?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Jack Bean tempeh, a highly nutritious protein source, is underutilized in the food industry. As a protein concentrate, the protein hydrolyzate has a limited shelf life, necessitating encapsulation for preservation. Encapsulation, mainly through freeze-drying, is a promising solution. This study, which explores the impact of different maltodextrin concentrations on the physical and chemical properties of encapsulated Jack Bean protein concentrate, has the potential to advance food technology and nutrition significantly. The methodology involved descriptive analysis and the addition of maltodextrin at three distinct concentrations: 10%, 20%, and 30% (w/w), relative to the weight of the ingredients. Meanwhile, the parameters investigated included protein content, yield, total solids, water and oil absorption, encapsulation effectiveness, and microstructure, which were studied using Scanning Electron Microscopy (SEM). The results revealed that the concentration of maltodextrin significantly influenced the encapsulation process. The protein content, water absorption, oil absorption, porosity, and encapsulation efficiency reached their peak at a maltodextrin concentration of 10%, with values of 27.26 ± 0.18%, 2.20 ± 0.05 g/g, 2.15 ± 0.02 ml/g, 0.52 ± 0.5%, and 73.19 ± 0.3%, respectively. Conversely, a 30% concentration of maltodextrin led to an increase in the yield to 61.33 ± 0.35% and the total solids to 92.52 ± 0.13%. The freeze-drying process resulted in non-uniform shapes in the microstructure in all treatments.
Introduction
Proteins are macromolecules composed of amino acids containing hydrogen, oxygen, carbon, and sulfur in the form of methionine, cysteine, and cystine connected through peptide bonds.[Citation1] Meanwhile, legumes are one of the primary vegetable protein sources, ranging from 20% to 35%.[Citation2,Citation3] One type of legume widely consumed in Indonesia is soybeans. However, the escalating demand has exceeded production, underscoring the need for alternative vegetable protein sources to replace soybeans. A common alternative currently being considered is Jack Bean, which contains high protein and carbohydrates in the range of 18%-25% and 50%-60%, while the fat content is deficient, only about 0.2%-3.0%.[Citation4]
Jack Beans are a viable source of plant protein, reaching approximately 24%, similar to soybeans. This similarity makes both plants suitable as alternate raw materials for producing tempeh. Notably, the process of fermenting protein in the form of tempeh has some advantages over beans since the tempeh mold produces digestive enzymes.[Citation5] The protease enzyme secreted by the mold facilitates protein hydrolysis into peptides and free amino acids, thereby enhancing digestibility.
Protein concentrate, with a minimum of 50% protein content, is produced by eliminating non-protein constituents from the raw material, such as fats, carbohydrates, minerals, and water.[Citation6] Protein concentrates are protein hydrolyzates resulting from the hydrolysis of protein during the fermentation process.[Citation7] Protein concentrate is encapsulated using maltodextrin and dried by freeze-drying. The method of encapsulation requires a core and transducer material. Meanwhile, the material inside the encapsulation is called a core, an internal phase, or a filler. At the same time, the core material can be an emulsion, a crystal, a solid suspension, or a gas.[Citation8,Citation9]
Maltodextrin is a viable option for a coating material that effectively masks flavor components, enhances volume, expedites drying, safeguards against heat-induced material damage, and improves solubility.[Citation10] It contains a Dextrose Equivalent (DE) value, representing the extent of starch reduction or modified product in percentage units. DE value is the sole factor that influences the qualities of maltodextrin. According to a previous report, maltodextrin with high and low DE values has excellent solubility and elevated viscosity.[Citation11] Ernawati et al.[Citation11] stated that encapsulating structure improves as the value of DE increases. The concentration of maltodextrin and DE value significantly affects the outcome of the encapsulation process. Notably, the drying process of encapsulating Jack Bean protein concentrate is crucial for extending storage duration and maintaining the overall quality. Furthermore, freeze drying, a non-thermal food processing technique[Citation12] is conducted by freezing to remove the water content in food products through sublimation, which converts the solid phase (water) into gas. The freeze-drying product is achieved by controlling the temperature and pressure during processing.[Citation13]
Studies on encapsulating Jack Bean tempeh protein concentrate in Indonesia still need more information. Therefore, this study aimed to encapsulate Jack Bean tempeh protein concentrate by varying concentrations of maltodextrin using a freeze-drying technique. Note that different amounts of maltodextrin used in encapsulation influenced the product’s physical, chemical, and structural features.
Materials and methods
Materials
The materials used were Jack Bean (Canavalia ensiformis) from Salatiga (Indonesia), distilled water, boric acid (H3BO3), NaOH, HCl, Tempeh Mold (Raprima, Indonesia),[Citation14] 70% Ethyl Ethanol solution, maltodextrin, Bradford buffer, and Kjeldahl tablets. Meanwhile, the tools used included an 80 mesh sieve, knife, grinder, Freeze dryer (Martin Christ 1.4- LD plus, Germany), Erlenmeyer, Scanning Electron Microscope (SEM) (SU3500, Japan), Spectrophotometer UV-Vis (100 DA-X, China), pH meter (Lutron YK 2001 PHA, Taiwan), magnetic stirrer, desiccator, scale, vortex, stirrer, oven, drying oven (Gemmyco China), beaker, thermometer, drop pipette, and centrifuges (Thermo Fisher, United States).
Jack Bean Tempeh
Jack Bean is thoroughly washed and subjected to a 30-minute boiling process, followed by a 24-hour soaking period in water. After soaking, the beans are peeled, cut, and washed thoroughly. Subsequently, Jack Beans are steamed for 45 minutes. Once Jack Beans are slightly heated, add mold tempeh with a concentration of 1.25%. Subsequently, Jack Bean, with mold tempeh inside a Polypropylene (PP) zip-lock plastic bag, was punctured with a knife or needle to create small openings. The Jack Beans were fermentation at a temperature of 27°C for 60 hours.[14]
Jack Bean Tempeh Flour
Produce the flour by cutting tempeh into slices 0.5 cm. Tempeh was dehydrated using a forced-air oven at 50°C for 12 hours. Subsequently, tempeh was pulverized using a grinder and filtered through an 80 mesh.[Citation7]
Jack bean protein concentrate
Dissolve Jack Bean tempeh flour in distilled water at a 1:10 (w/v) dissolving ratio and agitate using a stirrer. Initially, record the pH of the solution combination. Incubate the solution mixture (NaOH) at a temperature of 30°C and conduct the extraction process for 30 minutes. Subsequently, centrifuge the mixture at 4,000 rpm for 15 minutes and collect the liquid portion above the sediment. Mix this portion with a 2N Hydrochloric Acid (HCl) solution until the pH reaches 4.2, followed by agitation for 10 minutes. This is followed by the centrifugation process at 4,000 rpm for 15 minutes. Finally, the protein concentrate precipitate obtained is separated and weighed.[Citation7]
Purification method
The purification method can enhance the separation of fats from the protein matrix by defatting with ethyl alcohol. Consequently, 70% alcohol was added to the protein concentrate in a 1:3 (b/v) ratio, then homogenized with a stirrer for 1 hour. Subsequently, the solution was centrifugated at a temperature of 4°C and centrifuge (4,000 rpm/10 minutes). The residue obtained after subjecting Jack Bean tempeh protein concentrate to centrifugation was caused by purification.[Citation7]
Encapsulation process of jack bean tempeh protein concentrate
The encapsulation process of protein concentrate started with measuring the amount of maltodextrin based on the desired concentration, which included 10%, 20%, and 30% (w/w). Correspondingly, 100 ml of distilled water was added, and the mixture was swirled using a stirrer for 10 minutes. The mixture was agitated using a stirrer for 10 minutes, then subjected to a cooling process in a deep freezer at a temperature of −45°C for 60 minutes, then freeze-dried at a temperature of −45°C for 48 hours. Accordingly, Jack Bean tempeh protein concentrate was obtained through encapsulation.
Crude protein content
A 0.5 g sample of protein concentrate Jack Bean tempeh was added to the Kjeldahl flask, and subsequently, the Kjeldahl tablet and 10 mL of saturated H2SO4. Execute the destruction process for 2 hours, then elevate the Kjeldahl flask and allow it to cool. Once the substance has cooled down, add distilled water for dilution. About 30 mL of a solution containing 3% H3BO3, add two to three drops of methyl red indicator. Continue distilling until the resulting liquid is gathered in a H3BO3 solution. The distillate obtained in the Erlenmeyer flask was titrated using a 0.02 N HCl solution until the solution changed color to pink. Record the amount of HCl used.[Citation14,Citation15]
Mass yield
Determine the yield by comparing the encapsulation yield of Jack Bean tempeh protein concentrate encapsulation (a) with the sample’s initial weight (b).[Citation15]
where Jack Bean tempeh protein concentrate is encapsulated after the drying process (a), and a sample is recorded before the drying process (b).
Total solids
Calculate total solids using the water content data from the encapsulation of Jack Bean tempeh protein concentrate. The formula for total solids in the material was the constant weight of the dry material divided by the initial sample weight. The dry material is considered the total solids in the material.[Citation15]
where W0 = Initial Sample Weight, W1 = Weight of Constant Cup, W2 = Final weight after heating.
Water absorption
Weigh an empty, dry 50 ml centrifuge tube, noting that it weighs 1 g. Fill the centrifuge tube with 10 ml of distilled water and add a sample weighing 0.5 g (b grams). Vortex the mixture for 15 seconds, then incubate it for 1 hour. After incubation, centrifuge the mixture at 3,000 rpm for 10 minutes. Carefully extract the liquid portion and measure the remaining solid (c grams).[Citation16,Citation17] The water absorption was calculated using the formula:
where Weight of Empty Tube (a); Weight Sample (b); and Weight of solid + Tube (c)
Oil absorption
About 10 ml of oil was introduced into a centrifuge tube, and a 1 g sample was added. After mixing for 15 seconds and storing for 1 hour, the solution was centrifuged at 3,500 rpm for 30 minutes. Then, the supernatant volume was determined.[Citation18] The oil absorption was calculated using the formula:
Encapsulation efficiency
Evaluate encapsulation efficiency to determine the amount of protein concentrate used in the encapsulation formulation. Protein concentrates are protein hydrolyzates resulting from the hydrolysis of protein during the fermentation process. Prepare an encapsulation Jack Bean tempeh protein concentrate solution with 5 mg dissolved in 1 mL distilled water. Centrifuge the solution at 3,000 rpm for 5 minutes. Subsequently, about 1 mL of the remaining liquid after precipitation was used to measure the amount of protein present using the Bradford technique at a wavelength of 595 nm. This supernatant contains the non-encapsulated protein. Encapsulation efficiency was calculated using the formula[Citation19]:
Note: Total protein hydrolyzate = Total protein content, Free hydrolyzate = Encapsulated protein content
Scanning electron microscopy (SEM)
The sample holder was used to place the encapsulation sample of Jack Bean tempeh protein concentrate, followed by sealing it with silver paint and coating it with a pressure below 10 kV. Finally, a sample of 500× and 2000× magnification scales was taken.[Citation19]
Data analysis
The data analysis was performed using SPSS Version 25, and the means of different treatment groups were compared using Duncan’s Multiple Range Test (DMRT). The findings obtained from DMRT observations on the impact of variations in maltodextrin concentrations on the physical and chemical characteristics of the encapsulated Jack Bean tempeh protein concentrate.
Result and discussion
Crude protein content
displays the results of Jack Bean tempeh protein concentrate and encapsulation in terms of dry-weight protein content. The results revealed that the protein content of Jack Bean tempeh protein concentrate is approximately (50.41 ± 0.93)%. According to Wang et al.[Citation20] protein concentrate contains protein concentrations ranging from 50% to 70%. It is produced by extracting non-protein elements, including fat, minerals, carbs, and water. The protein content of Jack Bean tempeh was reduced when maltodextrin was added during encapsulation with 10%, 20%, and 30% concentrations, producing 27.26 ± 0.18%, 19.20 ± 0.28%, and 15.25 ± 0.39%.
Figure 1. The crude protein content of Jack Bean tempeh protein concentrate encapsulation with various percentages of maltodextrin.
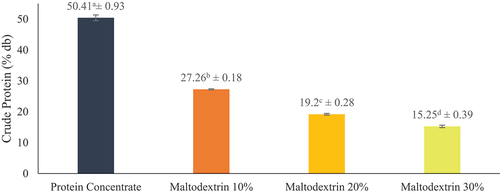
Therefore, incorporating maltodextrin decreased the protein level of Jack Bean tempeh protein concentrate since maltodextrin was derived from polysaccharide-based substances. Increasing concentration led to a proportional increase in the thickness of the carbohydrate-based coating.[Citation21] An excessive quantity of maltodextrin compounds may lead to residual maltodextrin, which occupies a portion of the powder’s overall dry matter content and decreases the total protein content.[Citation22] Moreover, these results are consistent with a study conducted by Abidin et al.[Citation23] indicating that the inclusion of maltodextrin led to a decrease in the protein content of the end product in the broth powder. The decrease was due to the inclusion of maltodextrin solids, which caused protein content to diminish as the amount of maltodextrin increased. Notably, the formulation contains a higher ratio of carbs than proteins, reducing the overall percentage of protein in the mixture.
Mass yield
Yield represents the proportion of the final product’s weight after drying to the weight of the initial raw material. This parameter quantifies the effectiveness of the processing procedure, allowing the determination of the quantity produced from the raw materials. Note that producing concentrate encapsulation started by formulating protein concentrate using Jack Bean tempeh flour. Protein loss of 34.4 g occurred during the extraction process. According to Wang et al.[Citation24] this can occur due to various factors that influence the protein extraction procedure, including the particle size of the material, the dissolution ratio, the pH level, and the temperature and duration. Thus, adding maltodextrin, a non-protein substance, resulted in a drop in protein content in the encapsulation of Jack Bean tempeh protein concentrate. illustrates the Jack Bean tempeh protein concentrate encapsulation yield for each treatment using varying quantities of maltodextrin.
Figure 2. Physical Properties Bean Tempeh protein concentrates encapsulation with various percentages of maltodextrin.
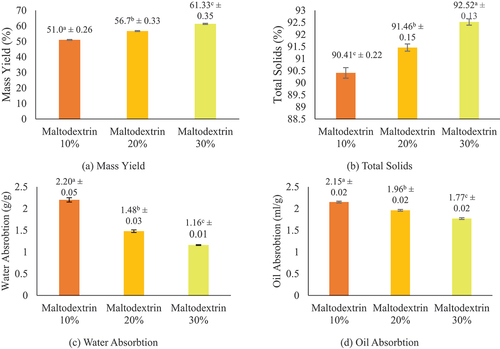
According to the results, elevating the maltodextrin content led to a higher yield of protein concentrate encapsulation. The yield of each treatment including 10%, 20%, and 30% concentration was 51.00 ± 0.26%, 56.70 ± 0.33%, and 61.33 ± 0.35%, respectively. The 30% concentration treatment produced the highest yield compared to others.
The increase in yield was influenced by maltodextrin added during encapsulation. Increasing concentration led to a better yield of the final product. This is attributable to maltodextrin being used to enhance the volume and total solids of the material.[Citation25] Similarly, Fuadi and Arianingrum[Citation26] discovered that adding more maltodextrin increased the quantity and weight of the substance, resulting in a higher yield.
The addition of 30% maltodextrin in the treatment increased the yield of encapsulated protein concentrate from 51% to 61.33%. This result is consistent with a study conducted by Srimiati et al.,[Citation27] 30% maltodextrin in strawberry extract produced higher yield, protein, fat, and carbohydrate levels than the 20% concentration treatment.
Total solids
displays the total solids results from the Jack Bean tempeh protein concentrate encapsulation. The results revealed that the total solids of the protein concentrate encapsulation increased in proportion to the concentration of maltodextrin added. The total solids of each treatment, including 10%, 20%, and 30% concentration, were 90.41 ± 0.22%, 91.46 ± 0.15%, and 92.52 ± 013%, respectively. Based on the results, total solids with the 30% treatment had the highest value compared to others.
According to Wartini and Putra,[Citation28] increasing the amount of maltodextrin added led to a higher concentration of solids in the final product after drying. This is due to the fact that maltodextrin expands the volume when used as a dressing material in encapsulation, raising the overall solids content.[Citation29] The material also facilitates the formation of a denser coating, influencing the overall amount of solids in the encapsulated protein concentrate. Based on the results, the treatment with a concentration of 30% increased the total solids from 90.41% to 92.52%.
Water absorption
Water absorption refers to the quantity of water absorbed by each g of a substance. illustrates the water absorption capacity of Jack Bean tempeh protein concentrate encapsulation at various maltodextrin concentrations. Based on the results, the encapsulant’s water absorption capacity reduced as the maltodextrin content increased. The values obtained for each treatment, including 10%, 20%, and 30% maltodextrin concentration, were 2.20 ± 0.05 g/g sample, 1.48 ± 0.03 g/g sample, and 1.16 ± 0.01 g/g sample, respectively. The water absorption was more significant in the treatment with a 10% maltodextrin concentration than others, while the 30% maltodextrin concentration produced the lowest water absorption.
The water absorption capacity of the product is related to the material and the amount of coating used. The addition of maltodextrin can affect the material’s water absorption process. According to Djaafar et al.[Citation30] adding maltodextrin can reduce a product’s water absorption after drying. Phoungchandang and Sertwasana[Citation31] also stated that adding maltodextrin reduced the water absorption of powder products. This occurs because maltodextrin reduces the cohesion between particles, leading to lower aggregation and decreased water-binding ability. These results are consistent with those of Jakubczyka et al.[Citation32] The water absorption capacity of the apple puree flour decreased as the amount of maltodextrin added increased.
The particle size of Jack Bean tempeh protein concentrate encapsulation also affected the water absorption value.[Citation33] When the encapsulation was treated with a 10% maltodextrin concentration, reduced particle size was observed compared to other treatments. Reducing the particle size tends to enhance the interface area between particles and water, augmenting the water absorption capacity. Accordingly, this condition leads to a more excellent water absorption value for the sample encapsulation with a 10% maltodextrin content compared to the other two treatments.
Oil absorption
Oil absorption is a process of physically binding oil by materials. displays the oil absorption of Jack Bean tempeh protein concentrate encapsulation at various maltodextrin concentrations. Based on the results, the higher the concentration of maltodextrin added, the lower the oil absorption of the sample. The oil absorption value of each treatment, including 10%, 20%, and 30% of maltodextrin concentration, was 2.15 ± 0.02 ml/g, 1.96 ± 0.02 ml/g, and 1.73 ± 0.02 ml/g, respectively. Meanwhile, the treatment with 10% maltodextrin produced a higher oil absorption than other treatments, while the addition of 30% concentration produced the lowest.
The oil absorption capacity of Jack Bean tempeh protein concentrates encapsulation decreased as maltodextrin concentration increased since the protein content of the ingredients influences oil absorption. The higher protein content demonstrated greater oil absorption. This was consistent with a study stating that protein content influenced the oil absorption capacity of flour material.[Citation34] Note that increasing the maltodextrin concentration in the treatment led to lower protein content. Therefore, the treatment containing 30% maltodextrin produced the most minor absorption compared to others. In addition, the sample’s size and quantity of pores also affect the water and oil absorption rates. As the number of pores/porosity rises, water and oil absorption also increases. The augmentation in water and oil absorption is due to the infiltration of water/oil into the specimen and subsequent entrapment within the pores.[Citation35]
Encapsulation efficiency
Encapsulation efficiency is one of the parameters used to measure the success of Jack Bean tempeh protein concentrate encapsulation.[Citation19] This parameter demonstrates the percentage of material successfully protected in the capsule. A high percentage of encapsulation efficiency characterized the 30% maltodextrin encapsulation. Therefore, it is crucial to consider this factor due to its significant role in evaluating the efficiency of the encapsulation process, as well as enhanced stability and extended shelf life.[Citation36] displays the encapsulation efficiency value of Jack Bean tempeh protein concentrate at various maltodextrin concentrations.
Figure 3. Encapsulation efficiency of Jack Bean tempeh protein concentrates encapsulation with various percentages of maltodextrin.
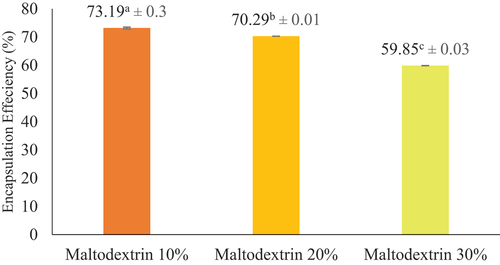
The effectiveness encapsulation of Jack Bean tempeh protein concentrate encapsulation decreased as the concentration of maltodextrin increased. The values obtained for each treatment, including 10%, 20%, and 30% concentration, were 73.19 ± 0.3%, 70.29 ± 0.00%, and 59.85 ± 0.3%, respectively. The efficiency value of the encapsulation was maximum when a 10% maltodextrin concentration was added compared to other treatments. Notably, a higher value of encapsulation efficiency corresponds to the excellent protection the coating material provides.[Citation10,Citation19]
Based on the results, encapsulation efficiency exhibited a decrease in value with an increase in maltodextrin content. According to Sucianti et al.[Citation37] increasing coating concentrations used to cover the core material led to decreased encapsulation efficiency. Meanwhile, Wanda et al.[Citation38] stated that the reduction with a higher concentration of maltodextrin is due to a loss in molecular efficiency, leading to easier diffusion of water molecules. Furthermore, the encapsulation efficiency value is influenced by several parameters, including the quantity of coated protein concentrate, the type of coating used, and the speed of the encapsulation process.[Citation14,Citation35]
Scanning electron microscopy (SEM)
The microstructure of protein concentrate from Jack Bean tempeh was evaluated to examine the morphological structure of the particles following the encapsulation procedure. Comprehending the encapsulation’s morphology, items that meet the desired characteristics can be easily selected. The SEM results were observed at magnifications of 500× and 2000 × . At the same time, the ImageJ tool was utilized to analyze the particles further and measure diameter and porosity, with the results presented in .
Table 1. SEM Microstructure results of Jack Bean tempeh protein concentrates with various percentages of maltodextrin with magnifications 500× and 2000 ×.
The 10% maltodextrin concentration treatment led to a thin, uneven, and elongated encapsulated structure. The mean particle diameter of encapsulation was 54.62 μm, while the maximum and minimum values were 77.88 μm and 29.86 μm, respectively. The encapsulation sample, with a concentration of 10% maltodextrin, demonstrated a more excellent water absorption due to the tiny particle size compared to others.
indicates that the 20% maltodextrin produced a particle size that exceeded that of the 10% treatment, with a coarse surface and more cohesion. The encapsulation had an average particle diameter of 83.55 μm, while the maximum and minimum values were 113.86 μm and 63.64 μm, respectively.
Jack Bean tempeh protein concentrate added with a 30% maltodextrin concentration had a delicate, uneven, and porous fragmented structure. The surface was coarse, and the mean particle diameter was 92.69 μm. Meanwhile, the maximum and minimum diameters were 144.56 μm and 53.86 μm, respectively. This maltodextrin treatment had a particle size that was 30% greater than previous treatments, primarily due to the increased number of transducers used in the encapsulation process. As the ratio of folding material to core materials rises, the size of the particles produced also increases due to the thicker wall of the micro-capsule.[Citation37]
The freeze-drying method can create tiny fractures in the protein concentration of Jack Bean tempeh. Rapid freezing leads to ice crystal development, impacting the maltodextrin wrapper’s structure. The drying method causes compression and pushing forces that lead to shrinkage of the cell wall.[Citation39]
Image segmentation
The SEM test was subsequently cut into a square of 550 × 550 pixels to analyze the image’s porosity. Porosity analysis was conducted by processing the image using image segmentation with the Otsu thresholding method (Image > Adjust > Otsu > Apply). Image segmentation of the treatment can be seen in .
Table 2. Image Segmentation Results Using ImageJ Application.
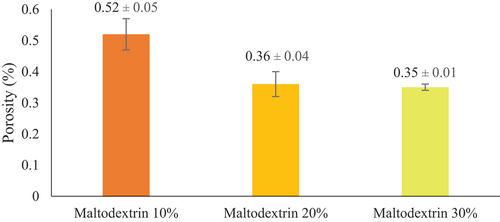
The porosity of a food product is a fraction of the volume of air space in a tissue.[Citation40] Based on the results obtained through the ImageJ application with the result presented in , the porosity of Jack Bean tempeh protein concentrates encapsulation with a 10%, 20%, and 30% maltodextrin concentration was 0.52 ± 0.5%, 0.36 ± 0.4%, and 0.35 ± 0.1%, respectively. The results were consistent with a study by Michalska and Lech,[Citation41] who stated that increasing maltodextrin concentration reduced the porosity of drying material. According to Dayal et al.[Citation42] maltodextrin has a high density, making penetration of the interparticle space easier. Thus, increasing maltodextrin concentration leads to an elevated material density and decreased porosity.
Conclusion
In conclusion, adding maltodextrin at different concentrations influenced Jack Bean tempeh protein concentrate encapsulation’s physical, chemical, and structural characteristics. Based on the results, protein content, water and oil absorption, porosity, and encapsulation efficiency were reduced following treatment. The 10% maltodextrin treatment produced chemical properties, such as a protein content of 50.41%, water absorption capacity of 2.20 ± 0.05 g/g, and oil absorption capacity of 2.15 ± 0.02 ml/g. At the same time, the physical properties include porosity of 0.52 ± 0.5% and encapsulation efficiency of 73.19 ± 0.3%. Furthermore, maltodextrin addition also improved the yield and total solidity to 51.00 ± 0.26% and 92.522 ± 013%, respectively. Nevertheless, SEM analysis of all treatments demonstrated small slices and irregularities caused by freeze-drying. The 30% concentration treatments produced a larger particle size compared to others.
Acknowledgments
Universitas Padjadjaran funded the research presented in this paper with the scheme Riset Percepatan Lektor Kepala (RPLK) with number grant 1485/UN6.3.1/PT.00/2024.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors must furnish comprehensive information regarding the datasets employed and/or examined in the present study. The data can be obtained from the associated author upon a reasonable request.
Additional information
Funding
References
- Çabuk, B.; Nosworthy, M. G.; Stone, A. K.; Korber, D. R.; Tanaka, T.; House, J. D.; Nickerson, M. T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56(2), 257–264. DOI: 10.17113/ftb.56.02.18.5450.
- Ekanayake, S.; Jansz, E. R.; Nair, B. M. Literature review of an underutilized legume: Canavalia gladiata L. Plant Foods Hum. Nutr. 2000, 55(4), 305–321. DOI: 10.1023/A:1008119107738.
- Patel, R.; Tyagi, V., Mallesha, P.R. Nutritional Evaluation of Canavalia Ensiformis (Jack Bean) Cultivated in North East Region of India. Int. J. Bot. Stud. 2016, 1(6), 18–21.
- Widaningrum; Sukasih, E.; Purwani, E. Y.; Widaningrum, N. Introductory Study on Processing of Fermented Jack Bean (Canavalia Ensiformis). J. Penelit. Pascapanen Pertan. 2017, 12(3), 129. DOI: 10.21082/jpasca.v12n3.2015.129-136.
- Yarlina, V. P.; Andoyo, R.; Djali, M.; Lani, M. N. Metagenomic Analysis for Indigenous Microbial Diversity in Soaking Process of Making Tempeh Jack Beans (Canavalia Ensiformis). Curr. Res. Nutr. Food Sci. 2022, 10(2), 620–632. DOI: 10.12944/CRNFSJ.10.2.18.
- Mao, X.; Hua, Y. Composition, Structure and Functional Properties of Protein Concentrates and Isolates Produced from Walnut (Juglans Regia L.). Int. J. Mol. Sci. 2012, 13(2), 1561–1581. DOI: 10.3390/ijms13021561.
- Yarlina, V. P.; Azzahra Rusmana, T.; Zaida, Z.; Djali, M.; Andoyo, R.; Debby Moody, S.; Lani, M. N. Characteristics of Jack Bean Tempeh Hydrolysate Protein as a Function of Spray Drying Temperature: Effect of Maltodextrin and Gum Arabic. Int. J. Food Prop. 2024, 27(1), 214–223. DOI: 10.1080/10942912.2024.2304289.
- Ezhilarasi, P. N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess. Technol. 2013, 6(3), 628–647. DOI: 10.1007/s11947-012-0944-0.
- Maqsoudlou, A.; Sadeghi Mahoonak, A.; Mohebodini, H.; Koushki, V. Stability and Structural Properties of Bee Pollen Protein Hydrolysate Microencapsulated Using Maltodextrin and Whey Protein Concentrate. Heliyon. 2020, 6(5), e03731. DOI: 10.1016/j.heliyon.2020.e03731.
- Giroldi, M.; Grambusch, I. M.; Schlabitz, C.; Kuhn, D.; Lehn, D. N.; Volken de Souza, C. F. Encapsulation of Protein Hydrolysates by Spray Drying: Feasibility of Using Buffalo Whey Proteins. Int. J. Food Sci. Technol. 2022, 57(6), 3419–3427. DOI: 10.1111/ijfs.15665.
- Ernawati, U. R.; Khasanah, L. U.; Anandito, R. B. K. The Effect of Variation Dextrose Equivalents Maltodextrin Values on the Microencapsulant Characteristic of Teak Leaves (Tectona Grandis L.F) Natural Dye. J. Teknol. Pertan. 2014, 15(2), 111–120.
- Rahman, M. M.; Das, R.; Hoque, M. M.; Zzaman, W. Effect of Freeze Drying on Antioxidant Activity and Phenolic Contents of Mango (Mangifera Indica). Int. Food Res. J. 2015, 22(2), 613–617.
- Habibi, N. A.; Fathia, S.; Utami, C. T. Changes in Food Material Characteristics in Fruit Chips by Freeze Drying Method (Review). JST. Jurnal Sains. Ter 2019, 5(2). DOI: 10.32487/jst.v5i2.634.
- Yarlina, V. P.; Nabilah, F.; Djali, M.; Andoyo, R.; Lani, M. N. Mold Characterization in “RAPRIMA” Tempeh Yeast from LIPI and Over-Fermented Koro Pedang (Jack Beans) Tempeh. Food Res. 2023, 7(Suppl. 1), 125–132. DOI: 10.26656/fr.2017.7(S1).27.
- AOAC. Official Method of Analysis. 18th ed.; Arlingt: Association of Official Analytical Collaboration (AOAC) International, 2005; pp 30–31.
- Amanah, Y. S.; Sya’di, Y. K.; Handarsari, E. Protein Content and Texture in Koro Benguk Tempeh with Black Soybean Substitution. J. Food Nutr. 2019, 9(2), 69. DOI: 10.26714/jpg.9.2.2019.69-78.
- Approved Methods of the AACC. AACC, 10th ed. 2000; Vol. 1. pp 22–23.
- Dewi, A. M. P.; Santoso, U.; Pranoto, Y.; Marseno, D. W. Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polym. (Basel). 2022, 14(6), 1–17. DOI: 10.3390/polym14061086.
- Kumari, A.; Kaushik, N.; Slizyte, R.; Khushboo K. Production and Microencapsulation of Protein Hydrolysate of Pink Perch (Nemipterus Japonicus) By-Products Obtained from Surimi Industry for Its Sustainable Utilization. Waste Biomass. Valor. 2022, 14(1), 209–226. DOI: 10.1007/s12649-022-01853-3.
- Wang, X.; Hunter, A. K.; Mozier, N. M. Host Cell Proteins in Biologics Development: Identification, Quantitation and Risk Assessment. Biotechnol. Bioeng. 2009, 103(3), 446–458. DOI: 10.1002/bit.22304.
- Novitasari, R. T. M.; Anggo, A. D.; Agustini, T. W. The Effect of Maltodextrin and Carrageenan Filler Combination on the Characteristics of Crab-Flavored Powder. J. Ilmu dan Teknol. Perikan. 2021, 3(1), 16–25. DOI: 10.14710/jitpi.2021.11407.
- Ozcelik, M.; Kulozik, U. The Role of Maltodextrin Concentration in Maintaining Storage Stability of Dried Fruit Foams Texturized Using Plant Protein–Polysaccharide Blends. Foods. 2023, 12(8), 1673. DOI: 10.3390/foods12081673.
- Abidin, A. F.; Yuwono, S. S.; Maligan, J. M. The Effect of Maltodextrin and Egg White on Characteristic of White Oyster Mushroom Broth Powder. J. Pangan. dan Agroindustr. 2019, 7(4), 53–61. DOI: 10.21776/ub.jpa.2019.007.04.6.
- Wang, M.; Jiang, L.; Li, Y.; Liu, Q.; Wang, S.; Sui, X. Optimization of Extraction Process of Protein Isolate from Mung Bean. Procedia. Eng. 2011, 15, 5250–5258. DOI: 10.1016/j.proeng.2011.08.973.
- Yuliawaty, S. T.; Susanto, W. H. Effect of Drying Time and Concentration of Maltodextrin on the Physical Chemical and Organoleptic Characteristic of Instant Drink Noni Leaf (Morinda Citrifolia. J. Pangan. dan Agroindustri. 2015, 3(1), 41–51.
- Fuadi, M.; Arianingrum, W. Study on the Production of High Calcium Eggshell Instant Beverage. Agrintech. J.Teknol Pangan. dan Has Pertan 2018, 2(1), 23–32. DOI: 10.30596/agrintech.v2i1.2607.
- Srimiati, M.; Zahra, A. D.; Harsanti, F.; Habibah, P.; Maharani, A. R. Effect of Maltodextrin Concentration on Physical Characteristics of Strawberry Extract That May Prevent COVID-19 in the Elderly. Amerta. Nutr. 2023, 7(4), 520–526. DOI: 10.20473/amnt.v7i4.2023.520-526.
- Wartini, N. M.; Putra, G. P. G. Characteristics of Pandan Fruit Color Encapsulates on the Treatment of Type and Concentration of Encapsulant. Media. Ilm Teknol. Pangan. 2018, 5(2), 139–148.
- Yuliawaty, S. T.; Susanto, W. H. The Effect of Drying Duration and Maltodextrin Concentration on the Physicochemical and Organoleptic Characteristics of Instant Noni Leaf (Morinda Citrifolia L). J. Pangan. dan Agroindustri. 2015, 3(1), 41–52.
- Djaafar, T. F.; Santoso, U.; Ariestyanta, A. Pengaruh Penambahan the Effect of Maltodextrin Addition and Inlet Temperature of Spray Dryer on the Physicochemical Characteristics of Kerandang Juice Powder (Canavalia Virosa). Agritech. 2018, 37(3), 334. DOI: 10.22146/agritech.10446.
- Phoungchandang, S.; Sertwasana, A. Spray-Drying of Ginger Juice and Physicochemical Properties of Ginger Powders. Science Asia. 2010, 36(1), 40–45. DOI: 10.2306/scienceasia1513-1874.2010.36.040.
- Jakubczyk, E.; Gondek, E.; Tambor, K. Characteristics of Selected Functional Properties of Apple Powders Obtained by the Foam-Mat Drying Method. Food Process Eng. a Chang World July, 2011, 1–6.
- Elleuch, M.; Bedigian, D.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre Characteristics and Antioxidant Activity of Sesame Seed Coats (Testae). Int. J. Food Prop. 2012, 15(1), 25–37. DOI: 10.1080/10942911003687231.
- Rohmah, M. Characterization of Physico-Chemical Properties of Kapas Banana (Musa comiculata) Flour and Starch. J. Teknol Pertan. Univ. Mulawarman 2012, 8(1), 20–24.
- Ammar, H. Production and Characterization of Lightweight Geopolymer Glass Fiber Composite. 1, 2022, 101–110.
- Akdeniz, B.; Sumnu, G.; Sahin, S. The Effects of Maltodextrin and Gum Arabic on Encapsulation of Onion Skin Phenolic Compounds. Chem. Eng. Trans. 2017, 57, 1891–1896. DOI: 10.3303/CET1757316.
- Sucianti; Nurhaeni; Hardi, J. Microencapsulation of Super Red Dragon Fruit Peel Extract (Hylocereus Costaricensis) with Various Maltodextrin Masses and Its Application as an Antioxidant. KOVALEN J. Ris. Kim. 2020, 6(3), 191–197. DOI: 10.22487/kovalen.2020.v6.i3.9889.
- Wanda, P.; Agus Wibowo, M.; Destiarti, L. Encapsulation and Stability Test of Methanol Extract of Papaya Leaves (Carica Papaya. Linn). Urnal. Kim. Khatulistiwa. 2017, 6(1), 25–29.
- Oyinloye, T. M.; Yoon, W. B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes. 2020, 8(3), 1–23. DOI: 10.3390/PR8030354.
- Karim, M. A.; Rahman, M. M.; Pham, N. D.; Fawzia, S. Food Microstructure as Affected by Processing and Its Effect on Quality and Stability; Elsevier Ltd., 2017. DOI: 10.1016/B978-0-08-100764-8.00003-4.
- Michalska, A.; Lech, K. The Effect of Carrier Quantity and Drying Method on the Physical Properties of Apple Juice Powders. Beverages. 2018, 4(1), 2. DOI: 10.3390/beverages4010002.
- Dayal, A.; Bhat, I. A.; Rashid, I. R.; Bhat, A.; Rashid, R. Effect of Maltodextrin on the Properties of Lyophilized Aloe Vera (Aloe Barbadensis Mill) Powder. Int. J. Curr. Microbiol. App. Sci. 2018, 7(4), 2071–2079. DOI: 10.20546/ijcmas.2018.704.237.