?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Kalanchoe pinnata (Lam.) Pers. is an ethnomedicinal plant known for its antioxidant, antibacterial, and therapeutic potential. The current study was an effort to explore the impact of extraction technique on the phytochemical, antioxidant, and antibacterial profile of the plant extracts. K. pinnata leaf extracts were prepared using conventional solvent extraction (100% methanol; 50% methanol: 50% water; 100% water), microwave-assisted extraction at 700-W power (3 min; 6 min; 9 min), and supercritical fluid extraction (SFE) (3500 psi; 4500 psi; 5500 psi). Best results were attained from SFE in terms of total phenolic content (240 ± 3 mg GAE/g), total flavonoid content (165.2 ± 0.4 mg QE/g), 2,2-diphenyl-1-picrylhydrazyl radical scavenging potential (91.9 ± 0.4%), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid assay value (8.04 ± 0.03 mMol TE/g) and ferric reducing antioxidant power assay value (471.7 ± 0.8 µg/mL). K. pinnata leaf extracts have been found to be most effective against Yersinia pseudotuberculosis (22.20 ± 0.95 mm) and least effective againstProteus vulgaris (0 ± 0 mm). High-performance liquid chromatography analysis of K. pinnata extract showed that it contains flavanol quercetin (2.0 ± 0.1 mg/g). It is evident from the findings of current study that K. pinnata extract can effectively treat bacterial infections and can cure metabolic disorders by reducing oxidative stress. Application of advance extraction techniques enhances the biological activity of plant extracts.
Introduction
In nature, every plant has tremendous diversity in secondary metabolites with protective potential. These chemical compounds possess antioxidant potential, that enables them to hinder and detain oxidation, and are thus referred as antioxidants. Hazardous nature of synthetic chemicals increases consumer inclination toward natural products. Increasing consumer awareness and changing food consumption trends, increase the demand of natural products to ward off safety risks associated with synthetic chemicals. Consumer demand urges the food processors to search for safer natural alternatives of synthetic chemical preservatives.[Citation1] Plants because of their diverse profile of bioactive compounds, exhibit similar perseverative potential as that of synthetic chemical additives. In addition to the preservative potential, these plants have health benefits due to free radical scavenging potential that relives human body from oxidative stress.[Citation2]
Genus Kalanchoe have 133 species out which 21 are renowned for the curative potential in folk medicine. Of these 21 species, 4 are of particular significance (K. pinnata, K. laciniata, K. crenata, and K. daigremontiana) due to their broad ethnopharmacological applications.[Citation3] K. pinnata (Lam.) Pers.. is a native species from Madagascar[Citation4] and is also found in West indies, Bangladesh, Pakistan, India, Australia, Polynesia, and Galapagos.[Citation5] K. pinnata is rich in bufadienolides (steroids), kaempferol, quercetin, alkaloids, lipids, triterpenes, and glycosides cardenolides. K. pinnata leaves contain a bioactive compound bufadienolides. Bufadienolides are responsible for anticancer, immunomodulator, sedative, CNS depressant, muscle relaxant, analgesic, insecticidal, antimicrobial, immunosuppressive, anti-inflammatory, gastroprotective, antiallergic, febrifuge, antianaphylactic, antiviral, antileishmanial, antihistamine, antitumorous, antifungal, antiulcerous, and antibacterial potential.[Citation6] Quercetin exhibits strong antioxidant and anti-inflammatory effect by oxidative, cell cycle, and kinase inhibition. This bioactive compound improves neuronal survival. Anticancer potential of quercetin is because of its apoptosis-inducing property.[Citation7] Another bioactive compound of K. pinnata, Bersaldegenin-1, 3, 5-orthoacetate, has been found to inhibited cancer cell growth against several cancer lines.[Citation8]
In Ayurveda, K. pinnata is also called Pasanabheda which literally means “dissolver of stone.” In ethnomedicinal practices, urinary inadequacy and renal stones are cured by utilizing this plant.[Citation9] In Asian countries, insect bites, wounds, swelling, and bruises are treated by topical application of grounded plant leaves of K. pinnata.[Citation10] A bitter tonic produced from K. pinnata leaves and stem is used to treat bowel astringency,[Citation11] vomiting muscular pain, diarrhea, indigestion, general weakness, small pox, seizures, otitis, migraine, asthma, and palpitation.[Citation10] There are research evidences that prove the effectiveness of K. pinnata against leg edema,[Citation12] gastrointestinal disorder, cough, ulcer, influenza, dysentery, utrine inflammation, diarrhea and cholera,[Citation13] bronchitis,[Citation14] ulcers,[Citation15] bacterial and viral and even contagious infections,[Citation16] influence tumors, hypertension, respiratory and breathing disorders, and diabetes.[Citation17] K. pinnata possess strong antitumor, anti-ulcer, anti-rheumatoid arthritis, intestinal worms, peripheral neuropathy, burns and bruises treating potential.[Citation14] Scientific studies also provide evidence of nematicide, hepatoprotective, nephroprotective, and hypocholesterolemic potential of K. pinnata extracts.[Citation18]
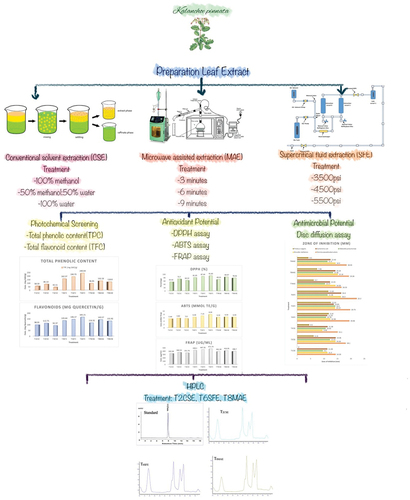
Numerous distinctive extraction techniques are practiced to attain K. pinnata (Lam.) Pers. leaf extract and essential oil. Conventional procedures for extraction include conventional solvent extraction (CSE), Soxhlet extraction (SE), steam distillation (SDE), hydro-distillation (HD), and combined application of steam and HD and maceration. Novel extraction techniques with improved yield and environment friendly approach include microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), subcritical fluid extraction, and supercritical fluid extraction (SFE). In order to extract biologically active components from plants, most practiced techniques include aqueous extraction, alcoholic solvent extraction, and CO2 SFE. Plant extract eminence entirely relies on extraction method and exposure to high temperature. The SFE technique is regarded as green revolution of plant extract preparation due to its nonsignificant negative impact on extract’s functional and biological properties and minimal solvent exposure.[Citation19]
The aim of the present work was to conduct a comparative assessment of antibacterial and antioxidant potential of K. pinnata leaf extracts prepared using different extraction techniques, i.e., CSE, MAE, and SFE. Bacteria used in current study are of vital medical significance, associated with liver, gastrointestinal, and kidney diseases. The study will provide scientific reference for commercial application of these extracts for the production of nutraceutical foods and health drinks in near future.
Materials and methods
Raw material procurement and authentication
K. pinnata (Lam.) Pers. plant was obtained from local market of Rahim Yar Khan, Punjab, Pakistan (28.4212° N, 70.2989° E). The plant species was confirmed by Fareed Biodiversity Conservation Centre, Department of Agricultural Engineering, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan (Approval Id FBCC-043), and a voucher specimen was stored in their laboratory. The plant roots, stems, and leaves were separated and cleaned to scrub off and remove any foreign matter and dust. Extraction efficiency of fresh stem, leaves, and roots was enhanced by grounding them using mechanical grinder. The ground plant parts were stored in air tight glass container separately, for further usage.
Proximate analysis
The moisture content in K. pinnata (stem, leaves, and roots) powder samples was evaluated by drying in Air-Forced Draft Oven (Memmert, Germany). According to the method No. 44–15.02 mentioned in AOAC,[Citation20] Ash content was estimated by following the procedure given in AOAC[Citation20] method No. 940.26. K. pinnata (stem, leaves, and roots) powder was analyzed for crude fat according to AOAC[Citation20] method No. 920.29. Kjeltech apparatus (DK-129,VELP Scientifica, Italy) was used to determine nitrogen percentage in K. pinnata (stem, leaves, and roots) powdered samples according to AOAC[Citation20] method No. 920.152. The crude fiber was estimated by following the method No. 962.09 outlined in AOAC.[Citation20]
Mineral determination
One gram of powdered sample (stem, leaves and roots) was taken in a 100-mL beaker, 20 mL of 1:3 HCl:HNO3 was added to it. The sample was then kept for overnight digestion, in a beaker with watch glass lid. For completion of digestion process, sample was heated at 110°C for 1 h, by direct heating on hot plate. After acid digestion, sample was cooled to room temperature. In a volumetric flask, 2% HNO3 solution was added to the mixture to make up the volume upto 50 mL; this stage completed the digestion process and sample was kept in close container for mineral determination.[Citation21] To get a baseline reading, a blank sample was prepared following the above-mentioned procedure excluding the plant material. The sample was analyzed using Flame Photometer (S20–100, Spectrolab analytical, UK, A250 plus spectrometer, Varian, Australia) for determination of calcium, sodium, potassium, iron, phosphorus, manganese, copper, zinc, lithium, and barium.
Preparation of K. pinnata leaves extract
K. pinnata leaf extracts were prepared using three different extraction techniques.
Conventional Solvent Extraction
Plant samples (1 g) was immersed in three different solvent combinations of methanol and water with different ratios (0:100 (T1CSE); 50:50 (T2CSE); 100:0 (T3CSE), volume/volume) for a time period of 24 h at 60°C. The mixtures were then homogenized at 60°C for 4 h using a homogenizer (HG-15D, Daihan Scientific Co., Ltd., Seoul, Korea). A simple filter paper assembly was used for removal of residual material from solvent, and then the samples were concentrated using a rotary evaporator at 60°C temperature and 150 rpm (WEV-1001 L, Daihan Scientific Co., Ltd., Seoul, Korea). Samples were then freeze-dried and stored at 4°C for further testing as described by Truong et al.[Citation22]
Super Critical Fluid Extraction (SFE)
The protocol designed by Lim et al.[Citation23] for SFE was followed with slight modification. Model SFT-150 of supercritical fluid extractor incorporation, USA was used for extraction. The particular model of SFE was equipped with a volume extractor, separator, and syphonated carbondioxide cylinder, to generate desired pressure of solvent as per requirement of the process; assembly also included a syringe pump. Vacuum-dried K. pinnata leaf powder (200 g) was placed in extractor vessel in each trial. In order to measure the extraction rate, weight of extract was calculated at regular intervals throughout the extraction process. The operating conditions were as follows: pressure; 3500 psi (T4SFE), 4500 psi (T5SFE), and 5500 psi (T6SFE); temperature 40°C; and extraction time of 2 h.
Microwave-assisted extraction
Different extracts were prepared by microwave heating at 700-W power for 3 min (T7MAE), 6 min (T8MAE) and 9 min (T9MAE), as per the method followed by Ghasemzadeh et al.[Citation24] with slight modifications. Two grams of K. pinnata powder was added into 10 mL of methanol. The mixture was taken in a 50-mL double-necked flask system with cooling jacket. The mixture in the flask was then exposed to microwave radiation at 700-W power, for extraction at different time intervals. After completion of extraction duration, flasks were then water-cooled to room temperature. Followed by extract filtration via basic filtration assembly. To concentrate the extract, solvent was removed using rotary evaporator at 40°C temperature and 150 rpm. For further testing, extracts were freeze dried and stored at −20°C.
Phytochemical screening assays
The phytochemical screening of K. pinnata leaf extracts was done by determining total phenolic content (TPC) and total flavonoid content (TFC).
Total Phenolic Content
Folin–Ciocalteu reagent assay was carried out to determine TPC using gallic acid equivalent (GAE).[Citation25] For the leaf extract analysis, 125 µL of extract with equivalent volume of Folin–Ciocalteau reagent was mixed. Followed by mixing of 500 µL distilled water and the resultant solution was allowed to stay at a temperature of 22°C, for the period of 5 min. In next step, 4.5 mL 7% sodium bicarbonate solution was added to the mixture. Absorbance was determined at 765 nm against blank sample. TPC was expressed in GAE, i.e., mg GAE/g for each extract.
Total Flavonoid Content
The procedure mentioned by Ghasemzadeh and Jaafar[Citation26] was used to determine TFC. For the analysis, 1 mL of K. pinnata leaf extract was added to 0.3 mL 5% NaNO2 solution, followed by the addition of 10% 0.3 mL AlCl3 after 5 min. Then, 2 mL of 1 molar sodium hydroxide was added to the mixture. The volume of the solution was raised to 10 mL by using distilled water. The absorbance was measured at 510 nm wavelength, and results were expressed as quercetin equivalent, i.e., mg QE/g
Antioxidant potential
DPPH Free radical scavenging potential
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity of K. pinnata leaf extract was measured as per the methodology described by Cheel et al.[Citation27] For analysis, 1 mL 0.5 Mm methanolic DPPH solution was prepared, followed by through mixing with 20 µL plant leaf extract. Prior to the spectroscopic measurement, the mixture was kept at 25°C for an incubation period of 30 min. Spectroscopic readings of absorbance were taken at 520 nm and calculations of DPPH assay were made using an expression:
where AB= Blank sample absorbance at 0 min, AA= Tested extract absorbance at 30 min.
2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid assay
The 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid assay (ABTS) of K. pinnata leaf extract was carried out as per the procedure followed by Rawson et al.[Citation28] 20 µL of diluted extract was added to 1 mL chromogen and the mixture was kept for incubation at 37°C. After incubation period, initial absorbance was taken. In next step, 200 µL H2O2 solution was added to the mixture followed by dark room incubation at a temperature of 37°C for the period 3 min. Subsequently, the final absorbance was taken at 734 nm. In order to determine resultant absorbance, initial absorbance was subtracted from the final absorbance. Antioxidant potential was determined via absorbance value comparison to synthetic antioxidant Trolox (0.1–0.4 mM) standard.
Ferric-reducing antioxidant power assay
The ferric reducing antioxidant power (FRAP) assay of K. pinnata leaf extracts was determined by analyzing the FeCl3 solution reducing ability of extract. And 2.5 mL of sodium phosphate buffer (200 mM) having pH 6.6 and 2.5 mL of potassium ferrocyanide (1% solution) was mixed with 500 µL of leaf extract. Followed by incubation of mixture for 20 min at a temperature of 50°C. Then 2.5 mL trichloroacetic acid was added to the incubated mixture. The prepared solution was then centrifuged at 650 rpm for the period 10 min. After centrifugation, 5 mL supernatant was collected and mixed with 5 mL distilled water followed by addition of 1 mL ferric chloride (0.1% solution). The absorbance was determined at a wavelength of 700 nm. Graphical plotting of absorbance of plant extract concentration and synthetic antioxidant Trolox standard was expressed as ferric-reducing power. All the experimental readings were taken in triplicate.[Citation29]
Antibacterial activity of K. pinnata extracts
The pure cultures of pathogenic bacteria Yersinia pseudotuberculosis (FCBP-WB-0082), Brucella melitensis (FCBP-SB-0237), Klebsiella pneumoniae (FCBP-PB-0047), Escherichia coli (FCBP-WB-0005), and Proteus vulgaris (FCBP-LB-0074) were procured from Fungal Culture Bank, Punjab University, Lahore, Pakistan. Bacterial stock cultures were maintained on Muller–Hinton Agar at a temperature of 4°C.
Preparation of culture
For activation bacterial culture was transferred to liquid Mueller–Hinton (MH) medium and incubate at 37°C for 24 h. After activation, decimal dilutions of each bacterial culture was prepared in 0.9% sterile saline (NaCl) until 0.5 McFarland (106–108 CFU/mL) turbidity was attained.[Citation30]
Disc diffusion assay
Disc diffusion assay was employed for determining the lengths of inhibition zones created by extracts against bacterial strain as done by Niculae et al.[Citation31] For the testing purpose, standardized inoculum of each strain was used to create a uniform bacterial bed on Mueller–Hinton Agar plates surface (Oxoid). Following that, Whatman, 6-mm diameter sterile filter paper discs containing 20 μL of K. pinnata extract were placed in the middle of plate with the help of sterile forceps, followed by overnight incubation of inverted plates at 37°C. The test against each strain was performed in triplicate. For the purpose of positive control, discs of amoxicillin + clavulanic Acid (20 μg +10 μg) were used, whereas sterile paper discs without herbal extract was considered as -ve control for the test. After the overnight incubation time, the inhibition zones were measured in terms of length using millimeter unit and compared to those of the positive control.
Quantification of active ingredients in selected treatments
Active ingredients of K. pinnata extract were analyzed through HPLC by the procedure of Pawar and Bhutani[Citation32] with few modifications. To quantify K. pinnata bioactive moieties, HPLC equipped with C-18 column and UV detector (PerkinElmer 200 series) was used. Sample (10 µL) aliquot was taken by auto sampler (PerkinElmer, USA) and on a reversed-phase C-18 column (250 × 4.6 mm ID, 5 µm particle size; Grace, Lokeren, Belgium). Temperature was maintained at 30°C throughout the analysis. The HPLC system consisted of two LC-20AT pumps. A mobile phase (70:30 mixture of acetonitrile: water) was used to elute derivatized compounds at a constant flow rate of 1 mL/min. Detection was done using a wavelength of UV detector 254 nm at ambient temperature.
Statistical analysis
Data collected from study parameters were handled using Microsoft excel (version 2016) and statistically analyzed using software Statplus. Two-way ANOVA was used to check the level of significance of TPC, TFC, DPPH assay, FRAP assay, and ABTS assay followed by mean comparisons through Fisher LSD significant difference test.[Citation33,Citation34]
Results and discussion
Proximate composition of K. pinnata
Plant material from stem, roots, and leaves was analyzed for compositional profiling. Leaves of K. pinnata had highest fiber, protein and lipid content, i.e., 2.98 ± 0.06, 3.67 ± 0.02, and 0.84 ± 0.01%, respectively. Ash content was maximum in the roots of plant, i.e., 1.83 ± 0.01%. exhibits proximate analysis (%) of root, stem, and leaves of K. pinnata. Human body requires a good amount of proteins for tissue replacement, energy supply, and developmental activities.[Citation35] Dietary protein deficiency could lead to growth retardation, stunted growth, muscular wasting, edema and fluid retention in peripheral body parts and abnormal belly swelling, particularly in kids.[Citation36] K. pinnata can be used as a plant protein source in routine diet and supplements designed to boost regulatory processes, enzyme synthesis, production of hormones and antibodies.[Citation37]
Table 1. Proximate analysis (%) of root, stem, and leaves of K. pinnata.
Dietary fiber facilitates proper digestion and smooth intestinal peristaltic movement of food by restraining enzyme action. However, our lower gut microbiota produces enzymes that can digest dietary fibers and produce primary and secondary metabolites that improves digestion, and reduce the incidence of health-related disorders like diabetes, obesity and high blood cholesterol levels. High fiber content of K. pinnata leaves can improve digestion, water absorption, softens the stool by bulk addition to prevent the risk of constipation.[Citation38] Improved defecation controls body weight and blood cholesterol levels and have a shielding effect against colon carcinoma.[Citation39] Minor percentage of fat content in plant material is an indicator of K. pinnata plant effectiveness in weight reduction diet.[Citation40]
Mineral analysis of K. pinnata
Root of plant have strongest mineral profile with calcium content 40.79 ± 0.08 ppm, sodium 48.7 ± 0.07 ppm, potassium 30.42 ± 0.03 ppm, magnesium 32.61 ± 0.06 ppm, and iron 1.89 ± 0.01 ppm. Calcium content of plant leaves was slightly lower than roots, i.e., 38.43 ± 0.08 ppm. The results of the current study depicted that K. pinnata is a rich mineral source. All plant parts particularly the roots are profound source of minerals like sodium, potassium, and calcium. There are numerous studies prove the occurrence of various adverse pathological conditions in human body is due to mineral deficiency.[Citation41] exhibits mineral analysis (ppm) of root, stem, and leaves of K. pinnata.
Table 2. Mineral analysis (ppm) of root, stem, and leaves of K. pinnata.
Sufficient amount of calcium in diet is essential for muscle contraction regulation in particularly in children and infants.[Citation42] It is also important for fetal bone and teeth development.[Citation43] To ensure the integrity of intracellular cementing, substance of bones and proper blood coagulation processes sufficient amount of extracellular calcium is necessary.[Citation44] Potassium regulates electrolytic balance of tissues and blood plasma, regulates water absorption in body, thus regulating body weight.[Citation45] While sodium in addition to playing major role in maintaining electrolytic balance in human body has been found to be effective in reducing the risk of cardiac ailments.[Citation46] Zinc from plant source plays a pivotal role in boosting their disease preventive and curative properties, due its infection restricting properties. Zinc ions have strong ability to bind bacterial membrane ions, resulting in an increase in time of bacterial adjustment phase (lag phase), that disturbs bacterial growth cycle. Organisms generation time lengthens upon such perturbations.[Citation47] Similarly, manganese plays a central role biological enzyme system. In addition, to the role of manganese as co-factor in enzyme activation process, it plays a significant role in protein metabolism, bone formation, and energy generation.[Citation48] Copper elevates death rate of infective agents resulting in stabilization of maternal health maintenance and infection eradication.[Citation49]
Phytochemical screening assays
TPC and TFC
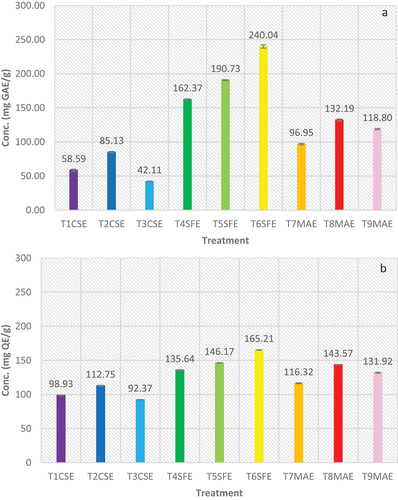
Extract prepared using different techniques exhibited highly significant difference (p < .05). In CSE, best results were attained in treatment where solvent was 50%water and 50%methanol combination, i.e., TPC 85.13 ± 1.14 mg GAE/g and TFC 112.75 ± 0.64 mg QE/g. Which proves that phenolic component of plant is of both polar and nonpolar nature, resulting in maximum extraction in mixture of water and methanol. In SFE technique, best results were attained at 5500 psi pressure in terms of TPC (240.04 ± 2.50 mg GAE/g) and TFC (165.21 ± 0.35 mg QE/g). The results exhibit that increase in pressure improves the solubility of phenolic and flavonoid content in supercritical CO2. In case of MAE, best results were attained from the extract attained after 6 min exposure to microwave radiation, i.e., TPC 132.19 ± 1.39 mg GAE/g and TFC 143.57 ± 0.28 mg QE/g. The results made it evident that exposure of microwaves impact the rate of extraction, however, less exposure reduces extraction rate but excessive exposure to microwaves can have negative impact on extraction rate. When different extraction techniques were compared, overall best phytochemical profile of plant leaf extract was attained using SFE. exhibits results of TPC and TFC of K. pinnata leaves extract.
The phenolic compound of plants plays a crucial role in strengthening the natural defense mechanism of human body against infections and metabolic stress, thus, providing auxiliary health benefits. Findings of Prasad Pandey et al.[Citation50] regarding metabolite profiling of K. pinnata (Bryophyllum) samples collected form Ramechhap (Nepal), provided evidential support to the outcomes of current study. Proving that bioactive compounds of K. pinnata exhibit strong antioxidant and enzyme inhibition potential; providing clear justification of medicinal significance of phenolic and flavonoid fractions of this plant. Another study, conducted by Portal et al.[Citation51] revealed that TFC of K. pinnata ethanolic extracts extract is 137.7 ± 2.81 mg/g. The extract exhibits strong antioxidant and UVA radiation protection, proving the photo-protective effect of the extract that can be used to treat skin disorders.
Antioxidant potential
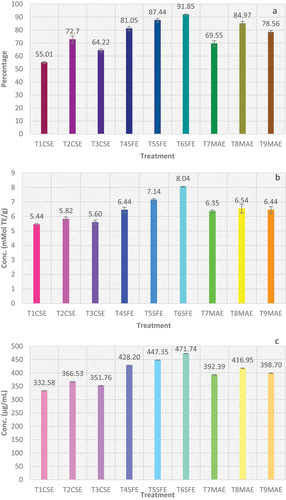
To determine antioxidant potential of K. pinnata leaf extract, three different tests were performed i.e., DPPH radical scavenging assay, ABTS assay, and FRAP assay. exhibits the DPPH, ABTS, and FRAP assay values of K. pinnata leaf extract.
DPPH free radical scavenging activity
DPPH free radical is nanometric structure that impedes the free radical generation by hydrogen donation.[Citation52] Highest DPPH value (91.85 ± 0.35%) was attained by T6SFE (5500 psi), while T1CSE (100% water) exhibited lowest value (55.01 ± 0.70%). In CSE, highest DPPH value (72.7 ± 2.3%) was seen in T2CSE followed by T3CSE (64.22 ± 1.22%). For supercritical extraction, DPPH scavenging activity of K. pinnata leaf extract increased with increase in pressure from 3500 to 5500 psi at a constant temperature (40°C). In microwave extraction, DPPH scavenging activity increased up to 6 min of exposure, then started to decrease. At 6 min of microwave heating, DPPH activity was highest (84.97 ± 1.70%) trailed by 9 min (78.56 ± 1.22%) and 3 min (69.55 ± 2.19%) exposure.
A similar study was conducted by Hamilton-Amachree and Uzoekwe[Citation53] for assessment of antioxidant and antibacterial potential of ethanolic and aqueous extract of K. pinnata from Niger Delta (Nigeria). DPPH assay exhibited percent inhibition ranges from 48.83% to 68.02% for K. pinnata extracts. A ready-to-drink (RTD) beverage was formulated using Aleo vera and K. pinnata and its functional characteristics were evaluated. DPPH radical scavenging potential of K. pinnata was found to be 80.33%. Results of the study proved that, K. pinnata leaf juice contain phytochemicals (alkaloids, phenols, carotene, saponin, flavonoids, etc.) and it can be used to prepare RTD nutraceutical beverage with antiurolithiatic, antioxidant, anti-inflammatory and antidiabetic properties, which can provide an effective herbal remedial solution to combat rising problem of non-communicable disease prevalence.[Citation54]
ABTS assay
Elimination of ABTS radicals signifies the presence of biologically active secondary metabolites like phenolics and flavonoids. Among the CSEs, absolute water showed least value (5.44 ± 0.07 mMol TE/g) of ABTS assay, while the combination of methanol and water resulted in highest (5.82 ± 0.14 mMol TE/g) ABTS assay value. SFE resulted in highest values among all the extraction techniques used in this study, while the microwave method showed ABTS values between solvent and SFE. T7MAE (3 min), T8MAE (6 min), and T9MAE (9 min) exhibited 6.32 ± 0.09 mMolTE/g, 6.54 ± 0.31 mMol TE/g, and 6.44 ± 0.22 mMol TE/g, accordingly.
Ojo et al.[Citation55] designed a similar experiment where leaf extract of K. pinnata, along with other fractions where tested for their inhibitory effect. The leaf extract exhibited IC50 94.97 ± 3.33 µg/mL value for ABTS radical scavenging. Abstemiously stable ABTS radical was used to test the inhibitory potential of various fractions of extract. Ethyl acetate fraction quenched ABTS radical at 66.82 ± 1.02 µg/mL, IC50 for aqueous extract was 96.65 ± 4.78 µg/mL for n-hexane 393.72 ± 23.02 µg/mL and for n-butanol 103.09 ± 10.12 µg/mL, which strongly support the results of current study. Enzyme inhibitory potential and radical scavenging ability of K. pinnata are because of high proportion of TPC. In addition to it, the study also elucidated the mechanism of bioavailability, enzyme inhibition action, concerned metabolic pathway and antioxidant potential, proving the effectiveness of extract to treat Alzheimer’s diseases and diabetes.
FRAP assay
FRAP assay works on the principle of reduction of ferric ion into ferrous ions due to reducing bioactive molecules present in plant isolates. Ferric-reducing antioxidant potential is a defense mechanism attained via two different pathways: electron transfer and hydrogen atom transfer.[Citation56] Significant (p < .05) variations were observed among the radical scavenging potential of extracts produced by different techniques. In case of CSE technique, best results were attained when combination of water and methanol was used as solvent, i.e., 366.53 ± 0.83 µg/mL, while the lowest value was attained from aqueous solvent, i.e., 332.58 ± 0.66 µg/mL. Overall, best results were attained from the extracts prepared using SFE at 5500 psi (471.71 ± 0.82 µg/mL). However, results of extracts prepared using MAE were better than solvent extraction technique while slightly lower than that of SFE. Moreover, extracts prepared using SFE pressure elevation exhibited a linear relationship with FRAP. While MAE showed previous trend was radiation exposure after a certain period it reduced FRAP values.
Daniel et al.[Citation57] published research on phytochemical, antioxidant, and antibacterial evaluation of Bryophyllum pinnatum extracts prepared using different solvents. Both stem and leaf extracts were prepared and evaluated separately. Plant extract showed a concentration dependent ferric reducing power with an IC50 ranged from 331.9 to 618.38 µg/mL. The results of this study evidently support the findings of current research. The reducing potential of K. pinnata extract is linked to reductones in the extract, which have the potential to break free radical chain through hydrogen atom donation. Reductones also prevent peroxide formation by reacting with peroxide precursors. These properties provide K. pinnata extract with strong antioxidant and broad-spectrum antibacterial potential.
Antibacterial activity of K. pinnata extracts
Y. pseudotuberculosis mount an oxidative stress response as first line of defense in host body, thus leading to infections like diarrhea, acute abdominal pain, and mesenteric lymphadenitis.[Citation58] B. melitensis can cause chronic hepatic suppurative disease.[Citation59] K. pneumoniae infection could lead to severe pneumonia, pyogenic liver abscess, endophthalmitis, meningitis and necrotizing fasciitis.[Citation60] E. coli can cause urinary tract infection, meningitis, abdominal and pelvic infection, bacteremia, and pneumonia.[Citation61] P. vulgaris can cause infection-related pyrexia, renal and vesical calculi, and septicemia.[Citation62]
To access antibacterial potential of K. pinnata extracts, disc diffusion assay was performed using Muller Hinton’s Agar. Five different pathogenic bacterial strains responsible for chronic infections were selected. Supercritical fluid extracts showed the best results among all in comparison to other extraction techniques. K. pinnata leaf extracts have been found to be most effective against Y. pseudotuberculosis and least effective against P. vulgaris. The results revealed that strongest zones of inhibition against Y. pseudotuberculosis was of T6SFE (22.20 ± 0.95 mm) and least effective was of T1CSE (14.33 ± 2.15 mm). Strongest zones of inhibition against B. melitensis was of T8MAE (15.16 ± 0.25 mm) and least effective was of T3CSE (9.46 ± 0.11 mm). Best results against K. pneumoniae were attained from T5SFE (14.20 ± 0.87 mm) and worst results were of T1CSE (9.60 ± 2.72 mm). T6SFE showed best inhibitory potential against E. coli i.e., (16.40 ± 0.43 mm) while T1CSE has been found to be least effective (11.26 ± 1.40 mm). Against Proteus vulgaris T5SFE exhibited best results (12.53 ± 0.37 mm) whereas T1CSE have zero inhibitory potential against the strain ().
Table 3. Zone of inhibition (mm) generated by K. pinnata leaf extracts against pathogenic bacteria.
The antibacterial potential of K. pinnata extracts prepared using different extraction techniques is due to presence of phenolic compounds in them. The antibacterial potential directly corresponds to the concentration of phenolic compounds, as the treatments from each extraction technique with strongest total phenolic profile exhibited most pronounced antibacterial potential.[Citation63] Similarly, supercritical fluid extracts had the highest percentage of TPC and exhibited robust bacterial hindering ability among all.[Citation64] Some authors have reported that the polyphenol chemistry; particularly number of OH-group and degree of polymerization impacts the microbial inhibitory effect of plant extracts. Furthermore, capacity of polyphenolics to inhibit the microbial growth is directly proportional to its concentration, this can also be explained as, higher the concentration greater will be penetration of phenolics in microbial cell membranes, stronger will be the metal ion, substrate, enzyme deprivation and degradation.[Citation65] Antibacterial activity of K. pinnata against these pathogenic bacterial provide evidential support that it can be effectively used in treating ailments like diarrhea, acute abdominal pain and mesenteric lymphadenitis, chronic hepatic suppurative disease, pneumonia, pyogenic liver abscess, endophthalmitis, meningitis and necrotizing fasciitis, urinary tract infection, meningitis, abdominal and pelvic infection, bacteremia, infection-related pyrexia, renal calculi, vesical calculi, and septicemia.
Quantification of active ingredients
Quercetin is a pigmenting flavonol (flavonoid) with antioxidant potential was quantified in the K. pinnata extracts of selected treatments. Quercetin is a pentahydroxy-flavone having the five hydroxy groups placed at the 3-, 3”-, 4”-, 5-, and 7-positions.[Citation66] Quercetin acts as antioxidant, anticancer, antifungal, and wound healing agent.[Citation67] Detoxification of reactive oxygen species in pathophysiology also confirm the cytoprotective role of quercetin.[Citation68] Accurate measurement of this bioactive compound is important to determine the effectiveness of different extraction techniques. HPLC analysis was carried out to quantify the quercetin from three selected treatments (TCSE, TSFE and TMAE).
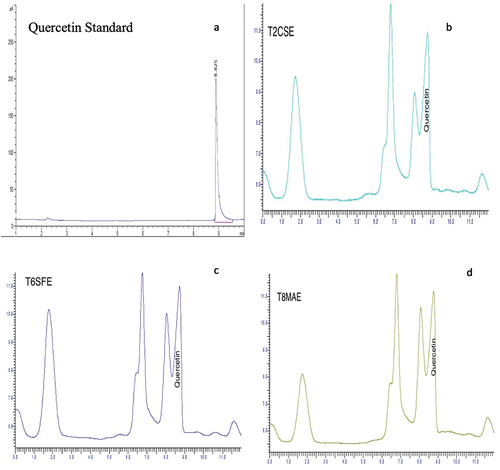
Multiple peaks in chromatogram signifies the presence of diverse range of metabolites particularly between 4 and 10 min. Fractions of TCSE, TSFE and TMAE exhibited similarity in chromatographic profile, particularly at retention time of 8 min. The available literature on chromatographic analysis points out that several bioactive compounds like flavonoids absorb at a wavelength of 254 nm. And 98% pure quercetin standard of HPLC grade (Sigma-Aldrich, St. Louis, MO; CAS No. 6151-25-3) was used to get standard peak which was attained at elution time of 8.825 min. Corresponding, peaks of samples eluted at 8.825 min exhibited that quercetin content differed significantly with the change of extraction method. Highest value for quercetin was quantified in SFE (1.98 ± 0.10 mg/g) while CSE resulted in least value (1.08 ± 0.04 mg/g). Whereas, significant difference was observed in quercetin value of TCSE(1.08 ± 0.04 mg/g) and TMAE(1.39 ± 0.21 mg/g). exhibits HPLC chromatogram of K. pinnata.
Figure 3. (a) Quercetin standard; (b) HPLC chromatogram of T2CSE; (c) HPLC chromatogram of T6SFE; and (d) HPLC chromatogram of T8MAE.
According to a study carried out by Araújo[Citation69] on chromatographic analysis of K. pinnata aqueous extract, the results exhibit the detection of compounds like quercetin-O-hexoside and quercetin-O-deoxyhexoside-O-pentoside. Presence of quercetin 3-O-α-l-arabinopyranosyl-(1→ 2)-α-l-rhamnopyranoside in K. pinnata extract was also confirmed by the findings of Coutinho et al.[Citation70]
Araújo et al.,[Citation71] in another study, verified the presence of quercetin in K. pinnata extract and proved antioxidant and anti-inflammatory properties of quercitrin. Glycolyzed quercetin exhibits gastroprotective potential.[Citation72] Chibli[Citation73] analyzed hexane fraction of K. pinnata and found an ultraviolet spectrum with characteristic bands of quercetin (flavonoid) at retention time 8.83 min, supports the findings of current study. Sobreira et al.[Citation74] also identified high quercetin peak at retention time of 8.5 min. The extracted fraction exhibited strong antiulcer properties.
Conclusion
Supercritical fluid extract of K. pinnata leaf prepared at 5500 psi, 40°C and extraction time of 2 h, showed best results among all the extraction techniques. In comparison with CSE, MAE and SFE have been found to be more effective and swift techniques to attain phenolic content with stronger and higher antioxidant capacity. In MAE, exposure of microwaves impacts the rate of extraction. However, less exposure reduces extraction rate but excessive exposure to microwaves can have slight negative impact on extraction rate. Increase in pressure in SFE improves the solubility of phenolic and flavonoid content in supercritical CO2, resulting in stronger antibacterial potential against infectious bacterial strains like Y. pseudotuberculosis (22.20 ± 0.95 mm), responsible for rare sepsis-associated illness in patients with chronic liver disease, B. melitensis (16.30 ± 0.50 mm) that can cause hepatic abbesses and chronic suppurative lesion, E. coli (16.40 ± 0.43 mm) produce toxins that can cause renal failure, K. pneumoniae (11.36 ± 0.58 mm) can cause pyogenic liver abscess, P. vulgaris (11.63 ± 0.45 mm) responsible for unrainy tract infection and antioxidant potential (TPC (240.04 ± 2.50 mg GAE/g), TFC (165.21 ± 0.35 mg QE/g), DPPH radical scavenging potential (91.85 ± 0.35%), ABTS value (8.04 ± 0.03 mMol TE/g) and FRAP value (471.74 ± 0.82 µg/mL) and can reduce oxidative stress levels in body. The current study provided an insight of the potential of bioactive compounds extracted from K. pinnata to treat hepatic, renal, and gastrointestinal diseases. Advance extraction techniques are unlocking the new possibilities in the field of phytotherapeutic, human health improvement and global resource sustainability, by escalating the ameliorative potential of bioactive compounds. However, further development in the field of metabolic engineering, microfluidics, optimization models, and nanoencapsulation is required to aggrandize the functionality and stability of these compounds.
Acknowledgment
The authors would like to thank Khwaja Fareed University Engineering and Information Technology, Rahim Yar Khan for scientific support and provide free asses to journals or books.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets utilized and analyzed during this investigation are available upon reasonable request from the corresponding author.
References
- Mesímesías, F. J.; Martín, A.; Hernández, A. Consumers’ Growing Appetite for Natural Foods: Perceptions Towards the Use of Natural Preservatives in Fresh Fruit. Food Res. Int. 2021, 150, 110749. DOI: 10.1016/j.foodres.2021.110749.
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods. 2019, 8(11), 579. DOI: 10.3390/foods8110579.
- Assis de Andrade, E.; Machinski, I.; Terso Ventura, A. C.; Barr, S. A.; Pereira, A. V.; Beltrame, F. L.; Strangman, W. K.; Williamson, R. T. A Review of the Popular Uses, Anatomical, Chemical, and Biological Aspects of Kalanchoe (Crassulaceae): A Genus of Plants Known As “Miracle Leaf”. Molecules 2023, 28(14), 5574. DOI: 10.3390/molecules28145574.
- Agüero-Hernández, A. L.; Rosales-López, C.; Herrera, C.; Vargas-Picado, A.; Muñoz, R.; Abdelnour-Esquivel, A. Hypoglycemic Effect of Kalanchoe Pinnata (Lam) Pers. Leaf Extract. Pharmacogn. J. 2020, 12(3), 557–561. DOI: 10.5530/pj.2020.12.84.
- Vandebroek, I.; Picking, D. Bryophyllum Pinnatum (Lam.) Oken (Crassulaceae). In Popular Medicinal Plants in Portland and Kingston, Jamaica; Vandebroek, I.; Picking, D.; Eds.; Springer: Cham, 2020; pp. 61–70.
- Rahman, R.; Al-Sabahi, J. N.; Ghaffar, A.; Nadeem, F.; Umar, A. Phytochemical, Morphological, Botanical, and Pharmacological Aspects of a Medicinal Plant: Kalanchoe Pinnata–A Review Article. Int. J. Chem. And Biochemical Sci. 2019, 16, 5–10.
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S. M.; Salem, M. A.; Cho, W. C.; El Mahdy, N. M.; Kılıç, C. S.; Sytar, O. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS. Omega 2020, 5(20), 11849–11872. DOI: 10.1021/acsomega.0c01818.
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological Activities of Leaf Extracts from Selected Kalanchoe Species and Their Relationship with Bufadienolides Content. Pharm. Biol. 2020, 58(1), 732–740. DOI: 10.1080/13880209.2020.1795208.
- Dighade, R.; Ingole, R.; Ingle, P.; Gade, A.; Hajare, S.; Ingawale, M. Nephroprotective Effect of Bryophyllum Pinnatum‐Mediated Silver Nanoparticles in Ethylene Glycol‐Induced Urolithiasis in Rat. IET Nanobiotechnol. 2021, 15(3), 266–276. DOI: 10.1049/nbt2.12011.
- Alishan Zia, D. C.; Kumar, P. S.; Maurya, V. K.; Selvakumar, P.; Verma, V. S. COMPERATIVE STUDY on HERBAL ANTIOXIDANT PRESENT in Bryophyllum Pinnatum PARTS (LEAVES, STEM and ROOTS). YMER Digit. 2022, 21(4), 319–331. DOI: 10.37896/YMER21.04/29.
- Adibe, M. K.; Gabriel, I. M.; Akintunde, A. A. M.; Esther, A. O. Chemical Compositions and Antioxidant Activity of Leaf and Stem Essential Oils of Bryophyllum Pinnatum (Lam.) Kurz. GSC Biol. And Pharm. Sci. 2019, 9(2), 057–064. DOI: 10.30574/gscbps.2019.9.2.0184.
- Latif, A.; Ashiq, K.; Qayyum, M.; Ashiq, S.; Ali, E.; Anwer, I. PHYTOCHEMICAL and PHARMACOLOGICAL PROFILE of the MEDICINAL HERB: BRYOPHYLLUM PINNATUM. JAPS: J. Anim. & Plant. Sci. 2019, 29(6), 1528–1534.
- Asfaw, T. B.; Esho, T. B.; Bachheti, A.; Bachheti, R. K.; Pandey, D. P.; Husen, A. Exploring Important Herbs, Shrubs, and Trees for Their Traditional Knowledge, Chemical Derivatives, and Potential Benefits. In Herbs, Shrubs, and Trees of Potential Medicinal Benefits; Husen, A.; Ed.; Boca Raton: CRC Press, 2022; pp. 1–26.
- Silva Ferreira, R. G. D.; Souza Fernandes, N. D.; Veiga-Junior, V. F. D. Kalanchoe Brasiliensis Camb. and Kalanchoe Pinnata (Lamk.) Pers. In Medicinal and Aromatic Plants of South America; Albuquerque, UP.; Patil, U.; Máthé, Á.; Eds.; Springer: Dordrecht, 2018; pp. 265–273.
- Christiana, K. A.; Mary, K. L.; Esther, A. A. PHYTOCHEMICAL, ANTIOXIDANT and ANTIMICROBIAL EVALUATION of NIGERIAN KALANCHOE PINNATA (LAM.). J. Chem. Soc. Of Nigeria 2019, 44(6), 1080–1085.
- Fongnzossie Fedoung, E.; Biwole, A. B.; Nyangono Biyegue, C. F.; Ngansop Tounkam, M.; Akono Ntonga, P.; Nguiamba, V. P.; Ngogang Yonkeu, J.; Forbi Funwi, P.; Tonga, C.; Nguenang, G. M. A Review of Cameroonian Medicinal Plants with Potentials for the Management of the COVID-19 Pandemic. Adv. Tradit. Med. 2021, 23(1), 1–26. DOI: 10.1007/s13596-021-00567-6.
- Elufioye, T. O.; Oyedeji, A. O.; Habtemariam, S. A Review of the Traditional Uses, Phytochemistry and Pharmacology of Bryophyllum Pinnatum (Lam.)(crassulaceae). J. Biologically Act. Products From Nat. 2022, 12(3), 190–222. DOI: 10.1080/22311866.2021.1988706.
- Mejía-Méndez, J. L.; Bach, H.; Lorenzo-Leal, A. C.; Navarro-López, D. E.; López-Mena, E. R.; Hernández, L. R.; Sánchez-Arreola, E. Biological Activities and Chemical Profiles of Kalanchoe Fedtschenkoi Extracts. Plants 2023, 12(10), 1943. DOI: 10.3390/plants12101943.
- Jha, A. K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. DOI: 10.1016/j.tifs.2021.11.019.
- AOAC. International Guidelines for Laboratories Performing Microbiological and Chemical Analyses of Food and Pharmaceuticals: An Aid to Interpretation of ISO/IEC 17025: 2005 AOAC International; Rockville, MD: AOAC International, 2006.
- Mohammed, M. I.; Ahmad, U. M. Mineral Elements Content of Some Coarse Grains Used As Staple Food in Kano Metropolis, Nigeria. Bayero J. Pure App. Sci. 2014, 7(1), 85–89. DOI: 10.4314/bajopas.v7i1.16.
- Truong, D. H.; Nguyen, D. H.; Ta, N. T. A.; Bui, A. V.; Do, T. H.; Nguyen, H. C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and in vitro Anti-Inflammatory Activities of Severinia Buxifolia. J. Food Qual. 2019, 2019, 1–9. DOI: 10.1155/2019/8178294.
- Lim, S.; Lee, K. T. Investigation of Impurity Tolerance and Thermal Stability for Biodiesel Production from Jatropha Curcas L. Seeds Using Supercritical Reactive Extraction. Energy. 2014, 68, 71–79. DOI: 10.1016/j.energy.2014.02.056.
- Ghasemzadeh, A.; Jaafar, H. Z.; Rahmat, A.; Swamy, M. K. Optimization of Microwave-Assisted Extraction of Zerumbone from Zingiber Zerumbet L. Rhizome and Evaluation of Antiproliferative Activity of Optimized Extracts. Chem. Cent. J. 2017, 11(1), 1–10. DOI: 10.1186/s13065-016-0235-3.
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total Phenolic Content, Antioxidant and Antimicrobial Activities of Some Medicinal Plants. Pak. J. Pharm. Sci. 2009, 22(1), 102–106.
- Ghasemzadeh, A.; Jaafar, H. Z. Profiling of Phenolic Compounds and Their Antioxidant and Anticancer Activities in Pandan (Pandanus Amaryllifolius) Extracts from Different Locations of Malaysia. BMC Complem. Altern. Med 2013, 13(1), 341. DOI: 10.1186/1472-6882-13-341.
- Cheel, J.; Theoduloz, C.; Rodríguez, J. A.; Caligari, P. D.; Schmeda-Hirschmann, G. Free Radical Scavenging Activity and Phenolic Content in Achenes and Thalamus from Fragaria Chiloensis Ssp. Chiloensis, F. Vesca and F. X Ananassa Cv. Chandler. Food Chem. 2007, 102(1), 36–44. DOI: 10.1016/j.foodchem.2006.04.036.
- Rawson, A.; Hossain, M. B.; Patras, A.; Tuohy, M.; Brunton, N. Effect of Boiling and Roasting on the Polyacetylene and Polyphenol Content of Fennel (Foeniculum vulgare) Bulb. Food Res. Int. 2013, 50(2), 513–518. DOI: 10.1016/j.foodres.2011.01.009.
- Jayaprakasha, G. K.; Singh, R. P.; Sakariah, K. K. Antioxidant Activity of Grape Seed (Vitis Vinifera) Extracts on Peroxidation Models in vitro. Food Chem. 2001, 73(3), 285–290. DOI: 10.1016/S0308-8146(00)00298-3.
- Ettahiri, W.; Salim, R.; Adardour, M.; Ech-Chihbi, E.; Yunusa, I.; Alanazi, M. M.; Taleb, M.; Barnossi, A. E.; Merzouki, O.; Iraqi Housseini, A.; Rais, Z. Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1, 2, 4-Triazolo [1, 5-A] Quinazolinone. Molecules 2023, 28(14), 5340. DOI: 10.3390/molecules28145340.
- Niculae, M. I. H. A. E. L. A.; Spînu, M. A. R. I. N. A.; Şandru, C. D.; Brudaşcă, F.; Cadar, D.; Szakacs, B. I. A. N. C. A.; Mateş, C. I. Antimicrobial Potential of Some Lamiaceae Essential Oils Against Animal Multiresistant Bacteria. Lucrări Ştinłifice Medicină Veterinară. 2009, 42(1), 170–175.
- Pawar, R.; Bhutani, K. Effect of Oleanane Triterpenoids from Terminalia Arjuna—A Cardioprotective Drug on the Process of Respiratory Oxyburst. Phytomed 2005, 12(5), 391–393. DOI: 10.1016/j.phymed.2003.11.007.
- Comuzzi, L.; Tumedei, M.; Pontes, A. E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 Pcf) Polyurethane Foam Blocks: Conical Vs Cylindrical Implants. Int. J. Environ. Res. And Public Health 2020, 17(8), 2617. DOI: 10.3390/ijerph17082617.
- Nishino, S.; Fujiki, Y.; Sato, T.; Kato, Y.; Shirai, R.; Oizumi, H.; Yamauchi, J.; Ohbuchi, K.; Miyamoto, Y.; Mizoguchi, K. Hesperetin, a citrus flavonoid, ameliorates inflammatory cytokine-mediated inhibition of oligodendroglial cell morphological differentiation. Neurol. Int. 2022, 14(2), 471–487. DOI: 10.3390/neurolint14020039.
- Sokolova, I. M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A. A. Energy Homeostasis As an Integrative Tool for Assessing Limits of Environmental Stress Tolerance in Aquatic Invertebrates. Mar. Environ. Res. 2012, 79, 1–15. DOI: 10.1016/j.marenvres.2012.04.003.
- Iorember, F. M. Malnutrition in Chronic Kidney Disease. Front. Pediatr. 2018, 6, 161. DOI: 10.3389/fped.2018.00161.
- Safder, I.; Khan, S.; Islam, I. U.; Ali, M. K.; Bibi, Z.; Waqas, M. Pichia Pastoris Expression System: A Potential Candidate to Express Protein in Industrial and Biopharmaceutical Domains. Biomed. Lett. 2018, 4(1), 1–14.
- Gill, S. K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18(2), 101–116. DOI: 10.1038/s41575-020-00375-4.
- Sueyoshi, M.; Fukunaga, M.; Mei, M.; Nakajima, A.; Tanaka, G.; Murase, T.; Narita, Y.; Hirata, S.; Kadowaki, D. Effects of Lactulose on Renal Function and Gut Microbiota in Adenine-Induced Chronic Kidney Disease Rats. Clin. Exp. Nephrol. 2019, 23(7), 908–919. DOI: 10.1007/s10157-019-01727-4.
- Properzi, C.; O’Sullivan, T. A.; Sherriff, J. L.; Ching, H. L.; Jeffrey, G. P.; Buckley, R. F.; Adams, L. A.; MacQuillan, G. C.; Garas, G.; Adams, L. A. Ad Libitum Mediterranean and Low‐Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68(5), 1741–1754. DOI: 10.1002/hep.30076.
- Dave, S. C.; Fisher, M. Relative Energy Deficiency in Sport (RED–S). Curr. Probl. In Pediatr. And Adolesc. Health Care. 2022, 52(8), 101242. DOI: 10.1016/j.cppeds.2022.101242.
- Shukla, G.; Subrahmanyam, C.; Yamuna, M.; Kumar, C. S. CC MATE Tablets: A Well-Balanced Calcium Supplement for Well-Functioning of Intestinal Ecosystem & for Healthy Body. Mediterranean J. Basic And Appl. Sci. (MJBAS) 2021, 5(4), 120–127. DOI: 10.46382/MJBAS.2021.5407.
- Farias, P. M.; Marcelino, G.; Santana, L. F.; de Almeida, E. B.; Guimarães, R. D. C. A.; Pott, A.; Freitas, K. D. C.; Freitas, K. D. C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25(23), 5630. DOI: 10.3390/molecules25235630.
- Shemishere, U. B.; Taiwo, J. E.; Erhunse, N.; Omoregie, E. S. Comparative Study on the Proximate Analysis and Nutritional Composition of Musanga Cercropioides and Maesobotyra Barteri Leaves. J. Appl. Sci. Environ. Manage. 2018, 22(2), 287–291. DOI: 10.4314/jasem.v22i2.22.
- Launonen, H.; Pang, Z.; Linden, J.; Siltari, A.; Korpela, R.; Vapaatalo, H. Evidence for Local Aldosterone Synthesis in the Large Intestine of the Mouse. J. Physiol. And Pharmacol. 2021, 72(5), 807–815.
- Baj, J.; Flieger, W.; Teresiński, G.; Buszewicz, G.; Sitarz, R.; Forma, A.; Karakuła, K.; Maciejewski, R. Magnesium, Calcium, Potassium, Sodium, Phosphorus, Selenium, Zinc, and Chromium Levels in Alcohol Use Disorder: A Review. J. Clin. Med. 2020, 9(6), 1901. DOI: 10.3390/jcm9061901.
- Mahamuni-Badiger, P. P.; Patil, P. M.; Badiger, M. V.; Patel, P. R.; Thorat-Gadgil, B. S.; Pandit, A.; Bohara, R. A. Biofilm Formation to Inhibition: Role of Zinc Oxide-Based Nanoparticles. Mater. Sci. Eng. C. 2020, 108, 110319. DOI: 10.1016/j.msec.2019.110319.
- Godswill, A. G.; Somtochukwu, I. V.; Ikechukwu, A. O.; Kate, E. C. Health Benefits of Micronutrients (Vitamins and Minerals) and Their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3(1), 1–32. DOI: 10.47604/ijf.1024.
- Germano, C.; Messina, A.; Tavella, E.; Vitale, R.; Avellis, V.; Barboni, M.; Masturzo, B.; Revelli, A.; Zola, P.; Manzoni, P. Fetal Brain Damage During Maternal COVID-19: Emerging Hypothesis, Mechanism, and Possible Mitigation Through Maternal-Targeted Nutritional Supplementation. Nutrients 2022, 14(16), 3303. DOI: 10.3390/nu14163303.
- Prasad Pandey, B.; Prakash Pradhan, S.; Adhikari, K. LC‐ESI‐QTOF‐MS for the Profiling of the Metabolites and in vitro Enzymes Inhibition Activity of Bryophyllum Pinnatum and Oxalis Corniculata Collected from Ramechhap District of Nepal. Chem. & Biodivers. 2020, 17(6), e2000155. DOI: 10.1002/cbdv.202000155.
- Portal, T.; Tavares, M.; Ataide, M.; Barbosa, N. R.; da Silva, J. R.; Moraes, W. P.; Vieira, J. L. Photoprotective Activity of Plants Used for Skin Disorders by the Native Population from the Brazilian Amazon Basin: A Preliminary Study. Pharmacogn. Res. 2023, 15(1), 112–118. DOI: 10.5530/097484900267.
- Mejía-Méndez, J. L.; Navarro-López, D. E.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Garcia-Amezquita, L. E.; Tiwari, N.; Juarez-Moreno, K.; Sanchez-Ante, G.; López-Mena, E. R. Lanthanide-Doped ZnO Nanoparticles: Unraveling Their Role in Cytotoxicity, Antioxidant Capacity, and Nanotoxicology. Antioxidants 2024, 13(2), 213. DOI: 10.3390/antiox13020213.
- Hamilton-Amachree, A.; Uzoekwe, N. M. Phytochemical Analysis and Assessment of the Antibacterial and Antioxidant Activities of the Aqueous and Ethanolic Leaf Extracts of Life Plant (Kalanchoe Pinnata L.) in the Niger Delta Nigeria. Fac. Of Nat. And Appl. Sci. J. Sci. Innovations 2022, 3(3), 1–10.
- Thaksala, H. K. S.; Hettiarachchi, C. O.; Gunathilake, K. D. P. P. Evaluation of Functional Properties in Ready-To-Drink Beverage Formulated with Kalanchoe Pinnata (Akkapana) and Aloe Vera. J. Food And Bioprocess Eng. 2022, 5(2), 168–177.
- Ojo, O. A.; Ojo, A. B.; Ajiboye, B. O.; Olaiya, O.; Akawa, A.; Olaoye, O.; Oyinloye, B. E.; Idowu, O.; Olasehinde, O.; Obafemi, T. Inhibitory Effect of Bryophyllum Pinnatum (Lam.) Oken Leaf Extract and Their Fractions on α-Amylase, α-Glucosidase and Cholinesterase Enzyme. Pharmacogn. J. 2018, 10(3), 497–506. DOI: 10.5530/pj.2018.3.82.
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules. 2020, 25(18), 4251. DOI: 10.3390/molecules25184251.
- Daniel, I. E.; Akpan, E. I.; Utam, E. C. Phytochemical Evaluation, Antioxidant and Antimicrobial Activities of Various Extracts from Leaves and Stems of Bryophyllum Pinnatum. Nepal J. Biotechnol. 2020, 8(1), 17–28. DOI: 10.3126/njb.v8i1.30206.
- Scheller, D.; Becker, F.; Wimbert, A.; Meggers, D.; Pienkoß, S.; Twittenhoff, C.; Knoke, L. R.; Leichert, L. I.; Narberhaus, F. The Oxidative Stress Response, in Particular the KatY Gene, Is Temperature-Regulated in Yersinia Pseudotuberculosis. PLOS Genet. 2023, 19(7), e1010669. DOI: 10.1371/journal.pgen.1010669.
- Kosmidou, M.; Klouras, E.; Rapti, I.; Filippas-Ntekouan, S.; Milionis, H.; Komatsu, H. Acute Brucellosis and Cirrhosis: The Triggering Event of Fatal Liver Decompensation. Case Rep. Hepatol. 1, 2020, 2020, 1–4. DOI: 10.1155/2020/8868001.
- Abbas, R.; Chakkour, M.; Zein El Dine, H.; Folorunsho Obaseki, E.; Obeid, S. T.; Jezzini, A.; Ghssein, G.; Ezzeddine, Z. General Overview of Klebsiella Pneumonia: Epidemiology and the Role of Siderophores in Its Pathogenicity. Biology 2024, 13(2), 78.
- Mueller, M.; Tainter, C. R. Escherichia coli Infection. In StatPearls [Internet]; Tainter, CR.; Ed.; Treasure Island (FL): StatPearls Publishing, 2023.
- Hammadi, K. M. Evaluation of Liver and Renal Function Tests Together with Histopathological Alterations in Rabbits Infected with a Virulent Strain of Proteus vulgaris. Biomedicine. 2023, 43(5), 1613–1620. DOI: 10.51248/.v43i5.3571.
- Parham, S.; Kharazi, A. Z.; Bakhsheshi-Rad, H. R.; Nur, H.; Ismail, A. F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants. 2020, 9(12), 1309. DOI: 10.3390/antiox9121309.
- Santos, P. H.; Kammers, J. C.; Silva, A. P.; Oliveira, J. V.; Hense, H. Antioxidant and Antibacterial Compounds from Feijoa Leaf Extracts Obtained by Pressurized Liquid Extraction and Supercritical Fluid Extraction. Food Chem. 2021, 344, 128620. DOI: 10.1016/j.foodchem.2020.128620.
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99(4), 1457–1474. DOI: 10.1002/jsfa.9357.
- Brovarets’, O. H. O.; Hovorun, D. M.; Salahub, D. Intramolecular Tautomerization of the Quercetin Molecule Due to the Proton Transfer: QM Computational Study. PLOS One. 2019, 14(11), e0224762. DOI: 10.1371/journal.pone.0224762.
- Nalini, T.; Khaleel Basha, S.; Mohamed Sadiq, A.; Sugantha Kumari, V. Fabrication and Evaluation of Nanoencapsulated Quercetin for Wound Healing Application. Polym. Bull. 2023, 80(1), 515–540. DOI: 10.1007/s00289-022-04094-5.
- Liu, X.; Song, L. Quercetin Protects Human Liver Cells from O, p’-DDT-Induced Toxicity by Suppressing Nrf2 and NADPH Oxidase-Regulated ROS Production. Food Chem. Toxicol. 2022, 161, 112849. DOI: 10.1016/j.fct.2022.112849.
- Araújo, E. R. D. Kalanchoe brasilienses Cambess e Kalanchoe pinnata (Lamarck) Persoon: caracterização química, avaliação gastroprotetora e antiinflamatória tópica. Dissertação de mestrado em Ciências Farmacêuticas. Natal: Universidade Federal do Rio Grande do Norte, 2017.
- Coutinho, M. A. S.; Casanova, L. M.; Nascimento, L. B. D. S.; Leal, D.; Palmero, C.; Toma, H. K.; Costa, S. S.; Nasciutti, L. E.; Costa, S. S. Wound Healing Cream Formulated with Kalanchoe Pinnata Major Flavonoid Is As Effective As the Aqueous Leaf Extract Cream in a Rat Model of Excisional Wound. Nat. Prod. Res. 2021, 35(24), 6034–6039. DOI: 10.1080/14786419.2020.1817012.
- Araújo, E. R. D.; Guerra, G. C. B.; Araújo, D. F.; de, S.; De Araújo, A. A.; Fernandes, J. M.; Júnior, R. F.; De, A.; Da Silva, V. C.; De Carvalho, T. G., et al. Gastroprotective and Antioxidant Activity of Kalanchoe Brasiliensis and Kalanchoe Pinnata Leaf Juices Against Indomethacin and Ethanol- Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 2018, 19(5), 1265. DOI: 10.3390/ijms19051265.
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Peng, C.; Cao, X.; Tang, Y.; Xie, X. Chemistry, Pharmacokinetics, Pharmacological Activities, and Toxicity of Quercitrin. Phytotherapy Res. 2022, 36(4), 1545–1575. DOI: 10.1002/ptr.7397.
- Chibli, L. A. Caracterização química e atividades biológicas de Bryophyllum pinnatum (Lam.) Oken. tópica. Dissertação de mestrado em Ciências Farmacêuticas. Brasil: Universidade Federal do Rio Grande do Norte, 2013.
- Sobreira, F.; Hernandes, L. S.; Vetore-Neto, A.; Díaz, I. E.; Santana, F. C.; Mancini-Filho, J.; Bacchi, E. M. Gastroprotective Activity of the Hydroethanolic Extract and Ethyl Acetate Fraction from Kalanchoe Pinnata (Lam.) Pers. Braz. J. Pharm. Sci. 2017, 53(1). DOI: 10.1590/s2175-97902017000116027.