Abstract
This article describes white-spot syndrome virus (WSSV) diagnostics in 50 shrimp frozen stocks imported to Mexico from the USA. Frozen stocks cover various shrimp species and have different origins. Routine histological techniques and polymerase chain reaction (PCR)-based molecular analysis were used to document this disease. One frozen shrimp stock containing only Penaeus aztecus from the USA was detected as WSSV-positive using these non-conventional samples. Feasibility of frozen shrimp analysis is discussed since current viral detection is conducted in fresh shrimps before their marketing and/or re-importation. The role of frozen commodities in viral mobility and introduction into Mexico was also discussed.
El presente trabajo describe el diagnóstico de la presencia de virus de la mancha blanca realizado en 50 lotes de camarones importados a México desde Estados Unidos de América (EUA). Los lotes congelados abarcan varios géneros de camarones y tienen orígenes diferentes. Se utilizaron técnicas histológicas rutinarias y métodos de análisis moleculares basados en el método de la reacción en cadena de la polimerasa (PCR) para documentar esta enfermedad. Únicamente un lote conteniendo individuos de la especie Penaeus aztecus proveniente de los EUA fue positivo al WSSV usando estas muestras no convencionales. El presente trabajo discute la utilidad de usar lotes congelados para el diagnóstico de enfermedades virales ya que comúnmente se utilizan muestras frescas antes de su comercialización y/o exportación. El papel que juegan las importaciones congeladas de camarones en la movilidad o introducción de virus a México es también discutido.
Keywords:
Palabras clave:
Introduction
White spot syndrome virus (WSSV) is the most virulent and the largest animal virus known to affect cultured shrimp (Lightner, Citation1996; Van Hulten et al., Citation2001). During the last few years, this disease has spread worldwide and caused large-scale mortalities and severe damage to shrimp culture, particularly in Asia, leading to massive economic losses (Lightner, Citation1996). It is estimated that in 2001, WSSV caused losses of 300,000 metric tons of shrimp, worth more than 1 billion US dollars (Rosenberry, Citation2001). WSSV infection often results in 100% mortality among affected penaeid populations within 3–10 days under farming conditions and the virus is known to infect shrimp of virtually all size classes (Lightner, Citation1996). In the absence of an effective treatment for WSSV, early and rapid diagnostics have a crucial role in its management (van Hulten et al., Citation2000). A diverse array of WSSV diagnostic techniques have been developed including histopathology, conventional and real time PCR, antibody-based tests, in situ hybridization, and dot-blot assays (Lightner, Citation2004). Molecular and histopathological diagnostic methods are internationally recommended (Walker & Subasinghe, Citation2000). Currently, samples of fresh shrimp collected from the field have been routinely and effectively used in shrimp disease diagnostics (Lightner, Citation1996; Mohan, Shankar, Kulkarni, & Sudha, Citation1998). Thus, many shrimp samples are regularly analyzed in the producing country but not by the importer counterpart. Risks of shrimp virus introduction during trading of live shrimp for culture have been described, but other potentially important sources of shrimp viruses such as ship ballast water or frozen seafood products have also been suggested (Chapman, Browdy, Savin, Prior, & Wenner, Citation2004; Flegel & Fegan, Citation2002; Hasson, Fan, Reisinger, Venuti, & Varner, Citation2006; Reville et al., Citation2005). Moreover, outbreaks of WSSV in different Americans countries have been reported (Ostrowski, Citation2004; Veterinary Services, Citation2003). Viral disease diagnostics on imported fresh and frozen stocks have been hardly implemented and have only included stock samples produced in Latin America or Asia (Escobedo-Bonilla et al., Citation2007; Hasson et al., Citation2006; Mijangos-Alquisires, Quintero-Arredondo, Castro-Longoria, Grijalva-Chon, & Ramos-Paredes, Citation2006). However, apparent viral isolate homogeneity, frequent WSSV outbreaks in shrimps across all the Americas (Veterinary Services, Citation2003) and frequent outbreaks in Mexico (Peinado-Guevara & López-Meyer, Citation2006) stimulated our interest in the mobility of this virus into Mexico. Therefore, the present study examines the WSSV presence in frozen shrimp samples imported to Mexico. These shrimps stocks were analyzed for WSSV by combined histological and PCR-based methods. The results detailed here suggested the occurrence of WSSV infection in Penaeus aztecus frozen stocks imported from USA. In this study, we have also discussed the feasibility of using these non-conventional samples in the WSSV diagnostics.
Materials and methods
Sample collection
Fifty small boxes of frozen shrimp (2 kg each) were obtained from a certified shrimp diagnostics laboratory (Genomic Biotechnology Center-IPN) adjacent to the Mexico-USA border. For WSSV diagnostic analysis ten frozen shrimp sections were randomly selected within each container of frozen shrimp. Frozen shrimp sections (500) were then numbered consecutively and classified according to their origin and species. In some cases, more than one shrimp was contained within each frozen section. Identification of individual shrimp ( ) within frozen sections was conducted according to taxonomic traits (Pérez-Farfante & Kensley, Citation1997). A preliminary WSSV diagnostic test was conducted by analyzing macroscopic WSSV symptoms as white spots dispersed all over and red discoloration of the body. In general, all shrimps were processed by removing the hepatopancreas tissue, then storing the remnant tissue and pleopodes at −20 °C, until DNA extraction was done as described below.
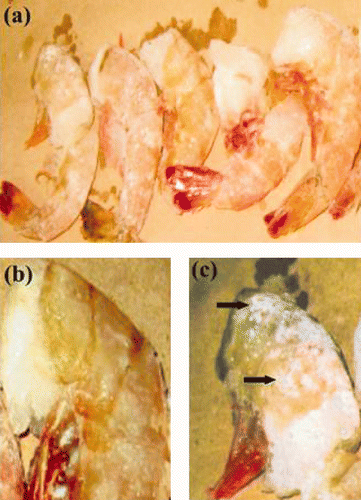
Figure 1. Preliminary frozen shrimp section analysis. (a): Frozen shrimp sections showed individual samples isolated. (b),(c): Primary viral infection evaluation by visual macroscopic WSSV symptoms in uninfected and infected shrimp samples, respectively. Arrows indicate the white spots characteristic of this disease.
Figura 1. Análisis preliminar de las secciones congeladas de camarón. (a): Sección de camarón congelada mostrando los camarones individuales aislados. (b),(c): Evaluación preliminar de la infección viral por síntomas de WSSV macroscópicos visuales en muestras no infectadas e infectadas, respectivamente. La flecha indica la característica mancha blanca de la enfermedad.
DNA extraction
DNA extraction was conducted using the commercially available Wizard™ Genomic DNA Purification kit (Promega, USA) with slight modifications. Approximately 100–150 mg of cut pleopode tissue were grinded and placed in a 1.5-mL tube containing 600 μl of lyses solution and incubated at 65 °C for 30 min. A second incubation step was conducted with 3 μL of RNAse at 37 °C for 30 min under gentle agitation. Tubes were placed at room temperature for 5 min, and 200 μL of precipitation solution was added, then vortexed for 20 sec and incubated for 5 min at 5 °C. After centrifugation for 4 min at 13,000 × g at 4 °C, the supernatant was carefully transferred to a new 1.5 mL tube containing 600 μL of isopropanol, mixed by inversion, and then incubated for 30 min at room temperature. DNA was recovered by centrifugation at 13,000 × g for 1 min. The pellet was washed with ethanol, left to dry at room temperature, then resuspended in 50 μL of the kit's commercial buffer solution at 65 °C for 1 h and stored at −20 °C until use.
PCR-based WSSV detection
Ten shrimp from each of 50 frozen boxes were individually tested by PCR for the presence of WSSV. Analysis for the presence of WSSV DNA was initially conducted using the single PCR procedure contained in certified kit ‘Simplex Primer Kit for WSSV’ (DiadXotics, Spain). Slight modifications were introduced by scaling down the reaction volume to 12.5 μL and by adjusting the annealing temperature to 60 °C in order to process a larger set of samples and to increase the WSSV specificity. Two PCR controls provided in the commercial kit were conducted: (i) an internal control yielding a 602 bp product and a WSSV-positive control yielding a 401 bp fragment.
Histopathology analysis
Pleopode tissue samples testing positive for WSSV via PCR were further tested by histopathological analysis. Samples were fixed by submersion in Davidson's fixative and sectioned and examined for histopathological evidence of WSSV infection. Histological observations were made according to the procedure outlined by Bell and Lightner (Citation1988) following staining with hematoxylin and eosin.
Results
Preliminary frozen shrimp characterization
The 50 boxes of imported frozen shrimps were categorized according to their origin and shrimp species. From a total of 50 frozen shrimp stocks analyzed, 62% were imported from USA, and the remaining 38% were also imported from USA but produced in Venezuela ( ). Only these last stocks were previously certified as specifically pathogen free. Frozen stocks were checked and held several days in custom office before diagnostic analysis was done and the registration sheets did not indicate capture date. Morphological shrimp identification from each frozen shrimp stock is summarized in . All shrimps isolated were headless by previous processing ( ). Most frozen shrimp boxes correspond to the species Litopenaeus vannamei with 42%, although other species such as Penaeus aztecus (32%) and P. duorarum (20%) were also well represented. Macroscopic WSSV symptom analysis revealed some organisms with potential WSSV disease. These organisms were isolated from frozen USA-produced stocks and exhibited red and brown body aspect and white spots, usual symptoms in this disease ( , ).
Table 1. Screening and incidence of PCR-positive reactions for white spot syndrome virus in frozen shrimp stocks imported to Mexico.
Tabla 1. Búsqueda e incidencia de reacciones de PCR positivas al síndrome de la mancha blanca en lotes de muestras congeladas de camarón importadas a México.
WSSV frozen stocks diagnostics
The PCR results indicated that only one frozen stock was WSSV positive by PCR-based diagnostics ( ). The WSSV positive frozen stock consisted of Penaeus aztecus shrimps produced in USA, which had not been certified as pathogen-free. Moreover, this was only frozen stock having shrimps possessing evident WSSV symptoms ( , ). The PCR-based diagnostics was confirmed in the pool of ten different frozen shrimp sections of the same stock. Correspondingly, shrimp analysis performed in frozen sections of other stocks showed no evidence of WSSV infection ( ). The results suggest that testing frozen shrimp sections can be effective in screening for WSSV.
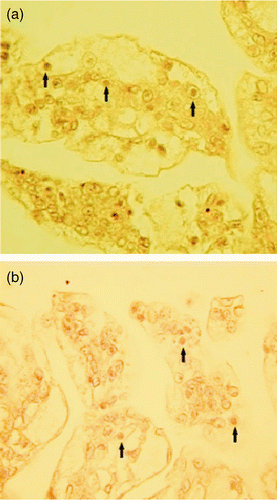
Histopathological analysis was conducted for isolated shrimps from different frozen section, in the case of both WSSV-presumptive and WSSV-negative stocks. All shrimps isolated from presumptive Penaeus aztecus stock produced in USA showed clear evidence of WSSV symptoms, in the form of hypertrophied nuclei with basophilic intranuclear viral inclusion bodies in the cells of ectodermal and mesodermal tissues such as the cuticular epidermis, connective tissue, pleopodes, gills, antennal gland, and other organs ( ). Frozen tissue changes did not obscure the characteristic WSSV diagnostic pathology. Intranuclear inclusion bodies of WSSV were still intact in the sloughed cellular debris in some of the shrimps isolated ( ).
Discussion
The present study is relevant to issues concerning (i) the feasibility of using frozen shrimp (Penaeus aztecus) stocks to diagnose the presence of virus and (ii) the presence of WSSV in shrimp commodity exported from USA. The movement of shrimp viral pathogens between countries has mainly been attributed to the international sale of infected nauplii, postlarvae and broodstock (Lightner, Citation2002). However, other unusual sources of viral pathogens have been documented, including imported bait-shrimp, ship ballast-water, shrimp-farm effluent, shrimp packing-plant wastes and frozen commodity shrimp (Durand, Tang, & Lightner, Citation2000; Flegel and Fegan, Citation2002; Lightner, Citation2002; Prior, Segars, & Browdy, Citation2001). Therefore, this study was conduced to explore frozen shrimp commodities exported from USA to Mexico.
Preliminary characterization of frozen shrimp sections ( ) allowed clear identification of WSSV symptoms although white spots are more apparent under surface of the carapace and the frozen shrimps were incomplete (Sangamaheswaran & Jeyaseelan, Citation2001). Thus, diagnosis of WSSV before it shows external symptoms is of practical significance. Preliminary diagnosis of infection in dead shrimps is of no use, due mainly to the risk of post-mortem and frozen-induced changes that can affect visual symptoms (Mohan et al., Citation2002). Moreover, for definitive diagnosis and certification of WSSV infection status, PCR technology has been recommended and used for live organisms (Walker & Subasinghe, Citation2000).
Similar sanitary regulation has been established in Mexico (NOM, Citation2004). Nevertheless, an early shrimp commodities diagnosis will provide an opportunity to help prevent the rapid spreading of the disease and must also be considered in virus screening regulations among different producing and consuming countries.
Viral positive diagnostics in imported shrimp stocks from USA ( and ) suggested revised policies governing importation of shrimp commodities into Mexico. Particularly, WSSV diagnostics from exported USA shrimp stocks must be recommended since historically several WSSV outbreaks in Carolina and Texas have occurred (Hasson et al., Citation2006). Virus-caused diseases include not only WSSV but also other pathogenic viruses such as Taura syndrome with an outbreak in the spring of 2004 in South Texas L. vannamei farms (Ostrowski, Citation2004). Moreover, it has been demonstrated that WSSV isolates from Texas cause higher mortality than other viral isolates (Chapman et al., Citation2004; Wang, Hassan, Shariff, Zamri, & Chen, Citation1999). Additional observations in the Gulf of Mexico suggest that WSSV is present in other species of shrimp (Hasson et al., Citation2006) and arthropods (Chang et al., Citation2001). However, so far, this virus has not been detected in P. aztecus. Interesting, the only WSSV-positive frozen stock consisted of Penaeus aztecus ( ). This shrimp species has been demonstrated to be more resistant to WSSV infection (27% mortality) than L. vannamei (100% mortality), the more commercially important species (Lightner, Hasson, White, & Redman, Citation1998). WSSV in P. aztecus shrimps was detected along the South Atlantic USA coast (Hasson et al., Citation2006). This study also showed that of a total of 32 shrimp stocks analyzed, only one corresponding to P. aztecus was WSSV-positive. The presence WSSV-positive P. aztecus may therefore suggest the presence of viral infection in other, more susceptible shrimp stock species. However, this study did not detect the presence of this virus in L. vannamei stocks although these comprised the majority of stocks analyzed ( ). Our detection of WSSV in only one P. aztecus stock may be the result of the PCR method used (single PCR) ( ) since many authors suggested use of nested PCR to increase to accuracy of viral diagnostics (Hasson et al., Citation2006; Peinado-Guevara and López-Meyer, Citation2006), or it could be due to an inadequate sample size since only 50 stocks were included in this study. The total volume of USA shrimp exports was valued at $94 million U.S. dollars ten years ago (Sangamaheswaran and Jeyaseelan, Citation2001). Generally, exportation statistics do not distinguish between farmed and wild-caught animals produced in USA or in other producer countries (Hasson et al., Citation2006). This study revealed different origins of stocks (Venezuela and USA) ( ). Moreover, USA stocks are not accompanied by a specific pathogen-free certification. Currently, there are no federal USA regulations that require shrimp virus screening of imported frozen shrimp intended for either sport fishing (as bait) or human consumption. However, such regulations already exist in other shrimp producing countries (i.e. Australia, Brazil, Nicaragua, Colombia and Mexico), and a comprehensive review of these policies is needed to further aid USA risk managers and stakeholders in evaluating this issue (Hasson et al., Citation2006). Viral diagnostic certification in Mexico was recently created and to our knowledge this is the first study conducted to examine North American shrimp exportations. Ideally, specific pathogen-free certification must accompany all frozen (uncooked) shrimp exports in all producing countries, particularly those that also possess higher levels of reprocessing and exchange in order to prevent viral disease mobility.
Figure 2. Representative WSSV electrophoretic band patterns from frozen shrimp sections. There are presented two representative WSSV analyses conduced in frozen sections from main and more abundant shrimps stocks of this study. Lanes M: 1 kb ladder; Lane 9: water template (negative control); Lane 10: WSSV-positive cDNA (401 bp-positive control); Lane 11: PCR-Reaction internal control (602 bp-positive control); Lanes 1 and 2: positive samples from P. aztecus imported from USA; Lanes 3 and 4: Samples from L. vannamei produced in Venezuela; Lanes 5 and 6: Samples from L. vannamei produced in USA; Lanes 7 and 8: Samples from Penaeus duorarum produced in USA.
Figura 2. Patrón de bandas electroforético característico del WSSV obtenido a partir de secciones de camarón congeladas. Se presentan dos análisis representativos del diagnostico WSSV realizados en las principales y más abundantes secciones de camarón congeladas. Carril M: Marcador 1 kb; Carril 9: Solo agua (testigo negativo); Carril 10: ADNc positivo al WSSV (Testigo positivo de 401 pb); Carril 11: Testigo interno de la reacción de PCR (Testigo positivo 602 pb). Carriles 1 y 2; Muestras positivas de P. aztecus importado desde los EUA. Carriles 3 y 4; Muestras de L. vannamei producidas en Venezuela. Carriles 5 y 6; Muestras de L. vannamei producidas en EUA. Carriles 7 y 8; Muestras de Penaeus duorarum producidos en EUA.
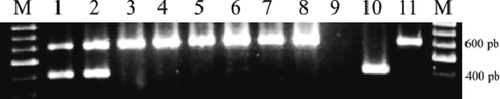
Figure 3. Cuticular epithelium of the pleopod infected with WSSV. (a) and (b): Paraffin sections from L. aztecus, × 400. Arrows indicate heavily infected cuticular epithelial cells showing large basophilic inclusions characteristic of WSSV infection.
Figura 3. Epitelio cuticular obtenido de tejido de pleopodos infectados con WSSV. (a) and (b): Secciones de parafina obtenidas de muestras de P. aztecus. × 400. Las flechas indican células de epitelio cuticular mostrando las grandes inclusiones basófilas características de la infección por WSSV.
Acknowledgements
This research was conducted under project SIP-20040248 supported by “Instituto Politécnico Nacional”. Jose Narvaez-Zapata is a fellow of COFAA, EDI and SNI and Miguel Angel Reyes-Lopez is a fellow of EDI. The authors want to thank Dr. Kenneth L. Beattie for help with English spelling and styling.
References
- Bell , T. and Lightner , D. V. 1988 . A handbook of normal penaeid shrimp histology , Baton Rouge, LA : World Aquaculture Society .
- Chang , Y. S. , Peng , S. E. , Wang , H. C. , Hsu , H. C. , Ho , C. H. , Wang , C. H. , Wang , S. Y. , Lo , C. F. and Kou , G. H. 2001 . Sequencing and amplified restriction fragment length polymorphism analysis of ribonucleotide reductase large subunit gene of the white spot syndrome virus in blue crab (Callinectes sapidus) from American Coastal Waters . Marine Biotechnology , 3 : 163 – 171 .
- Chapman , R. W. , Browdy , C. L. , Savin , S. , Prior , S. and Wenner , E. 2004 . Sampling and evaluation of white spot syndrome virus in commercially important Atlantic penaeid shrimp stocks . Diseases of Aquatic Organisms , 59 : 179 – 185 .
- Durand , S. V. , Tang , K. F. and Lightner , D. V. 2000 . Frozen commodity shrimp: Potential avenue for introduction of white spot syndrome virus and yellow head virus . Journal of Aquatic Animal Health , 12 : 128 – 135 .
- Escobedo-Bonilla , C. M. , Wille , M. , Sanz , V. A. , Sorgeloos , P. , Pensaert , M. B. and Nauwynck , H. J. 2007 . Pathogenesis of a Thai strain of white spot syndrome virus (WSSV) in juvenile, specific pathogen-free Litopenaeus vannamei . Disease of Aquatic Organisms , 74 : 85 – 94 .
- Flegel , T. W. and Fegan , D. F. 2002 . Strategies for preventing the spread of fish and shellfish diseases . Fisheries Science , 68 : 776 – 788 .
- Hasson , K. W. , Fan , Y. , Reisinger , T. , Venuti , J. and Varner , P. W. 2006 . White-spot syndrome virus (WSSV) introduction into the Gulf of Mexico and Texas freshwater systems through imported, frozen bait-shrimp . Diseases of Aquatic Organisms , 71 : 91 – 100 .
- Lightner , D. V. Shrimp diseases and exotic pathogens . Proceedings of the Shrimp Virus Disease Workshop . Nov 28–29 2001 , Panama City, FL. pp. 6 – 10 . New Orleans, LA : National Marine Fisheries Service . Panama City Laboratory Contribution 02–05
- Lightner , D. V. 2004 . Biosecurity in shrimp farming: pathogen exclusion through use of SPF stock and routine surveillance . Aquaculture , 36 : 229 – 248 .
- Lightner , D. V. 1996 . A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp , Baton Rouge, LA : World Aquaculture Society .
- Lightner , D. V. , Hasson , K. W. , White , B. L. and Redman , R. M. 1998 . Experimental infection of Western hemisphere penaeid shrimp with asian white spot syndrome virus and Asian yellow head virus . Aquatic Animal Health , 10 : 271 – 281 .
- Mijangos-Alquisires , Z. , Quintero-Arredondo , N. , Castro-Longoria , R. , Grijalva-Chon , J. M. and Ramos-Paredes , J. 2006 . White spot syndrome virus (WSSV) in Litopenaeus vannamei captured from the Gulf of California near an area of extensive aquaculture activity . Disease of Aquatic Organisms , 71 : 87 – 90 .
- Mohan , C. V. , Shankar , K. M. , Kulkarni , S. and Sudha , P. M. 1998 . Histopathology of cultured shrimp showing gross signs of yellow head syndrome and white spot syndrome during 1994 Indian epizootics . Diseases of Aquatic Organisms , 34 : 9 – 12 .
- Mohan , C. V. , Corsin , F. , Thakur , P. C. , Padiyar , P. A. , Madhusudan , M. , Turnbull , J. F. , Hao , N. V. and Morgan , K. L. 2002 . Usefulness of dead shrimp specimens in studying the epidemiology of white spot syndrome virus (WSSV) and chronic bacterial infection . Diseases of Aquatic Organisms , 50 : 1 – 8 .
- NOM . 2004 . NOM-EM-006-PESC-2004 , Mexico : Official Mexican Regulation DF .
- Ostrowski , A. C. 2004 . Consortium research update FY2004. The latest report from the USMSFP consortium . Waimanolo , : 1 – 6 . HI
- Peinado-Guevara , L. I. and López-Meyer , M. 2006 . Detailed monitoring of white spot syndrome virus (WSSV) in shrimp commercial ponds in Sinaloa, Mexico by nested PCR . Aquaculture , 251 : 33 – 45 .
- Pérez-Farfante , I. and Kensley , B. F. 1997 . Penaeoid and sergestoid shrimps and prawns of the world, key and diagnosis for the families and genera . Mémoires du Muséum Nacional d'Histoire Naturelle , 175 : 1 – 233 .
- Prior , S. , Segars , A. and Browdy , C. L. 2001 . “ A preliminary assessment of live and frozen bait shrimp as indicators and/or vectors for shrimp viruses ” . In Aquaculture 2001. Book of abstracts , 541 Baton Rouge, LA : World Aquaculture Society .
- Reville , C. , Al-Beik , J. , Meehan-Meola , D. , XU , Z. , Goldsmith , M. L. , William , R. and Alcivar-Warren , A. 2005 . White spot syndrome virus in frozen shrimp sold at Massachusetts supermarkets . Journal of Shellfisheries Research , 24 : 11 – 22 .
- Rosenberry , B. 2001 . World shrimp farming 2001 , San Diego : Shrimp News International .
- Sangamaheswaran , A. P. and Jeyaseelan , M. J.P. 2001 . White spot viral disease in Penaeid Shrimp – A Review. Naga . The International Center for Living Aquatic Resources Management Quarterly , 24 : 3 – 4 .
- van Hulten , M. C.W. , Tasi , M. F. , Schipper , C. A. , Lo , C. F. , Kou , G. H. and Vlak , J. M. 2000 . Analysis of a genomic segment of white spot syndrome virus of shrimp containing ribonucleotide redutase genes and repeated region . Journal of Genetic Virology , 81 : 307 – 316 .
- van Hulten , M. C.W. , Witteveldt , J. , Peters , S. , Kloosterboer , N. , Tarchini , R. , Fiers , M. , Sandbrink , H. , Lankhorst , R. K. and Vlak , J. M. 2001 . The White Spot Syndrome Virus DNA genome sequence . Virology , 286 : 7 – 22 .
- Veterinary Services . 2003 . Outbreak of Shrimp Viral Disease in Central America: Situation Report. Aqua KE Government Documents, 5210020 . Aquaculture Magazine , 25 ( 3 )
- Walker , P. and Subasinghe , R. P. 2000 . DNA-based molecular diagnostic techniques: Research needs for standardization and validation of the detection of aquatic animal pathogens and diseases , 93 Rome : FAO . FAO Fisheries Technical Paper 395
- Wang , Y. G. , Hassan , M. D. , Shariff , M. , Zamri , S. M. and Chen , X. 1999 . Histopathology and cytopathology of white spot syndrome virus (WSSV) in cultured Penaeus monodon from peninsular Malaysia with emphasis on pathogenesis and the mechanism of white spot formation . Diseases of Aquatic Organisms , 39 : 1 – 11 .