 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The citrus processing industry generates around 50% fruit waste, encompassing peels, pulp, seeds, and other residues. Citrus peel, among these by-products, contains significant amounts of bioactive compounds, representing a sustainable and renewable source of phenolics. In this context, the present study was conducted to probe phytochemical profile and antioxidant potency of orange peel extracts. Moreover, the quantification of hesperidin and nobiletin was carried out using HPLC. The extracts were obtained using water and 50% aqueous-methanol and aqueous-ethanol, separately alongside varying time intervals, i.e. 30, 45 and 60 min. The obtained extracts were then investigated for their phytochemical and antioxidant profile. Afterwards, three best treatments, one from each extracts (aqueous-ethanol, methanol, and water), were chosen based on their phytochemical profiling. The phytochemical analyses and in vitro antioxidant assays showed the highest total phenolics (2010 mg GAE/100 g), flavonoids (90 mg/100 g), and flavonols (2.2 mg/100 g) in methanolic extracts obtained at 60 min. Likewise, the highest DPPH (60.55%), antioxidant activity (51.7 %), FRAP (13.6 mg TE/g), and ABTS (7.4 µmol TE/g) were also attributed to methanolic extract of orange peel obtained at 60 min except for antioxidant activity which was obtained at 30 min. Likewise, HPLC analysis depicted maximum content of hesperidin and nobiletin (133.70 and 8.50 mg/g) in methanol extract as compared to ethanol (98.80 and 5.50 mg/g) and water (61.90 and 1.25 mg/g), correspondingly. It was concluded that aqueous-methanol could be used as solvent of choice for isolation of orange peel flavonoids with extraction duration of 60 min. Flavonoid and antioxidant levels in orange peel extracts are significantly influenced by extraction time and solvent type.
Introduction
Globally, health claims for foods containing phytochemicals such as fruits and vegetables have invigorated consumer’s belief in diet-based therapies as a disease remedial tool. The chemical diversity and antioxidant properties of these polyphenols revitalized the concept of functional foods and nutraceuticals.[Citation1] Among various fruits, citrus fruits have plethora of polyphenols with the major contributions of flavanones especially hesperidin and nobiletin as powerful free radical scavengers.[Citation2,Citation3]
The term “citrus” is usually linked to family “Rutaceae,” characterized by genus of “flowering plants,” providing diversity of constituents that are of cardinal importance in human nutrition including flavonoids, folic acid, potassium, pectin, vitamin C, and dietary fiber.[Citation4] It is one of the chief fruit-bearing crops in Pakistan consisting of orange, kinnow, sweet orange, grapefruit, lime, lemon, etc. The yearly production of citrus is estimated at about 2.33 million tons.[Citation5] On utilization, nearly half (50%) of the fruit is left as uneatable waste comprising peels, pulp, seeds, and other residues.[Citation6] This unhandled waste is a source of environmental pollution and dangerous to ecosystem owing to high biological oxygen demand (BOD) but still contains substantial quantities of bioactive compounds.[Citation7]
Due to agronomic practices and climatic condition, the total flavonoids of citrus peel may vary accordingly. In orange peel, there are two unique classes of flavones i.e. glycosylated flavanones and polymethoxylated flavones.[Citation8] Flavanones are the most abundant group of flavonoids present in citrus peel comprising hesperidin (0.066 to 66.09 mg/g) and narirutin (0.03 to 26.5 mg/g), rarely present in other plants.[Citation8–10] Various epidemiological studies have associated the intake of phytochemicals from orange peel to curtail dyslipidemia, hypertension, and diabetes. Moreover, citrus fruit extracts have shown a significant antioxidant, antitumor, and blood clot inhibition activities. It is noteworthy that citrus flavonoids (flavanones and polymethoxylated flavones) were found to have numerous pharmaceutical properties that can delay rate of morbidity and mortality.[Citation11]
Isolation of antioxidants from citrus peels is of great interest in functional food business as they can delay oxidative damages, hence improving the quality and nutritional attributes of foods. The established health benefits of flavonoids have necessitated their extraction and quantification in different food items.[Citation2] Therefore, various extraction techniques are used to maximize mass transfer and yield of citrus peel bioactives. The current exploration is designed at varying extraction parameters i.e. solvent and time to acquire maximum antioxidant capacity of the orange peel extracts.
Materials and methods
Raw materials
Citrus samples (Orange sinensis L.) were taken from the fruits and vegetables market at Faisalabad, Punjab, Pakistan. The standards and chemicals were acquired from Merck (Merck KGa, Darmstadt, Germany) and Sigma-Aldrich (Sigma-Aldrich Tokyo, Japan) through local vendor.
Sample preparations
Initially, oranges were washed and cleaned in order to confiscate adherent dirt and dust and then peel was removed from the fruit. Afterwards, the peels were dried in sunlight (40°C–45°C) by spreading on a porous steel tray covered with thin muslin cloth for 3 days until crisp and finely ground to powder by electrical grinder. This orange peel powder was placed in sealed jars for further analyses.
Preparation of citrus peel extracts
The peel extracts were acquired employing water as well as two binary organic solvents: aqueous ethanol (50% v/v) and aqueous-methanol (50% v/v) as extraction solvents at three different time intervals, i.e., 30, 45 and 60 min at 50°C.[Citation12,Citation13] To obtain extract, 40 g of powdered peel was evenly distributed in 400 mL of the corresponding solvent, maintaining a 1:10 w/v ratio. This mixture was placed in a conical flask and subjected to agitation on an orbital shaker for respective time intervals. The resulting solution was then filtered through Whatman filter paper No. 1 and concentrated via Rotary Evaporator (Eyela, Japan). The resulting extract was then employed for subsequent analyses.
Phytochemical screening tests
Total phenolics
The polyphenol content (TPC) in orange peel extracts were estimated using the Folin–Ciocalteu method.[Citation14] Accordingly, peel extract (50 µL) was taken to each test tube containing Folin–Ciocalteu (250 µL), 20% solution of sodium carbonate (750 µL) and finally the volume was fulfilled to 5 mL using distilled water. Finally, the absorbance was recorded at 765 nm after 2 h with UV/visible light spectrophotometer (CECIL, CE7200) compared to control that ensures the entire reagents for reaction excluding extract. Total phenolics were assessed as mg of gallic acid equivalent/100 g by subsequent expression.
where C is total phenolic contents in mg/g plant extract, GAE, c is gallic acid concentration in mg/mL, V is extract volume (mL), and m is weight of citrus peel extract (g).
Total flavonoids
Total flavonoids were estimated using the methodology based on the development of a flavonoid-aluminum complex.[Citation15] As a standard, quercetin was employed in order to quantify total flavonoids in peel extracts. Briefly, 1 mL extract of citrus peel was appended to a volumetric flask (10 mL) and volume was fulfilled to 5 mL using distilled water trailed by inclusion of 5% sodium nitrite (0.3 mL). Subsequently, 0.6 mL of AlCl3(10% w/v) was taken following a time duration of 5 min and 2 mL of NaOH (1 M) after 6 min followed by inclusion of distilled water (2.1 mL). Immediately, absorbance was noted at 510 nm on UV/visible light spectrophotometer. The collected data were recorded as mg of quercetin equivalents (QE)/100 g of extract.
Total flavonol contents
The flavonol contents were calculated following the procedure reported by Anagnostopoulou et al.[Citation16] For the purpose, 1 mL of peel extract was mixed with 1 mL of 5% sodium acetate and 1 mL of 2% aluminum trichloride. After 150 min, absorption was taken spectrophotometrically at 440 nm. The quercetin standard curve was achieved by blending 1 mL of various strengths of quercetin ranging from 0.05 to 0.15 mg/mL with same solvent as used for sample extraction except the inclusion of peel extract. Total flavonols were recorded as mg of quercetin/100 g of extract.
Antioxidant assays
DPPH assay
For the evaluation of DPPH ((1,1-diphenyl-2-picrylhydrazyl)) free radical scavenging activity, the procedure reported by Ebrahimzadeh et al.[Citation14] was followed. The samples for the analysis were prepared by diluting 0.025 mL of peel extract with 10 mL of extraction solvent. Further, 3 mL of freshly made DPPH reagent (6 × 10−5M) was added in 77 μL orange peel extract. The samples were retained in darkness for 15 min at room temperature. Afterwards, absorbance was measured at 517 nm using UV/visible light spectrophotometer. Likewise, the absorbance of control sample containing the equal content of DPPH reagent and solvent excluding extract was measured at the same wavelength. The DPPH value of each citrus peel extract is represented as percent reduction (%) in DPPH radicals.
A is absorbance of control and B is absorbance of tested sample.
Antioxidant activity (AA)
Antioxidant potential of orange peel extract was assessed according to the methodology of Shahsavari et al.[Citation17] In this regard, β-carotene (2 mg) was mixed with chloroform (20 mL) in a flask. Moreover, 3 mL of aliquot (β-carotene) was added in a flask having 40 mg linoleic acid together with 400 mg Tween 20 and the resultant mixture was vaporized at 40°C for 10 min with rotary evaporator in order to eliminate chloroform. The mixture was further diluted using 100 mL distilled water and was properly homogenized via vortex mixer to formulate emulsion. Then, 3 mL of β-carotene emulsion and 0.12 mL of citrus peel extracts were added in respective vials and were properly mixed. Subsequently, vials were kept in a water bath at 50°C for 30 min. Lastly, the absorbance of the resultant samples was recorded at 470 nm on UV/visible light spectrophotometer. The degradation rate of each peel extract was measured conferring to the first order kinetic reaction following the equation.
ln is natural log, a is initial absorbance on 470 nm at t = 0, b is absorbance on 470 nm at t = 30 min, t is time in minutes. The antioxidant activity (AA) was calculated as percent inhibition (%) comparative to the control by using following formula.
FRAP
The reducing potency of sample extracts was calculated by assessing competence to reduce ferric tripyridyltriazine into blue shaded ferrous, detectable at 593 nm as reported by Xu et al.[Citation18] FRAP chemical was made by mixing together 25 mL acetate buffer (0.1 M, pH 3.6), 2.5 mL TPTZ (10 mM) along with 2.5 mL ferric chloride (20 mM) trailed by its incubation at 30°C for 10 min. To analyze reducing power of citrus peel extracts, 1.5 mL of FRAP solution was added in 100 µL of peel extract/control along with 100 µL of distilled water. The absorbance was recorded at 593 nm on UV/visible light spectrophotometer. The standard curve of ferrous sulfate (0–500 µM/mL) was drawn, and value recorded was expressed as μM Fe2+ equivalents/g of sample.
ABTS
According to Xu et al.,[Citation18] the ABTS assay is a decolorizing process in which ABTS radicals were freshly prepared by the addition of 5 mL of potassium persulfate (4.9 mM) to 5 mL ABTS solution (14 mM) and holding this combination in the dark for 16 h. The mixture was additionally diluted with relevant extraction solvent to have an absorbance value 0.7 at wavelength of 734 nm and was further utilized in antioxidant screening test. The ultimate reaction mixture (1 mL) containing only ABTS solution (950 µL) and peel extract (50 µL) was uniformly mixed for at least 30 s and stayed at room temperature for 5 min. Afterwards, the absorbance of sample was measured at 734 nm using UV-visible spectrophotometer (Shimadzu UV-160A, Kyoto, Japan) in comparison to control containing ABTS without any extract sample. The standard curve was expressed by preparing numerous strengths of Trolox varying from 780 to 1000 µL/mL. The ABTS free radical scavenging potency was measured as µM trolox equivalent antioxidant capacity (TEAC)/g of sample.
HPLC analysis of bioactive components from citrus peel
From nine treatments, the best three treatments, one from every extract types (aqueous-ethanol, -methanol, and water), were used for high-performance liquid chromatography (HPLC) analysis on the basis of their phytochemical profiling and in vitro analysis. Preparation of stock and standard solutions: For the preparation of stock solutions of hesperidin and nobiletin, 1 mg of each accurately weighed standard (hesperidin and nobiletin) was dissolved in methanol:water (1:1), separately, and filled up to volume for stock solution preparation. Further, five different concentrations of standard solutions were prepared from each stock solution in 5 ml volumetric flasks for the establishment of calibration curves by dilution. Prior to analysis, the prepared standard solutions of each analyte were filtered using 0.45 μm membrane filter. The calibration curve for each bioactive was designed by plotting the peak area (y) against the concentration of each standard (x, mg/mL).
HPLC quantification of active ingredients: Best selected orange peel extracts were scrutinized for quantification of hesperidin and nobiletin; the main bioactives of orange peel following procedure of Xu et al.[Citation18] by HPLC (PerkinElmer, Series 200, USA) was equipped with shim-pack CLC-ODS C18 column (15 cm x 4.6 mm, 5.0 μm particle size) along with auto sampler. The mobile phase contained isocratic HPLC grade water (dd H2O) at a flow rate of 1.0 mL/min. However, the 10 µL of each extract was used as sample and during complete analysis, the column temperature was kept constant at 40°C. The eluent was explored at 345 nm for hesperidin and 330 nm for nobiletin using UV detector. Quantification of both active ingredients was executed by comparison of peaks retention time collected for each sample to those of standards; hesperidin and nobiletin.
Statistical analysis
The collected data for each parameter were analyzed statistically by using Statistix (8.1) software. The basic principle included two factors under a completely randomized design, followed by least significant difference test (p < 0.05) for post-hoc comparison.
Results and discussion
Phytochemical screening test
Polyphenols are secondary metabolites widely distributed in fruits and vegetables with potent antioxidant capacity. These compounds are probed for their scavenging/quenching free radicals and chelating metal ions, thus confirming health boosting potential against various degenerative disparities. The citrus peel is a host of active phytoceutics with promising biological properties owing to their phenolics and antioxidant activity. It is better to identify the phenolics prior to utilize them as natural antioxidant in designer foods.
Total phenolics
The associated health claims of phenolics necessitated their detection in food stuff. For phenolic analysis, Folin–Ciocalteu methodology was employed centered on phosphotungstic acid reduction into phosphotungstic blue, resulted in an increment in absorbance owing to increase in quantity of aromatic phenolics. Rise in intensity of blue color depicts more phenolics in the sample. For total polyphenols, pooled means concerning to solvents i.e., aqueous-methanol, aqueous-ethanol, and water were 189.6 ± 5.2, 184.4 ± 5.1 and 156.1 ± 4.2 mg (GAE)/100 g, separately while pooled means relating to time intervals were 175.2 ± 7.00, 171.55 ± 6.9 and 183.3 ± 6.9 mg GAE/100 g at 30, 45 and 60 min, correspondingly (). Maximum phenolics (209.7 ± 6.3 mg GAE/100 g) were found in methanolic orange peel extract obtained at 60 min.
Table 1. Phytochemicals in citrus peel extracts.
The existing outcomes are in line with study of Al-Juhaimi[Citation19] who reported total phenolic content in citrus peel as 178.9 mg GAE/100 g using ethanol (80%) as solvent at 70°C for 180 min. Previously, Ramful et al.[Citation20] evaluated phenolics and antioxidant potential of citrus peel to formulate phytoceutics-enriched edibles. Accordingly, total phenolics in peel of different citrus varieties changed from 188.2 to 766.7 mg GAE/100 g.
Various extraction approaches have significant impact on extraction yield of solvents as it was elucidated by Khan et al.[Citation21] They determined total phenolics of orange peel by ultrasound-assisted extraction and solvent extraction. They observed that sonication increased phenolic extraction (152 to 275 mg GAE/100 g) as compared to solvent extraction (103 to 208 mg GAE/100 g). Similarly, the results of Wang et al.[Citation22] were also in line with present results, as they reported antioxidant strength of citrus peel extract. Accordingly, total polyphenol of peel extract was 112.2 ± 2.9 mg GAE/100 g. Barros et al.[Citation23] reported that phenolic contents of peels are 2.5 to 4 times higher than pulp portion due to low amount of vitamin C.
It is worthy to mention that abstraction of polyphenols from citrus peel is affected by its solubility in the extraction solvent. Additionally, solvent polarity plays a pivotal role in enhancing the extract contents. Also, ethanol and methanol were better than water alone for extracting polyphenols due to their good solubility and higher polarity for phenolics.
Total flavonoids
Citrus peel contains a considerable number of natural antioxidants that deter progression of chronic disorders. These components are hydroxybenzoic and hydroxycinnamic acids, anthocyanins, proanthocyanidins, flavonoids, and lignans.[Citation24] Based on the results obtained, the highest flavonoid contents were recorded in methanolic extract (80.05 ± 5.2 mg/100 g) trailed by ethanol and water (74.0 ± 5.1 and 50.5 ± 4.2 mg QE/100 g), correspondingly as depicted by their pooled means in . Additionally, effect of solvent and time interaction for total flavonoids has depicted the maximum flavonoids (89.5 ± 3.5 mg QE/100 g) in methanol at 60 min. Pooled means for time intervals showed that time also affected flavonoid contents as maximum amount was extracted at 60 min 76.8 ± 3.1 mg QE/100 g while minimum at 30 min 62.6 ± 2.5 mg QE/100 g.
The present findings are in accordance with those of Oluremi et al.[Citation25] who evaluated total flavonoids present in different varieties of citrus peel. Quantitatively, flavonoids found in citrus peel varied from 25 to 45.5 mg/100 g. Wang et al.[Citation26] determined total flavonoid contents in eight different varieties of citrus with maximum content in lemon while minimum in kumquat. The most important glycosylated flavonone in citrus peel was hesperidin that has been reported for its therapeutic effects. Furthermore, Xu et al.[Citation18] elaborated the influence of temperature and time on two major classes of flavonoids; glycosylated flavonones and polymethoxylated flavones (PMF). Conclusively, glycosylated flavonones (62.0 mg/g) were easier to be extracted at 100°C for 30 min as compared to PMF (0.3 mg/g).
It appeared that thermal energy has liberated flavonoids by damaging the structural network inside cell walls ultimately, leading to their rapid release.[Citation27] In view of Azman et al.,[Citation24] flavonoids isolated from citrus peels in frozen state are more than the fresh ones. The reason was found to be the deactivation of hydrolytic and oxidative enzymes through freezing.
Flavonol contents: Citrus peel flavonoids are categorized into three major classes: flavonones, flavones, and flavonols that are responsible for antioxidant activity of peel in addition to vitamin C. The pooled means () for flavonol indicated maximum amount in methanolic fractions (2.0 ± 0.1 mg/100 g) but lowest in aqueous extracts (1.6 ± 0.05 mg/100 g). Mean values represented that maximum flavonols were noted in methanol at 60 min (2.15 ± 0.1 mg/100 g), while it was minimum in water (1.75 ± 0.1 mg/100 g). Nevertheless, pooled means for time intervals depicted highest flavonols at 60 min (2.0 ± 0.2 mg/100 g) while the lowest contents were observed at 30 min (1.8 ± 0.2 mg/100 g).
The trends found in the current study are in accordance with previous report showing highest flavonol content in aqueous methanol extract (1.07 µg/g citrus peel) in contrast to water and methanol (0.9 and 0.5 µg/g citrus peel) alone, respectively.[Citation2] Formerly, Wang et al.[Citation26] demonstrated quercetin (6.1 to 57.3 mg/100 g) and kaempferol (0.9 to 103.5 mg/100 g) as the main flavonols quantified in citrus peel on dry basis. However, Anwar et al.[Citation28] have evaluated antioxidant capacity of extracts from various agricultural by-products. It was revealed that myricetin was present in highest amount 110.2 mg/100 g, while quercetin and kaempferol were undetectable. Moreover, Chen et al.[Citation29] evaluated the heating effect (50°C, 60°C, 70°C, 80°C, 90°C and 100°C) on the antioxidant potential of citrus flavonoids. They concluded that concentration of flavonoids content was significantly higher at 100°C that decreased at lower temperature.
Antioxidant profile
Amongst bioactive compounds, major portion of our diet consists of flavonoids. There are more than 4000 naturally occurring flavonoids that have been recognized and categorized for their structure and health-enhancing properties. These phytoceutics may synergistically enhance the antioxidant capability based upon the environment of their phenolic groups, mechanisms of action and working conditions employed for quantification of antioxidant activity. Moreover, antioxidant activity may also be decreased by intervention from non-antioxidant components. For the reason, a single assay will never reproduce the entire antioxidant profile of a sample. Therefore, a number of results from different assays were combined together to achieve more consistent information about the phytochemicals present in orange peel and their allied antioxidant capacity.
DPPH assay
DPPH scavenging capacity assay is generally utilized for the estimation of antioxidant activity. The DPPH is a stable and intense colored oxidizing radical that forms yellow colored hydrazine associated with abstraction of free hydrogen atoms from phenolic antioxidants. Pooled means regarding DPPH () illuminated highest value for methanolic extract 58.5 ± 2.5% followed by ethanolic and water extracts as 55.5 ± 2.15% and 42.8 ± 1.85%, correspondingly. Among various citrus peel extracts, antioxidant power of methanolic peel extract was maximum 60.55 ± 2.4% at 60 min but minimum at 30 min 56.8 ± 2.2%.
Table 2. Antioxidant attributes of citrus peel extracts.
The present finding regarding DPPH of citrus peel is in collaboration with study of Khan[Citation30] who studied peel antioxidant potency of Citrus maxima by DPPH radical-scavenging activity using maceration and Soxhlet extraction modes. Accordingly, the percentage scavenging activity of Citrus maxima peel against free radicals varied from 11.85% to 78.6%. The logic behind this alteration is more the phenolics present in peel extract, the lower will be optical density (OD) recorded owing to number of DPPH free radicals scavenged by the phytonutrients isolated from the peel of Citrus maxima.
Previously, Khan et al.[Citation21] assessed radical scavenging potential of orange peel by ultrasound supported extraction and conventional solvent extraction techniques. They concluded that using sonication method, citrus peel potency to scavenge free radical increased by 30% in contrast to solvent extraction. Furthermore, Abd El-aal and Halaweish[Citation31] has determined antioxidant potential of two different citrus varieties. According to them, baladi peel extract has more DPPH activity as compared to navel orange peel extract that ranged from 65% to 72% for both varieties. Similarly, Wang et al.[Citation22] quantified antioxidant capacity of citrus peel extract (46%) in terms of DPPH assay. Barros et al.[Citation23] demonstrated free radical scavenging activity of four citrus varieties for pulp and peel. They observed that 70% aqueous methanolic extract of mandarin peel has more potency to scavenge free radical (825.4 µmol of TE/100 g of fruit weight) as compared to pulp (265.6 µmol of TE/100 g of fruit weight) of same fruit.
Antioxidant activity (AA)
Pooled means for antioxidant activity relevant to solvents, i.e., methanol, ethanol, and water, has exposed highest activity in methanol (49.3 ± 2.3%) followed by ethanol (46.1 ± 2.1%) and water (43.1 ± 1.8%) as depicted in . Likewise, extraction time also influenced bleaching rate of each extract for ß-carotene that was more at 60 min 47.4 ± 2.2% but reduced to 45.7 ± 2.0% at 45 min. In the model of β-carotene linoleate, the linoleic acid-free reactive species are produced by carrying out coupled oxidation between linoleic acid as well as β-carotene; thus, the free radicals generated would invade at the unsaturated β-carotene resultantly and discolor it by oxidizing. The existence of antioxidants in the reaction media suspend bleaching of β-carotene. Similarly, presence of antioxidant in citrus peel extraction media inhibit β-carotene bleaching by deactivating activities of free radicals liberated in the system.[Citation32] The results of the current study regarding antioxidant power of peel extracts were assured by Chatha et al.[Citation33] who explored antioxidant activity of grapefruit, lemon, and mussambi by using various strengths of methanol as solvent. Results interpretation has revealed maximum linoleic acid oxidation inhibition as 91.8% for mussambi peel (70% methanol) while minimum as 68.2% for grapefruit peel extract (90% methanol). Moreover, Asikin et al.[Citation34] reported the effect of different extraction approaches: steam distillation or a cold-press system on antioxidant potential of citrus peel extract. Antioxidant activity calculated by β-carotene bleaching inhibition assay has proved that cold pressed extraction was better than steam distillation to retain phenolic content of extract more efficiently.
However, Anwar et al.[Citation28] have quantified antioxidant capacity of extracts from various agricultural by-products. According to them, citrus peel extract has 86.9% inhibition for linoleic acid oxidation. Similarly, Gursoy et al.[Citation35] have analyzed antioxidant properties of citrus peel oil by β-carotene and linoleic acid assay. They observed that citrus oil has potency of 96.8% to inhibit oxidation of linoleic acid due to polyphenolic constituents.
Ferric-Reducing Antioxidant Potential (FRAP) assay
Another method to interpret antioxidant capacity of orange peel is reliant on the of Fe3+ TPTZ composite (colorless) reduction to Fe2+-tripyridyltriazine (blue colored) obtained due to the action of electron donating antioxidants at acidic pH. The reaction is examined by recording the alterations in absorbance at 593 nm.[Citation36] The current results () have demonstrated that methanolic extract has maximum FRAP as 11.9 ± 2.3 mg TE/g that decreased in water extract 8.9 ± 0.2 mg TE/g peel. In the same way, time significantly affected antioxidant power of each extract that was highest (11.5 ± 0.4 mg TE/g) at 60 min while lowest (8.8 ± 0.25 mg TE/g) at 30 min.
The present results are in corroboration with those of Ghafar et al.[Citation37] who inferred that among various citrus varieties, FRAP value for Citrus hystrix was highest as 89.0 TEAC mg/g, whilst lowest (48.2 TEACmg/g) for Citrus microcarpa extract. The antioxidant potential of citrus species was in increasing order of Citrus hystrix > Citrus aurantifolia > Citrus sinensis > Citrus microcarpa. Further, Barros et al.[Citation23] have observed in vitro antioxidant capacity of various citrus parts. Results relating to FRAP assay has revealed that antioxidant potential of peel extract was higher as compared to pulp of same fruit. Among five cultivars, mandarin peels have maximum ferric reducing potential 3897.9 µmol TE/100 g rather than pulp 744.0 µmol TE/100 g of fruit weight.
ABTS assay
As far as ABTS assay of citrus peel () is concerned, a significant impact of solvent and time was observed on antioxidant potency of extracts to scavenge the ABTS radical cation (ABTS+). The pooled means regarding ABTS depicted maximum value as 7.2 ± 0.35 µmol TE/g in methanolic extracts as compared to 6.5 ± 0.25 and 5.45 ± 0.1 µmolTE/g in ethanol and water extracts, correspondingly. Furthermore, it was also predicted that ABTS value increased to 7.4 ± 0.4 in methanolic extract at 60 min while decreased to 6.8 ± 0.25 µmol TE/g in ethanolic extract and 5.7 ± 0.3 µmol TE/g (water extract) at same time.
Nutraceutics are renowned for their properties to assuage the mounting rate of various life-threatening disorders. In this regard, plant-based bioactive compounds are acknowledged for their prophylactic role. Current findings were consistent to Mehmood et al.,[Citation38] characterized various phytochemicals to assess antioxidant indices of citrus processing waste (grapefruit, oranges, and mussambi using aqueous ethanol, aqueous methanol, and water as solvents. Accordingly, the maximum antioxidant capacity using ABTS was found in grapefruit ethanolic extract (10.35 µmol TE/g) trailed by oranges and mussambi peel. Earlier, Oboh and Ademosun[Citation39] assessed ABTS+ scavenging capability of orange, grapefruit, and shaddock. They concluded that bound phenolics of oranges have the highest antioxidant potential (6.05 mmolTEAC/g of peel) as compared to shaddock that has least (1.23 mmol TEAC/g of peel). Regarding free phenolics, grapefruit and orange peels have maximum free radical scavenging capacity as 5.9 and 5.7 mmol TEAC/g of peel, respectively followed by shaddock peel as 4.9 mmol TEAC/g of dry matter. Later on, Siahpoosh and Javedani[Citation40] evaluated antioxidant potential of Iranian citrus peel that supported present exploration. According to them, ABTS value changed from 8.5 to 7.3 μmol Trolox/g peel weight, highest for grapefruits while lowest for sweetie.
Quantification of hesperidin and nobiletin via HPLC
The three best obtained extract of orange peel one from each solvent (aq. -ethanol, -methanol and water) were analyzed using HPLC and chromatograms were attained under the similar conditions as for analysis of standard hesperidin and nobiletin. The obtained resultant peaks from HPLC were put in comparison with the standard retention time, peak area and for spectral assessment. Statistical differences were found (p < .01) regarding the effect of solvents on hesperidin and nobiletin contents. The results of comparative quantitative analysis indicated maximum hesperidin and nobiletin as 133.70 and 8.5 mg/g in methanol extract followed by ethanol (98.80 & 5.50 mg/g) and water (61.90 & 1.25 mg/g), correspondingly. It was revealed that methanol was the most compatible solvent to separate maximum amount of glycosylated flavonones and polymethoxylated flavones owing to its polar and organic nature in contrast to ethanol and water. Moreover, the results revealed that hesperidin was present in higher amounts content in citrus peel than nobiletin ().
Figure 1. HPLC chromatograph of hesperidin in (A) methanolic (B) ethanolic and (C) water extract of citrus peel.
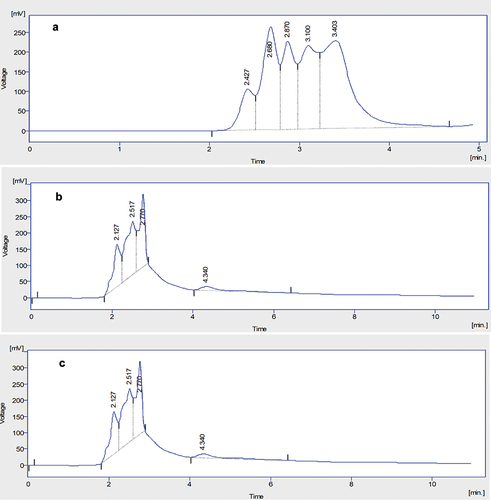
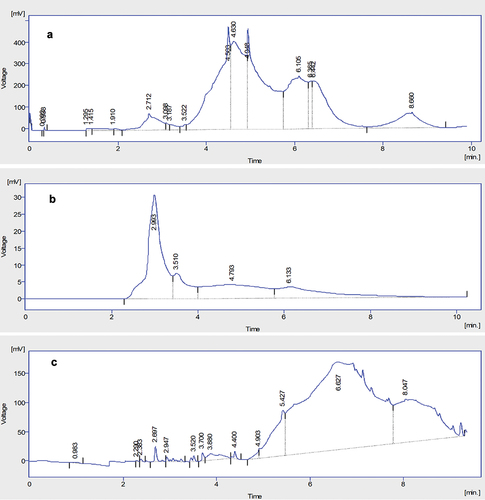
Figure 2. HPLC chromatograph of nobiletin in (A) methanolic (B) ethanolic and (C) water extract of citrus peel.
Nutraceutics are renowned to alleviate the hostile impact of unhealthy living style especially plant-linked bioactive components that are acknowledged for many health advantages. The aim of this study work was to characterize and quantify the selected phytochemicals; hesperidin and nobiletin, and further evaluate their antioxidant potential. The bioactives present in orange peel are flavonoids for example hesperidin, naringin, nobiletin, synephrine, and alkaloids which have prophylactic potential on health stratum of human. According to Seoka et al. (2020), the flavonoid accumulation in various citrus varieties depends on abiotic factors such as state of maturation, genetic variations, and environmental changes.
In an investigation, conducted on flavonoids accumulation in Ponkan and Satsuma – mandarins, Seoka et al.[Citation41] analyzed high content of flavanones in Satsuma mandarin but quantity of polymethoxy flavone including nobiletin was significantly low accounted 6% less than the total flavonoids. Previously, Garcia-Castello et al.[Citation42] probed that various ethanol concentrations along with dilution with water altered the hesperidin concentration from 0.25 to 0.75 mg/g in grapefruit peel. Formerly, Loizzo et al.[Citation43] analyzed methanolic extracts of citrus peel and its leaves for antioxidant activity. The analyses were carried on HPLC system (HP 1100) employed with UV-detector (280 nm) and showed higher flavonol contents (apigenin: 88.70, kaempferol: 64.20 and rutin: 134.60 mg/100 gram) in leaves with the exception of quercetin that was maximum in peel. On the other hand, citrus fruit peel was higher in nobiletin (0.20 mg/100 gram) and hesperidin (0.05 mg/100 gram) but very low in tangeretin.
Conclusion
The present results showed that there is a significant influence on type of solvent and extraction time on phytochemical content and antioxidant activity of citrus peel extracts. Furthermore, a combined effect of solvent and extraction time can momentously affect the phytochemical densities and abilities of extract to scavenge free radicals. Conclusively, the present findings have affirmed the methanol as “solvent of choice” to valorize citrus peel active moieties due to its compatibility with its bioflavonoids. Based on phytochemical screening tests, it is suggested that citrus peel and its extracts may be employed as functional ingredients in designer foods. Also, experimental comparison of the drying methods with controlled conditions can be made since sunlight drying may have affected some of the light sensitive bioactive present in citrus peel. Hence, further investigations with varying other parameters like temperature, drying method, and extract purification could be explored for more potent antioxidant abilities.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP2024R5) King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Suwal, S.; Marciniak, A. Technologies for the Extraction, Separation and Purification of Polyphenols – a Review. Nepal J Biotechnol. 2019, 6(1), 74–91. DOI: 10.3126/njb.v6i1.22341.
- Park, J.-H.; Lee, M.; Park, E. Antioxidant Activity of Orange Flesh and Peel Extracted with Various Solvents. Prev. Nutr. Food Sci. 2014, 19(4), 291–298. DOI: 10.3746/pnf.2014.19.4.291.
- Senevirathne, M.; Jeon, Y.-J.; Ha, J.-H.; Kim, S.-H. Effective Drying of Citrus By-Product by High Speed Drying: A Novel Drying Technique and Their Antioxidant Activity. J. Food Eng. 2009, 92(2), 157–163. DOI: 10.1016/j.jfoodeng.2008.10.033.
- Ezejiofor, T.; Eke, N.; Okechukwu, R.; Nwoguikpe, R.; Duru, C. Waste to Wealth: Industrial Raw Materials Potential of Peels of Nigerian Sweet Orange (Citrus sinensis</I. Afr. J. Biotechnol. 2011, 10, 6257–6264.
- GOP. 50-Years of Pakistan in Statistics Volume. Federal Bureau Of Statistics. Islamabad, Pakistan. 2012.
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M. H. Citrus Waste Derived Nutra-/pharmaceuticals for Health Benefits: Current Trends and Future Perspectives. J. Funct. Foods. 2018, 40, 307–316. DOI: 10.1016/j.jff.2017.11.015.
- Safdar, M. N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A. A. Extraction and Quantification of Polyphenols from Kinnow (Citrus Reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25(3), 488–500. DOI: 10.1016/j.jfda.2016.07.010.
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46(6), 2123–2129. DOI: 10.1021/jf9709562.
- Cheynier, V.; Sarni-Manchado, P. Les polyphénols en agroalimentaire. Lavoisier Techniques And Documentation, Paris. P. 2006, 50–59.
- Sawalha, S. M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of Main Phenolic Compounds in Sweet and Bitter Orange Peel Using CE–MS/MS. Food Chem. 2009, 116(2), 567–574. DOI: 10.1016/j.foodchem.2009.03.003.
- Ernawita; Wahyuono, R. A.; Hesse, J.; Hipler, U.-C.; Elsner, P.; Böhm, V. Vitro Lipophilic Antioxidant Capacity, Antidiabetic And Antibacterial Activity Of Citrus Fruits Extracts From Aceh, Indonesia, Antioxidants (Basel). 2017, 6(1), 11. DOI: 10.3390/antiox6010011
- Abdelbaky, M.; Elmehiry, H.; Ali, N., 2009.
- Amin, I.; Mukhrizah, O. Antioxidant Capacity of Methanolic and Water Extracts Prepared from Food-Processing By-Products. J. Sci. Food Agric. 2006, 86(5), 778–784. DOI: 10.1002/jsfa.2414.
- Ebrahimzadeh, M. A.; Pourmorad, F.; Bekhradnia, A. R. Iron Chelating Activity, Phenol and Flavonoid Content of Some Medicinal Plants from Iran. Afr. J. Biotechnol. 2008, 7(18), 3188–3192.
- Yoo, K. M.; Lee, C. H.; Lee, H.; Moon, B.; Lee, C. Y. Relative Antioxidant and Cytoprotective Activities of Common Herbs. Food Chem. 2008, 106(3), 929–936. DOI: 10.1016/j.foodchem.2007.07.006.
- Anagnostopoulou, M. A.; Kefalas, P.; Papageorgiou, V. P.; Assimopoulou, A. N.; Boskou, D. Radical Scavenging Activity of Various Extracts and Fractions of Sweet Orange Peel (Citrus sinensis. Food Chem. 2006, 94(1), 19–25. DOI: 10.1016/j.foodchem.2004.09.047.
- Shahsavari, N.; Barzegar, M.; Sahari, M. A.; Naghdibadi, H. Antioxidant Activity and Chemical Characterization of Essential Oil of Bunium Persicum. Plant Foods Human Nutr. 2008, 63(4), 183–188. DOI: 10.1007/s11130-008-0091-y.
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55(2), 330–335. DOI: 10.1021/jf062517l.
- Al-Juhaimi, F. Y. Citrus Fruits By-products as Sources of Bioactive Compounds with Antioxidant Potential. Pak. J. Bot. 2014, 46, 1459–1462.
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O. I. Bioactive Phenolics and Antioxidant Propensity of Flavedo Extracts of Mauritian Citrus Fruits: Potential Prophylactic Ingredients for Functional Foods Application. Toxicology. 2010, 278(1), 75–87. DOI: 10.1016/j.tox.2010.01.012.
- Khan, M. K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone Glycosides) from Orange (Citrus sinensis L.) Peel. Food Chem. 2010, 119(2), 851–858. DOI: 10.1016/j.foodchem.2009.08.046.
- Wang, A.-Y.; Zhou, M.-Y.; Lin, W.-C. Antioxidative and Anti-Inflammatory Properties of Citrus Sulcata Extracts. Food Chem. 2011, 124(3), 958–963. DOI: 10.1016/j.foodchem.2010.07.035.
- Barros, H. R. D. M.; Ferreira, T. A. P. D. C.; Genovese, M. I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134(4), 1892–1898. DOI: 10.1016/j.foodchem.2012.03.090.
- Azman, N. F. I. N.; Azlan, A.; Khoo, H. E.; Razman, M. R. Antioxidant Properties of Fresh and Frozen Peels of Citrus Species. Curr. Res. Nutr. Food Sci. 2019, 7(2), 331–339. DOI: 10.12944/CRNFSJ.7.2.03.
- Oluremi, O.; Ngi, J.; Andrew, I. Phytonutrients in citrus fruit peel meal and nutritional implication for livestock production. Livestock Res. Rural Dev. 2007, 19, 1–5.
- Wang, Y.-C.; Chuang, Y.-C.; Ku, Y.-H. Quantitation of Bioactive Compounds in Citrus Fruits Cultivated in Taiwan. Food Chem. 2007, 102(4), 1163–1171. DOI: 10.1016/j.foodchem.2006.06.057.
- Sung, J.; Suh, J. H.; Wang, Y. Effects of Heat Treatment of Mandarin Peel on Flavonoid Profiles and Lipid Accumulation in 3T3-L1 Adipocytes. J. Food Drug Anal. 2019, 27(3), 729–735. DOI: 10.1016/j.jfda.2019.05.002.
- Sultana, F.; Anwar, B.; Rafique Asi, M.; Ali Shahid Chatha, S. Antioxidant Potential of Extracts from Different Agro Wastes: Stabilization of Corn Oil. Grasas y Aceites. 2008, 59(3), 59. DOI: 10.3989/gya.2008.v59.i3.510.
- Chen, M.-L.; Yang, D.-J.; Liu, S.-C. Effects of Drying Temperature on the Flavonoid, Phenolic Acid and Antioxidative Capacities of the Methanol Extract of Citrus Fruit (Citrus sinensis (L.) Osbeck) Peels. Int. J. Food Sci. Technol. 2011, 46(6), 1179–1185. DOI: 10.1111/j.1365-2621.2011.02605.x.
- Khan, N. H. Phytochemical screening, antimicrobial and antioxidant activity determination of citrus maxima peel. Pharm Pharmacol Int J. 2018, 6(4), 6. DOI: 10.15406/ppij.2018.06.00187.
- Abd El-Aal, H.; Halaweish, F. Food Preservative Activity of Phenolic Compounds in Orange Peel Extracts (Citrus sinensis L. Lucrări Ştiinţifice-Seria Zootehnie. 2010, 53, 233–240.
- Divya, P. J.; Jamuna, P.; Jyothi, L. A.; Yildiz, F. Antioxidant Properties of Fresh and Processed Citrus aurantium Fruit. Cogent Food Agric. 2016, 2(1), 2. DOI: 10.1080/23311932.2016.1184119.
- Chatha, S. A. S.; Hussain, A. I.; Asi, M. R. Evaluation of antioxidant potential of citrus peel extracts. J. Chem. Soc. Pak. 2011, 33, 863–868.
- Asikin, Y.; Taira, I.; Inafuku, S.; Sumi, H.; Sawamura, M.; Takara, K.; Wada, K. Volatile Aroma Components and Antioxidant Activities of the Flavedo Peel Extract of Unripe Shiikuwasha (Citrus depressa Hayata). J. Food Sci. 2012, 77(4), C469–C475. DOI: 10.1111/j.1750-3841.2011.02604.x.
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluation of the Chemical Composition and Antioxidant Activity of the Peel Oil ofCitrus Nobilis. Int. J. Food Prop. 2010, 13(5), 983–991. DOI: 10.1080/10942910902927136.
- Benzie, I. F. F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. Measurement Of Antioxidant Activity & Capacity. 2017, 77–106. DOI: 10.1002/9781119135388.ch5.
- Ghafar, M. F. A. G.; Prasad, K. N.; Weng, K. K.; Ismail, A. Flavonoid, Hesperidine, Total Phenolic Contents and Antioxidant Activities from Citrus Species. Afr. J. Biotechnol. 2010, 9(3), 326–330.
- Mehmood, T.; Khan, M. R.; Shabbir, M. A.; Anjum, M. Phytochemical profiling and HPLC quantification of citrus peel from different varieties. Prog. Nutr. 2018, 20, 279–288.
- Oboh, G.; Ademosun, A. O. Characterization of the Antioxidant Properties of Phenolic Extracts from Some Citrus Peels. J. Food Sci. Technol. 2012, 49(6), 729–736. DOI: 10.1007/s13197-010-0222-y.
- Siahpoosh, A.; Javedani, F. Antioxidative capacity of Iranian Citrus deliciosa peels. Free Radic. Antioxid. 2012, 2(2), 62–67. DOI: 10.5530/ax.2012.2.2.11.
- Seoka, M.; Ma, G.; Zhang, L.; Yahata, M.; Yamawaki, K.; Kan, T.; Kato, M. Expression and Functional Analysis of the Nobiletin Biosynthesis-Related Gene CitOMT in Citrus Fruit. Sci. Rep. 2020, 10(1), 1–11. DOI: 10.1038/s41598-020-72277-z.
- Garcia-Castello, E. M.; Rodriguez-Lopez, A. D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of Conventional and Ultrasound Assisted Extraction of Flavonoids from Grapefruit (Citrus Paradisi L.) Solid Wastes. LWT Food Sci. Technol. 2015, 64(2), 1114–1122. DOI: 10.1016/j.lwt.2015.07.024.
- Loizzo, M. R.; Tundis, R.; Bonesi, M.; Menichini, F.; De Luca, D.; Colica, C.; Menichini, F. Evaluation of Citrus Aurantifolia Peel and Leaves Extracts for Their Chemical Composition, Antioxidant and Anti‐Cholinesterase Activities. J. Sci. Food Agric. 2012, 92(15), 2960–2967. DOI: 10.1002/jsfa.5708.
