Abstract
Iron-functionalized titanium dioxide (TiO2) composites with various Fe-to-Ti ratios were prepared on flexible glass fibers (GF-Fe-TiO2) via a sol-gel method, followed by a dip-coating process. The photocatalytic ability of these composites in degrading selected volatile organic compounds (VOCs; benzene, toluene, ethylbenzene, and o-xylene [BTEX]) at indoor concentration levels was examined. The GF-Fe-TiO2 composites were characterized using scanning electron microscopy, energy-dispersive X-ray elemental analysis, ultraviolet (UV)-visible spectroscopy, and X-ray diffraction. The GF-Fe-TiO2 composites showed superior photocatalytic performance to that of a reference glass fiber–supported TiO2 photocatalyst for the treatment of BTEX under visible light. However, this trend was reversed under UV irradiation. Specifically, the average BTEX photocatalytic efficiencies of the 0.01-GF-Fe-TiO2 composite in a 3-hr visible-light photocatalytic process were 4%, 33%, 51%, and 74%, respectively. Conversely, the average BTEX photocatalytic efficiencies obtained for GF-TiO2 were close to 0%, 5%, 16%, and 29%, respectively. These findings demonstrated that the GF-Fe-TiO2 composites could be applied to photocatalytically purify BTEX, especially under visible-light exposure. Moreover, the GF-Fe-TiO2 composites prepared with different Fe-to-Ti ratios displayed different BTEX photocatalytic decomposition efficiencies under visible or UV light, allowing for optimization of the Fe-to-Ti ratio (which was found to be 0.01).
Implications: The application of nanomaterials for air purification necessitates a supporting material to stabilize them while in contact with the treated air in the photocatalytic chamber. Glass fibers have an obvious advantage over other supporting materials mainly because of its flexibility, which makes it much easier to handle. However, the applications of glass fiber–supported, visible light–activated photocatalysts to the treatment of air pollutants are rarely reported in literature. Accordingly, this study aimed to investigate the applicability of glass fiber–supported Fe-TiO2 for the purification of VOCs under visible- as well as UV-light exposure.
Introduction
Photocatalysis over titanium dioxide (TiO2) has recently received increasing attention for its ability to degrade a range of environmental pollutants. There are several advantages associated with the usage of TiO2, including low cost, high chemical and thermal stability, and good oxidation capacity (Gaya and Abdullah, Citation2008; Paz, Citation2010; Folli et al., Citation2012; Lan et al., Citation2013). However, environmental applications for stand-alone TiO2 nanopowders have been limited by the low photocatalytic efficiency resulting from the high recombination effects of their charge carriers. TiO2 nanopowders are also limited to photocatalytic activation under ultraviolet (UV) irradiation, because of the broad band gap of TiO2 (Paola and García-López, Citation2012). Consequently, many surface modification techniques have been used for enhancing the performance of TiO2 photocatalysts (Park et al., Citation2013). Doping of transition or noble metals into TiO2 is a promising method for improving the photocatalytic performance in the treatment of environmental contaminants (Bouras et al., Citation2007; Vargas et al., Citation2012; Yu et al., Citation2013; Inturi et al., Citation2014). Iron (Fe)-functionalized TiO2 composites (Fe-TiO2) are among the most popular transition metal–functionalized TiO2 photocatalysts. Their popularity is a result of the reduced electron-hole pair recombination and narrow band gaps relative to conventional TiO2 photocatalysts. Consequently, Fe-TiO2 has been effectively used for the degradation of a number of environmental pollutants under visible-light irradiation (Cui et al., 2009; Tieng et al., Citation2011; Sun et al., Citation2012; Vargas et al., Citation2012). Moreover, Fe is less expensive than noble metals such as Pd, Pt, Rh, Ag, and Au, which allows for its widespread usage.
The application of nanomaterials for air purification necessitates the use of a supporting material to stabilize them while in contact with the treated air in a photocatalytic chamber (Bianchi et al., Citation2012). A variety of materials have been explored as supports for TiO2 nanopowders for the purification of airborne pollutants. These include powdered activated carbon (Matos et al., Citation2010), activated carbon fibers (Jo et al., Citation2011), glass tubes (Jo and Kim, Citation2009), polymer materials (Kedem et al., Citation2009), Teflon (Bianchi et al., Citation2012), steel (Bianchi et al., Citation2012), and glass fibers (GFs) (Lin et al., Citation2013). GFs have an obvious advantage over other supporting materials because of their flexibility, making them much easier to handle (Panniello et al., Citation2012; Lin et al., Citation2013). Moreover, Lin et al. (Citation2013) reported that GF-supported Fe-TiO2 (GF-Fe-TiO2), synthesized by a dip-coating sol-gel process, exhibited photocatalytic performance superior to that of the fiber-supported pure TiO2 for the decomposition of phenol in an aqueous medium. However, the application of GF-Fe-TiO2 to the treatment of airborne pollutants is relatively unknown. In fact, the light absorbance kinetics and reaction mechanisms of contaminants differ between the water-solid and air-solid boundaries (Henderson, Citation2011). Accordingly, extrapolation of the photocatalytic performance of GF-Fe-TiO2 from solvent applications to gas applications may not be sound. Therefore, specific investigation of the application of GF-Fe-TiO2 to the decomposition of airborne pollutants is required.
In this study, Fe-TiO2 photocatalysts with different Fe-to-Ti ratios were synthesized using tetrabutyl titanate (TnBT) and iron(III) nitrate as the Ti and Fe sources, respectively. The photocatalysts were then dip-coated on to flexible GFs to prepare GF-Fe-TiO2 composites, and their photocatalytic activity was determined for the degradation of low-concentration toxic air pollutants (benzene, toluene, ethylbenzene, and o-xylene [BTEX]). These pollutants were selected based on their prevalence in urban indoor and outdoor environments (Esplugues et al., Citation2010) as well as their associated health hazards (Ramírez et al., Citation2012). Atmospheric BTEX mainly originates from anthropogenic sources such as motor vehicle exhaust gases, combustion emissions, and a variety of industrial handling and storage processes (Leuchner and Rappenglück, Citation2010). In addition, there are many indoor BTEX sources, such as household products, paints, furniture, and building finishing materials (Shin and Jo, Citation2012). Additionally, a GF-TiO2 photocatalyst was synthesized, and its photocatalytic activity was determined and compared with those of the GF-Fe-TiO2 composites.
Methods
Synthesis and characterization of photocatalysts
GF-Fe-TiO2 composites with different Fe-to-Ti ratios were prepared using a sol-gel procedure, followed by a dip-coating process. First, 17 mL of TnBT (Ti[O(CH2)3CH3]4; 97%; Aldrich, St. Louis, MO), 0.5 mL of nitric acid (98%; Aldrich), and 1.5 mL of deionized water were added sequentially to 136 mL of ethanol (99.9%; Aldrich). This was followed by the addition of either 0.001, 0.002, 0.01, 0.02, or 0.1 g of iron(III) nitrate (Fe(NO3)3; 99.99%; Aldrich) to the solution, resulting in Fe-TiO2 composites with Fe-to-Ti ratios of 0.005, 0.01, 0.05, 0.1, and 0.5, respectively. A clean GF sheet (11.5 × 20 cm; C120-3K; Hyundai Fiber Co., Hanam, Korea) was subsequently dipped into the mixture for 10 min and then pulled out slowly at a rate of 1 cm min−1. Following this, the coated GF was dried at room temperature for 3 hr. This coating procedure was repeated three times to obtain high Fe-TiO2 loading of the samples. The final products were calcined in an electric furnace, in which the temperature was increased to 400 °C at a rate of 2 °C min−1, and was then held constant for 1 hr before finally being cooled to room temperature over 24 hr. The GF-Fe-TiO2 composites with Fe-to-Ti ratios of 0.005, 0.01, 0.05, 0.1, and 0.5 were designated as 0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2, respectively. In addition, a reference photocatalyst (0.05-GF-TiO2) was prepared, following the same procedure used for the preparation of GF-Fe-TiO2, but without the addition of Fe(NO3)3. The characteristics of the GF-Fe-TiO2 and GF-TiO2 photocatalysts were determined via field emission scanning electron microscopy (FE-SEM) equipped with an energy-dispersive X-ray (EDX) spectrometer (Hitachi S-4300 and EDX-350 FE-SEM; Tokyo, Japan), UV-visible spectroscopy (Varian CARY 5G; Santa Clara, CA), and X-ray diffraction (XRD; Rigaku D/max-2500 diffractometer; Tokyo, Japan). Pure GF was also investigated as a control substrate for FE-SEM characteristics.
Determination of photocatalytic efficiency
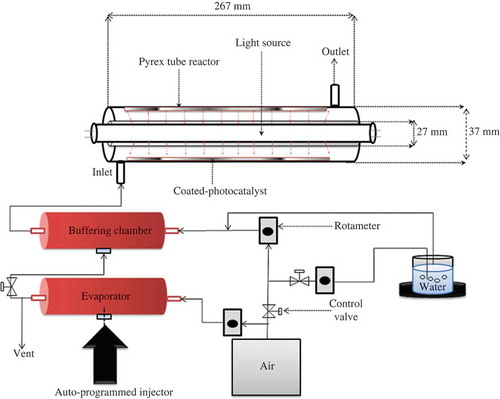
The photocatalytic efficiencies of the GF-Fe-TiO2 photocatalysts with different Fe-to-Ti ratios and GF-TiO2 were investigated for their decomposition of BTEX using a cylindrical-shaped photocatalytic reactor. shows a schematic diagram of the experimental system. The reactor was prepared using a Pyrex tube (length: 26.5 cm, inner diameter: 3.8 cm), containing a cylindrical-shaped daylight lamp (F8T5DL; Youngwha Lamp Co., Seoul, Korea) or a cylindrical-shaped black-light lamp (F8T5BL; Youngwha Lamp Co.) with a peak spectral intensity of 352 nm. The inside wall of the Pyrex tube was covered with either a GF-Fe-TiO2 or a GF-TiO2 sheet. Gas manufacturer-dried zero-grade air was supplied by an air cylinder and was allowed to flow through a carbon filter for repurification. The dried air stream was humidified by passing it through a humidification apparatus, which consisted of water-containing impingers. Standard gases were prepared by mixing the humidified air with vaporous BTEX. Pure liquid BTEX mixture was infused into a syringe, and then the syringe was loaded to an autoprogrammed syringe pump (KdScientific model 210; Holliston, MA) following its operating procedure. Vaporous BTEX standard gases were generated by injecting the liquid BTEX into a heated Pyrex bulb via the syringe pump. For experimental convenience, the input concentration of the model gas was fixed at 0.1 ppm for all target compounds, which was within typical residential indoor air quality concentration levels (Shin and Jo, Citation2013), whereas the air flow rate was fixed to 1 L min−1 and maintained during the 3-hr photocatalytic process. The space velocity was 1.7 cm sec−1, which was calculated by dividing the flow rate by the cross-sectional area of the reactor. The relative humidity was fixed at 45%, which is within a comfortable range for humans. Finally, the model gas was transferred into the photocatalytic reactor to determine the photocatalytic decomposition activity of the photocatalysts. The light intensities provided by the visible- and UV-light sources were 2.7 and 0.4 mW cm−2, respectively, which were determined at the distance between the lamps and the inside wall of the cylindrical-shaped reactor. Air sampling was conducted every hour during the 3-hr photocatalytic process. Air samples were collected at upstream and downstream portions of the reactor using a stainless steel trap containing Tenax GC adsorbent. The gas-phase species adsorbed on the Tenax GC adsorbent trap were analyzed via a gas chromatography/mass spectrometer (GC/MS) (PerkinElmer Clarus SQ 8; Santa Clara, CA) combined with a thermal desorbing device (PerkinElmer ATD 350). The chemical species were qualitatively analyzed based on both their retention times and mass spectra (Wiley software library 275, Palisade Corporation, Ithaca, NY). Quantification of the chemical species was conducted using calibration curves obtained using five concentrations normalized to an internal standard chemical (fluorobenzene). The calibration curve was established using standard gases. For quality control, laboratory blank and spiked standard samples were analyzed. For each experimental day, one laboratory blank trap was analyzed to check for any trap contamination. To check the quantitative response, known standards were daily injected into a trap to transfer the target compounds to the analytical system through the thermal desorbing device. The entire experimental procedure was repeated three times in order to give reliable data, and the mean values are reported in this study.
Results and Discussion
Characteristics of GF-Fe-TiO2 composites with different Fe-to-Ti ratios
The characteristics of pure GF (FE-SEM analysis only), a reference GF-TiO2 photocatalyst, and GF-Fe-TiO2 composites with different Fe-to-Ti ratios were examined by FE-SEM, EDX, XRD, and UV-visible analyses. illustrates the FE-SEM images of the pure GF, reference GF-TiO2, and five Fe-TiO2 composites (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2). There was a noticeable difference between images of the pure GF and photocatalyst-covered GFs. The results confirmed that, although there were some cracks in the coatings, GF was successfully coated with TiO2 and Fe-TiO2 for the GF-TiO2 and GF-Fe-TiO2 composites, respectively. Similarly, Panniello et al. (Citation2012) observed the occurrence of certain cracks during the coating processes of nanostructured TiO2 on silica fibers prepared using a sol-gel process. The gaps between neighboring GFs may allow light to penetrate deep into the photocatalyst-coated GF nets. Moreover, the EDX images of all the GF-Fe-TiO2 composites displayed bands corresponding to O, Ti, Fe, Si, and Pt, whereas GF-TiO2 did not exhibit any band corresponding to Fe (). The bands attributed to O and Ti were ascribed to TiO2, whereas the peak corresponding to Fe was attributed to Fe embedded into the TiO2 lattice. This result is consistent with that of Azad and Hershey (Citation2010), who reported that the EDX spectra of electrospun Fe-TiO2 nanofibers revealed the presence of Fe atoms. The Pt bands were ascribed to the coating of GFs with Pt during a pretreatment step. Additionally, the Fe-to-Ti ratios observed in the EDX images of the GF-Fe-TiO2 composites (apart from 0.05-GF-Fe-TiO2) were consistent with their calculated values, indicating precise synthesis of the composites (). Even for 0.05-GF-Fe-TiO2, the observed Fe-to-Ti ratio (0.06) differed slightly from the calculated ratio (0.05).
Figure 2. Scanning electron microscopy of pure GF, a reference photocatalyst (GF-TiO2), and GF-Fe-TiO2 composites with different Fe-to-Ti ratios (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2).
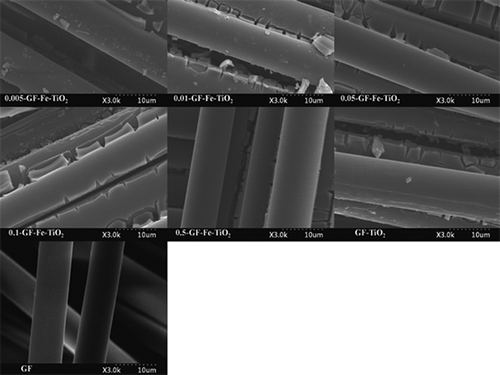
Figure 3. Energy-dispersive X-ray images of a reference photocatalyst (GF-TiO2) and GF-Fe-TiO2 composites with different Fe-to-Ti ratios (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2).
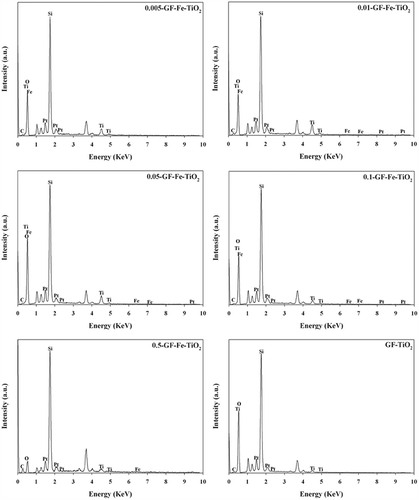
Table 1. Surface characteristics of a reference photocatalysts (GF-TiO2) and GF-Fe-TiO2 composites with various Fe-to-Ti ratios
The XRD images of the reference GF-TiO2 photocatalyst and five Fe-TiO2 composites (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2) are shown in . Both the GF-Fe-TiO2 composites and GF-TiO2 displayed only anatase peaks, with a main peak at 2θ = 25.3°. This was different to the Degussa P25 TiO2 photocatalyst, which revealed both anatase and rutile crystal phase peaks (Jo and Kim, Citation2009). However, our study is consistent with other reports (Cui et al., 2009; Tieng et al., Citation2011), which observed only anatase peaks in their XRD spectra. In addition, no Fe-matched peaks were observed in . Liu et al. (Citation2012) reported a Fe-matched peak at 2θ = 36.9° in the XRD spectra of Fe-TiO2 composites synthesized with an Fe-to-Ti ratio of 0.03. However, they did not observe any Fe-matched peaks for Fe-TiO2 composite prepared with a lower Fe-to-Ti ratio (0.01). Therefore, the low amounts of Fe ions embedded into TiO2 in the GF-Fe-TiO2 composites might be undetectable by the instrument used in this study owing to a limited detection capacity.
Figure 4. X-ray diffraction patterns of a reference photocatalyst (GF-TiO2) and GF-Fe-TiO2 composites with different Fe-to-Ti ratios (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2).
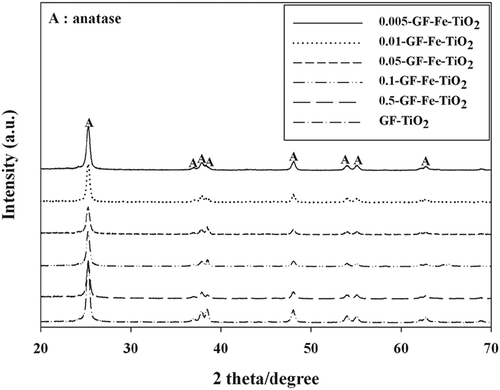
The ratios of anatase crystal and the sizes of crystal phases were calculated using the Spurr (Spur and Myers, Citation1957) and Scherrer (Cullity and Atock, Citation2001) equations, respectively. The Spurr equation states that CA = 1/(1 + 1.265IR/IA), where CA indicates the content of anatase in a catalyst, and IA and IR indicate the intensities of major XRD peaks for the anatase and rutile phases, respectively. Using the Scherrer formula, D = kλ/β cos θ, where k indicates the shape coefficient (0.9), λ indicates the wavelength (Cu Ka = 0.15405 nm), β indicates the full width at half maximum of the most intense peak (in radians), and θ indicates the peak location. As shown in , both the reference GF-TiO2 and the GF-Fe-TiO2 composites revealed only an anatase crystal phase. The diameters of TiO2 and Fe-TiO2 (calculated using the Scherrer formula) were comparable to the diameters determined from their corresponding XRD patterns. In addition, the calculated diameter of TiO2 (21.5 nm) was similar to the calculated diameters of the Fe-TiO2 composites (20.9−21.7 nm). These results indicated that Fe3+ ions did not significantly influence the diameter of the photocatalysts. In contrast, Cui et al. (2009) reported that the diameter of a laboratory-made unmodified TiO2 photocatalyst (12.0 nm) was smaller than the diameters of Fe-TiO2 composites with Fe-to-Ti ratios of 0.001, 0.005, 0.010, and 0.015 (13.4, 12.6, 16.4, and 12.3 nm, respectively). The differences between the two studies were attributed to the different synthetic routes used in preparing the photocatalysts. Cui et al. (2009) used a thermal hydrolysis method, followed by a calcination treatment, whereas we used dop-coating, followed by calcination.
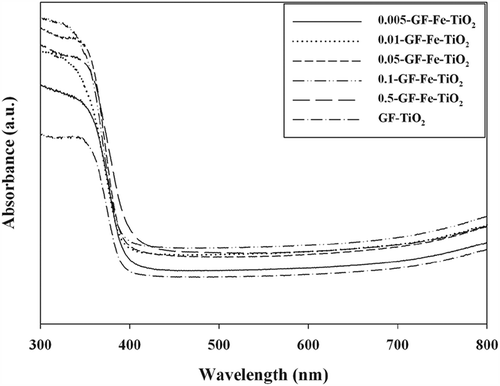
The UV-visible absorbance spectra of the samples (GF-Fe-TiO2 composites and GF-TiO2) are illustrated in . The reference GF-TiO2 photocatalyst displayed an absorption edge at λ ≈ 410 nm, which was consistent with that reported by other researchers (Jo and Kim, Citation2009; Sun et al., Citation2012). However, the absorption spectrum of the GF-Fe-TiO2 composite was substantially shifted toward the visible-light region. This observation was in good agreement with the results reported by Sun et al. (Citation2012). Moreover, the GF-Fe-TiO2 composites showed larger red shifts with increasing amounts of Fe impregnated into TiO2. These results were attributed to rapid charge transfer between TiO2 and Fe resulting from the electron levels created by the embedded Fe; this charge transfer could cause impurities within the band gap range of TiO2 (Cui et al., Citation2010). Therefore, the Fe-TiO2 composites could be used under both visible- and UV-light irradiation.
Photocatalytic decomposition efficiency
The BTEX photocatalytic decomposition efficiencies of the reference TiO2 photocatalyst and as-prepared GF-Fe-TiO2 composites were measured under visible- or UV-light irradiation after 3-hr adsorption processes in the absence of irradiation. In the control experiments (using noncoated GF), the decomposition of BTEX was negligible under both visible and UV light (0% to ≤1%). In addition, the effect of adsorption of BTEX on the surface of the GF-Fe-TiO2 was also negligible under both visible- and UV-light irradiation. illustrates the time-series BTEX photocatalytic efficiencies of the GF-Fe-TiO2 composites and GF-TiO2 under visible-light activation conditions. Point (0,0) indicates that the photocatalytic efficiencies of the GF-Fe-TiO2 composites were nearly zero prior to activation by the light sources. All of the GF-Fe-TiO2 composites showed higher photocatalytic efficiencies for BTEX relative to the reference photocatalyst, GF-TiO2. Specifically, the average BTEX photocatalytic efficiencies of the 0.01-GF-Fe-TiO2 composite over a 3-hr photocatalytic process were 4%, 33%, 51%, and 74%, respectively. Moreover, those obtained for GF-TiO2 were close to 0%, 5%, 16%, and 29%, respectively. In a study of aqueous 4-nitrophenol, Zhao et al. (Citation2010) found that the photocatalytic efficiencies of Fe-TiO2 composites (prepared by embedding Fe(NO3)3 into the anatase phase of TiO2) were higher than those of the pure TiO2. The improved photocatalytic activity of the GF-Fe-TiO2 composites over GF-TiO2 was ascribed to the Fe3+ ions. It was found that Fe3+ would serve as electron and hole trap (Fe3+ + e− → Fe2+ and Fe3+ + h+ → Fe4+), thereby decreasing charge carrier recombination and elevating the photocatalytic activity of the composites (Zhao et al., Citation2010; Sun et al., Citation2012). The presence of Fe3+ ions is further supported by the EDX spectrum (). Fe3+ ions could easily embed in the crystal lattice of TiO2 owing to similar diameters of Fe3+ (0.64 Å) and Ti4+ (0.68 Å) ions (Lu et al., Citation2011). The GF-Fe-TiO2 composites showed lower photocatalytic activity for benzene than for the other target compounds. This was ascribed to the lower photocatalytic oxidation rate of benzene compared with other target chemicals (Strini et al., Citation2005).
Figure 6. Photocatalytic efficiencies (PEs; %) of benzene, toluene, ethylbenzene, and o-xylene as determined via a reference photocatalyst (GF-TiO2) and GF-Fe-TiO2 composites with different Fe-to-Ti ratios (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2) under visible-light irradiation.
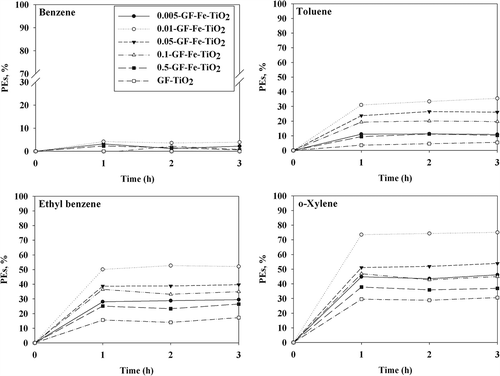
also shows that the BTEX photocatalytic efficiencies for the GF-Fe-TiO2 composites (under visible-light exposure) increased as the Fe-to-Ti ratio increased from 0.005 to 0.010. This was also due to the positive effect of Fe3+ ions as described previously. However, the photocatalytic efficiencies decreased gradually as the Fe-to-Ti ratio increased from 0.010 to 0.50. This was attributed to excess Fe3+ ions, which might serve as centers for charge carrier recombination (Lu et al., Citation2011). Excess Fe3+ ions could also produce Fe(OH)2+, which could interfere with the absorption of photons over the wavelength range 290−400 nm, and subsequently lower photocatalytic performance (Zhao et al., Citation2010). Additionally, it is noteworthy that, although their peak areas were not high enough to be quantified, there were some peaks for compounds other than the target aromatics on the GC chromatogram. This could possibly indicate the formation of by-products. Our preliminary tests also demonstrated that there was no generation of benzene during the photocatalysis of individual compounds of toluene, ethylbenzene, and o-xylene.
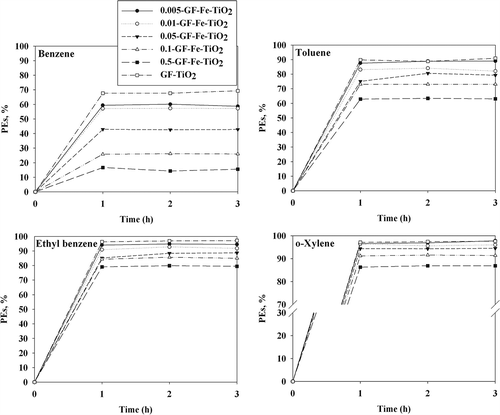
illustrates the BTEX photocatalytic efficiencies of the Fe-TiO2 composites exposed to UV light. Unlike the visible-light conditions, the BTEX photocatalytic efficiencies of GF-Fe-TiO2 composites were lower than those obtained for GF-TiO2. Specifically, the average BTEX photocatalytic efficiencies of the 0.5-GF-Fe-TiO2 composite were 15%, 63%, 79%, and 86%, respectively. Conversely, those obtained for GF-TiO2 were 68%, 90%, 96%, and 97%, respectively. Moreover, the GF-Fe-TiO2 composites displayed different BTEX photocatalytic efficiencies (under visible and UV light) between the various Fe-to-Ti ratios. The BTEX photocatalytic efficiencies of the GF-Fe-TiO2 composites decreased as the Fe-to-Ti ratios increased. These results were also ascribed to Fe ions acting as a recombination center for the charge carriers generated under the UV-light conditions (Cui et al., Citation2010).
Figure 7. Photocatalytic efficiencies (PEs; %) of benzene, toluene, ethylbenzene, and o-xylene as determined via a reference photocatalyst (GF-TiO2) and GF-Fe-TiO2 composites with different Fe-to-Ti ratios (0.005-GF-Fe-TiO2, 0.01-GF-Fe-TiO2, 0.05-GF-Fe-TiO2, 0.1-GF-Fe-TiO2, and 0.5-GF-Fe-TiO2) under UV irradiation.
Conclusion
Flexible glass fiber–supported Fe-TiO2 composites were synthesized using TnBT and Fe(NO3)3 as Ti and Fe sources, respectively. The Fe-TiO2 composites were applied to the photocatalytic treatment of BTEX at indoor concentration levels. FE-SEM results demonstrated that the GF was coated with TiO2 and Fe-TiO2 for the GF-TiO2 and Fe-TiO2 composites, respectively. EDX spectroscopy of the GF-Fe-TiO2 composites demonstrated that Fe ions were embedded into the crystal lattice of TiO2. Furthermore, EDX spectroscopy showed that the desired compositions of the Fe-TiO2 composites were achieved during synthesis. UV-visible spectroscopy demonstrated that the GF-Fe-TiO2 composites could be used under both visible- and UV-light conditions. In addition, the visible light–activated GF-Fe-TiO2 composites displayed a photocatalytic performance superior to that of the reference GF-TiO2 photocatalyst for the treatment of BTEX. However, the results were reversed for the UV-activated GF-Fe-TiO2 composites. Because visible-light sources consume a lower energy compared with UV-light sources, when considering energy efficiency, use of GF-Fe-TiO2 that shows a higher photocatalytic activity with a low-energy visible-light source is preferred. In addition, the BTEX photocatalytic decomposition efficiencies for the GF-Fe-TiO2 composites varied with Fe-to-Ti ratio, suggesting that a Fe-to-Ti ratio of 0.01 was optimal.
Acknowledgment
The authors would like to thank Mr. Joon Yeob Lee, who is a Ph.D. student in the Department of Environmental Engineering, Kyungpook National University, for his experimental works.
Funding
This work was supported by the Rural Development Administration Korea (PJ00923504) and the National Research Foundation of Korea (NRF) grant funded through GCRC-SOP (no. 2011-0030013).
Additional information
Funding
Notes on contributors
Sung-Bong Yang
Sung-Bong Yang is a professor at the Department of Chemistry, University of Ulsan, Ulsan, Republic of Korea.
Ho-Hwan Chun
Ho-Hwan Chun is a professor at the Department of Naval Architecture and Ocean Engineering, Pusan National University, Busan, Republic of Korea.
Rajesh J. Tayade
Rajesh J. Tayade is a senior technical officer at the Discipline of Inorganic Materials and Catalysis, Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research (CSIR-CSMCRI), Gujarat, India.
Wan-Kuen Jo
Wan-Kuen Jo is a professor at the Department of Environmental Engineering of Kyungpook National University, Daegu, Republic of Korea.
References
- Azad, A.-M., and R. Hershey. 2010. Bactericidal efficacy of electrospun pure and Fe- doped titania nanofibers. J. Mater. Res. 25:1761–1770. doi:10.1557/JMR.2010.0237
- Bianchi, C., C. Pirola, E. Selli, and S. Biella. 2012. Photocatalytic NOx abatement: The role of the material supporting the TiO2 active layer. J. Hazard. Mater. 211–212:203–207. doi:10.1016/j.jhazmat.2011.10.095
- Bouras, P., E. Stathatos, and P. Lianos. 2007. Pure versus metal-ion-doped nanocrystalline titania for photocatalysis. Appl. Catal. B 73:51–59. doi:10.1016/j.apcatb.2006.06.007
- Cui, L., F. Huang, M. Niu, L. Zeng, J. Xu, and Y. Wang. 2010. A visible light active photocatalyst: Nano-composite with Fe-doped anatase TiO2 nanoparticles coupling with TiO2 (B) nanobelts. J. Mol. Catal. A 326:1–7. doi:10.1016/j.molcata.2010.04.013
- Cullity, B.D., and S.R. Stock. 2001. Elements of X-ray Diffraction, 3rd ed., 167–171. Upper Saddle River, NJ: Prentice Hall.
- Esplugues, A., F. Ballester, M. Estarlich, S. Llop, V. Fuentes-Leonarte, E. Mantilla, and C. Iñiguez. 2010. Indoor and outdoor air concentrations of BTEX and determinants in a cohort of one-year old children in Valencia, Spain. Sci. Total Environ. 409:63–69. doi:10.1016/j.scitotenv.2010.09.039
- Folli, P., C. Pade, T.B. Hansen, T. De Marco, and D.E. Macphee. 2012. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cement Concrete Res. 42:539–548. doi:10.1016/j.cemconres.2011.12.001
- Gaya, U.I., and A.H. Abdullah. 2008. Heterogeneous photocatalytic decomposition of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 9:1–12. doi:10.1016/j.jphotochemrev.2007.12.003
- Henderson, M.A. 2011. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 66:185–297. doi:10.1016/j.surfrep.2011.01.001
- Inturi, S.N.R., T. Boningari, M. Suidan, and P.G. Smirniotis. 2014. Visible-light- induced photodecomposition of gas phase acetonitrileusing aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B 144:333–342. doi:10.1016/j.apcatb.2013.07.032
- Jo, W.K., and J.T. Kim. 2009. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 164:360–366. doi:10.1016/j.jhazmat.2008.08.033
- Jo, W.K., S.H. Shin, and E.S. Hwang. 2011. Removal of dimethyl sulfide utilizing activated carbon fiber-supported photocatalyst in continuous-flow system. J. Hazard. Mater. 191:234–239. doi:10.1016/j.jhazmat.2011.04.069
- Kedem, S., D. Rozen, Y. Cohen, and Y. Paz. 2009. Enhanced stability effect in composite polymeric nanofibers containing titanium dioxide and carbon nanotubes. J. Phys. Chem. C 113:14893–14899. doi:10.1021/jp9007366
- Lan, Y., Y. Lu, and Z. Ren. 2013. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energ. 2: 1031–1045. doi:10.1016/j.nanoen.2013.04.002
- Leuchner, M., and B. Rappenglück. 2010. VOC source–receptor relationships in Houston during TexAQS-II. Atmos. Environ. 44:4056–4067. doi:10.1016/j.atmosenv.2009.02.029
- Lin, S., X. Zhang, Q. Sun, T. Zhou, and J. Lu. 2013. Fabrication of solar light induced Fe-TiO2 immobilized on glass-fiber and application for phenol photocatalytic decomposition. Mater. Res. Bull. 48:4570–4575. doi:10.1016/j.materresbull.2013.07.063
- Liu, L., F. Chen, F. Yang, Y. Chen, and J. Crittenden. 2012. Photocatalytic decomposition of 2,4-dichlorophenol using nanoscale Fe/TiO2. Chem. Eng. J. 181– 182:189–195. doi:10.1016/j.cej.2011.11.060
- Lu, X., Y. Ma, B. Tian, and J. Zhang. 2011. Preparation and characterization of Fe- TiO2 films with high visible photoactivity by autoclaved-sol method at low temperature. Solid State Sci. 13:625–629. doi:10.1016/j.solidstatesciences.2010.12.036
- Matos, J., E. García-López, L. Palmisano, A. García, and G. Marcì. 2010. Influence of activated carbon in TiO2 and ZnO mediated photo-assisted decomposition of 2- propanol in gas–solid regime. Appl. Catal. B 99: 170–180. doi:10.1016/j.apcatb.2010.06.014
- Panniello, A., M.L. Curri, D. Diso, A. Licciulli, V. Locaputo, A. Agostiano, R. Comparelli, and G. Mascolo. 2012. Nanocrystalline TiO2 based films onto fibers for photocatalytic decomposition of organic dye in aqueous solution. Appl. Catal. B 121–122:190–197. doi:10.1016/j.apcatb.2012.03.019
- Paola, A.D., and E. García-López. 2012. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 211–212:3–29.
- Park, H., Y. Park, W. Kim, and W. Choi. 2013. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C 15:1–20. doi:10.1016/j.jphotochemrev.2012.10.001
- Paz, Y. 2010. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B 99:448–460. doi:10.1016/j.apcatb.2010.05.011
- Ramírez, N., A. Cuadras, E. Rovira, F. Borrull, and R.M. Marcé. 2012. Chronic risk assessment of exposure to volatile organic compounds in the atmosphere near the largest Mediterranean industrial site. Environ. Int. 39: 200–209. doi:10.1016/j.envint.2011.11.002
- Shin, S.H., and W.K. Jo. 2012. Volatile organic compound concentrations, emission rates, and source apportionment in newly-built apartments at pre-occupancy stage. Chemosphere 89:569–578. doi:10.1016/j.chemosphere.2012.05.054
- Shin, S.H., and W.K. Jo. 2013. Longitudinal variations in indoor VOC concentrations after moving into new apartments and indoor source characterization. Environ. Sci. Pollut. Res. 20:3696–3707. doi:10.1007/s11356-012-1296-z
- Spurr, R.A., and H. Myers. 1957. Quantitative analysis of anatase-rutile mixtures with a X-ray diffractometer. Anal. Chem. 29:760–762. doi:10.1021/ac60125a006
- Strini, A., S. Cassese, and L. Schiavi. 2005. Measurement of benzene, toluene, ethyl benzene and o-xylene gas phase photodecomposition by titanium dioxide dispersed in cementitious materials using a mixed flow reactor. Appl. Catal. B 61:90–97. doi:10.1016/j.apcatb.2005.04.009
- Sun, S., J. Ding, J. Bao, C. Gao, Z. Qi, X. Yang, B. He, and C. Li. 2012. Photocatalytic decomposition of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 258:5031–5037. doi:10.1016/j.apsusc.2012.01.075
- Tieng, S., A. Kanaev, and K. Chhor. 2011. New homogeneously impregnated Fe(III)- TiO2 photocatalyst for gaseous pollutant decomposition. Appl. Catal. A 399:191–197. doi:10.1016/j.apcata.2011.03.056
- Vargas, X., E. Tauchert, J.-M. Marin, G. Restrepo, R. Dillert, and D. Bahnemann. 2012. Fe-impregnated titanium dioxide synthesized: Photocatalytic activity and mineralization study for azo dye. J. Photochem. Photobiol. A 243: 17–22. doi:10.1016/j.jphotochem.2012.06.001
- Yu, C., F. Cao, G. Li, R. Wei, J.C. Yu, R. Jin, Q. Fan, and C. Wang. 2013. Novel metal (Rh, Pd, Pt)/BiOX(Cl, Br, I) composite photocatalysts with enhanced photocatalytic performance in dye decomposition. Sep. Purif. Technol. 120:110–122. doi:10.1016/j.seppur.2013.09.036
- Zhao, B., G. Mele, I. Pio, J. Li, L. Palmisano, and G. Vasapollo. 2010. Degradation of 4-nitrophenol (4-NP) using Fe-TiO2 as a heterogeneous photo-Fenton catalyst. J. Hazard. Mater. 176:569–574. doi:10.1016/j.jhazmat.2009.11.066