?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The presented work proposes that the self-assembly of amphiphilic compounds in Xiaoqu Baijiu occurs at the distillation and condensation stage of Chinese Baijiu production for the first time. Such phenomena have similitude with the self-assembly nano-particles conducted by evaporation and condensation approaches. Hydrogen-bonding interactions prompting the formation of molecular clusters in the system. Herein, we characterized the variation of dipole moment, hydrogen bond strength, and cluster morphology in Xiaoqu Baijiu, based on electrochemical impedance spectroscopy, Raman spectroscopy, atomic force microscopy, and molecular dynamics simulation. The effects of major aroma substances on the self-assembly of Xiaoqu Baijiu were fully investigated, it turns out ethyl lactate had the strongest effect on resulting in self-assembled clusters.
Introduction
Xiaoqu Baijiu is one of the most popular products in the Chinese liquor market. It has the characteristics of natural aroma, purity and elegance, a mellow body, dryness and clarity, and an easy taste that slides down the throat.[Citation1] Xiaoqu Baijiu contains a variety of substances, among which ethanol and water account for 98%, and small molecular organics such as acids, alcohols, esters, and aldehydes account for 2%.[Citation2] The majority of aroma substances in Xiaoqu Baijiu are acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol, and isoamyl alcohol. Since the molecular structure of these aroma substances has hydrophilic heads and hydrophobic tails, most of them belong to amphiphilic molecules.[Citation3] The distinctive quality and style of Xiaoqu Baijiu are intricately linked to the precise balance of aromatic compounds and the intricate self-assembly structures they form.[Citation4] In sensory evaluation, on account of the distribution of different taste perception areas at the tip of the tongue, each aroma substance acts as a proton donor and binds to the corresponding taste receptor,[Citation5] resulting in a comprehensive taste of sourness, sweetness, saltiness and bitterness. This indicates that the synergistic effect in the structure of aroma substances affects the overall sensory quality of Baijiu. Zhuang[Citation6] elucidated that Chinese Baijiu is a colloidal solution formed by colloidal particles in the sub-nanometer range. The specific self-assembly status of the aroma substances that constitute the colloidal particles dominates the sensory characteristics of Baijiu.
Researches regarding the self-assembly process, which obtains structures formed through precursor evaporation and subsequent condensation, is becoming an emerging approach to systematically control the formation of complex, multicomponent materials with structural features orders of magnitude larger than the constituent colloidal nanocrystals.[Citation7] The study of such a self-assembled system has found its significance role in food science and technology,[Citation8] polymer material synthesis,[Citation9] environmental science and engineering.[Citation10] Xiaoqu Baijiu is a colloidal ethanol aqueous solution obtained through distillation and condensation of fermented grains. As mentioned above, the structure of colloidal particles in Baijiu is dominated by the species and quantities of aroma compounds, which in turn affects its sensory evaluation. Hence, elucidating a detailed mechanism by which the primary aroma compounds, for instance, acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol, and isopentanol, in Xiaoqu Baijiu influence the structures of colloidal particles, which formed during the distillation and condensation stage holds crucial theoretical and practical significance.[Citation11]
This work proposes and characterizes the super-molecular self-assembly phenomena of amphiphilic in Xiaoqu Baijiu. Such self-assembly behavior originates from the distillation process of Xiaoqu Baijiu production,[Citation12] as shown in . The fermented grains are heated in distillers. When the temperature reaches the boiling point of each component, amphiphilic molecules are vaporized and exist in a disordered manner. Then, freshly brewed Baijiu is produced in the condenser. Strong inter-molecular forces are generated due to molecular spacing plunged within the gas to liquid-phase transition.[Citation13] Hydrogen bonding is the core driving force for the self-assembly of amphiphilic in Xiaoqu Baijiu.[Citation14] It is essentially a process of self-assembly of ethanol, water, and amphiphilic aroma substances interacted into associated multi-molecular clusters. During the distillation process, the entropy of the whole system is maximized due to the temperature rise.[Citation15] The condensation process leads to an ordered spatial configuration of molecules.[Citation16] The objective of this work is to investigate how the ingredients of aroma substances affect the self-assembly status of the co-blended system during the distillation and condensation of Xiaoqu Baijiu. We characterized the extent of self-assembly by tracing the impedance evolutions of the sample after standard addition of the above six major aroma substances in Xiaoqu Baijiu. The evolution of dielectric constants within the system was analyzed through molecular dynamics simulation calculations. Raman spectroscopy was used to reveal the trend of the overall hydrogen bond strength in each sample. The morphological characteristics of the self-assembled clusters were visually observed by atomic force microscopy. This paper illustrates the microscopic mechanism of self-assembly of amphiphilic in Xiaoqu Baijiu during the distillation, condensation, and blending stages from various perspectives for the first time. The influence of aroma substance content on the extent of self-assembly are investigated thoroughly.
Materials and metods
Chemicals and materials
Deionized water is purified by ultra-pure water machine (Jinan Aiken Environmental Protection Technology Co. Ltd., Shandong, China). Ethanol, acetic acid, ethyl acetate, isoamyl alcohol, n-propanol are obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
Apparatus and software
The electrochemical equipment consists of a CorrTest electrochemical workstation (Wuhan, China) with a common three-electrode system, including the Glassy carbon electrode as the working electrode, two platinum wire electrodes serving as the reference electrode, and the assistant electrode. Other apparatuses include a thermostatic water bath (Shanghai, China); a DTC-8 ultrasonic cleaning machine (Hubei, China); Raman spectra were recorded using a Horiba-Jobin Yvon LabRam spectrograph HR-800 (Horiba, Ltd., Kyoto, Japan); Atomic Force Microscopy (AFM) (Bruker Dimension ICON, Beijing, China).
Solution preparation
Aroma substance content in Baijiu as the concentration range of aroma substances in the experiment. Two sets of ethanol solutions at 50% concentration were prepared as simulated Baijiu. Each set contains six 50% ethanol solution samples. Ethyl acetate, n-propanol, isoamyl alcohol, lactic acid and ethyl lactate were separately added to each sample, with a flavor substance concentration gradient fixed at 0, 2, 4, 6, 8, 10 g/L. Six samples from one group were subjected to condensation reflux to simulate the distillation process in the Baijiu production process, while the other group was left untreated. The above experimental steps were repeated for acetic acid, ethyl acetate, n-propanol, isoamyl alcohol, lactic acid, and ethyl lactate.
Electrochemical measurements
All electrochemical experiments were conducted using a CorrTest electrochemical workstation and a glass three-electrode cell, with the Pt wire serving as the counter electrode and reference electrode. The working electrode employed was the glassy carbon electrode. A sinusoidal voltage ranging from 0.1 M Hz to 10 Hz was applied to the Baijiu samples. A sampling rate of 10 frequency points per decade was adopted. To ensure temperature stability, a thermostatic water bath maintained the measuring system at 25°C ±0.1°C.
Raman spectra
Raman spectra were recorded using a Horiba-Jobin Yvon LabRam spectrograph HR-800 (Horiba, Ltd., Kyoto, Japan). The spectrometer is equipped with a 1800 g/mm holographic grating. Excitation for the Raman study was provided by a 532 μm argon laser. The detector was an ISA aircooled CCD. All sample solutions containing aroma substances were measured by Raman spectroscopy.
Atomic force microscope
Bruker Dimension FastScan atomic force microscope has an observable area 180 mm × 150 mm, digital camera of five million pixels, 180um to 1465um visual range, with digital zoom and autofocus function, NanoScope V controller, and a Nanoscope (IIIa) type scanning probe microscope, tip length 225 μm (the elastic constant is between 20 N/m to 70 N/m). All images were obtained in Tapping mode. The contact force was controlled within the order of 3-4NN.
The self-assembled clusters were fixed to a conductive silicon wafer with a smooth surface by electrochemical deposition. The silicon wafer is 20 mm long and 2 mm wide. Deposition was carried out with electrochemical potentiostatic impedance mode, AC amplitude 200 mv,6 h. All samples were observed by atomic force microscope.
Molecular dynamics simulation model construction
The number of ethanol and water molecules is fixed in each calculation system, and the concentration of the same aroma substance is varied. Compass force field is used in forcite module for simulation dynamics calculation.[Citation17] The simulation employs three-dimensional cube boxes and periodic boundary conditions to maintain a constant particle population and eliminate boundary effects. Structural optimization is performed for acetic acid, water, and aroma substances. The final result is output under NPT condition, and the calculation time is 500ps. High performance computing system was used for simulation, and 32 cores were used for each simulation. Repeat the above calculation steps for ethyl acetate, n-propanol, isoamyl alcohol, lactic acid, and ethyl lactate.
Results and discussion
Electrochemical impedance spectroscopy(EIS) characteristics of Xiaoqu Baijiu self-assembly phenomenon
We focused on characterizing the imaginary part of the impedance spectrum resulting from the standard addition of six major aroma substances EIS Measurements. Since the system is entropy-driven by inter-molecular hydrogen bonding in solution, temperature fluctuation varies the energy within the solution, which may affect molecular motion.[Citation18] Consequently, all EIS measurements were conducted at a constant temperature of 298.15K (±0.1K). The presence of self-assembled clusters in the system causes an increase in the inter-molecular dipole moment, resulting in an increased dielectric constant of the system. The relationship between the dielectric constant and impedance is shown in Equationequations (1(1)
(1) ) and (Equation2
(2)
(2) ).
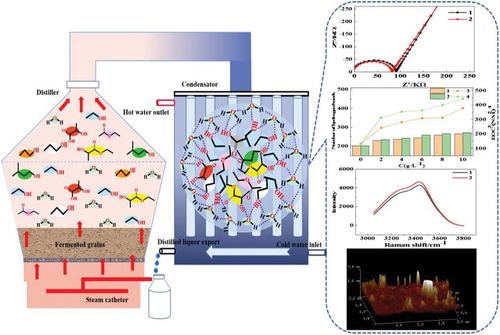
In these equations, C denotes the capacitance value, ω represents the current angular frequency, and Z’’ signifies the imaginary part of impedance. Thus, the negative reciprocal of the imaginary part of impedance is positively correlated with the dielectric constant.[Citation19] shows representative electrochemical impedance spectra of acetic acid addition to 50% ethanol with corresponding concentration gradient before and after condensation reflux. The virtual impedance of each aroma substance decreased notably after condensation reflux, indicating an increase in the dipole moment of colloidal particles and a consequent rise in the dielectric constant, resulting in a higher assembly degree after condensation reflux. To compare the impact of different aroma substances on particle assembly, illustrate the influence of differing amounts of each aroma substance on the imaginary part of impedance reflux. It is evident that water-soluble aroma substances such as acetic acid, lactic acid, and n-propanol exhibit a marked reduction in system impedance after reflux. However, the virtual impedance of less water-soluble aroma substances ethyl acetate, ethyl lactate, and isopentanol increases significantly with concentration after reflux. These alcohol-soluble aroma substances form relatively compact hydrogen bonds with ethanol, releasing more free water into the system.[Citation20,Citation21] Due to solvent effects, this leads to a reduction in the system’s apparent dielectric constant, effectively increasing the assembly degree of ethanol with esters and isoamyl alcohols. Additionally, ethyl lactate exhibits the most significant change in impedance before and after reflux, indicating its pronounced influence on the self-assembly behavior.
Figure 2. A shows the representative electrochemical impedance spectra of acetic acid addition to 50% ethanol with corresponding concentration gradient before and after condensation reflux. b, c and d are corresponding curve of virtual impedance with each aroma substance concentration variation. AA, LA, EA, LA, NP and IP represent acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol and isopentanol, respectively.
Raman spectra of simulate Xiaoqu Baijiu sample
Raman spectroscopy is one of the most powerful techniques for studying liquid water because it provides direct information about inter- and intra-molecular vibrational patterns.[Citation22] To further elucidate the interaction of hydrogen bonds in Xiaoqu Baijiu, we measured all the sample with the standard addition of six major aroma substances using Raman spectroscopy.
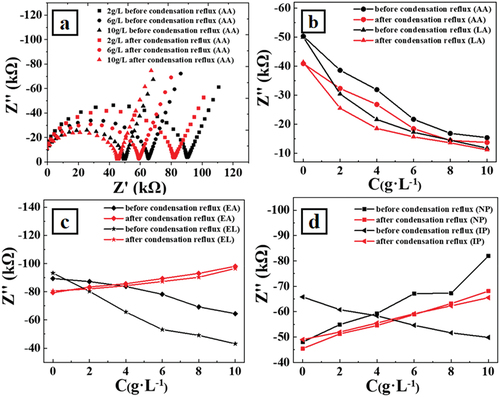
shows representative Raman spectra for an ethyl lactate addition sample, the dashed curves show the Gaussian peak splitting on the original Raman data. After Gaussian peak splitting of the original data, three peaks emerge.[Citation23] The peak at 3286 cm−1 is generated by symmetric hydroxyl vibrations, the peak at 3434 cm−1 is generated by asymmetric hydrogen bonding vibrations, and the one at 3580 cm−1 is generated by free water hydrogen bonding vibrations. Hence, illustrates a notable enhancement in hydrogen bonding for the sample supplemented with ethyl lactate after condensation reflux. As shown in , after condensation reflux, it is observed that the asymmetric Raman hydrogen bonding vibrational peaks in simulated Xiaoqu Baijiu, with the addition of different aroma substances had different degrees of redshift in the range of 0.5–2.2 cm−1. During the self-assembly process, hydrogen bonds connect the molecules, restricting the stretching vibration frequency of symmetrical hydroxyl. This leads to the reduction of all the vibration levels of symmetrical hydroxyl and attenuation of energy released during the level transition. So the peak position moves to the long-wavelength direction (redshift).[Citation24] The higher the number of hydrogen bonds formed, the wider the redshift amplitude. Among all flavor substances, ethyl lactate exhibits the most significant Raman redshift after reflux, suggesting its strongest promotion of self-assembly during the distillation stage of Xiaoqu Baijiu.This result is consistent with the electrochemical impedance spectroscopy conclusion.
Figure 3. A shows a representative Raman spectra of ethyl lactate addition sample, dashed curves show the Gaussian peak splitting on the original Raman data. b shows the red shifts of the peaks located at 3250 cm−1 before and after condensation reflux for each of the six aroma substances addition. AA, LA, EA, EL, NP and IP represent acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol and isopentanol, respectively.
AFM characterization of self-assembled clusters
We observed the self-assembled cluster morphology of the standard addition of six major aroma substances using Atomic Force Microscopy (AFM). AFM is one of the most advanced detection machines for probing surface topography at the molecular level, providing information on structural evolution in self-assembled cluster aggregates, including length, width, and pitch.[Citation25] Moreover, it fully illustrates the formation and structural characteristics of self-assembled particles.[Citation26]
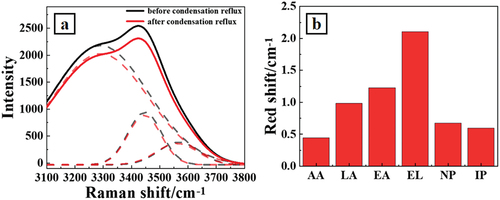
The self-assembled clusters were fixed on monocrystalline silicon wafers by electrodeposition and observed under an atomic force microscope. show the representative AFM image of the sample containing isoamyl alcohol before and after reflux, while shows the particle size variation of the system containing six aroma substances respectively. As can be seen from , after the operation of condensation, the maximum particle size of self-assembled clusters in the system increases from 15 nm to 32 nm in height and from 194 nm to 305 nm in diameter, indicating that in the process of self-assembly of amphiphilic molecules, the extent of intermolecular aggregation becomes tighter and tighter. The self-assembled clusters with a larger particle range are generated.
Figure 4. Shows the representative atomic force micro graph of assembled clusters which were electrochemically deposited on polished silica wafer electrodes before(4a) and after(4b) the condensation reflux operation by adding isoamyl alcohol; presents the variation of obtained clusters in diameter and height based on atomic force micro graph. AA, LA, EA, EL, NP and IP stand for acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol and isopentanol, respectively.
The remaining five substances all facilitate the self-assembly process of the simulated Baijiu in varying amplitudes, with the largest particle size of the self-assembled clusters after the addition of ethyl lactate to the blended system, reaching a size of 40 nm in height and 580 nm in diameter after thermal treatment. This is due to the presence of diversified oxygen-containing groups in the molecular structure of ethyl lactate. These groups act as hydrogen bond acceptors or donors during the self-assembly process, forming various hydrogen bonds in the co-mingled system, allowing the aroma substances to bind more tightly and the self-assembled particles to become larger in size.
Molecular dynamics simulation
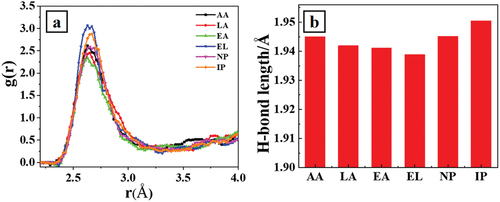
To understand how aroma substances affect the self-assembled clusters at the molecular level. We performed molecular dynamics simulations of the standard addition of six major aroma substances. is a representative radial distribution function of calculated Xiaoqu Baijiu with the same concentration of six aroma substances addition. Among these aroma substances, ethyl lactate addition was calculated to have the highest probability of occurrence at a given radius, which indicates that ethyl lactate exhibits the most promotion of self-assembly in Xiaoqu Baijiu during the distillation and condensation stage, yielding the highest number of self-assembled particles. Consequently, the AFM image shows the largest deposition of self-assembled particles on the electrode surface. presents the corresponding average hydrogen bonds length of the samples with consistent aroma substance addition. The one with ethyl lactate addition has shortest hydrogen bond length of about 1.94 Å, which indicating its strongest promotion of hydrogen bond formation in the self-assembly process during the distillation condensation of Xiaoqu Baijiu, depending on the hydrophobic force and numerous hydrogen bond action sites, aligning with the results characterized by Raman spectroscopy.
Figure 5. A is a representative radial distribution function of calculated Xiaoqu Baijiu with same concentration of six aroma substances addition. presents the corresponding average hydrogen bonds length of the samples in . AA, LA, EA, EL, NP and IP stand for acetic acid, lactic acid, ethyl acetate, ethyl lactate, n-propanol and isopentanol, respectively.
Conclusion
The presented work confirmed that the self-assembly behavior originated from the distillation process of Xiaoqu Baijiu production. The steep gas to liquid-phase transition during distillation and condensation results in strong inter-molecular forces, which lead to the self-assembly of amphiphilic aroma substances driven by hydrogen bonding. The distillation process of Baijiu production was simulated by condensation reflux of all samples. The samples with various aroma substances, were characterized by multiple analytical methods. Electrochemical impedance spectroscopy showed that the dipole moment of the self-assembled Baijiu cluster increased after distillation and condensation. The red shift of the Raman spectrum suggested an enhancement of the hydrogen bond strength during self-assembly. Molecular dynamics calculations traced the evolution trend of increasing dielectric constant and hydrogen bonding number in the system. As the self-assembly behavior proceeds, the permittivity of the system and the number of hydrogen bonds increase gradually, which is consistent with the results of electrochemistry and Raman spectroscopy. The AFM micrograph directly reflects the morphological variation of self-assembled particles. In the observed results, the particle size of the clusters is larger than that without reflux. The above characterization results fully support that the self-assembly process indeed originated from the distillation process of Xiaoqu Baijiu production.
The effect of major aromas on the self assembly of Xiaoqu Baijiu was investigated. Acetic acid, ethyl acetate, lactic acid, ethyl lactate, n-propanol, and isopentanol were investigated. Among the six aroma substances, ethyl lactate has diversified oxygen-containing groups in its molecular structure, which results in effective participation in the self-assembly process as a hydrogen bond acceptor or donor. It strongly suggests that the self-assembly behavior is effectively promoted by ethyl lactate. This work has revealed the self-assembly mechanism of amphiphilic aroma substances in Xiaoqu Baijiu so far. However, the microscopic molecular configuration of the self-assembled cluster structure remains unknown. Subsequent projects will focus on solving the microscopic molecular structures of self-assembled clusters. Distillation and condensation are inevitable and necessary steps in the production of Baijiu. This paper provides powerful evidence to elucidate the microscopic mechanism of self-assembled molecules. The study of self-assembly behavior in the condensation stage of distillation of Xiaoqu Baijiu contributes a scientific and theoretical basis for regulating and optimizing the baijiu production process.
Credit statement
Ruicong Liu and yuqun xie wrote this paper. Xinyue Jiang, Yulin zheng, Guodong Jiang, Shengzhi Yange, and Xiang Cheng reviewed this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Jiahuan, L.; Quanquan, Q.; Xizhen, S.; Shuxia, W.; Qianqian, X.; Yaqing, X.; Qiang, Y.; Xixuan, Y. J. Food Chem. 2022, 384. DOI: 10.1016/j.foodchem.2022.132452.
- Shenxi, C., Wei, J., Qun, L., Bin, L., Yuancai, L., Zhixin, S., Jie, T., Qiang, Y., Long, Z., Liping, Z. J. World J. Microbiol. Biotechnol. 2022, 38(10). DOI: 10.1007/S11274-022-03353-X.
- Arion Vladimir, B.; Danica, B.; Bijelić Ana, P.; Ana, V. J. Gels 2022, 9(1). DOI: 10.3390/GELS9010014.
- Shuang, C.; Shanshan, F.; Lulu, W.; Yan, X.; Yan, Y.; Liang, Y. Characterization of Potent Odorants Causing A Pickle-like Off-odor in Moutai-aroma Type Baijiu by Comparative Aroma Extract Dilution Analysis, Quantitative Measurements, Aroma Addition, and Omission Studies. J. Agric. Food Chem. 2020, 68(6), 1666–1677. DOI: 10.1021/acs.jafc.9b07238.
- Sheikh Umar, A.; Yili, C.; Lijun, K.; Shitian, L. J. Front. Cell. And Dev. Biol. 2023, 2023. DOI: 10.3389/FCELL.2023.1133890.
- Lu-Wen, G.; Zhong-Hui, T. J.Liquor-Mak. Sci.amp; Technol. 2002. DOI: 10.13746/j.njkj.2002.04.047.
- Shenxi, C.; Wei, J.; Bin, L.; Yuancai, L.; Jie, T.; Qiang, Y.; Hao, Z. J. World J. Microbiol. And Biotechnol. 2021, 38(1). DOI: 10.1007/S11274-021-03183-3.
- Guangxue, C.; Jinhua, H.; Xueyao, H.; Jin, L.; Yongxue, L.; Dingrong, L.; Geok Leng, S.; Cheng, T. Protein Gel with Designed Network and Texture Regulated via Building Blocks to Study Dysphagia Diet Classifications. J. Food Hydrocolloids 2023, 140. DOI: 10.1016/J.FOODHYD.2023.108640.
- Jing, C.; Xinan, C.; Jingwei, H.; Chan Kyung, K.; Jun, L.; Zongchang, L.; Wendong, L.; Xiaojun, W.; Yueyang, W.; Xinhui, Z., et al. J. J. Mol. Graphics and Modell. 2022, 116. DOI: 10.1016/J.JMGM.2022.108235.
- Mingzhang, L.; Hui, L.; Song, N.; Jianfeng, W.; Mingfeng, X.; Qiaowei, Z.; Jian, Z. J. Cem. And Concr. Compos. 2023, 139. DOI: 10.1016/J.CEMCONCOMP.2023.105053.
- Jiang, B.; Pan, H.; Chen, J.; Jin, Z. J. Food Chem. 2013, 151. DOI: 10.1016/j.foodchem.2013.11.075.
- Brodkorb, A.; Oboroceanu, D.; Wang, L.; Magner, E.; Auty, M. A. E. Characterization of β-Lactoglobulin Fibrillar Assembly Using Atomic Force Microscopy, Polyacrylamide Gel Electrophoresis, and in situ Fourier Transform Infrared Spectroscopy. J. Agric. Food Chem. 2010, 58(6), 3667–3673. DOI: 10.1021/jf9042908.
- Hongxun, H.; Xin, H.; Xiongtao, J.; Xin, L.; Yingjie, M.; Na, W.; Ting, W.; Yihan, Z.; Lina, Z. J. Particuology 2023. DOI: 10.1016/J.PARTIC.2022.03.001.
- Xinyue, J. I. A. N. G.; Duanji, W. A. N.; Yuqun, X. I. E.; Fuping, Z. H. E. N. G. Ionization Equilibrium of Water Molecule Dominated Ethanol-Water Binary Solution Self-Assemble. J. Electrochemistry 2022, 90(6), 067007–067007. DOI: 10.5796/ELECTROCHEMISTRY.22-00054.
- Daniel Maria, B.; Deepak, G.; Amos, M.; Prajwal, P. J. Phys. Rev. 2023, 107(1–1). DOI: 10.1103/PHYSREVE.107.014129.
- Amadi, E. V.; Venkataraman, A.; Papadopoulos, C. Nanoscale Self-Assembly: Concepts, Applications and Challenges. Nanotechnology 2022, 33(Number 13). DOI: 10.1088/1361-6528/ac3f54.
- Roux, B.; Lamoureux, G.; Noskov, S. Y. Molecular Dynamics Study of Hydration in Ethanol−water Mixtures Using a Polarizable Force Field. J. Phys. Chem. B 2005, 109(14), 6705–6713. DOI: 10.1021/jp045438q.
- Kim, K. A.; Kim, K. S.; Lee, D. Y. J. J. Korean Magnetic Reson. Soc. 2004, 8(1), 1–18. DOI: 10.1226/6531(pISSN).
- Duanji, W.; Zheng, F.; Wang, J.; Jiang, X.; Xie, Y. J. Sensors 2020, 20(3). DOI: 10.3390/s20030901.
- Yu, A. C.; Lian, H.; Kong, X.; Lopez Hernandez, H.; Qin, J.; Eric, A. Appel.Nat. Commun. 2021, 12(Article number: 746). DOI: 10.1038/s41467-021-21024-7.
- Syamala, P. P. N.; Würthner, F. Chemistry Europe; May 4, 2020. DOI: 10.1002/chem.202000995.
- Stoker, J. M.; Rowley, R. L. Molecular Dynamics Simulation of Real-Fluid Mutual Diffusion Coefficients with the Lennard-Jones Potential Model. J. J. Chem. Phys. 1989, 91(6), 3670–3676. DOI: 10.1063/1.456847.
- Egashira, K.; Nishi, N. (4998) J. J. Phys. Chem. B 1998, 102(21). DOI: 10.1021/jp9806359.
- Li, F.; Li, S.; Men, Z.; Wang, S.; Li, Z.; Sun, C. Study of Hydrogen Bonding in Ethanol-Water Binary Solutions by Raman Spectroscopy. Spectrochim. Acta Part A: Mol. And Biomol. Spectrosc. 2017, 189, 621–624. DOI: 10.1016/j.saa.2017.08.077.
- Ando, T. Functional Implications of Dynamic Structures of Intrinsically Disordered Proteins Revealed by High-Speed AFM Imaging. J. Biomolecules 2022, 12(12), 1876. DOI: 10.3390/BIOM12121876.
- Obeid, S.; Guyomarc’h, F.; Tanguy, G.; Leconte, N.; Rousseau, F.; Dolivet, A.; Leduc, A.; Wu, X.; Cauty, C.; Jan, G., et al. Frédéric Gaucheron, Christelle Lopez. J. Food Res. Int. 2020, 129(C), 108847. DOI: 10.1016/j.foodres.2019.108847.