ABSTRACT
Neurological and cardiovascular illnesses, cancers, metabolic disorders, and microbial infections result in deaths of millions of people globally. Recent researches have intensely been directed at evaluating the therapeutic potential of herbal plants as alternative medicines. One such species is chamomile (Matricaria chamomilla), from the Asteraceae family. It elicits protective properties against inflammation and oxidative stress, bacterial and fungal infections, cancers, ulcers, neuropathologies, etc. The present review focuses on various medical/ethnomedicinal uses of chamomile. Moreover, we emphasize on the therapeutic actions of constituents from chamomile, along with their underlying mechanism(s). Literature was systematically searched on electronic databases using keywords; “Matricaria chamomilla/chamomile” in combination with “medicinal,” and “therapeutic.” Our analyses of the retrieved articles indicate tremendous therapeutic potential of chamomile, and establish it as an alternative multimodal herbal medicine. The individual active constituents (e.g., geraniol, caffeic acid, chlorogenic acid, umbelliferone, rutin) were also found to elicit significant beneficial properties in various disorders. Collectively, chamomile and its bioactive seem to hold great promises as miraculous herbal drugs with multiple medical uses. In conclusion, based upon its beneficial antibacterial, antioxidant and anti-inflammatory actions, Matricaria chamomilla is strongly implicated in protection against skin photo-damage, aging, neuropathologies and cancers.
Introduction
The study of herbs dates back to over 5,000 years to the ancient Sumerians, who initiated the description of medicinal uses for plants. Since then, traditional medicine has been used to treat a variety of illnesses. Modern research has also been increasingly focused on the therapeutic potential of various traditional medicinal plants and their bioactives. One such plant is Matricaria chamomilla, a popular therapeutic plant species from the Asteraceae family (). Its medicinal and fragrant qualities have earned it the nickname “the star of medical plants.” Due to its apple-like scent, it gets the name “chamomile” (the Greek word “chamaimlon” means “earth-apple”)[Citation1]. Chamomile was first mentioned in ancient Egyptian writings, wherein crushed chamomile flower heads were evidenced to be used in cosmetic formulations, and for the treatment skin conditions and dermatitis.[Citation2] Chamomile’s therapeutic potential has also been mentioned by Greek physicians, Asclepius, Hippocrates, and Galen.[Citation3] . Interestingly, chamomile was included as a member of the nine sacred herbs in Anglo-Saxon culture. Medieval Arab herbalists have also noted the numerous traditional remedies of chamomile based upon its hepatoprotective, anti-inflammatory, anti-flatulence, anti-diarrheal, spasmolytic, laxative and diuretic, and anti-epileptic properties.[Citation1]
Chamomile remains one of the most popular medicinal herbs used in the modern age. In fact, it is included in the pharmacopoeia of 26 countries.[Citation4] Interestingly, in 1987, Germany designated it as “the medicinal plant of the year.” Chamomile is cultivated commercially in many nations for the extraction of its blue essence, production of herbal tea, and for pharmacological and cosmeceutical uses.[Citation4] Its flower heads and essential oils are employed for anti-inflammatory, antibacterial, antiseptic, antispasmodic, and sedative uses.[Citation5] Herbal tea containing chamomile extract is regularly consumed by significant number of individuals across the globe. When the mucous membranes of the mouth and throat become inflamed, the tea infusion may be used as a wash or gargle. Chamomile flower in form of a tincture is used to treat diarrhea in children. In situations of external swelling, like facial swelling linked to an underlying illness or abscess, chamomile flowers are often used as a poultice or hot foment. Chamomile extract is also used in pharmacological formulations to to treat toothaches, earaches, and neuralgia.[Citation6] Additionally, it has been reported to be utilized as a bath addition and is suggested to reduce anogenital irritation.[Citation7] It is advised to breathe in the vaporized essential oils made from chamomile flowers to treat general sadness and anxiety. Chamomile oil is often used as a component in aromatherapy and hair care products.[Citation6]
While the traditional and modern medicinal uses of chamomile are well-known, researchers are only beginning to establish the molecular and cellular mechanisms of actions of the whole chamomile extract as well as its individual phytochemicals. Thus, studies have indicated their potent antioxidant functions of scavenging reactive oxygen species and inhibiting oxidative damage.[Citation8] The purpose of this timely review is to summarize the utilities of chamomile and its bioactives in the numerous pathological conditions, with a particular focus on the underlying mechanisms of action.
Chemical constituents of Matricaria chamomilla and their therapeutic potentials
Matricaria chamomilla includes various therapeutically valuable active chemical constituents. These include sesquiterpenes, flavonoids, coumarins, and polyacetylenes. Bioactive compounds of chamomile extract are summarized in . In the following sections, we discuss the therapeutic potential of the major active constituents of Matricaria chamomilla.
Table 1. Various bioactive chemical constituents of Matricaria chamomile.
α-bisabolol
Bisabolol is a sesquiterpene alcohol that was first isolated from Matricaria chamomilla in the 20th century. Due to its low toxicity, the US Food and Drug Administration (FDA) has classified bisabolol as generally recognized as safe (GRAS) chemical, allowing it to be utilized as an active ingredient in a number of commercial products.[Citation12] Bisabolol has shown to harbor significant anti-inflammatory, analgesic, antibacterial, anticancer, antibacterial, antiseptic, and skin-soothing properties.[Citation39]
Gastroprotective actions
Bisabolol reduces pepsin levels without changing the pH of stomach acid, making it a great alternative for treating stomach and upper intestinal dysfunctions.[Citation3]
Leishmanicidal activities
Bisabolol has been shown to elicit strong leishmanicidal action, possibly via activation of programmed cell death in the promastigote stage of the parasite. It result in damages of cellular membranes and reduce mitochondrial membrane potential and ATP production in this parasite.[Citation40] Neuroprotective and cardioprotective actions: α-bisabolol has been shown to induce neuroprotection by preventing the formation of amyloid oligomers and prevent their fibrillization.[Citation41] Further, it can inhibit inflammatory signaling through the NLRP3 inflammasome and toll-like receptor-4/nuclear factor kappa B/mitogen-activated protein kinase (TLR4-NFκB/MAPK) pathway signalling, rectify lysosomal dysfunction, rescue dysregulations in autophagic flux, and mitigate oxidative stress.[Citation42]
Antioxidant functions
Antioxidant activity of α-bisabolol has been determined in both cell culture and cell-free systems. It has been reported that bisabolol robustly inhibits the formation of reactive oxygen species (ROS) and stimulates antioxidant systems.[Citation12] Nonetheless, the antioxidant mechanisms of bisabolol need to be further tested.
Anti-inflammatory
Bisabolol-enriched plant extract have been reported to elicit antiphlogistic effects because of their anti-inflammatory properties [40]. The traditional uses of α-bisabolol in treating various skin irritations including dermatitis and eczema may also depend, at least in part, on its anti-inflammatory actions of inhibiting leukotriene synthesis.[Citation12] This is confirmed by an in vitro study which showed that α-bisabolol inhibits 5-lipoxygenase (5-LOX).[Citation43]
Anticancer effects
α-Bisabolol may also harbor anti-tumorigenic effects by promoting apoptosis of cancer cells via modulation of kisspeptin receptor 1 signaling,[Citation10] however this needs to be further researched.
β-farnesene
β-farnesene is a sesquiterpenoid with several industrial applications and is commercially synthesized using engineered Saccharomyces cerevisiae strains.[Citation44]
Antioxidant actions
β-farnesene has been reported to show antioxidant free radical scavenging activity in in vitroiDPPH assays.[Citation45]
Antimicrobial potential
β-farnesene-enriched extracts have shown robust antibacterial activity against both gram-positive and gram-negative bacteria including Staphylococcus aureus.[Citation46] β-farnesene-mediated inhibition of Staphylococcus epidermidis and E. coli has also been reported in the literature.[Citation47]
Aphicidal activity
Aphids are a cause of concern for serious damage to agricultural products. β-farnesene is known as a biological agent in repellents for controlling aphids.[Citation48] In aphicidal bioassay systems, farnesene and its analogs have shown appreciable aphicidal activity against Myzus persicae[Citation49]. In silico analyses have been performed to evaluate the inhibitory mechanism of β-farnesene against molecular targets in aphids.[Citation50]
Germacrene D
Germacrene D is a flavorful chemical obtained from the flower of Matricaria chamomile. It is also extracted from herbs such as Artemisia annua and Hypericum .
Antimicrobial actions
Germacrene D containing essential oil extracted from Artemisia annua has been shown to elicit antimicrobial activity against Enterococcus hirae (gram-positive) and certain fungal species.[Citation51] Germacrene D also harbors potential for inhibiting gram-negative bacterial species such as Klebsiella pneumoniae, Salmonella enteritidis, gram-positive bacteria like Staphylococcus aureus and Bacillus subtilis, and fungi such as Aspergillus niger and Candida albicans.[Citation22,Citation52]
Antioxidant potential
The extracted oil having Germacrene D as a major compound exhibits antioxidant activity using DPPH and showed inhibition compared with BHA and ascorbic acid moreover the compound was characterized by GC-MS analysis. Furthermore, the ABTS Assay also showed the inhibition of 28% measuring the absorbance by UV at 415 nm.[Citation53]
Anti-inflammatory activities
Germacrene D has been evidenced to show immunotherapeutic effects by modulatory neutrophil functions.[Citation54] Essential oil extracted from Verbesina macrophylla (Cass.) containing germacrene D as the major component was shown to beneficially alter the pro-inflammatory actions of immune mediators, tumor necrosis factor alfa (TNF-α) and interleukin 1 beta (IL-1β).[Citation55]
Occidol acetate
Occidol is another relevant bioactive which can be extracted from Matricaria chamomile. It is also enriched in dichloromethane extract of the Fagraea fragrans.
Anti-microbial actions
Occidol has antibacterial activity against both gram-positive and -negative bacteria. Fagraea fragrans extract containing occidol acetate, as determined by GC-MS has been illustrated to show significant antibacterial activity against gram-negative bacteria, Serratia marcescens, Escherichia coli, and Salmonella typhi as well as gram-positive bacterial species, Micrococcus luteus, Staphylococcus aureus and Listeria monocytogenes.[Citation56]
Chamazulene
Chamazulene is derived from chamomile flower heads. It has been shown to elicit potent herbicidal effects and phytotoxic action against weed species, such as Amaranthus chlorostachys (A. cruentus), Chenopodium album, Cirsium arvense, Persicaria lapathifolia (Polygonum lapathifolium), Sonchus oleraceus, and Stellaria medium.[Citation57]
Antitumorigenic actions
Chamazulene showed anticancer activity through apoptotic pathway on cancerous cell.[Citation58] Other in vitro studies have also confirmed its robust antiproliferative and antitumorigenic actions.[Citation59]
Anti-inflammatory and anti-oxidant
Chamazulene is implicated as a potent anti-inflammatory agent.[Citation20] In addition, it is also an antioxidant and free radical scavenger, and can suppress leukotriene B4 synthesis.[Citation60,Citation61] The sturdy anti-oxidant and anti-inflammatory properties of chamazulene have been proposed as mechanisms for treating osteoarthritis, as evidenced by animal studies.[Citation58]
Apigenin
Apigenin is a naturally occurring compound of the flavone family, sometimes referred to by its chemical name 4,’5,7-trihydroxyflavone. It is an aglycone derivative of many naturally occurring glycosides with the generic molecular formula C15H10O5 and molecular weight 270.24. Numerous biological effects including antioxidant, anticancer, anti-inflammatory, anti-allergic, anti-genotoxic, cardioprotective, neuroprotective, and antibacterial have been shown for apigenin or and its synthetic flavone derivatives. Because of its yellow color, apigenin is also used as a dye for wool.[Citation62]
Anti-inflammatory and anti-oxidant actions
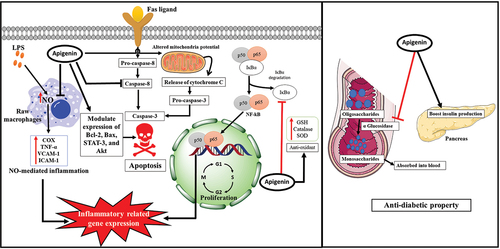
Apigenin can promote anti-inflammatory pathways such as p38/MAPK and PI3K/Akt, preventing IκB degradation and nuclear translocation of the NF-κB via inhibiting COX-2 activity.[Citation63,Citation64] Apigenin also has the potential to increase the cellular antioxidant defense system via increasing activities of glutathione, catalase, and superoxide dismutase (SOD).[Citation65] Apigenin-7-glucoside (APG), a chemical constituent of Matricaria chamomilla, has been demonstrated to have anti-inflammatory properties by reducing TNF-α production in mouse macrophages.[Citation66]
Anti-diabetic potential
Apigenin has been reported to harbor significant anti-diabetic activities due to its ability to suppress α-glucosidase enzyme, boost insulin production,[Citation67] and neutralize ROS in the cell.[Citation68] Additionally, it reduced glycolysis and glucose uptake while boosting mitochondrial function.[Citation69] Apigenin has also provided endothelial cells with a modest amount of NO, reducing the risk of endothelial cell damage and dysfunction induced by hyperglycemia.[Citation70]
Anticancer properties
The anticancer properties of apigenin rely on its ability to arrest cell cycle progression and induce apoptosis.[Citation25,Citation25,Citation25] It causes cell cycle arrest at the G1/S and G2/M phases via altering the expression of cyclin dependent kinases (CDKs) and other cell cycle related genes.[Citation71–73] It also influences intrinsic apoptotic pathways by altering mitochondrial membrane potential, triggering cytoplasmic release of cytochrome c, and activating caspase 3.[Citation74] Additionally, it has been reported to modulate apoptotic pathways by activating caspase-8 and modulating the expression of various other proteins, including Bcl-2, Bax, STAT-3, and Akt, which drive death in cancer cells.[Citation75] Additionally, Chiang et al. showed that apigenin possesses anti-hepatoma properties through an apoptotic mechanism mediated by the p53-dependent pathway and the increase of p21 expression, which causes cell cycle arrest in the G2/M phase.[Citation76]
Neuroprotective properties
Apigenin has been implicated as a neuroprotectant. For instance, it can reduce GABA-activated chloride current in a dose-dependent manner in picrotoxin-induced convulsions.[Citation77] Apigenin has been proposed as a potential natural treatment for neuroinflammation-related diseases, including multiple sclerosis. Indeed, it was found that apigenin decreased neuroinflammatory signaling in LPS-treated dendritic cells (DCs) [69]. Further, apigenin may also be beneficial in ameliorating motor deficits by interacting with various neurotransmission systems.[Citation77,Citation78] demonstrates the different mechanism(s) of apigenin.
Geraniol (GE)
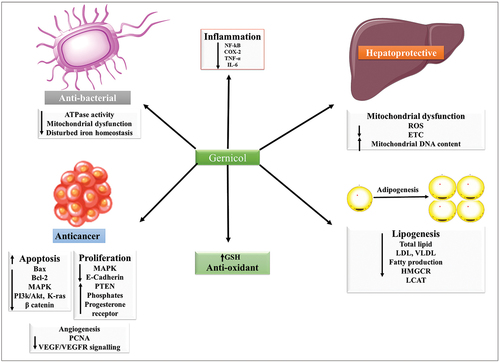
Geraniol is a monoterpene extracted from the shoot primordia of Matricaria chamomilla and possesses dose and time-dependent apoptosis-inducing activity.[Citation79] Its pharmacological properties include anticancer, anti-inflammatory, antioxidative, antibacterial activity, hepatoprotective, cardioprotective, and neuroprotective.[Citation80]
Anticancer functions
Geraniol possesses strong antitumorigenic and cytotoxic effects. For instance, in colon cancer colo-205 cells, geraniol suppresses cell growth and promotes apoptosis via upregulation of Bax and downregulation of Bcl-2, as well as by inducing DNA damage and cell cycle arrest.[Citation81,Citation82] Geraniol may also inhibit transcription of K-ras, MAPK, PI3K, NF-κB, COX-2 and catenin while increasing the expression of phosphatase, PTEN, progesterone receptors and E-cadherin protein.[Citation83] It also has anti-angiogenic properties in the endothelioma eEND2 cells, which are probably mediated by decreases in PCNA production and inhibition of the VEGF/VEGFR-2 signaling pathway.[Citation84]
Anti-inflammatory and anti-oxidant actions
Inhibition of COX-2 and stimulation of IL-10 are thought to underlie the anti-inflammatory action of geraniol.[Citation85,Citation86] Further, it has also been reported to increase glutathione levels, thus contributing to the endogenous anti-oxidant mechanisms.
Hepatoprotective functions
Geraniol has been reported to harbor significant hepatoprotective potential. It suppresses activity of alanine aminotransferase and aspartate aminotransferase.[Citation87] It is also reported to maintain hepatic mitochondrial function by decreasing hepatic mitochondrial ROS stimulating mitochondrial electron transport chain enzyme activity, and protecting mitochondrial DNA,[Citation88] Further, it can stimulate the production of iNOS and cytochrome P450 2E1 in hepatocytes, and reduce malondialdehyde and 3-nitrotyrosine synthesis.[Citation89] Furthermore, Geraniol also inhibits liver inflammation by inhibiting cytokines, TNF-α and IL-6.[Citation88] It also lowers blood fat levels by inhibiting lipogenesis and downregulating HMGCR, and LCAT lowers cholesterol esters levels and increases C-reactive protein (CRP) activity.[Citation88] Additionally, geraniol therapy also suppresses the synthesis of hepatic fatty acids, total lipids, and non-saponifiable lipids, lowering total cholesterol and triglyceride in the plasma. Geraniol can also induce production of LDL mRNA and VLDL receptor mRNA and promote blood LDL uptake. Finally, it reduces fatty acid production while suppressing triglyceride synthesis.[Citation90]
Anti-fungal actions
Studies indicate that geraniol is s robust antifungal agent. Treatment with geraniol has been shown to retard growth of Candida albicans and other Candida species,[Citation91] possibly by altering mitochondrial function, disrupting iron homeostasis, and reducing genotoxicity.[Citation92] It also disrupts cell wall function by lowering ergosterol levels and decreasing plasma membrane ATPase activity.[Citation93] Another study reported its antimycotic action in a concentration-dependent manner, with the lowest inhibitory concentration against C. albicans at 16 g/mL. In addition, GE also suppresses the growth of Pseudohyphae and Chlamydoconidia.[Citation94] summarizes the potential underlying mechanisms of apigenin-mediated therapeutic actions.
Herniarin
Herniarin is chemically known as 7-methoxycoumarin and is natural coumarin derivative found in various plant species.
Antioxidant and anti-inflammatory actions
Herniarin provides beneficial effects in the middle cerebral artery occlusion (MCAO) ischemic model in rats, reducing IL-1 and TNF-α and inhibiting oxidative stress and inflammation.[Citation95] It has also been shown to robustly reduce oxidative stress and damage in streptozotocin-induced diabetic neuropathy.[Citation96]
Anti-cytotoxicity actions
Herniarin also elicits significant chemoprotection against cisplatin-induced genotoxicity. In bone marrow cells, it reduced the proportion of apoptotic and necrotic cells as well as attenuates generation of ROS.[Citation97]
Umbelliferone
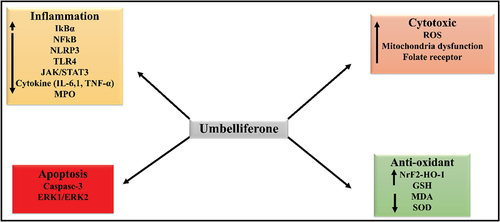
Umbelliferone is chemically known as 7-hydroxycoumarin and is present in multiple plant species including chamomile, bitter orange, carrot and golden apple. It has potent antioxidant, anticancer and antidiabetic properties.[Citation98]
Antioxidant activities
In aqueous environment, umbelliferone elicits significant antioxidant actions.[Citation99] In addition, it is also known to stimulate SOD activities, which complement its antioxidant potency.[Citation98] Further, umbelliferone mitigates lead-induced testicular toxicity by suppressing oxidative damage, inflammation, and cell death and increasing endogenous antioxidant defense systems via nuclear factor erythroid-2 related factor 2/heme oxygenase 1 (Nrf2/HO-1) signaling[Citation100]
Anti-inflammatory actions
Umbelliferone has been shown to regulate hyperactivated inflammatory signaling in LPS-induced model of acute lung injury by regulating the TLR4/MyD88/NF-κB signaling axis. Thus, it significantly reduces infiltration of immune cells in the lung tissues, represses the levels of proinflammatory mediators, myeloperoxidase monocyte chemoattractant protein-1 (MCP-1), IL-1, and 6, TNF-α, in bronchoalveolar lavage fluid, diminishes oxidative damage and the formation of malondialdehyde[Citation101,Citation102]
Antitumorigenic functions
Umbelliferone has shown anticancer activity in form of a mesoporous silica nanoparticle (MSN) containing pH-sensitive poly acrylic acid (PAA). This formulation has demonstrated robust cytotoxic activity in human breast cancer cell MCF-7.[Citation103]
Nephroprotective potential
Umbelliferone is thought to attenuate kidney dysfunction. It exhibits nephroprotective benefits possibly by inhibiting the pro-inflammatory and pro-apoptotic NF-κB, TLR-4, NLRP3 inflammasome, JAK1/STAT3, and caspase-3 pathways. It may also have significant modulatory effects on the ERK1/ERK2 and p38MAPK cell signaling pathways.[Citation101] Umbelliferone was recently shown to significantly improve gentamicin-induced renal damage. The nephroprotective effects mediated by umbelliferone may be ascribed to their anti-inflammatory properties and capacity to inhibit TLR-4/NF-κB/NLRP-3 inflammasome and activate the JAK1/STAT3 pathway.[Citation104] presents the mechanisms of action behind the therapeutic actions of umbelliferone.
Chlorogenic acid (CA)
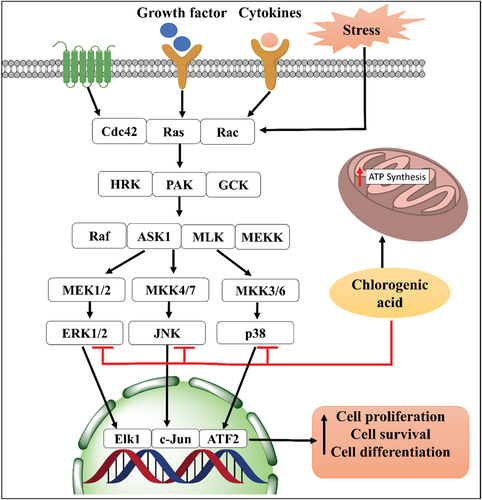
Chlorogenic acid is another interesting phenolic phytochemical isolated from the Matricaria chamomilla. It is also present in coffee, hawthorn, honeysuckle, Chrysanthemum and Eucommia.[Citation105]
Antioxidant actions
Chlorogenic acid is an important antioxidant component of the Matricaria chamomilla extract and has been implicated in protecting cells from oxidative damage and lipid peroxidation.[Citation106,Citation107] It has also been evidenced to beneficially modulate gut bacteria homeostasis, thereby affecting multiple aspects of the host metabolism pathophysiology.[Citation108]
Cardioprotective and antiobesity functions
Chlorogenic acid has been shown to rescue elevated systolic blood pressure and attenuate left ventricular diastolic stiffness in obese conditions, probably by reducing deposition of collagen proteins and preventing infiltration of inflammatory cells infiltration.[Citation109] In rat models, it reduces inflammation and fat deposition in the liver and attenuation dysregulation of plasma liver enzymes.[Citation109,Citation110] Dihydrocaffeic acid, a metabolite of chlorogenic acid, also possesses antioxidant, cardioprotective, neuroprotective, and anticancer action.
Anticancer effects
Chlorogenic acid robustly suppresses tumor cell mitochondrial ATP synthesis, proliferation, motility, and invasiveness in solid tumors.[Citation111] It has also been shown to enhance the stability and expression of the mRNA coding for small ubiquitin-like modifier (SUMO1), resulting in SUMOylation of c-Myc. It further regulates the c-Myc SUMOylation/miR-17/p21 signaling axis downregulates the expression of miR-20a, miR-93, and miR-106b, with consequent enhancement of p21 expression leading to attenuation of cancer cell malignancy and reduction of tumor load.[Citation111] Chlorogenic acid may also drastically decrease tissue inflammation and death in DSS-induced ulcerative colitis in rodents, via its effects on MAPK/ERK/JNK cellular signaling pathway, thereby rescuing colonic mucosal damage and inflammation, oxidative stress, and cellular apoptosis.[Citation111] Lastly, dihydrocaffeic acid, a chlorogenic acid derivative has been elicited to harbor potent anticancer activities in cancer cell lines, MCF-7, Hep-G2, PC-3, and HCT-116.[Citation112]
Neuroprotective potential
Chlorogenic acid has been demonstrated to have neuroprotective properties in both in vitro and in vivo studies. For instance, it reduces ROS generation and restores mitochondrial membrane potential via activating the endogenous enzyme antioxidant systems and regulating intracellular Ca2+ concentrations in a glutamate-induced excitotoxicity model.[Citation113] Further, it has been implicated as a an antioxidant and cognition-stimulating agent for protection against neurodegenerative pathologies.[Citation114] It’s neuroprotective potential has also been reported neurotoxicity induced by 6-hydroxydopamine (6-OHDA), a catecholaminergic neurotoxin.[Citation115] Further, chlorogenic acid may also protect against cerebral ischemia/reperfusion injury. Thus, it has been reported that chlorogenic acid decreases cerebral infarction area, mitigated Evans blue extravasation, and restores cerebral water content. These effects may be dependent on anti-inflammatory and antiapoptotic actions of chlorogenic acid and its ability to modulate signaling via the cellular mediators, TNF-α, iNOS, and caspase-3.[Citation116,Citation117] represents the potential molecular targets and pathways of chlorogenic acid-mediated therapy against different pathologies.
Caffeic acid
Caffeic acid is a well-known therapeutic phytochemical which is also present in Matricaria chamomilla. Multiple in vitro and in vivo studies have provided irrefutable evidences supporting its actions as a treatment agency for a variety of malignancies.[Citation118]
Antibacterial activities
Caffeic acid elicits strong antibacterial potency against S. aureus strains with minimum inhibitory concentration (MIC) between 256 g/mL to 1024 g/mL. Further, it enhances the bactericidal efficiencies of antibiotics such as erythromycin, clindamycin, and cefoxitin. Caffeic-acid’s synergy with antibiotics highlights its promise as an effective new antibacterial strategy against multidrug-resistant bacteria.[Citation119]
Anticancer functions
Caffeic acid has been illustrated to induce toxicity and promote intrinsic apoptotic signaling pathways in breast cancer cells, by influencing the expression levels apoptotic genes, p53, p21 and myeloid cell leukemia sequence 1 (Mcl-1) activated the.[Citation120] In its esterified caffeic acid phenethyl ester (CAPE) form, it has been reported to retard metabolic activity and biofilm formation of S. mutans. CAPE may also block critical virulence factors of S. mutans which are associated with carcinogenicity, such as acid tolerance and generation of extracellular polysaccharides. Indeed, because of its ability to inhibit cariogenic bacteria, CAPE has been implicated a potent anticariogenic drug.[Citation121]
Antiviral potential
Interestingly, caffeic-acid derivatives have been proposed as antagonists of SARS-CoV-2 viral proteins responsible for the viral infectivity[Citation122] In silico analyses of molecular interaction and dynamics with open and closed states of the SARS-CoV-2 spike S2 subunit have uncovered khainaoside C, 6-O-caffeoylarbutin, khainaoside B, khainaoside C and vitexfolin A as the most potent inhibitors of the SARS-CoV-2 virus.[Citation123]
Luteolin
Luteolin is a yellow colored bioactive dye which is chemically recognized as 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4 H–1-benzopyran-4-one.
Anticancer actions
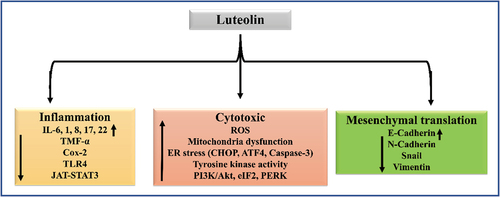
Luteolin has been shown to inhibit epithelial-to-mesenchymal transition by promoting the expression of epithelial marker protein; E-cadherin, altering cytoskeletal organization, and down-regulating mesenchymal marker proteins; N-cadherin, snail, and vimentin. In glioblastoma cells, it has been evidenced to increase intracellular reactive oxygen species (ROS) levels by activating endoplasmic reticulum stress response systems and inducing mitochondrial dysfunction.It also acts as an antiproliferative agent by inhibiting receptor tyrosine-kinase and PI3K/Akt signaling pathways in breast cancer cells.[Citation124] Luteolin has also shown anti-cancer potential in human hepatoma HepG2 cells by initiating G1 cell cycle arrest, triggering apoptosis, and modulating the expression levels of p21, Bax, and caspase-3.[Citation125] Further, its anticancer benefits in colorectal cancer have been proposed to be mediated by the Wnt/catenin signaling pathway.[Citation126]
Cardioprotective effects
Luteolin also elicits significant cardioprotective effects by preventing ROS generation, and mitigating mitochondrial membrane potential loss.[Citation127] It has been revealed that among the multiple bioactives present in methanolic extract of chamomile, luteolin and apigenin have the highest capacity for stimulating angiogenesis.[Citation128]
Anti-inflammatory activities
Luteolin can reduce negative photobiological effects on the skin keratinocytes, fibroblasts, and immunological cells by acting as a potent anti-oxidant and anti-inflammatory agent. It inhibits proinflammatory mediators, including IL-1, IL-6, IL-8, IL-17, IL-22, TNF-α, and COX-2, and regulating cellular signaling through NF-κB, JAK-STAT, and TLR pathways.[Citation129]
Neuroprotective functions
Lastly, luteolin is implicated as a neuroprotective agent as it has been shown to retard neuroinflammatory responses, attenuate glial activation, and control oxidative stress and damage in pathologies such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and traumatic brain injury.[Citation130] summarizes the therapeutic actions of luteolin at the molecular and cellular levels.
Quercetin
Quercetin can be utilized as a nutraceutical for various ailments. It is beneficial in treating and preventing human illnesses principally because it beneficially affects redox and cell signal transduction pathways.[Citation131] Quercetin has been proposed to serve as a promising lead compound for synthetic analogues in medical research programs.[Citation132]
Antioxidant and anti-inflammatory actions
Upon its supplementation, quercetin and its metabolites in systemic circulation following operate as powerful antioxidants and anti-inflammatory agents, indicating the benefits of a quercetin-rich diet.[Citation133] Quercetin and its six derivatives (quercetin-3-O-glucuronide, tamarixetin, isorhamnetin, isorhamnetin-3-O-glucoside, quercetin-3,4′-di-O-glucoside, quercetin-3,5,7,3′,4′-pentamethylether) have been recently proposed to elicit significant antioxidant and anti-inflammatory activity.[Citation134] Interestingly, while the antioxidant actions of quercetin are reduced upon derivatization of its hydroxyl groups, the number of free hydroxyl groups in quercetin do not alter its anti-inflammatory functions.[Citation135]
Cardioprotective potential
Several lines of evidences have implicated quercetin as a robust therapeutic drug for the treatment of cardiovascular illnesses with the abilities to modulate the effects of aging, hypertension, angiotensin-converting enzyme, and endothelial-dependent and independent pathways.[Citation132]
Antitumorigenic actions
The tremendous antioxidant capacity of quercetin is thought to drive its preventive actions in several human malignancies. Further, quercetin has direct proapoptotic effects on tumor cells, retarding the progression of cancers. Indeed, its anticancer functions have been demonstrated in several investigations using a variety of cell lines and animal models.[Citation136]
Neuroprotective actions
Lastly, the protective effects of quercetin against neuropathologies such as Alzheimer’s disease may be because of its multimodal abilities to regulate neuroinflammatory, cell signaling kinase, and redox pathways.[Citation137]
Rutin
Rutin is also known as rutoside, quercetin-3-O-rutinoside, and sophorin. It is a flavonoid found in chamomile, Asparagus, buckwheat, apricots, apples, cherries, grapes, grapefruit, plums, oranges and tea.[Citation138]
Antioxidant and anti-inflammatory functions
Rutin acts as an therapeutic agent by reducing the levels of proinflammatory cytokines, stimulating antioxidant enzyme activities, activating MAPK cascade, downregulating expression of cell death and proapoptotic genes, upregulating cell survival and antiapoptotic genes, and restoring mitochondrial bioenergetic activities.[Citation139] In wound healing models, rutin has also been proposed to promote neovascularization, reduce oxidative stress, and decrease inflammation.[Citation139,Citation140] Its downstream targets include antioxidant enzymes induced by NRF2, matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF).[Citation139]
Anticancer actions
Rutin’s abilities to regulate cellular signaling pathways such as Wnt/β-catenin, PI3K/Akt, JAK/STAT, MAPK, p53 and NF-cascades have been hypothesized to contribute to its anti-tumorigenic actions.[Citation141] Indeed, it has been shown that rutin modulates a plethora of pathophysiological aspects of inflammatory, apoptotic, autophagic, and angiogenic signaling in cancer progression.[Citation138]
Neuroprotective potential
Rutin has also been implicated as a viable pharmacological option for the treatment of neurodegenerative conditions such as Alzheimer’s disease due to its protective effects against Aβ and tau pathologies, and its antioxidant and anti-inflammatory actions.[Citation139,Citation142]
Medicinal uses of Matricaria Chamomilla extracts
Antibacterial functions
In Europe, Latin America, Asia, Nepal, and Africa, Matricaria species are frequently used to treat the signs and symptoms of bacterial infections against Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger.[Citation143] Inhalational and oral treatments are used to treat respiratory infections, whereas topical applications are used to treat skin infections. Although the genus has a wide range of therapeutic applications, the species are most commonly used for treating gastrointestinal diseases. Interestingly, Matricaria chamomilla extracts have antimicrobial properties with the minimum inhibitory concentration (MIC) which are amongst the lowest for plant-based formulations ().[Citation144]
Table 2. Minimum inhibitory concentration (MIC) of Chamomile extract.
Antifungal activity
Antimicrobial peptides from Matricaria chamomilla have been reported to have significant antifungal potential against Candida albicans and Aspergillus species. Since the antifungal activity remains stable at high temperatures (30–50°C) and pH (7–11), these peptides may have notable therapeutic applications.[Citation145] Matricaria chamomilla flower’s essential oil has demonstrated antifungal action against Aspergillus niger, preventing fungal contamination and destruction of stored food and other vulnerable materials.[Citation146] Similarly, Matricaria chamomilla has also shown strong antifungal potential against Aspergillus flavus, a common food contaminant, possibly via inhibiting the synthesis of aflatoxin B1 which is also known for its carcinogen activities.[Citation147] Matricaria chamomilla has a wide range of antifungal potency as evidenced by its actions against development of fungal molds in museum gypsum board antiques (Aspergillus flavus), archeological manuscripts (Aspergillus niger), museum organic materials (Fusarium culmorum), and museum archeological tissues (Aspergillus terreus).[Citation148]
Anti-cancer actions
Several studies indicate the potency of Matricaria chamomilla extracts as a potent therapeutic agent for oncological uses. When combined with silver nanoparticles, phytochemicals in Matricaria chamomilla have been found to be efficient in retarding the tumorigenicity of lung cancer A549 cells in a dose- and time-dependent manner.[Citation149] Similarly, hydroalcoholic extract inhibits cellular motility and colonization of human breast cancer cell lines, MCF-7 and MDA-MB-468. The extract contains anti-cancer bioactives such as bisabolol, germacrene D, β-farnesene, and geraniol.[Citation150,Citation151] Organic solubilized extracts of Matricaria chamomilla have been found to effectively inhibited Caco-2 colon cancer cell migration, possibly in a VEGFR2-dependent manner.[Citation152] Methanolic extract of M. chamomilla inhibits the growth of several human cancer cell lines, including PZ-HPV-7, LNCaP, and PC-3, at the concentration of 100–400 μg/ml.[Citation6] As an essential oil, Matricaria chamomilla strongly inhibits the growth of cancer cell lines such as HCT116 and human colon carcinoma.[Citation153] Matricaria chamomilla is a powerful nutritional chemoprotective agent for colorectal cancer induced by 1,2-dimethylhydrazine.[Citation154] In agreement, Matricaria chamomilla and its chief chemical constituents, such as apigenin and luteolin, have shown protective effects against carcinogenic agent 1,2-dimethylhydrazine.[Citation155] Apigenin may rely on its ability to regulate Wnt/catenin signaling pathway for its anti-cancer actions.[Citation155] All these beneficial outcomes suggest the potential applications of using Matricaria chamomilla for preventing and retarding the progression of different cancer types.
Antioxidant and anti-inflammatory activities
Aqueous extraction of Matricaria chamomilla at 80°C is a reasonable health-promoting agent because of its maximal antioxidant actions, however its use should be limited to guarantee safe consumption.[Citation105] Other in vivo and in vitro studies have confirmed the antioxidant properties of Matricaria chamomilla extract. For example, in adherent cells, chamomile reduces oxidative stress and enhances cell growth and division.[Citation156] It is also known to prevent oxidative damage in paraquat-induced lung injury.[Citation157] Further, glycoconjugates of Matricaria chamomilla are advocated as novel natural-based pharmaceuticals and nutritional supplements for the prevention and treatment of oxidative stress-related illnesses.[Citation158] In rats, Matricaria chamomilla extract decreases tissue oxidative stress and damage induced by carbon tetrachloride toxicity. It may also elicit beneficial effects on lipid peroxidation, antioxidant enzymes (SOD, glutathione synthetase, and glutathione peroxidase), and liver enzymes (aspartate aminotransferase; AST, and alanine aminotransferase; ALT).[Citation159] Active ingredients of Matricaria chamomilla, such as fraxidin, matriisobenzofuran, apigenin, scopoletin, palmatoside A, apigenin 7-O-b-glucopyranoside, and p-hydroxyacetophenone, have been reported to regulate glutathione S-transferase (GST) activity, thereby contributing to the redox-associated GSH signaling.[Citation160] Lastly, scavenging of 2,2-diphenyl-1-picrylhydrazylhydrate (DPPH) radical has been shown to be effectively performed by various Matricaria chamomilla extracts, most effectively by its methanolic extract.[Citation144]
Co-administration of non-steroidal anti-inflammatory drugs (NSAIDs), diclofenac and indomethacin in complementation with an ethanolic extract of Matricaria chamomilla extract has been shown to elicit strong synergistic anti-inflammatory efficacies in a carrageenan-induced paw inflammation and stomach damage rat model.[Citation161] An independent study has confirmed the anti-inflammatory actions of Matricaria chamomilla in another rodent model of carrageenan-induced pedal edema, possibly by regulating the levels of inflammatory mediators, prostaglandin E2 (PGE2) and nitric oxide (NO).[Citation162,Citation163] Further, Matricaria chamomilla also attenuated xylol-induced ear edema\and HAC-induced celiac capillary vascular permeability in mice.
Neuroprotective potential
Numerous neuropharmacological agents and strategies have been proposed against central nervous system (CNS) illnesses, but most of these only offer symptomatic alleviation. Another drawback in neuropharmacological applications is the inability of the drugs to effectively pass the blood brain barrier (BBB). In these regards, researchers have proposed the usage of bioactives from natural sources such as medicinal plants like Matricaria chamomilla . Indeed, ethanolic extract of Matricaria chamomilla has been evidenced to enhance memory and cognition by stimulating cholinergic activity, retarding neuroinflammation and inducing antioxidant systems in the rat hippocampus.[Citation32] Similar results have reported in a model of formaldehyde-induced memory loss.[Citation164] Additionally, Matricaria chamomilla extract prevents scopolamine-induced oxidative damage to hippocampal neuronal morpho-functional characteristics.[Citation165] Morris water maze and passive avoidance tests have revealed that supplementation of Matricaria chamomilla ethanolic extract has free radical scavenging and memory-boosting actions, indicating that it may be a feasible treatment option against AD pathogenesis.[Citation166]
Interestingly, Matricaria chamomilla has been proposed to elicit significant anxiolytic and antidepressant actions in patients with generalized anxiety disorder and concomitant depression.[Citation167] Chamomile supplementation also decreases irregular sleep patterns in postmenopausal women, possibly due to the high content of apigenin which acts as a sedative and sleep-promoting compound.[Citation168] Matricaria chamomilla extract has also been evidenced to greatly lower neuropathic pain associated with chemotherapeutics such as cisplatin and vincristine.[Citation169] In agreement, Matricaria chamomilla and morphine show synergistic analgesic effects in a mouse model of vincristine-induced peripheral neuropathy.[Citation170] Likely, in a scopolamine-induced toxicity model in rats, chamomile extract administration serves as an anxiolytic and antidepressant agent, possibly via its cholinergic stimulatory actions.[Citation171] Matricaria chamomilla-based therapeutic formulation (CH12) has been utilized in bovine species to decrease stress and prevent cortisol production, as well as to induce relaxation.[Citation172] Lastly, Matricaria chamomilla is also implicating as a stimulant of neuromuscular circuitry, indicating its potential in improving motor coordination in neuropathological states.[Citation165]
Antiparasitic activity
Due to high cost and severity, leishmaniasis is a parasitic illness with few therapies.[Citation173] Chitosan nano capsules containing essential oil of Matricaria chamomilla from oil-in-water emulsions modified with tetradecyl chains hold great potential as anti-leishmanial therapy.[Citation174] Anti-parasitic actions of Matricaria chamomilla have been confirmed in Leishmania promastigotes and axenic amastigotes,[Citation175] mite Psoroptes cuniculi,[Citation176] and Limnatis nilotica.[Citation177]
Beneficial actions in dysmenorrhea or endocrinal deregulation
Chamomile has been demonstrated to inhibit cyclooxygenase and stop the formation of prostaglandins and leukotrienes, decreasing pain and inflammation. A study in female students using the premenstrual syndrome symptoms screening questionnaire revealed that supplementation of chamomile powder for 7 days before menstruation effectively reduces the intensity of menstrual pain, with consequent reductions in physical and psychological deficits. Phytoestrogens in chamomile appear to neutralize estrogen’s detrimental effects on endorphin levels by binding to estrogen receptors and lowering its actions, which may underlie chamomile’s beneficial actions in enhancing mood.[Citation178] Matricaria chamomilla flower extract has been observed to modulate testosterone secretion in rats.[Citation179] In male adult rats, Matricaria chamomilla extract can reduce spermatozoa count and motility, spermatozoon tail length, serum testosterone, and serum estradiol level.[Citation180]
Amelioration of skin inflammation, eczema and physical injuries
Generation of cytokines and eicosanoids are the major cause of eczema. These are inhibited by Matricaria chamomilla and are instrumental in preventing inflammatory reaction cascade associated with skin burn, itching, and abrasion.[Citation181] The stimulatory effects of chamomile extract on leukocytes, such as macrophages and B lymphocytes, has been hypothesized to treat eczema and skin irritation.[Citation182] Topically, it is used to cure swelling, dermatitis, and infectious diseases as rubs, while its oily formulation has been utilized for healing burn wounds.[Citation2] A herbal cream-based formulation containing extracts from marshmallow (Althaea officinalis), chamomile (Matricaria chamomilla), walnuts (Juglans regia), and fenugreek (Trigonella foenum) has been shown to considerably reduce the symptoms of itching, burning, bumps, redness, fissures, and scaling in eczema, notably with minimum side effects.[Citation183] In conclusion, natural therapies for many skin diseases, including wound healing, include Matricaria chamomilla, as a prominent natural therapeutic with potent anti-inflammatory and antioxidant effects.[Citation184] Anti-inflammatory and antioxidant actions of Matricaria chamomilla have also been utilized for the protection of testis tissue from torsion/detorsion-induced injury.[Citation185] Similarly, chamomile extract in rubbing oil formulation speeds up burn wound healing.[Citation186]
Treatment of diabetes mellitus
Ethanolic extract of Matricaria chamomilla shows significant antihyperglycemic activities, indicating that it can serve as a powerful agent to regulate blood glucose levels.[Citation187] Simultaneous antioxidant and anti-hyperglycemic actions of Matricaria chamomilla have also been reported in a rodent model of streptozotocin-induced diabetes.[Citation188] In diabetic rats, Matricaria chamomilla has shown substantial hypoglycemic impact, lowering blood levels of glucose, urea, creatinine, uric acid, AST, and ALT, among other metabolites.[Citation189] In concurrence, Matricaria chamomilla has been elicited to significantly improve diabetes-associated cardiac complications by decreasing lipid peroxidation, oxidative stress, and blood glucose levels.[Citation190] Synergistic application of endurance training and Matricaria chamomilla has been reported to improve diabetic symptoms and memory functions in a rodent model of hyperglycemia.[Citation191] In conclusion, chamomile extracts enriched in terpenoid and phenolic compounds are more effective against chronic conditions of hyperglycemia and oxidative injury in diabetes mellitus, as confirmed by a systemic review of literature.[Citation192]
Hepatoprotective functions
As a natural antioxidant, Matricaria chamomilla may be useful in preventing oxidative liver damage in paraquat poisoning.[Citation193] The anti-tumorigenic, anti-inflammatory and antioxidant nature of phytochemicals present in Matricaria chamomilla extract may also contribute to its hepatoprotective actions against dimethylhydrazine-induced carcinogenic liver damage.[Citation155] Matricaria chamomilla has been demonstrated to have significant hepatoprotective impacts in a chemically induced model of liver injury and oxidative stress, by positively affecting the endogenous antioxidant enzyme systems.[Citation194] Aqueous extract of Matricaria chamomilla has similarly shown attenuation of liver tissue damage caused by methomyl as evidenced by rescue of weight loss and liver enzyme activities.[Citation195]
Nephroprotective functions
Matricaria chamomilla is a potential nephroprotective molecule that reduces cisplatin-induced nephrotoxicity by inhibiting gamma-glutamyl transferase (GGT) and stimulating endogenous antioxidant pathways. It boosts body weight, normalizes renal functions, improves apoptotic indicators, decreases oxidative stress markers, and rectifies hypocalcemic conditions caused by cisplatin-induced nephrotoxicity.[Citation196] Moreover, co-supplementation of Matricaria chamomilla and Nigella sativa has been proposed to harbor significantly improved nephroprotective properties and attenuating cisplatin-induced toxicity, most likely via stimulation of antioxidant and anti-apoptotic pathways.[Citation197]
Treatment of colic pain, diarrhea and intestinal worm infestation
In vivo studies show that Matricaria chamomilla has antidiarrheal and antispasmodic effects, presumably mediated its ion modulation of K+ and Ca2+ ions.[Citation198] Matricaria chamomilla essential oil and its two components, chamazulene and α-bisabolol inhibit the growth of nematode larvae, resulting in their enhanced mortality.[Citation199] Similarly, the anti-helminthic potency of Matricaria chamomilla is well-known. An investigation of the effects of Matricaria chamomilla on ostertagia infection in lambs indicated its robust actions in promoting weight gain as an antihelminthic agent.[Citation200] Likewise, Matricaria chamomilla flower’s extracts elicit potent inhibitory actions against the intestinal parasitic helminths, Acanthamoeba castellanii[Citation201] and Haemonchus contortus.[Citation162,Citation202]
Antiulcer activity
Matricaria chamomilla’s antiulcerogenic effects on ethanol-induced stomach mucosal lesions are mediated via positive regulation of antioxidant systems and concomitant reduction of oxidative stress and damage.[Citation203] In mice, supplementation of Matricaria Chamomilla results in a dose-dependent attenuation of stomach ulcer.[Citation204] Oral treatment of Matricaria chamomilla extract has been found to inhibit stomach ulcers.[Citation205] Similarly, ingestion of an aqueous extract of Matricaria chamomilla flowers is associated with antiulcer activity against stress-induced stomach ulceration in rabbits.[Citation206] Moreover, its flower head extracts were found to have antiulcer efficacy in rats with indomethacin-induced ulcers.[Citation207] The beneficial effects of different Matricaria chamomilla extracts are summarized in .
Table 3. Various studies with different extract of Matricaria chamomilla and their beneficial outcomes.
Future perspectives and conclusion
Matricaria chamomilla has been used as an herbal medication since ancient times. It is still popular today and probably will continue to be used in the future as it contains various bioactive phytochemicals with potent multimodal therapeutic activities. In light of this, therapies against the various human pathological conditions such as cancers, ulcers, inflammation, microbial infections, diabetes, and neurological illnesses may be suitably directed utilizing Matricaria chamomilla and its phytotherapeutics, either alone or in complementation of standard agents. However, there are certain concerns which must be addressed in order to establish Chamomilla as a clinically relevant therapeutic agent. First, protocols for the production of Matricaria chamomilla extract and the purification of its active ingredients must be standardized. Second, assessment of the molecular and cellular mechanisms underlying the therapeutic actions of Matricaria chamomilla in a disease-specific manner must be further extended. Moreover, animal and cellular studies can be used for repurposing Matricaria chamomilla as a treatment alternative for related diseases with overlapping etiologies. The initial information of the appropriate dosages requirement must also be addressed using animal studies. Finally, clinical trials for the utilities of Matricaria chamomilla and its phytotherapeutics against human disease-specific cases are warrantied. Indeed, randomized clinical trials are absolutely required to validate Matricaria chamomilla as a multimodal diet-based therapeutic intervention. In conclusion, while the present review concludes that Matricaria chamomilla is a safe dietary herbal therapeutic agent that shows multifaceted beneficial activities with minimal side effects in numerous pathologies, more studies, particularly in human subjects are required to demonstrate its actual clinical potential.
Ethical statement
There are no ethical requirements for this article as it does not involve primary research on animals or humans.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- El Joumaa, M. M.; Borjac, J. M. Matricaria Chamomilla: A Valuable Insight into Recent Advances in Medicinal Uses and Pharmacological Activities. Phytochem. Rev. 2022, 21(6), 1913–1940. DOI: 10.1007/s11101-022-09817-0.
- Marković, M.; Pljevljakušić, D.; Kojičić, K.; Cupara, S. Ethnopharmacological Application of Chamomile (Matricaria Chamomilla L.) in the Pirot County of Southeastern Serbia. Arh. Farm. (Belgr). 2020, 70(4), 238–247. DOI: 10.5937/arhfarm2004238M.
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5(9), 82. DOI: 10.4103/0973-7847.79103.
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M. F. B.; Rocha, J. E.; Coutinho, H. D. M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M., et al. Matricaria Genus as a Source of Antimicrobial Agents: From Farm to Pharmacy and Food Applications. Microbiol. Res. 2018, 215, 76–88. DOI: 10.1016/j.micres.2018.06.010.
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A Comprehensive Review on Biology, Genetic Improvement, Agro and Process Technology of German Chamomile (Matricaria Chamomilla L.). Plants. 2021, 11(1), 29. DOI: 10.3390/plants11010029.
- Srivastava, J. K.; Shankar, E.; Gupta, S. Chamomile: A Herbal Medicine of the Past with a Bright Future (Review). Mol. Med. Rep. 2010, 3(6), 895–901. DOI: 10.3892/mmr.2010.377.
- Kyokong, O.; Charuluxananan, S.; Muangmingsuk, V.; Rodanant, O.; Subornsug, K.; Punyasang, W. Efficacy of Chamomile-Extract Spray for Prevention of Post-Operative Sore Throat. J. Med. Assoc. Thai. 2002, 85(1), S180–5.
- Togar, B.; Turkez, H.; Hacimuftuoglu, A.; Tatar, A.; Geyikoglu, F. Guaiazulene: biochemical activity and cytotoxic and genotoxic effects on rat neuron and N2a neuroblastom cells. J. Intercult. Ethnopharmacol. 2015, 4(1), 29. DOI: 10.5455/jice.20141124062203.
- Attia, M.; Kim, S.-U.; Ro, D.-K. Molecular Cloning and Characterization of (+)-Epi-α-Bisabolol Synthase, Catalyzing the First Step in the Biosynthesis of the Natural Sweetener, Hernandulcin, in Lippia Dulcis. Arch. Biochem. Biophys. 2012, 527(1), 37–44. DOI: 10.1016/j.abb.2012.07.010.
- Eddin, L. B.; Jha, N. K.; Goyal, S. N.; Agrawal, Y. O.; Subramanya, S. B.; Bastaki, S. M. A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients. 2022, 14(7), 1370. DOI: 10.3390/nu14071370.
- Farias, K. S.; Kato, N. N.; Boaretto, A. G.; Weber, J. I.; Brust, F. R.; Alves, F. M.; Tasca, T.; Macedo, A. J.; Silva, D. B.; Carollo, C. A. Nectandra as a Renewable Source for (+)-α-Bisabolol, an Antibiofilm and Anti-Trichomonas Vaginalis Compound. Fitoterapia. 2019, 136, 104179. DOI: 10.1016/j.fitote.2019.104179.
- Kamatou, G. P. P.; Viljoen, A. M. A Review of the Application and Pharmacological Properties of α -Bisabolol and α -Bisabolol-Rich Oils. J. Am. Oil Chem. Soc. 2010, 87(1), 1–7. DOI: 10.1007/s11746-009-1483-3.
- Aziz, Z. A. A.; Ahmad, A.; Setapar, S. H. M.; Karakucuk, A.; Azim, M. M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M. A.; Ashraf, G. M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential - A Review. Curr. Drug Metab. 2018, 19(13), 1100–1110. DOI: 10.2174/1389200219666180723144850.
- Deligeorgopoulou, A.; Allemann, R. K. Evidence for Differential Folding of Farnesyl Pyrophosphate in the Active Site of Aristolochene Synthase: A Single-Point Mutation Converts Aristolochene Synthase into an (E)-β-Farnesene Synthase. Biochemistry. 2003, 42(25), 7741–7747. DOI: 10.1021/bi034410m.
- Amara, U.; Mashwani, Z. U. R.; Khan, A.; Laraib, S.; Wali, R.; Sarwar, U.; Ain, Q. T.; Shakeel, S.; Rahimullah, S. Conservation Status and Therapeutic Potential of Saussurea lappa: An Overview. AJPS. 2017, 08(3), 602–614. DOI: 10.4236/ajps.2017.83041.
- Telascrea, M.; de Araújo, C. C.; Marques, M. O. M.; Facanali, R.; de Moraes, P. L. R.; Cavalheiro, A. J. Essential Oil from Leaves of Cryptocarya Mandioccana Meisner (Lauraceae): Composition and Intraspecific Chemical Variability. Biochem. Syst. Ecol. 2007, 35(4), 222–232. DOI: 10.1016/j.bse.2006.09.015.
- Fahlbusch, K.; Hammerschmidt, F.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2003; pp. 1131–1145. DOI:10.1002/14356007.a11_141.
- Mahdavi, B.; Ghezi, S.; Maleki, B. Chemical Composition and Bioactivity of Essential Oil and Extracts of Salvia Limbata C.A. Mey (Lamiaceae). Curr. Bioact. Compd. 2020, 16(9), 1299–1305. DOI: 10.2174/1573407216666200218111906.
- Salehi, A.; Hazrati, S. How Essential Oil Content and Composition Fluctuate in German Chamomile Flowers During the Day? J. Essent. Oil Bear. Plants. 2017, 20(3), 622–631. DOI: 10.1080/0972060X.2017.1351895.
- Flemming, M.; Kraus, B.; Rascle, A.; Jürgenliemk, G.; Fuchs, S.; Fürst, R.; Heilmann, J. Revisited Anti-Inflammatory Activity of Matricine in Vitro: Comparison with Chamazulene. Fitoterapia. 2015, 106, 122–128. DOI: 10.1016/j.fitote.2015.08.010.
- Héthelyi, É.; Dános, B.; Tétényi, P. Phytochemical Studies on the Essential Oils of Species Belonging to theAchillea Genus by Gas Chromatography/Mass Spectrometry. Biol. Mass Spectrom. 1989, 18(8), 629–636. DOI: 10.1002/bms.1200180821.
- Stanojevic, L. P.; Marjanovic-Balaban, Z. R.; Kalaba, V. D.; Stanojevic, J. S.; Cvetkovic, D. J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria Chamomilla L.). J. Essent. Oil Bear. Plants. 2016, 19(8), 2017–2028. DOI: 10.1080/0972060X.2016.1224689.
- Rauter, A. P.; Ennis, M.; Hellwich, K.-H.; Herold, B. J.; Horton, D.; Moss, G. P.; Schomburg, I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90(9), 1429–1486. DOI: 10.1515/pac-2013-0919.
- Redaelli, C.; Formentini, L.; Santaniello, E. Apigenin 7-Glucoside and Its 2″- and 6″-Acetates from Ligulate Flowers of Matricaria Chamomilla. Phytochemistry. 1980, 19(5), 985–986. DOI: 10.1016/0031-9422(80)85160-0.
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20(6), 1305. DOI: 10.3390/ijms20061305.
- Chen, W.; Viljoen, A. M. Geraniol — a Review of a Commercially Important Fragrance Material. South African J. Bot. 2010, 76(4), 643–651. DOI: 10.1016/j.sajb.2010.05.008.
- Farkoosh, S. S.; Ardakani, M. R. R.; Darzi, F.; Faregh, M. T. Effect of Mycorrhizal Symbiosis and Bacillus Coagolance on Qualitative and Quantitative Traits of Matricaria Chamomilla Under Different Levels of Phosphorus. Middle-East J. Sci. Res. 2011, 8, 01–09.
- Yonzon, M.; Lee, D. J.; Yokochi, T.; Kawano, Y.; Nakahara, T. Antimicrobial Activities of Essential Oils of Nepal. J. Essent. Oil Res. 2005, 17(1), 107–111. DOI: 10.1080/10412905.2005.9698846.
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia Dracunculus (Tarragon): A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Front. Pharmacol. 2021, 12, 383–426. DOI: 10.3389/fphar.2021.653993.
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of Various Techniques for the Extraction of Umbelliferone and Herniarin in Matricaria Chamomilla Processing Fractions. Chem. Cent. J. 2017, 11(1), 78. DOI: 10.1186/s13065-017-0308-y.
- Mazimba, O. Umbelliferone: Sources, Chemistry and Bioactivities Review. Bull. Fac. Pharmacy, Cairo Univ. 2017, 55(2), 223–232. DOI: 10.1016/j.bfopcu.2017.05.001.
- Ionita, R.; Postu, P. A.; Mihasan, M.; Gorgan, D. L.; Hancianu, M.; Cioanca, O.; Hritcu, L. Ameliorative Effects of Matricaria Chamomilla L. Hydroalcoholic Extract on Scopolamine-Induced Memory Impairment in Rats: A Behavioral and Molecular Study. Phytomedicine. 2018, 47, 113–120. DOI: 10.1016/j.phymed.2018.04.049.
- Nováková, L.; Vildová, A.; Mateus, J. P.; Gonçalves, T.; Solich, P. Development and Application of UHPLC–MS/MS Method for the Determination of Phenolic Compounds in Chamomile Flowers and Chamomile Tea Extracts. Talanta. 2010, 82(4), 1271–1280. DOI: 10.1016/j.talanta.2010.06.057.
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F. J.; Prohens, J. Breeding for Chlorogenic Acid Content in Eggplant: Interest and Prospects. Not. Bot. Horti Agrobot. Cluj-Napoca. 2013, 41(1), 26. DOI: 10.15835/nbha4119036.
- Espíndola, K. M. M.; Ferreira, R. G.; Narvaez, L. E. M.; Silva Rosario, A. C. R.; da Silva, A. H. M.; Silva, A. G. B.; Vieira, A. P. O.; Monteiro, M. C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 67–77. DOI: 10.3389/fonc.2019.00541.
- Colunga Biancatelli, R. M. L.; Berrill, M.; Catravas, J. D.; Marik, P. E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 231–255. DOI: 10.3389/fimmu.2020.01451.
- Jucá, M. M.; Cysne Filho, F. M. S.; de Almeida, J. C.; Mesquita, D. D. S.; Barriga, J. R. D. M.; Dias, K. C. F.; Barbosa, T. M.; Vasconcelos, L. C.; Leal, L. K. A. M.; Ribeiro, J. E., et al. Flavonoids: biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34(5), 692–705. DOI: 10.1080/14786419.2018.1493588.
- Ganeshpurkar, A.; Saluja, A. K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25(2), 149–164. DOI: 10.1016/j.jsps.2016.04.025.
- Ma, Y.; Li, W.; Mai, J.; Wang, J.; Wei, Y.; Ledesma-Amaro, R.; Ji, X.-J. Engineering Yarrowia Lipolytica for Sustainable Production of the Chamomile Sesquiterpene (−)-α-Bisabolol. Green Chem. 2021, 23(2), 780–787. DOI: 10.1039/D0GC03180A.
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I. A.; Bazzocchi, I. L.; Valladares, B.; Akkari, H.; Lorenzo-Morales, J.; Piñero, J. E. Leishmanicidal Activity of α-Bisabolol from Tunisian Chamomile Essential Oil. Parasitol. Res. 2018, 117(9), 2855–2867. DOI: 10.1007/s00436-018-5975-7.
- Shanmuganathan, B.; Suryanarayanan, V.; Sathya, S.; Narenkumar, M.; Singh, S. K.; Ruckmani, K.; Pandima Devi, K. Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018, 143, 1196–1207. DOI: 10.1016/j.ejmech.2017.10.017.
- Nagoor Meeran, M. F.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S. N.; Adeghate, E.; Ojha, S. α-Bisabolol Protects Against β-Adrenergic Agonist-Induced Myocardial Infarction in Rats by Attenuating Inflammation, Lysosomal Dysfunction, NLRP3 Inflammasome Activation and Modulating Autophagic Flux. Food Funct. 2020, 11(1), 965–976. DOI: 10.1039/C9FO00530G.
- Baylac, S. Inhibition of 5-Lipoxygenase by Essential Oils and Other Natural Fragrant Extracts. Int. J. Aromather. 2003, 13(2–3), 138–142. DOI: 10.1016/S0962-4562(03)00083-3.
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the Oleaginous Yeast Yarrowia Lipolytica for β‐Farnesene Overproduction. Biotechnol. J. 2021, 16(7), 63. DOI: 10.1002/biot.202100097.
- Brantner, A. H.; Pfeifhofer, H. W.; Ercegovac, O.; Males, Z.; Plazibat, M. Essential Oil Composition and Antioxidant Activity ofThymus Bracteosus Vis. Ex Benth. Flavour Fragr. J. 2005, 20(6), 596–600. DOI: 10.1002/ffj.1494.
- Qadir, M.; Maurya, A. K.; Agnihotri, V. K.; Shah, W. A. Volatile Composition, Antibacterial and Antioxidant Activities of Artemisia Tournefortiana Reichb. from Kashmir, India. Nat. Prod. Res. 2021, 35(1), 152–156. DOI: 10.1080/14786419.2019.1613990.
- Oliveira, A.; França, H.; Kuster, R.; Teixeira, L.; Rocha, L. Chemical Composition and Antibacterial Activity of Brazilian Propolis Essential Oil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16(1), 121–130. DOI: 10.1590/S1678-91992010005000007.
- Qin, Y.; Yang, Z.; Song, D.; Wang, Q.; Gu, S.; Li, W.; Duan, H.; Zhou, J.; Yang, X. Bioactivities of Synthetic Salicylate-Substituted Carboxyl (E)-β-Farnesene Derivatives as Ecofriendly Agrochemicals and Their Binding Mechanism with Potential Targets in Aphid Olfactory System. Pest Manag. Sci. 2020, 76(7), 2465–2472. DOI: 10.1002/ps.5787.
- Zhang, J.-P.; Qin, Y.-G.; Dong, Y.-W.; Song, D.-L.; Duan, H.-X.; Yang, X.-L. Synthesis and Biological Activities of (E)- β -Farnesene Analogues Containing 1,2,3-Thiadiazole. Chinese Chem. Lett. 2017, 28(2), 372–376. DOI: 10.1016/j.cclet.2016.10.030.
- Qin, Y.-G.; Yang, Z.-K.; Zhou, J.-J.; Zhang, S.-Y.; Pan, S.-X.; Liu, Y.; Gu, S.-H.; Duan, H.-X.; Yang, X.-L. Effects of Carboxyl and Acylamino Linkers in Synthetic Derivatives of Aphid Alarm Pheromone (E)-β-Farnesene on Repellent, Binding and Aphicidal Activity. J. Mol. Struct. 2022, 1268, 133658. DOI: 10.1016/j.molstruc.2022.133658.
- Juteau, F.; Masotti, V.; Bessière, J. M.; Dherbomez, M.; Viano, J. Antibacterial and Antioxidant Activities of Artemisia Annua Essential Oil. Fitoterapia. 2002, 73(6), 532–535. DOI: 10.1016/S0367-326X(02)00175-2.
- Montanari, R. M.; Barbosa, L. C. A.; Demuner, A. J.; Silva, C. J.; Carvalho, L. S.; Andrade, N. J. Chemical Composition and Antibacterial Activity of Essential Oils from Verbenaceae Species: Alternative Sources of (E)-Caryophyllene and Germacrene-D. Quim. Nova. 2011, 34(9), 1550–1555. DOI: 10.1590/S0100-40422011000900013.
- Laouer, H.; Yabrir, B.; Djeridane, A.; Yousfi, M.; Beldovini, N.; Lamamra, M. Composition, Antioxidant and Antimicrobial Activities of the Essential Oil of Marrubium Deserti. Nat. Prod. Commun. 2009, 4(8), 1934578X0900400. DOI: 10.1177/1934578X0900400824.
- Schepetkin, I.; Özek, G.; Özek, T.; Kirpotina, L.; Khlebnikov, A.; Quinn, M. Chemical Composition and Immunomodulatory Activity of Hypericum Perforatum Essential Oils. Biomolecules. 2020, 10(6), 916. DOI: 10.3390/biom10060916.
- de Veras, B. O.; de Oliveira, J. R. S.; de Menezes Lima, V. L.; Do Amaral Ferraz Navarro, D. M.; de Oliveira Farias de Aguiar, J. C. R.; de Medeiros Moura, G. M.; da Silva, J. W.; de Assis, C. R. D.; Gorlach-Lira, K.; de Assis, P. A. C., et al. The Essential Oil of the Leaves of Verbesina Macrophylla (Cass.) S.F.Blake Has Antimicrobial, Anti-Inflammatory and Antipyretic Activities and is Toxicologically Safe. J. Ethnopharmacol. 2021, 265, 113248. DOI: 10.1016/j.jep.2020.113248.
- Pripdeevech, P.; Saansoomchai, J. Antibacterial Activity and Chemical Composition of Essential Oil and Various Extracts of Fagraea Fragrans Roxb. Flowers. Chiang Mai J. Sci. 2013, 40, 214–223.
- Solymosi, P. Herbicidal Activity of Chamazulene. Növényvédelem. 2000, 36, 119–123. DOI: 10.1016/B978-0-12-818188-1.00031-1.
- Akram, W.; Tagde, P.; Ahmed, S.; Arora, S.; Emran, T. B.; Babalghith, A. O.; Sweilam, S. H.; Simal-Gandara, J. Guaiazulene and Related Compounds: A Review of Current Perspective on Biomedical Applications. Life. sci. 2023, 316, 121389. DOI: 10.1016/j.lfs.2023.121389.
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and Antioxidant Activity of the Chamazulene‐Rich Essential Oil Obtained from Artemisia Arborescens L. Growing on the Isle of La Maddalena, Sardinia, Italy. Chem. Biodivers. 2013, 10(8), 1464–1474.
- Capuzzo, A.; Occhipinti, A.; Maffei, M. E. Antioxidant and Radical Scavenging Activities of Chamazulene. Nat. Prod. Res. 2014, 28(24), 2321–2323. DOI: 10.1080/14786419.2014.931393.
- Safayhi, H.; Sabieraj, J.; Sailer, E.-R.; Ammon, H. Chamazulene: An Antioxidant-Type Inhibitor of Leukotriene B 4 Formation. Planta. med. 1994, 60(5), 410–413. DOI: 10.1055/s-2006-959520.
- Ali, F.; Rahul, N.; Jyoti, F.; Siddique, S. Health Functionality of Apigenin: A Review. Int. J. Food. Prop. 2017, 20(6), 1197–1238. DOI: 10.1080/10942912.2016.1207188.
- Lapchak, P. A.; Boitano, P. D. Effect of the Pleiotropic Drug CNB-001 on Tissue Plasminogen Activator (tPa) Protease Activity in Vitro: Support for Combination Therapy to Treat Acute Ischemic Stroke. J. Neurol. Neurophysiol. 2014, 5, 1–9. DOI: 10.1155/2014/525141.
- Lee, J.-H.; Zhou, H. Y.; Cho, S. Y.; Kim, Y. S.; Lee, Y. S.; Jeong, C. S. Anti-Inflammatory Mechanisms of Apigenin: Inhibition of Cyclooxygenase-2 Expression, Adhesion of Monocytes to Human Umbilical Vein Endothelial Cells, and Expression of Cellular Adhesion Molecules. Arch. Pharm. Res. 2007, 30(10), 1318–1327. DOI: 10.1007/BF02980273.
- Telange, D. R.; Patil, A. T.; Pethe, A. M.; Fegade, H.; Anand, S.; Dave, V. S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. DOI: 10.1016/j.ejps.2016.12.009.
- Miguel, F. G.; Cavalheiro, A. H.; Spinola, N. F.; Ribeiro, D. L.; Barcelos, G. R. M.; Antunes, L. M. G.; Hori, J. I.; Marquele-Oliveira, F.; Rocha, B. A.; Berretta, A. A. Validation of a RP-HPLC-DAD Method for Chamomile (Matricaria Recutita) Preparations and Assessment of the Marker, Apigenin-7-Glucoside, Safety and Anti-Inflammatory Effect. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–9. DOI: 10.1155/2015/828437.
- Pamunuwa, G.; Karunaratne, D. N.; Waisundara, V. Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia Dulcis. Evidence-Based Complement. Altern. Med. 2016, 2016, 1–11. DOI: 10.1155/2016/8243215.
- Shay, J.; Elbaz, H. A.; Lee, I.; Zielske, S. P.; Malek, M. H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. OXID. MED. CELL LONGEV. 2015, 2015, 1–13. DOI: 10.1155/2015/181260.
- Bijani, S.; Dizaji, R.; Sharafi, A.; Hosseini, M.-J. Neuroprotective Effect of Apigenin on Depressive-Like Behavior: Mechanistic Approach. Neurochem. Res. 2022, 47(3), 644–655. DOI: 10.1007/s11064-021-03473-0.
- Wang, Q.-Q.; Cheng, N.; Yi, W.-B.; Peng, S.-M.; Zou, X.-Q. Synthesis, Nitric Oxide Release, and α-Glucosidase Inhibition of Nitric Oxide Donating Apigenin and Chrysin Derivatives. Bioorg. Med. Chem. 2014, 22(5), 1515–1521. DOI: 10.1016/j.bmc.2014.01.038.
- Iizumi, Y.; Oishi, M.; Taniguchi, T.; Goi, W.; Sowa, Y.; Sakai, T.; Fei, P. The Flavonoid Apigenin Downregulates CDK1 by Directly Targeting Ribosomal Protein S9. PLoS One. 2013, 8(8), e73219. DOI: 10.1371/journal.pone.0073219.
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin Impairs Oral Squamous Cell Carcinoma Growth in vitro Inducing Cell Cycle Arrest and Apoptosis. Int. J. Oncol. 2013, 43(5), 1675–1682. DOI: 10.3892/ijo.2013.2072.
- Takagaki, N.; Sowa, Y.; Oki, T.; Nakanishi, R.; Yogosawa, S.; Sakai, T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int. J. Oncol. 2005, 25, 5257. DOI: 10.3892/ijo.26.1.185.
- SEO, H.-S.; KU, J. M.; CHOI, H.-S.; WOO, J.-K.; JANG, B.-H.; GO, H.; SHIN, Y. C.; KO, S.-G. Apigenin Induces Caspase-Dependent Apoptosis by Inhibiting Signal Transducer and Activator of Transcription 3 Signaling in HER2-Overexpressing SKBR3 Breast Cancer Cells. Mol. Med. Rep. 2015, 12(2), 2977–2984. DOI: 10.3892/mmr.2015.3698.
- Seo, H.-S.; Choi, H.-S.; Kim, S.-R.; Choi, Y. K.; Woo, S.-M.; Shin, I.; Woo, J.-K.; Park, S.-Y.; Shin, Y. C.; Ko, S.-K. Apigenin Induces Apoptosis via Extrinsic Pathway, Inducing p53 and Inhibiting STAT3 and Nfκb Signaling in HER2-Overexpressing Breast Cancer Cells. Mol. Cell. Biochem. 2012, 366(1–2), 319–334. DOI: 10.1007/s11010-012-1310-2.
- Chiang, L.-C.; Ng, L. T.; Lin, I.-C.; Kuo, P.-L.; Lin, C.-C. Anti-Proliferative Effect of Apigenin and Its Apoptotic Induction in Human Hep G2 Cells. Cancer Lett. 2006, 237(2), 207–214. DOI: 10.1016/j.canlet.2005.06.002.
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological Profile of Apigenin, a Flavonoid Isolated from Matricaria Chamomilla. Biochem. Pharmacol. 2000, 59(11), 1387–1394. DOI: 10.1016/S0006-2952(00)00264-1.
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral Characterisation of the Flavonoids Apigenin and Chrysin. Fitoterapia. 2000, 71, S117–S123. DOI: 10.1016/S0367-326X(00)00186-6.
- Izumi, S.; Takashima, O.; Hirata, T. Geraniol is a Potent Inducer of Apoptosis-Like Cell Death in the Cultured Shoot Primordia of Matricaria Chamomilla. Biochem. Biophys. Res. Commun. 1999, 259(3), 519–522. DOI: 10.1006/bbrc.1999.0813.
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol – a Review. Planta. med. 2019, 85(1), 48–55. DOI: 10.1055/a-0750-6907.
- Chaudhary, S. C.; Siddiqui, M. S.; Athar, M.; Alam, M. S. Geraniol Inhibits Murine Skin Tumorigenesis by Modulating COX‐2 Expression, Ras‐ERK1/2 Signaling Pathway and Apoptosis. J. Appl. Toxicol. 2013, 33(8), 828–837. DOI: 10.1002/jat.2739.
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and Geranyl Acetate Induce Potent Anticancer Effects in Colon Cancer Colo-205 Cells by Inducing Apoptosis, DNA Damage and Cell Cycle Arrest. J. Buon. 2018, 23(2), 346–352. DOI: 10.1177/1934578X1701200519.
- Khan, A. Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M. U.; Tahir, M.; Ali, F.; Hamiza, O. O.; Hasan, S. K.; Sultana, S. Geraniol Attenuates 12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Oxidative Stress and Inflammation in Mouse Skin: Possible Role of p38 MAP Kinase and NF-Κb. Exp. Mol. Pathol. 2013, 94(3), 419–429. DOI: 10.1016/j.yexmp.2013.01.006.
- Wittig, C.; Scheuer, C.; Parakenings, J.; Menger, M. D.; Laschke, M. W.; Addison, C. L. Geraniol Suppresses Angiogenesis by Downregulating Vascular Endothelial Growth Factor (VEGF)/VEGFR-2 Signaling. PLoS One. 2015, 10(7), e0131946. DOI: 10.1371/journal.pone.0131946.
- Murbach Teles Andrade, B. F.; Conti, B. J.; Santiago, K. B.; Fernandes, A.; Sforcin, J. M. C ymbopogon martinii essential oil and geraniol at noncytotoxic concentrations exerted immunomodulatory/anti-inflammatory effects in human monocytes. J. Pharm. Pharmacol. 2014, 66(10), 1491–1496. DOI: 10.1111/jphp.12278.
- Su, Y.-W.; Chao, S.-H.; Lee, M.-H.; Ou, T.-Y.; Tsai, Y.-C. Inhibitory Effects of Citronellol and Geraniol on Nitric Oxide and Prostaglandin E 2 Production in Macrophages. Planta. med. 2010, 76(15), 1666–1671. DOI: 10.1055/s-0030-1249947.
- El Azab, F.; Elguindy, E.; Yacout, N. M.; Elgamal, G. A. Hepatoprotective Impact of Geraniol Against CCl4-Induced Liver Fibrosis in Rats. Pakistan J. Biol. Sci. 2020, 23(12), 1650–1658. DOI: 10.3923/pjbs.2020.1650.1658.
- Chen, J.; Fan, X.; Zhou, L.; Gao, X. Treatment with Geraniol Ameliorates Methionine‐Choline‐Deficient Diet‐Induced Non‐Alcoholic Steatohepatitis in Rats. J. Gastroenterol. Hepatol. 2016, 31(7), 1357–1365. DOI: 10.1111/jgh.13272.
- Ibrahim, S. M.; El- Denshary, E. S.; Abdallah, D. M.; Hribal, M. L. Geraniol, Alone and in Combination with Pioglitazone, Ameliorates Fructose-Induced Metabolic Syndrome in Rats via the Modulation of Both Inflammatory and Oxidative Stress Status. PLoS One. 2015, 10(2), e0117516. DOI: 10.1371/journal.pone.0117516.
- Galle, M.; Kladniew, B. R.; Castro, M. A.; Villegas, S. M.; Lacunza, E.; Polo, M.; de Bravo, M. G.; Crespo, R. Modulation by Geraniol of Gene Expression Involved in Lipid Metabolism Leading to a Reduction of Serum-Cholesterol and Triglyceride Levels. Phytomedicine. 2015, 22(7–8), 696–704. DOI: 10.1016/j.phymed.2015.04.005.
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S.; Sturtevant, J. Fungicidal action of geraniol against Candida albicans is potentiated by abrogated CaCdr1p drug efflux and fluconazole synergism. PLoS One. 2018, 13(8), e0203079. DOI: 10.1371/journal.pone.0203079.
- Singh, S.; Fatima, Z.; Hameed, S. Insights into the Mode of Action of Anticandidal Herbal Monoterpenoid Geraniol Reveal Disruption of Multiple MDR Mechanisms and Virulence Attributes in Candida albicans. Arch. Microbiol. 2016, 198(5), 459–472. DOI: 10.1007/s00203-016-1205-9.
- Sharma, Y.; Khan, L. A.; Manzoor, N. Anti-Candida Activity of Geraniol Involves Disruption of Cell Membrane Integrity and Function. J. Med. Mycol. 2016, 26(3), 244–254. DOI: 10.1016/j.mycmed.2016.04.004.
- Leite, M. C. A.; de Brito Bezerra, A. P.; de Sousa, J. P.; de Oliveira Lima, E. Investigating the Antifungal Activity and Mechanism(s) of Geraniol Against Candida albicans Strains. Med. Mycol. 2015, 53(3), 275–284. DOI: 10.1093/mmy/myu078.
- Asgharzade, S.; Khorrami, M. B.; Forouzanfar, F. Neuroprotective Effect of Herniarin Following Transient Focal Cerebral Ischemia in Rats. Metab. Brain Dis. 2021, 36(8), 2505–2510. DOI: 10.1007/s11011-021-00841-1.
- Shaibani, Z. The Effect of Herniarin on Oxidative Stress in the Hippocampus in Streptozotocin-Induced Diabetic Rats. J. Anim. Biol. 2021, 14, 55–63. DOI: 10.22034/ASCIJ.2021.684776.
- Salehcheh, M.; Safari, O.; Khodayar, M. J.; Mojiri-Forushani, H.; Cheki, M. The Protective Effect of Herniarin on Genotoxicity and Apoptosis Induced by Cisplatin in Bone Marrow Cells of Rats. Drug Chem. Toxicol. 2022, 45(4), 1470–1475. DOI: 10.1080/01480545.2020.1842883.
- Sim, M.-O.; Lee, H.-I.; Ham, J. R.; Seo, K.-I.; Kim, M.-J.; Lee, M.-K. Anti-Inflammatory and Antioxidant Effects of Umbelliferone in Chronic Alcohol-Fed Rats. Nutr. Res. Pract. 2015, 9(4), 364. DOI: 10.4162/nrp.2015.9.4.364.
- Boulebd, H. Are Thymol, Rosefuran, Terpinolene and Umbelliferone Good Scavengers of Peroxyl Radicals? Phytochemistry. 2021, 184, 112670. DOI: 10.1016/j.phytochem.2021.112670.
- Alotaibi, M. F.; Al-Joufi, F.; Abou Seif, H. S.; Alzoghaibi, M. A.; Djouhri, L.; Ahmeda, A. F.; Mahmoud, A. M. Umbelliferone Inhibits Spermatogenic Defects and Testicular Injury in Lead-Intoxicated Rats by Suppressing Oxidative Stress and Inflammation, and Improving Nrf2/HO-1 Signaling. Drug Des. Devel. Ther. 2020, 14, 4003–4019. DOI: 10.2147/DDDT.S265636.
- Wang, D.; Wang, X.; Tong, W.; Cui, Y.; Li, X.; Sun, H. Umbelliferone Alleviates Lipopolysaccharide-Induced Inflammatory Responses in Acute Lung Injury by Down-Regulating TLR4/MyD88/NF-Κb Signaling. Inflammation. 2019, 42(2), 440–448. DOI: 10.1007/s10753-018-00953-4.
- Wang, H.-Q.; Wang, S.-S.; Chiufai, K.; Wang, Q.; Cheng, X.-L. Umbelliferone Ameliorates Renal Function in Diabetic Nephropathy Rats Through Regulating Inflammation and TLR/NF-Κb Pathway. Chin. J. Nat. Med. 2019, 17(5), 346–354. DOI: 10.1016/S1875-5364(19)30040-8.
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P. C. Tumor Targeted Delivery of Umbelliferone via a Smart Mesoporous Silica Nanoparticles Controlled-Release Drug Delivery System for Increased Anticancer Efficiency. Mater. Sci. Eng. C. 2020, 116, 111239. DOI: 10.1016/j.msec.2020.111239.
- Hassanein, E. H. M.; Ali, F. E. M.; Kozman, M. R.; Abd El-Ghafar, O. A. M. Umbelliferone Attenuates Gentamicin-Induced Renal Toxicity by Suppression of TLR-4/nf-Κb-p65/NLRP-3 and JAK1/STAT-3 Signaling Pathways. Environ. Sci. Pollut. Res. 2021, 28(9), 11558–11571. DOI: 10.1007/s11356-020-11416-5.
- Sotiropoulou, N. S.; Megremi, S. F.; Tarantilis, P. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria Chamomilla L.) and Sage (Salvia Officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10(7), 2270. DOI: 10.3390/app10072270.
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice Through MAPK/ERK/JNK Pathway. Biomed Res. Int. 2019, 2019, 1–14. DOI: 10.1155/2019/6916189.
- Kováčik, J.; Klejdus, B. Dynamics of Phenolic Acids and Lignin Accumulation in Metal-Treated Matricaria Chamomilla Roots. Plant Cell Rep. 2008, 27(3), 605–615. DOI: 10.1007/s00299-007-0490-9.
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. (Lausanne). 2021, 12, 237–244. DOI: 10.3389/fendo.2021.762691.
- Bhandarkar, N. S.; Brown, L.; Panchal, S. K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. DOI: 10.1016/j.nutres.2018.11.002.
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32(4), 1200–1209. DOI: 10.1007/s11095-014-1526-9.
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G., et al. Chlorogenic Acid Effectively Treats Cancers Through Induction of Cancer Cell Differentiation. Theranostics. 2019, 9(23), 6745–6763. DOI: 10.7150/thno.34674.
- Santana-Gálvez, J.; Villela Castrejón, J.; Serna-Saldívar, S. O.; Jacobo-Velázquez, D. A. Anticancer potential of dihydrocaffeic acid: a chlorogenic acid metabolite. CyTA - J. Food. 2020, 18(1), 245–248. DOI: 10.1080/19476337.2020.1743762.
- Rebai, O.; Belkhir, M.; Sanchez-Gomez, M. V.; Matute, C.; Fattouch, S.; Amri, M. Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and Its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem. Res. 2017, 42(12), 3559–3572. DOI: 10.1007/s11064-017-2403-9.
- Heitman, E.; Ingram, D. K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20(1), 32–39. DOI: 10.1179/1476830514Y.0000000146.
- Shan, S.; Tian, L.; Fang, R. Chlorogenic Acid Exerts Beneficial Effects in 6-Hydroxydopamine-Induced Neurotoxicity by Inhibition of Endoplasmic Reticulum Stress. Med. Sci. Monit. 2019, 25, 453–459. DOI: 10.12659/MSM.911166.
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Singh, S. S.; Birla, H.; Singh, S. P.; Krishnamurthy, S.; Patnaik, R. Neuroprotective Effect of Chlorogenic Acid in Global Cerebral Ischemia-Reperfusion Rat Model. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392(10), 1293–1309. DOI: 10.1007/s00210-019-01670-x.
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Nutr. Neurosci. 2020, 20, 32–39. DOI: 10.1179/1476830514Y.0000000146
- Alam, M.; Ahmed, S.; Elasbali, A. M.; Adnan, M.; Alam, S.; Hassan, M. I.; Pasupuleti, V. R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 989–1010. DOI: 10.3389/fonc.2022.860508.
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R. D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T. J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed Res. Int. 2018, 2018, 1–9. DOI: 10.1155/2018/7413504.
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine. 2019, 9, 574–586. DOI: 10.22038/AJP.2019.13475.
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64(9), 789. DOI: 10.1128/AAC.00251-20.
- Malik, J. A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 Mutations versus Vaccine Effectiveness: New Opportunities to New Challenges. J. Infect Public Health. 2022, 15(2), 228–240. DOI: 10.1016/j.jiph.2021.12.014.
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A. F.; Ali, M.; Abdalla, M.; Ibrahim, I. M.; Elfiky, A. A. Caffeic Acid Derivatives (CAFDs) as Inhibitors of SARS-CoV-2: CAFDs-Based Functional Foods as a Potential Alternative Approach to Combat COVID-19. Phytomedicine. 2021, 85, 153310. DOI: 10.1016/j.phymed.2020.153310.
- Cook, M. T. Mechanism of Metastasis Suppression by Luteolin in Breast Cancer. Breast Cancer Targets Ther. 2018, 10, 89–100. DOI: 10.2147/BCTT.S144202.
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M. A.; Khan, I. A.; Imran, A.; Orhan, I. E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. DOI: 10.1016/j.biopha.2019.108612.
- Ashokkumar, P.; Sudhandiran, G. Luteolin Inhibits Cell Proliferation During Azoxymethane-Induced Experimental Colon Carcinogenesis via Wnt/β-Catenin Pathway. Invest. New Drugs. 2011, 29(2), 273–284. DOI: 10.1007/s10637-009-9359-9.
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020, 11, 10990–10998. DOI: 10.3389/fphys.2020.00113.
- Guimarães, R.; Calhelha, R. C.; Froufe, H. J. C.; Abreu, R. M. V.; Carvalho, A. M.; Queiroz, M. J.; Ferreira, R. P. Wild Roman Chamomile Extracts and Phenolic Compounds: Enzymatic Assays and Molecular Modelling Studies with VEGFR-2 Tyrosine Kinase. Food Funct. 2016, 7(1), 79–83. DOI: 10.1039/C5FO00586H.
- Gendrisch, F.; Esser, P. R.; Schempp, C. M.; Wölfle, U. Luteolin as a Modulator of Skin Aging and Inflammation. BioFactors. 2021, 47(2), 170–180. DOI: 10.1002/biof.1699.
- Kempuraj, D.; Thangavel, R.; Kempuraj, D. D.; Ahmed, M. E.; Selvakumar, G. P.; Raikwar, S. P.; Zaheer, S. A.; Iyer, S. S.; Govindarajan, R.; Chandrasekaran, P. N., et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors. 2021, 47(2), 190–197. DOI: 10.1002/biof.1687.
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S. M.; Salem, M. A.; Merghany, R. M.; El Mahdy, N. M.; Kılıç, C. S.; Sytar, O., et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020, 5(20), 11849–11872. DOI: https://doi.org/10.1021/acsomega.0c01818.
- Patel, R. V.; Mistry, B. M.; Shinde, S. K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. DOI: 10.1016/j.ejmech.2018.06.053.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods. 2018, 40, 68–75. DOI: 10.1016/j.jff.2017.10.047.
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F. R. S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2023, 80(1), 241–262. DOI: 10.1007/s00289-022-04091-8.
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J.; Olejarz, K.; Zieliński, H.; Piskuła, M. K. Metabolites of Dietary Quercetin: Profile, Isolation, Identification, and Antioxidant Capacity. J. Funct. Foods. 2014, 11, 121–129. DOI: 10.1016/j.jff.2014.09.013.
- Rauf, A.; Imran, M.; Khan, I. A.; Ur‐Rehman, M.; Gilani, S. A.; Mehmood, Z.; Mubarak, M. S. Anticancer potential of quercetin: A comprehensive review. Phyther. Res. 2018, 32(11), 2109–2130. DOI: 10.1002/ptr.6155.
- Zaplatic, E.; Bule, M.; Shah, S. Z. A.; Sahab Uddin, M.; Niaz, K. Corrigendum to “Molecular Mechanisms Underlying Protective Role of Quercetin in Attenuating Alzheimer’s disease” [Life Sci. 221 (2019) 109–119]. Life. sci. 2019, 231(231), 116616. DOI: 10.1016/j.lfs.2019.116616.
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C. E.; Farzaei, M. H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers (Basel). 2020, 12(8), 2276. DOI: 10.3390/cancers12082276.
- Enogieru, A. B.; Haylett, W.; Hiss, D. C.; Bardien, S.; Ekpo, O. E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. OXID. MED. CELL LONGEV. 2018, 2018, 1–17. DOI: 10.1155/2018/6241017.
- Irfan, F.; Jameel, F.; Khan, I.; Aslam, R.; Faizi, S.; Salim, A. Role of Quercetin and Rutin in Enhancing the Therapeutic Potential of Mesenchymal Stem Cells for Cold Induced Burn Wound. Regen. Ther. 2022, 21, 225–238. DOI: 10.1016/j.reth.2022.07.011.
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phyther. Res. 2021, 35(5), 2500–2513. DOI: 10.1002/ptr.6977.
- Sun, X.; Li, L.; Dong, Q.-X.; Zhu, J.; Huang, Y.; Hou, S.; Yu, X.; Liu, R. Rutin Prevents Tau Pathology and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. J. Neuroinflammation. 2021, 18(1), 131. DOI: 10.1186/s12974-021-02182-3.
- Satyal, P.; Shrestha, S.; Setzer, W. N. Composition and Bioactivities of an (E)-β-Farnesene Chemotype of Chamomile (Matricaria Chamomilla) Essential Oil from Nepal. Nat. Prod. Commun. 2015, 10(8), 1934578X1501000. DOI: 10.1177/1934578X1501000835.
- Roby, M. H. H.; Sarhan, M. A.; Selim, K. A.-H.; Khalel, K. I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. DOI: 10.1016/j.indcrop.2012.10.012.
- Seyedjavadi, S. S.; Khani, S.; Zare‐Zardini, H.; Halabian, R.; Goudarzi, M.; Khatami, S.; Imani Fooladi, A. A.; Amani, J.; Razzaghi‐Abyaneh, M. Isolation, Functional Characterization, and Biological Properties of MCh‐AMP1, a Novel Antifungal Peptide from Matricaria Chamomilla L. Chem. Biol. Drug Des. 2019, 93(5), 949–959. DOI: 10.1111/cbdd.13500.
- Tolouee, M.; Alinezhad, S.; Saberi, R.; Eslamifar, A.; Zad, S. J.; Jaimand, K.; Taeb, J.; Rezaee, M.-B.; Kawachi, M.; Shams-Ghahfarokhi, M.; et al. Effect of Matricaria Chamomilla L. Flower Essential Oil on the Growth and Ultrastructure of Aspergillus Niger van Tieghem. Int. J. Food Microbiol. 2010, 139(3), 127–133.
- Enas, M. A. Phytochemical Composition, Antifungal, Antiaflatoxigenic, Antioxidant, and Anticancer Activities of Glycyrrhiza Glabra L. and Matricaria Chamomilla L. Essential Oils. J. Med. Plants Res. 2013, 7(29), 2197–2207. DOI: 10.5897/JMPR12.5134.
- EL-Hefny, M.; Abo Elgat, W.; Al-Huqail, A.; Ali, H. Essential and Recovery Oils from Matricaria Chamomilla Flowers as Environmentally Friendly Fungicides Against Four Fungi Isolated from Cultural Heritage Objects. Processes. 2019, 7(11), 809. DOI: 10.3390/pr7110809.
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M. J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Biomimetic Synthesis of Silver Nanoparticles Using Matricaria Chamomilla Extract and Their Potential Anticancer Activity Against Human Lung Cancer Cells. Mater. Sci. Eng. C. 2018, 92, 902–912. DOI: 10.1016/j.msec.2018.07.053.
- Kamali, A. M.; Nikseresht, M.; Delaviz, H.; Barmak, M. J. S.; Ardakani, M.; Mahmoudi, M. T. In vitro Cytotoxic Activity of Matricaria Chamomilla Root Extract in Human Breast Cancer Cell Line MCF-7. Life Sci. J. 2014, 11, 403–406.
- Nikseresht, M.; Kamali, A.; Rahimi, H.; Delaviz, H.; Toori, M.; Kashani, I.; Mahmoudi, R. The Hydroalcoholic Extract of Matricaria Chamomilla Suppresses Migration and Invasion of Human Breast Cancer MDA-MB-468 and MCF-7 Cell Lines. Pharmacognosy. Res. 2017, 9(1), 87. DOI: 10.4103/0974-8490.199778.
- Shaaban, M.; El-Hagrassi, A. M.; Osman, A. F.; Soltan, M. M. Bioactive Compounds from Matricaria Chamomilla: Structure Identification, in vitro Antiproliferative, Antimigratory, Antiangiogenic, and Antiadenoviral Activities. Zeitschrift für Naturforsch. C. 2022, 77(3–4), 85–94. DOI: 10.1515/znc-2021-0083.
- Mitoshi, M.; Kuriyama, I.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; Kobayashi, Y.; Jippo, T.; Kanazawa, K.; Yoshida, H.; Mizushina, Y. Effects of Essential Oils from Herbal Plants and Citrus Fruits on DNA Polymerase Inhibitory, Cancer Cell Growth Inhibitory, Antiallergic, and Antioxidant Activities. J. Agric. Food. Chem. 2012, 60(45), 11343–11350. DOI: 10.1021/jf303377f.
- El Joumaa, M. M.; Taleb, R. I.; Rizk, S.; Borjac, J. M. Protective effect of Matricaria chamomilla extract against 1,2-dimethylhydrazine-induced colorectal cancer in mice. J. Complement Integr. Med. 2020, 17(3), 20190143. DOI: 10.1515/jcim-2019-0143.
- Shebbo, S.; El Joumaa, M.; Kawach, R.; Borjac, J. Hepatoprotective Effect of Matricaria Chamomilla Aqueous Extract Against 1,2-Dimethylhydrazine-Induced Carcinogenic Hepatic Damage in Mice. Heliyon. 2020, 6(6), e04082. DOI: 10.1016/j.heliyon.2020.e04082.
- Hassanpour, H.; Ghanbarzadeh, M. Induction of cell division and antioxidative enzyme activity of Matricaria chamomilla L. cell line under clino-rotation. Plant Cell Tissue Organ Cult. 2021, 146(2), 215–224. DOI: 10.1007/s11240-021-02060-z.
- Ranjbar, A.; Mohsenzadeh, F.; Chehregani, A.; Khajavi, F.; Zijoud, S.-M.; Ghasemi, H. Ameliorative Effect of Matricaria Chamomilla.L on Paraquat: Induced Oxidative Damage in Lung Rats. Pharmacognosy. Res. 2014, 6(3), 199. DOI: 10.4103/0974-8490.132595.
- Kolodziejczyk-Czepas, J.; Bijak, M.; Saluk, J.; Ponczek, M. B.; Zbikowska, H. M.; Nowak, P.; Tsirigotis-Maniecka, M.; Pawlaczyk, I. Radical Scavenging and Antioxidant Effects of Matricaria Chamomilla Polyphenolic–Polysaccharide Conjugates. Int. J. Biol. Macromol. 2015, 72, 1152–1158. DOI: 10.1016/j.ijbiomac.2014.09.032.
- Aksoy, L.; Sözbilir, N. B. Effects of Matricaria Chamomilla L. on Lipid Peroxidation, Antioxidant Enzyme Systems, and Key Liver Enzymes in CCl 4 -Treated Rats. Toxicol. Environ. Chem. 2012, 94(9), 1780–1788. DOI: 10.1080/02772248.2012.729837.
- Iverson, C. D.; Zahid, S.; Li, Y.; Shoqafi, A. H.; Ata, A.; Samarasekera, R. Glutathione S-Transferase Inhibitory, Free Radical Scavenging, and Anti-Leishmanial Activities of Chemical Constituents of Artocarpus Nobilis and Matricaria Chamomilla. Phytochem. Lett. 2010, 3(4), 207–211. DOI: 10.1016/j.phytol.2010.07.008.
- Ortiz, M. I.; Cariño‐Cortés, R.; Ponce‐Monter, H. A.; González‐García, M. P.; Castañeda‐Hernández, G.; Salinas‐Caballero, M. Synergistic Interaction of Matricaria Chamomilla Extract with Diclofenac and Indomethacin on Carrageenan‐Induced Paw Inflammation in Rats. Drug Dev. Res. 2017, 78(7), 360–367. DOI: 10.1002/ddr.21401.
- El Mihyaoui, A.; Esteves da Silva, J. C. G.; Charfi, S.; Candela Castillo, M. E.; Lamarti, A.; Arnao, M. B. Chamomile (Matricaria Chamomilla L.): A Review of Ethnomedicinal Use. Phytochemistry And Pharmacological Uses. Life. 2022, 12(4), 479. DOI: 10.3390/life12040479.
- Wu, Y.; Xu, Y.; Yao, L. Anti-inflammatory and Anti-allergic Effects of German Chamomile (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants. 2012, 15(1), 75–83. DOI: 10.1080/0972060X.2012.10644022.
- Sayyar, Z.; Yazdinezhad, A.; Hassan, M.; Jafari Anarkooli, I. Protective Effect of Matricaria Chamomilla Ethanolic Extract on Hippocampal Neuron Damage in Rats Exposed to Formaldehyde. OXID. MED. CELL LONGEV. 2018, 2018, 1–10. DOI: 10.1155/2018/6414317.
- Asgharzade, S.; Rabiei, Z.; Rafieian-Kopaei, M. Effects of Matricaria Chamomilla Extract on Motor Coordination Impairment Induced by Scopolamine in Rats. Asian Pac. J. Trop. Biomed. 2015, 5(10), 829–833. DOI: 10.1016/j.apjtb.2015.06.006.
- Alibabaei, Z.; Rabiei, Z.; Rahnama, S.; Mokhtari, S.; Rafieian-Kopaei, M. Matricaria Chamomilla Extract Demonstrates Antioxidant Properties Against Elevated Rat Brain Oxidative Status Induced by Amnestic Dose of Scopolamine. Biomed. Aging Pathol. 2014, 4(4), 355–360. DOI: 10.1016/j.biomag.2014.07.003.
- Amsterdam, J. D.; Li, Q. S.; Xie, S. X.; Mao, J. J. Putative Antidepressant Effect of Chamomile (Matricaria Chamomilla L.) Oral Extract in Subjects with Comorbid Generalized Anxiety Disorder and Depression. J. Altern. Complement. Med. 2020, 26(9), 815–821. DOI: 10.1089/acm.2019.0252.
- Abbasinia, H.; Alizadeh, Z.; Vakilian, K.; Jafari, Z.; Poor, P. M.; Ranjbaran, M. Effect of Chamomile Extract on Sleep Disorder in Menopausal Women. Iran. J. Obstet. Gynecol. Infertil. 2016, 19, 1–7. DOI: 10.22038/IJOGI.2016.7631.
- Abad, A. N. A.; Nouri, M. H. K.; Gharjanie, A.; Tavakoli, F. Effect of Matricaria chamomilla Hydroalcoholic Extract on Cisplatin-induced Neuropathy in Mice. Chin. J. Nat. Med. 2011, 9, 126–131. DOI: 10.3724/SP.J.1009.2011.00126.
- Nouri, K. Antinociceptive effect of Matricaria chamomilla on vincristine-induced peripheral neuropathy in mice. Afr J. Pharm. Pharmacol. 2012, 6(1), 24–29. DOI: 10.5897/AJPP11.340.
- Ioniță, R. Anxiolytic and Antidepressant Effects of Matricaria Chamomilla Hydroalcoholic Extract in a Rat Model of Scopolamine. Farmacia. 2019, 67(1), 68–72. DOI: 10.31925/farmacia.2019.1.9.
- Reis, L. S. L. D. S.; Pardo, P. E.; Oba, E.; Kronka, S. D. N.; Frazatti-Gallina, N. M. Matricaria chamomilla CH 12 decreases handling stress in Nelore calves. J. Vet. Sci. 2006, 7(2), 189. DOI: 10.4142/jvs.2006.7.2.189.
- Moore, E.; Lockwood, D. Treatment of Visceral Leishmaniasis. J. Glob. Infect. Dis. 2010, 2(2), 151. DOI: 10.4103/0974-777X.62883.
- Karam, T. K.; Ortega, S.; Ueda Nakamura, T.; Auzély-Velty, R.; Nakamura, C. V. Development of Chitosan Nanocapsules Containing Essential Oil of Matricaria Chamomilla L. for the Treatment of Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. DOI: 10.1016/j.ijbiomac.2020.06.149.
- Luize, P. S.; Tiuman, T. S.; Morello, L. G.; Maza, P. K.; Ueda-Nakamura, T.; Dias Filho, B. P.; Cortez, D. A. G.; Mello, J. C. P. D.; Nakamura, C. V. Effects of Medicinal Plant Extracts on Growth of Leishmania (L.) Amazonensis and Trypanosoma Cruzi. Rev. Bras. Cienc. Farm. 2005, 41(1), 85–94. DOI: 10.1590/S1516-93322005000100010.
- Macchioni, F.; Perrucci, S.; Cecchi, F.; Cioni, P. L.; Morelli, I.; Pampiglione, S. Acaricidal activity of aqueous extracts of camomile flowers, Matricaria chamomilla, against the mite Psoroptes cuniculi. Med. Vet. Entomol. 2004, 18(2), 205–207. DOI: 10.1111/j.0269-283X.2004.00488.x.
- Bahmani, M.; Saki, K.; Gholami-Ahangaran, M.; Parsaei, P.; Mohsenzadegan, A.; Zia-Jahromi, N. Evaluating the Anti-Leech Activity of Methanolic Extract of Matricaria Chamomilla L. Comparing with Ivermectin, Mebendasole, Praziquantel, Rafoxanide, Febantel and Albendasole. Middle-East J. Sci. Res. 2012, 12, 260–263. DOI: 10.5829/idosi.mejsr.2012.12.2.6446.
- Mollabashi, E. N.; Ziaie, T.; Khalesi, Z. B.; Leili, E. K.; Bekhradi, R. Effect of Chamomile capsule on premenstrual syndrome symptoms relief. Iran. J. Obstet. Gynecol. Infertil. 2018, 21, 72–80. DOI: 10.22038/IJOGI.2018.11799.
- Johari, H.; Khavarian, M.; Mokhtari, M.; Kamali, M.; Kargar Jahromi, H. The Effects of Hydro Alcoholic Extract of Matricaria Chamomilla Flower on Testosterone and Gonadotropins Hormone in Adult Male Rat. Pars Jahrom Univ. Med. Sci. 2014, 12(4), 37–41. DOI: 10.29252/jmj.12.4.6.
- Karbalay-Doust, S.; Noorafshan, A.; Dehghani, F.; Panjehshahin, M. R.; Monabati, A. Effects of Hydroalcoholic Extract of Matricaria Chamomilla on Serum Testosterone and Estradiol Levels, Spermatozoon Quality, and Tail Length in Rat. Iran. J. Med. Sci. 2010, 35, 122–128.
- Shadi, T. Z.; Talal, A. Z. A Review of Four Common Medicinal Plants Used to Treat Eczema. J. Med. Plants Res. 2015, 9(24), 702–711. DOI: 10.5897/JMPR2015.5831.
- Dolati, S. A Review of the Therapeutic Effects of Chamomile (Matricaria Chamomile) in Traditional and Modern Medicine. Res. Sport Sci. Med. Plants. 2021, 1, 1–12. DOI: 10.30495/VARZESH.2021.1918705.1003.
- Boroujeni, H.; Parvin, N.; Mirzaeian, P.; Marandi, S. Formulation and Clinical Trial Study of AJMT Cream in Treatment of Eczema. Iioab. J. 2017, 8, 46–50.
- Niknam, S.; Tofighi, Z.; Faramarzi, M. A.; Abdollahifar, M. A.; Sajadi, E.; Dinarvand, R.; Toliyat, T. Polyherbal Combination for Wound Healing: Matricaria Chamomilla L. and Punica Granatum L. DARU J. Pharm. Sci. 2021, 29(1), 133–145. DOI: 10.1007/s40199-021-00392-x.
- Soltani, M.; Moghimian, M.; Abtahi-Eivari, S. H.; Shoorei, H.; Khaki, A.; Shokoohi, M. Protective Effects of Matricaria Chamomilla Extract on Torsion/detorsion-Induced Tissue Damage and Oxidative Stress in Adult Rat Testis. Int. J. Fertil. Steril. 2018, 12(3), 242–248. DOI: 10.22074/ijfs.2018.5324.
- Jarrahi, M. An Experimental Study of the Effects of Matricaria Chamomilla Extract on Cutaneous Burn Wound Healing in Albino Rats. Nat. Prod. Res. 2008, 22(5), 422–427. DOI: 10.1080/14786410701591713.
- Cemek, M.; Kağa, S.; Şimşek, N.; Büyükokuroğlu, M. E.; Konuk, M. Antihyperglycemic and Antioxidative Potential of Matricaria Chamomilla L. in Streptozotocin-Induced Diabetic Rats. J. Nat. Med. 2008, 62(3), 284–293. DOI: 10.1007/s11418-008-0228-1.
- Mahadeva Rao, U. S.; Subramanian, S. Biochemical Evaluation of Antihyperglycemic and Antioxidative Effects of Morinda Citrifolia Fruit Extract Studied in Streptozotocin-Induced Diabetic Rats. Med. Chem. Res. 2009, 18(6), 433–446. DOI: 10.1007/s00044-008-9140-1.
- Najla, O. A.; Olfat, A. K.; Kholoud, S. R.; Enas, N. D.; Hanan, I. Hypoglycemic and Biochemical Effects of Matricaria Chamomilla Leave Extract in Streptozotocin-Induced Diabetic Rats. J. Heal. Sci. 2012, 2, 43–48. DOI: 10.5923/j.health.20120205.01.
- Soliman, G. A. The Potential Cardioprotective Effect of Matricaria Chamomilla Extract Against Diabetes-Induced Oxidative Stress in Rats. Farmacia. 2020, 68(2), 269–279. DOI: 10.31925/farmacia.2020.2.12.
- Heidarianpour, A.; Mohammadi, F.; Keshvari, M.; Mirazi, N. Ameliorative Effects of Endurance Training and Matricaria Chamomilla Flowers Hydroethanolic Extract on Cognitive Deficit in Type 2 Diabetes Rats. Biomed. Pharmacother. 2021, 135, 111230. DOI: 10.1016/j.biopha.2021.111230.
- Hajizadeh-Sharafabad, F.; Varshosaz, P.; Jafari-Vayghan, H.; Alizadeh, M.; Maleki, V. Chamomile (Matricaria recutita L.) and diabetes mellitus, current knowledge and the way forward: A systematic review. Complement Ther. Med. 2020, 48, 102284. DOI: 10.1016/j.ctim.2019.102284.
- Tavakol, H.; Farzad, K.; Fariba, M.; Abdolkarim, C.; Hassan, G.; Seyed-Mostafa, H.; Akram, R. Hepatoprotective Effect of Matricaria Chamomilla.L in Paraquat Induced Rat Liver Injury. Drug Res. (Stuttg). 2014, 65(2), 61–64. DOI: 10.1055/s-0033-1363999.
- Madrigal-Santillán, E. Review of Natural Products with Hepatoprotective Effects. World J. Gastroenterol. 2014, 20(40), 14787. DOI: 10.3748/wjg.v20.i40.14787.
- Chani, J. M.; Jasim, N. Z. Hepatoprotective Effect of Matricaria Chamomilla Hot Aqueous Extract Against Methomyl 90%- Induced Hepatotoxicity in Mice. Al-Kufa Univ. J. Biol. 2016, 8, 185–195.
- Salama, R. M. Matricaria chamomilla attenuates cisplatin nephrotoxicity. Saudi J. Kidney Dis. Transplant. 2012, 23(4), 765. DOI: 10.4103/1319-2442.98158.
- Salama, R. H. M.; Abd-El-Hameed, N. A.; Abd-El-Ghaffar, S. K.; Mohammed, Z. T.; Ghandour, N. M. A. Nephroprotective Effect of Nigella Sativa and Matricaria Chamomilla in Cisplatin Induced Renal Injury—Supportive Treatments in Cisplatin Nephrotoxicity. Int. J. Clin. Med. 2011, 02(3), 185–195. DOI: 10.4236/ijcm.2011.23031.
- Mehmood, M. H.; Munir, S.; Khalid, U. A.; Asrar, M.; Gilani, A. H. Antidiarrhoeal, Antisecretory and Antispasmodic Activities of Matricaria Chamomilla are Mediated Predominantly Through K±channels Activation. BMC Complement. Altern. Med. 2015, 15(1), 75. DOI: 10.1186/s12906-015-0595-6.
- Romero, M. D. C.; Valero, A.; Martín-Sánchez, J.; Navarro-Moll, M. C. Activity of Matricaria Chamomilla Essential Oil Against Anisakiasis. Phytomedicine. 2012, 19(6), 520–523. DOI: 10.1016/j.phymed.2012.02.005.
- Bahrami, A. M.; Doosti, A.; Moosavi, A. B. Effect of Matricaria Chamomilla L. Plant Extraction on Experimental Infected Lamb with Ostertagia Ostertagi Parasits. Int. J. Pharmacol. 2010, 6(5), 712–718. DOI: 10.3923/ijp.2010.712.718.
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I. A.; Bazzocchi, I. L.; Valladares, B.; Pinero, J. E.; Lorenzo-Morales, J.; Akkari, H. Correlation of Radical-Scavenging Capacity and Amoebicidal Activity of Matricaria Recutita L. (Asteraceae). Exp. Parasitol. 2017, 183, 212–217. DOI: 10.1016/j.exppara.2017.09.011.
- Váradyová, Z.; Pisarčíková, J.; Babják, M.; Hodges, A.; Mravčáková, D.; Kišidayová, S.; Königová, A.; Vadlejch, J.; Várady, M. Ovicidal and Larvicidal Activity of Extracts from Medicinal-Plants Against Haemonchus Contortus. Exp. Parasitol. 2018, 195, 71–77. DOI: 10.1016/j.exppara.2018.10.009.
- Cemek, M.; Yilmaz, E.; Büyükokuroğlu, M. E. Protective Effect of Matricaria Chamomilla on Ethanol-Induced Acute Gastric Mucosal Injury in Rats. Pharm. Biol. 2010, 48(7), 757–763. DOI: 10.3109/13880200903296147.
- Morshedi, M.; Gol, A.; Mohammadzadeh, A. The Effect of Matricaria Chamomilla on the Treatment of Ibuprofen-Induced Gastric Ulcers in Male Rats. Hormozgan Med. J. 2014, 20, 270–275.
- Karbalay-Doust, S.; Noorafshan, A. Antiulcerogenic Effects of Matricaria Chamomilla Extract in Experimental Gastric Ulcer in Mice. Iran. J. Med. Sci. 2009, 34, 198–203.
- Naema, F.; J, N.; Ali, A.; Ahmed, A. Effect of the Aqueous Extract of Matricaria Chamomilla on Stress-Ethanol Induced Acute Gastric Ulceration in Rabbits. Med. J. Basrah Univ. 2006, 24(1), 66–69. DOI: 10.33762/mjbu.2006.46423.
- El Souda, S. S. E. D.; Ahmed, K. M.; Grace, M. H.; Elkherassy, E. E. A.; Farrag, A. R. H.; Abdelwahab, S. M. Flavonoids and Gastroprotective Effect of Matricaria Chamomilla Against Indomethacin-Induced Ulcer in Rats. J. Herbs. Spices Med. Plants. 2015, 21(2), 111–117. DOI: 10.1080/10496475.2014.919372.
- Gomaa, A.; Hashem, T.; Mohamed, M.; Ashry, E. Matricaria Chamomilla Extract Inhibits Both Development of Morphine Dependence and Expression of Abstinence Syndrome in Rats. J. Pharmacol. Sci. 2003, 92(1), 50–55. DOI: 10.1254/jphs.92.50.
- de Souza Reis, L. S. L.; Frazatti-Gallina, N. M.; de Lima Paoli, R.; Giuffrida, R.; Albas, A.; Oba, E.; Pardo, P. E. Efficiency of Matricaria chamomilla CH12 and number of doses of rabies vaccine on the humoral immune response in cattle. J. Vet. Sci. 2008, 9(4), 433. DOI: 10.4142/jvs.2008.9.4.433.
- Dada, R.; Toghyani, M.; Tabeidian, S. A. The Effect of Chamomile Flower (Matricaria Chamomilla L.) Extract and Powder as Growth Promoter on Growth Performance and Digestive Organs of Broiler Chickens. Res. Opin. Anim. Vet. Sci. 2015, 5, 290–294.