the Immune Response. Mediators Inflamm. Mar, 3, 2022, 8011988. DOI: 10.1155/2022/8011988. PMID: 35369029; PMCID: PMC8966747.
- Zhu, S.; Yu, W.; Bi, L.; Qin, F.; Li, J.; Zeng, H.; Lu, L. Quercetin Induces Apoptosis of Human Breast Cancer Cells by Activating PTEN and Inhibiting PI3K/AKT and JNK Signaling Pathways. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. Aug 2022, 38(8), 714–720. Chinese. PMID: 35851085.
- Zulkefli, N.; Che Zahari, C. N.; Sayuti, N. H.; Kamarudin, A. A.; Saad, N.; Hamezah, H. S.; Bunawan, H.; Baharum, S. N.; Mediani, A.; Ahmed, Q. U., et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. Feb 27, 2023, 24 5, 4607. DOI: 10.3390/ijms24054607.