ABSTRACT
Real-time measurements of short-chain (C < 8) per- and polyfluoroalkyl substances (PFAS) were performed in Central New Jersey air using chemical ionization mass spectrometry (CIMS). The CIMS was calibrated for C2 – C6 perfluorinated carboxylic acids, and 4:2 and 6:2 fluorotelomer alcohols. Of these, only trifluoroacetic acid (TFA) was detected in ambient air above instrumental detection limits. However, instrumental sensitivities (and thus ambient mixing ratios) were estimated for other detected PFAS including C3H2F6O and C6HF11O3. TFA mixing ratios reached up to 0.7 parts-per-trillion by volume (pptv). Estimated C3H2F6O and C6HF11O3 mixing ratios reached the single pptv level. These latter two formulas are consistent with hexafluoroisopropanol (HFIP) and hexafluoropropylene oxide dimer acid (HFPO-DA) respectively, though they may potentially represent multiple isomers. Diel profiles of detected PFAS, along with local meteorological data can provide information on potential local sources of these compounds. However, only limited discussion of potential sources was provided here given the sparse detection of these compounds above instrument detection limits. These results demonstrate the potential of online CIMS instrumentation for measuring certain PFAS in ambient outdoor air in real time at or below the pptv level. This technique also has potential for fenceline monitoring and other near-source applications.
Implications: Online chemical ionization mass spectrometry (CIMS) has potential for fast, real-time measurements of certain airborne per- and polyfluoroalkyl substances (PFAS). Three short-chain (C < 8) PFAS were detected by CIMS in Central New Jersey ambient air near or above the parts-per-trillion by volume (pptv) level. This technique also has potential for fenceline monitoring and other near-source applications for airborne PFAS.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
Per‐ and polyfluoroalkyl substances (PFAS) include a wide variety of manufactured compounds commonly used to make products more resistant to stains, grease, water, and heat (1). They are also key chemicals associated with the production of fluorinated polymers (1). PFAS compounds are known to be released to the environment. PFAS compounds can be extremely persistent in the environment and in the human body (1, 2). Coupling these facts with their ubiquitous persistent nature makes PFAS an important class of contaminants of emerging concern for the U.S. Environmental Protection Agency (EPA), and other environmental regulatory organizations.
Several PFAS are sufficiently volatile under ambient conditions to exist as airborne gas-phase pollutants (3, 4). The outdoor atmosphere thereby serves as an important environmental transport and exposure route for these pollutants (4, 5). Despite this, environmental PFAS measurements have historically focused on water and soil sampling. Previous efforts to measure airborne PFAS have typically involved offline measurement techniques (6), which are subject to low time resolution, and require external laboratory analyses of collected samples, which is often intensive and time-consuming.
Recently, online chemical ionization mass spectrometry (CIMS) instrumentation has been applied to measuring PFAS in the gas-phase (3, 7-12). Iodide (I−) is a common reagent ion used for the chemical ionization of polar atmospheric analytes. CIMS utilizing iodide reagent ions (hereinafter “iodide CIMS”) has historically been used for fast (~1 Hz), online atmospheric measurements of oxygenated and/or nitrogenated organics, and certain inorganic nitrogen (e.g., N2O5, HONO) and halogenated (e.g., Cl2, ClNO2) compounds (13, 14). Riedel et al. (3) demonstrated the sensitivity and selectivity of iodide CIMS toward fluorotelomer alcohols (FTOHs), perfluorinated carboxylic acids (PFCAs), and perfluoroether carboxylic acids (PFECAs). Other recent studies have used iodide CIMS to measure gas-phase PFAS as volatile emissions from aqueous film-forming foams (AFFFs) (10), fluoropolymer coating (9), calcium oxide thermal treatment of FTOHs (7), photochemical oxidation of polyfluorinated ethers (11), and laboratory-generated aqueous aerosols containing perfluorooctane sulfonic acid (PFOS) and perfluoroalkyl phosphoric acid diesters (diPaPs) (12).
This work discusses a recent deployment of an iodide CIMS instrument to the EPA/Office of Research and Development (ORD)/Center for Environmental Solutions and Emergency responses (CESER) facility in Edison, New Jersey for sampling gas-phase PFAS in ambient air during July – August 2023. In-field calibrations for C2 – C6 PFCAs, 4:2 FTOH, and 6:2 FTOH, plus post-field estimations of instrumental sensitivities (and ambient mixing ratios) for a limited set of other gas-phase PFAS detected by the CIMS were performed. While only a limited number of short-chain PFAS (C < 8) were detected and quantified in ambient air for brief periods, results presented here nonetheless demonstrate the potential for measuring certain PFAS in ambient air at or below the parts-per-trillion by volume (pptv) level.
Experimental methods
Field site description and sampling setup
Field sampling took place on a grassy field located on the EPA/Office of Research and Development (ORD)/Center for Environmental Solutions and Emergency responses (CESER) facility in Edison, New Jersey (40.508638, -74.356864). The iodide CIMS and other technology relevant to field sampling were housed in an air-conditioned instrument trailer parked on the field. Field sampling took place between 20 July and 10 August 2023. Field site photos are provided in .
Ambient air outside the trailer was continuously sampled by the iodide CIMS for the online, real-time detection of gas-phase PFAS. Sampled ambient air flowed through a 10-foot length of ¼” outer diameter (OD), high-density polyethylene (HDPE) tubing controlled to 40 °C (Clayborn Lab) to mitigate condensation of ambient water to tubing surfaces leading to potential loss of water-soluble analytes. HDPE has been demonstrated to have relatively short passivation times (and therefore measurement delays) compared to stainless steel and Silcosteel tubing for measuring various short-chain PFAS (8). Unlike perfluoroalkoxy alkane (PFA), fluorinated ethylene propylene (FEP), or other fluorinated polymers commonly used as tubing materials for air sampling, HDPE did not outgas small, fluorinated molecules (e.g., trifluoroacetic acid) that may interfere with CIMS measurements of PFAS in ambient air. Fluoropolymer materials were avoided in all other parts of the sampling setup wherever possible per this reasoning. Sample flow through the HDPE inlet was controlled by the sampling flow rate into the iodide CIMS inlet (~2 L min−1), and a bypass pump (GAST) added in-line to increase total sample flow rate to ~4 L min−1. Total inlet residence time was ~1 s. An overflow of ultra-zero grade air (UZA; Airgas) was delivered through 1/8” OD stainless steel tubing to the top of the sampling inlet to monitor instrument background signals of detected analytes (e.g., zeroing). These instrument zeroes were performed for 10 minutes once every two hours. UZA flow rates were controlled via mass-flow controller (MFC; Alicat Scientific), which were automated using FlowVision 2.0 software (Alicat Scientific).
Chemical ionization mass spectrometry (CIMS) operation and data analysis
Measurements of gas-phase PFAS (and other analytes) in ambient air were performed using an online time-of-flight (TOF) CIMS (Tofwerk AG and Aerodyne Research Inc.) operating in iodide ionization mode (13, 14). To generate reagent ions, ~2 L min−1 ultra-high purity nitrogen (UHP N2; Airgas) flowed through a temperature-controlled oven (40 °C; Aerodyne Research Inc.) containing an iodomethane (CH3I) permeation device to yield a gaseous N2 + CH3I mixture. This mixture then flowed through a 210Po ionizer (NRD) to generate I− and I(H2O)− reagent ions, which subsequently entered the ion-molecule reactor (IMR) region of the CIMS. Sampled ambient air entered the IMR orthogonally to reagent ion flow at ~2 L min−1. Analytes of interest (M) within sampled ambient air reacted with reagent ions to yield iodide-analyte adducts ([I+M]−), which then entered the long time-of-flight (LTOF) region of the CIMS for detection. We did not observe substantial formation of deprotonated ([M-H]−) PFAS as product ions during ambient sampling or calibrations. Given a lack of sample pre-separation (e.g., chromatography), detected chemical formulas may represent a sum of multiple isomers. TOF-CIMS spectra were collected between 30-1500 mass-to-charge (m/z) units, averaged to 0.3 Hz data using Acquility data acquisition software (v.2.3.13; Tofwerk AG) operating in Igor Pro 7 (WaveMetrics Inc.). Mass spectral peak resolution (m/dm) was approximately 12000, and mass accuracy was typically <5 ppm.
Iodide CIMS data were processed using Tofware (v3.2.3; Tofwerk AG) operating in Igor Pro 7, which performed mass calibrations to convert time-of-flight arrival times to m/z, mass spectral baseline calculation and subtraction, and high-resolution peak fitting algorithms to extract CIMS signal time series from individual mass spectral peaks. NO3−, I(H2O)−, I(HNO3)−, I2−, and I3− were used as mass calibration peaks, as they were fully resolved and ubiquitous as CIMS background peaks. All further (e.g., post-processing) analyses of CIMS data took place in Igor Pro 7, including CIMS data normalization, background subtraction using bi-hourly zero measurement data, and additional statistical calculations. Raw and normalized MS signals are in units of ion counts per second (or Hz). CIMS data were normalized to the total reagent ion signal (e.g., multiplied by 106 Hz/[I− + I(H2O)−]) to ensure variations in CIMS signal was not driven by variability in reagent ion signal (13).
CIMS calibrations and sensitivity estimations
Iodide CIMS calibrations were performed for trifluoroacetic acid (TFA), perfluoropropanoic acid (PFPrA), perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), 4:2 FTOH, and 6:2 FTOH following methods of Riedel et al. (3). Briefly, calibrant solutions of these analytes were prepared in the concentration range of 1 – 10 ng µL−1 in ethyl acetate. Aliquots of 1-5 µL of these solutions were injected onto and subsequently desorbed from a PTFE filter into the IMR region of the instrument. These calibrations were performed in-field but following conclusion of field measurements to prevent emissions of TFA and other small PFAS molecules from the PTFE from compromising quantification of ambient PFAS. Any signals attributable to small PFAS from the calibration apparatus were captured during background measurements while flowing only UZA through the apparatus, and subtracted from calibrant signals. Integrated calibrant signals detected by the CIMS were then related to total desorbed mass of calibrant for each injection to determine CIMS sensitivities toward these compounds (). Instrumental limits of detection (LODs) were calculated following methods in Riedel et al. (3) (). An LOD value was not assigned to PFHxA given its relatively large error (~270%) in calculated sensitivity.
Iodide CIMS instrumental response toward N2O5 is often used to estimate a theoretical maximum instrument sensitivity. This is because N2O5 undergoes ion-molecule reactions with iodide at the collision limit, and thus yields product ions (and therefore CIMS signal) at maximum efficiency (15). Thus, the iodide CIMS was calibrated for N2O5 post-campaign following methods in Bertram et al. (16) to determine a theoretical upper limit sensitivity, which can be used to conservatively estimate lower-bound mixing ratios for detected analytes (such as PFAS) without available calibration standards. This yielded a theoretical maximum sensitivity of 24 ± 2 Hz pptv−1. CIMS sensitivities from in-field PFCA calibrations were within experimental error of the theoretical maximum (except for PFPrA; ) suggesting these PFAS molecules also underwent collision-limited reactions with iodide to form strongly-bound adducts (15). PFPrA had a sensitivity of 60 ± 10 Hz pptv−1. This may be due to improper PFPrA solution preparation, or another unknown cause. We additionally note this theoretical maximum sensitivity was not corrected for mass-dependent ion transmission effects, which may not be uniform across the full range of m/z detected herein (17). The theoretical maximum sensitivity derived from N2O5 calibrations was used to conservatively estimate mixing ratios of C3H2F6O and C6HF11O3—two uncalibrated PFAS detected in ambient air.
Table 1. Iodide CIMS sensitivities and LODs for PFCAs and FTOHs
Figure 1. Photos from the field site. (A) The instrument trailer located on a grassy field at the EPA/ORD/CESER facility in Edison, NJ. (B) A view of the air sampling inlet extending from the ceiling of the trailer. (C) A view of the inlet delivering sampled ambient air to the iodide CIMS instrument inside the trailer.

Results and discussion
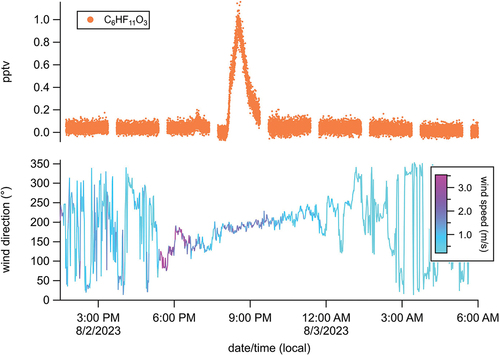
With the exception of TFA, the iodide CIMS did not detect any of the calibrated PFAS significantly above instrument background levels. However, it detected above-background levels of other uncalibrated PFAS compounds including C3H2F6O and C6HF11O3. Thus, this discussion will be limited to measured mixing ratios of TFA, and estimated mixing ratios of C3H2F6O, and C6HF11O3.
Ambient mixing ratios of TFA are reported in . Less than 1% of TFA measurement data were above the instrument LOD (0.4 pptv; 20 ng m−3). Data above the LOD reached up to ~0.7 pptv (~30 ng m−3). Previous measurements of monthly average TFA concentrations in urban air in Beijing reached up to the single ng m−3 level (18, 19), which is considerably below the reported detection limit of the iodide CIMS. Surface deposition of TFA contributes to its persistence as an environmental pollutant in water and soil (20-22). Its emission to the air may also impact atmospheric aerosol nucleation (23).
Relative maxima in TFA mixing ratios typically took place between the hours of 08:00 and 12:00 local time (). This may be consistent with a primary morning rush-hour emission source from nearby automobile traffic, and/or some degree of photochemical aging of an airborne precursor. For instance, the photochemical aging of 2,3,3,3-tetrafluoropropene (known commercially as “HFO-1234yf” or “R-1234yf”) is believed to be an important photochemical precursor to atmospheric TFA (20). HFO-1234yf is used as a contemporary automotive refrigerant, replacing the previously used R-134a (1,1,1,2-tetrafluoroethane) due to its lower global warming potential. These diel profiles also indicate that TFA mixing ratios begin to rise around 06:00, when air temperature starts to rise and prior to any substantial near-surface photochemistry (), suggesting volatilization of deposited TFA from outdoor surfaces may be a more important local source than photochemistry. Binned TFA data used to evaluate these diurnal trends include data below instrument LOD and thus may be of limited reliability. Given this, further speculation of local atmospheric sources of TFA is not provided.
Estimated mixing ratios of C3H2F6O reached the single pptv (~102 ng m−3) level several times throughout the measurement campaign (). These concentration maxima were typically short lived (1-2 hours) and did not follow any obvious diel trends (). This may be consistent with primary emissions from nearby source(s) transported to the measurement site. However, there was also no obvious relationship between these concentration spikes and wind speed/direction data provided from a nearby meteorological station (Rutgers PAM site; http://pamsite.rutgers.edu/), suggesting these emissions came from no one particular direction at random times of day.
The chemical formula C3H2F6O is consistent with hexafluoro-2-propanol (or hexafluoroisopropanol; HFIP), a fluoroalcohol and solvent used in various chemical syntheses and industrial processes (24, 25). The high vapor pressure of HFIP (16 kPa at 20 °C) would lend to its volatilization and subsequent transport in the atmosphere. To our knowledge, there are no existing measurements of HFIP in the atmosphere, though it is a likely greenhouse gas with a calculated global warming potential hundreds of times greater than CO2 (26). This formula may also correspond to 1,2,2,2-tetrafluoroethyl difluoromethyl ether (commercially known as “desflurane”), though iodide CIMS is typically not sensitive toward organic ethers when compared to hydrogen-bonding moieties (15, 27). Confirmation via iodide CIMS calibrations using authentic standards is suggested to evaluate relative instrument sensitivities toward there isomers.
Estimated ambient mixing ratios for C6HF11O3 are reported in . This chemical formula is consistent with hexafluoropropylene oxide dimer acid (HFPO-DA), a PFECA and chemical component of Chemours’ GenX process (4, 28, 29). Except for one brief period, C6HF11O3 was not measured substantially above instrument background levels. A spike in ambient C6HF11O3 levels was observed on 2 August 2023 between 20:00 and 22:00 local time, where estimated mixing ratios reached approximately 1 pptv (or ~100 ng m−3) (). This period coincided with prevailing southerly winds, suggestive of an emission source in that direction (). However, given that lack of other HFPO-DA observations throughout the campaign, further speculation about sources is not provided. D’Ambro et al. (4) recently estimated that primary emissions of GenX compounds may be detectible in the atmosphere at ng m−3 quantities at distances of 10 km or further from chemical production facilities using and releasing these chemicals. Airborne concentrations are expected to decrease substantially with distance due to deposition and other loss processes (4). Emission stack height and plume temperature are likely influential in the dispersion of this compound downwind of a source. Evaluation of dilution distances for PFAS from emission sources has not been experimentally evaluated, and field exploration is warranted.
Figure 2. Time series of TFA mixing ratios (top panel) and estimated mixing ratios of C3H2F6O (middle panel) and C6HF11O3 (bottom panel). Mixing ratios are in units of parts-per-trillion by volume (pptv).
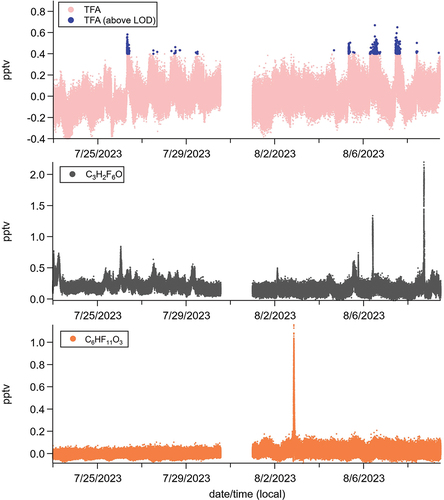
Figure 3. Diel profiles of TFA mixing ratios (top panel), estimated C3H2F6O mixing ratios (middle panel), relative CIMS signals for C5H10O3 (bottom panel; left axis), and air temperature near the field site (bottom panel; right axis). C5H10O3 likely represents a sum of isoprene hydroxyhydroperoxides (ISOPOOH) and epoxydiols (IEPOX), which are tracers for near-surface secondary photochemical production (30). Shaded area represents ± one standard deviation of binned (averaged) mixing ratio data. Binned averages of TFA mixing ratios include data below instrumental LOD, and thus may not be reliable. Air temperature data are from the Rutgers PAM meteorological station (http://pamsite.rutgers.edu/).
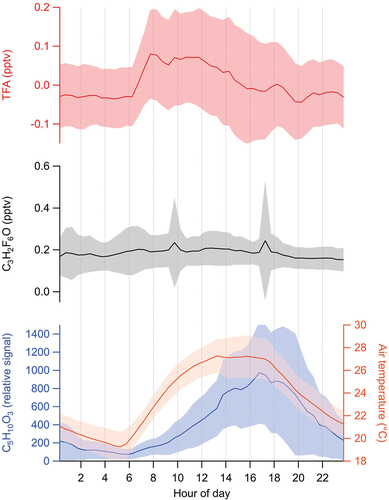
Figure 4. Estimated C6HF11O3 mixing ratios (top panel) and wind speed and direction (bottom panel) between 13:30 on 8/2/2023 and 06:00 on 8/3/2023. Wind direction data are reported in units of degrees, with 0° representing northerly winds, 90° representing easterly winds, 180° representing southerly winds, and 270° representing westerly winds. Wind speed and direction data are from the Rutgers PAM meteorological station (http://pamsite.rutgers.edu/).
Conclusions
This field deployment demonstrated the potential of online iodide CIMS instrumentation for measuring certain PFAS in ambient outdoor air at or below the parts-per-trillion by volume (pptv) level. TFA was measured at quantities up to 0.7 pptv (~30 ng m−3), and C3H2F6O and C6HF11O3 were estimated to reach up to single parts-per-trillion levels (~102 ng m−3). Nearby point, area, and mobile sources potentially contributed to these observations (or precursors thereof). While the detection of these short-chain PFAS at reliably quantifiable levels was sparse, this work represents a crucial step forward in performing fast, real-time measurements of airborne PFAS to better understand their atmospheric sources and sinks, and subsequent environmental and health impacts.
Future field sampling efforts may improve upon this work considerably:
Directly calibrating the CIMS for a greater number of PFAS will provide more certainty in quantified mixing ratios of detected species. Use of online CIMS for measuring airborne PFAS is still a developing technique. Thus, developing robust, field-ready CIMS calibration methods is essential for reliable real-time measurements of PFAS in ambient air. Additional optimizations aimed at lowering instrument detection limits (e.g., longer signal averaging periods (31)) and further mitigating potential loss within sampling inlet lines will enhance the utility of online CIMS for measuring airborne PFAS at atmospherically-relevant levels (e.g., ng m−3 or lower).
While Central New Jersey may be an opportune place to attempt measuring airborne PFAS (5), the field site discussed herein may have been distant from any nearby sources of PFAS, and was surrounded by several tall trees and other foliage onto which airborne PFAS may deposit or at paired upwind/downwind locations surrounding an anticipated source. Future studies may benefit from deploying in locations more representative of urban and/or industrial areas with airsheds directly impacted by nearby sources of PFAS emissions. For instance, Washington et al. (5) recently measured chloroperfluoropolyether carboxylates in New Jersey soils, which they attributed to airborne transport of fluorochemical industrial emissions. While these compounds were not observed in this study, their detection in ambient air (as free acids) may be possible with iodide CIMS under optimized sampling conditions. Future field sampling efforts using this technique could also include fenceline and other near-source measurements of airborne PFAS.
While the meteorological data presented herein were fairly close to the sampling site (~8 km away), the air temperature and wind speed and direction data collected at the nearby station may not accurately represent meteorological conditions at the location of CIMS operation. Co-locating meteorological measurements with online CIMS instrumentation is essential for reliable analysis of directional sources and diel analysis of airborne PFAS.
response to reviewers.docx
Download MS Word (18.8 KB)Mattila 2024 - Measuring PFAS in NJ air using CIMS - Revision (track changes).docx
Download MS Word (27.3 MB)Acknowledgements
The authors thank Michael Borst and James Faircloth of U.S. EPA Office of Research and Development (ORD) for their assistance with field site setup and logistics, Ryan Fulgham of U.S. EPA ORD for assistance with N2O5 calibration methods, Jonathan Krug of U.S. EPA ORD for project support, and Matthew Drews of Rutgers Photochemical Assessment Monitoring Station (PAMS) for providing meteorological data collected at the PAMS Site. The U.S. EPA through its Office of Research and Development supported the research described here. It has NOT been subjected to Agency administrative review and is NOT yet approved for publication and may not reflect official Agency policy. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the U.S. Environmental Protection Agency. EPA and its employees do not endorse any commercial products, services, or enterprises. J.M.M. was supported by the Oak Ridge Institute for Science and Education (ORISE) Research Participation Program for the U.S. Environmental Protection Agency (EPA).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available at https://doi.org/10.23719/1530551.
Additional information
Notes on contributors
James M. Mattila
James M. Mattila was an Oak Ridge Institute for Science and Education (ORISE) postdoctoral fellow at the U.S. Environmental Protection Agency (EPA) Office of Research and Development (ORD) at the time of this work. He received his PhD in Chemistry from Colorado State University in 2021. His research focuses on studying airborne PFAS using online mass spectrometry instrumentation. He now works for U.S. EPA Office of Air and Radiation developing air pollution emission regulations.
John H. Offenberg
John H. Offenberg is a Research Chemist at U.S. EPA ORD. His research focuses on photochemical transformations in the atmosphere.
References
- J. Glüge et al. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22, 2345–2373 (2020).
- De Silva, A.O., J.M. Armitage, T.A. Bruton, C. Dassuncao, W. Heiger‐Bernays, X.C. Hu, A. Kärrman, B. Kelly, C. Ng, A. Robuck, et al., PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem.. 40, 631–57 (2021). 3 10.1002/etc.4935
- T. P. Riedel, J. R. Lang, M. J. Strynar, A. B. Lindstrom, J. H. Offenberg, Gas-Phase Detection of Fluorotelomer Alcohols and Other Oxygenated Per-and Polyfluoroalkyl Substances by Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett.. 6, 289–293 (2019).
- E. L. D’Ambro et al. Characterizing the air emissions, transport, and deposition of per-and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environ. Sci. Technol.. 55, 862–870 (2021).
- Washington, J.W., C.G. Rosal, J.P. McCord, M.J. Strynar, A.B. Lindstrom, E.L. Bergman, S.M. Goodrow, H.K. Tadesse, A.N. Pilant, B.J. Washington, et al., Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science 368, 1103–07 (2020). 6495 10.1126/science.aba7127
- S. F. Nakayama et al. Worldwide trends in tracing poly-and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends Anal. Chem. 121, 115410 (2019).
- T. P. Riedel et al. Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide. Chemosphere 272, 129859 (2021).
- J. M. Mattila, E. Y. Li, J. H. Offenberg, Tubing material considerably affects measurement delays of gas-phase oxygenated per-and polyfluoroalkyl substances. Journal of the Air & Waste Management Association 73, 335–344 (2023).
- Wickersham, L.C., J.M. Mattila, J.D. Krug, S.R. Jackson, M.A.G. Wallace, E.P. Shields, H. Halliday, E.Y. Li, H.K. Liberatore, S.(. Farrior, et al., Characterization of PFAS Air Emissions from Thermal Application of Fluoropolymer Dispersions on Fabrics. Journal of the Air & Waste Management Association 73, 533–52 (2023). 7 10.1080/10962247.2023.2192009
- J. M. Mattila et al. Characterizing Volatile Emissions and Combustion Byproducts from Aqueous Film-Forming Foams Using Online Chemical Ionization Mass Spectrometry. Environ. Sci. Technol.. 10.1021/acs.est.1023c09255 (2024).
- A. P. Folkerson, S. R. Schneider, J. P. Abbatt, S. A. Mabury, Avoiding Regrettable Replacements: Can the Introduction of Novel Functional Groups Move PFAS from Recalcitrant to Reactive? Environ. Sci. Technol.. 57, 17032–17041 (2023).
- B. B. Bowers, J. A. Thornton, R. C. Sullivan, Evaluation of iodide chemical ionization mass spectrometry for gas and aerosol-phase per-and polyfluoroalkyl substances (PFAS) analysis. Environ Sci Process Impacts 25, 277–287 (2023).
- B. H. Lee et al. An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: application to atmospheric inorganic and organic compounds. Environ. Sci. Technol.. 48, 6309–6317 (2014).
- B. H. Lee et al. Flight deployment of a high‐resolution time‐of‐flight chemical ionization mass spectrometer: observations of reactive halogen and nitrogen oxide species. J. Geophys. Res. Atmos. 123, 7670–7686 (2018).
- F. D. Lopez-Hilfiker et al. Constraining the sensitivity of iodide adduct chemical ionization mass spectrometry to multifunctional organic molecules using the collision limit and thermodynamic stability of iodide ion adducts. Atmos. Meas. Tech.. 9, 1505–1512 (2016).
- Bertram, T., J. Thornton, and T. Riedel, An experimental technique for the direct measurement of N2O5 reactivity on ambient particles. Atmos. Meas. Tech.. 2, 689–723 (2009).10.5194/amt-2-231-2009
- M. Heinritzi et al. Characterization of the mass-dependent transmission efficiency of a CIMS. Atmos. Meas. Tech.. 9, 1449–1460 (2016).
- H. Xia, W. Jing, Z. Zi-Han, B.-Y. Zhang, J.-B. Zhang, Determination of gaseous and particulate trifluoroacetic acid in atmosphere environmental samples by gas chromatography-mass spectrometry. Chinese Journal of Analytical Chemistry 41, 1140–1145 (2013).
- Zhang, B., Z. Zhai, and J. Zhang, Distribution of trifluoroacetic acid in gas and particulate phases in Beijing from 2013 to 2016. Sci. Total Environ. 634, 471–77 (2018). 10.1016/j.scitotenv.2018.03.384
- Joudan, S., A.O. De Silva, and C.J. Young, Insufficient evidence for the existence of natural trifluoroacetic acid. Environ Sci Process Impacts 23, 1641–49 (2021). 11 10.1039/D1EM00306B
- D. A. Ellis et al. The fate and persistence of trifluoroacetic and chloroacetic acids in pond waters. Chemosphere 42, 309–318 (2001).
- Richey, D., C. Driscoll, and G. Likens, Soil retention of trifluoroacetate. Environ. Sci. Technol.. 31, 1723–27 (1997). 6 10.1021/es960649x
- Liu, L., F. Yu, K. Tu, Z. Yang, and X. Zhang, Influence of atmospheric conditions on the role of trifluoroacetic acid in atmospheric sulfuric acid–dimethylamine nucleation. Atmos. Chem. Phys.. 21, 6221–30 (2021). 8 10.5194/acp-21-6221-2021
- Dreveton, A., Overview of the fluorochemicals industrial sectors. Procedia Eng. 138, 240–47 (2016).10.1016/j.proeng.2016.02.081
- I. Colomer, A. E. Chamberlain, M. B. Haughey, T. J. Donohoe, Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 1, 0088 (2017).
- P. J. Godin, K. Le Bris, K. Strong, Conformational analysis and global warming potentials of 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol from absorption spectroscopy. J Quant Spectrosc Rad Transfer 203, 522–529 (2017).
- Iyer, S., F. Lopez-Hilfiker, B.H. Lee, J.A. Thornton, and T. Kurtén, Modeling the detection of organic and inorganic compounds using iodide-based chemical ionization. J Phys Chem A 120, 576–87 (2016). 4 10.1021/acs.jpca.5b09837
- M. Beekman et al. “Evaluation of substances used in the GenX technology by Chemours, Dordrecht,” (National Institute for Public Health and the Environment, 2016).
- S. Lerner, NEW TEFLON TOXIN CAUSES CANCER IN LAB ANIMALS, The Intercept, (2016), https://theintercept.com/2016/03/03/new-teflon-toxin-causes-cancer-in-lab-animals/ accessed 4 January 2024).
- F. Paulot et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 325, 730–733 (2009).
- T. H. Bertram et al. A field-deployable, chemical ionization time-of-flight mass spectrometer. Atmos. Meas. Tech.. 4, 1471–1479 (2011).
