Abstract
In the present study, the characterization and properties of silver nanoparticles from Yucca shilerifera leaf extract (AgNPs) were investigated using UV–visible spectroscopic techniques, zeta potential, and dynamic light scattering. The UV–visible spectroscopic analysis showed the absorbance peaked at 460 nm, which indicated the synthesis of silver nanoparticles. The experimental results showed silver nanoparticles had Z-average diameter of 729 nm with lower stability (195.1 mV). Additionally, our dates revealed that AgNPs showed broad spectrum antagonism (p ≤ .05) against Fusarium solani (83.05%) and Macrophomina phaseolina (67.05%) when compared to the control after nine days of incubation. Finally, AgNPs from leaf extracts of Y. shilerifera may be used as an agent of biocontrol of microorganism of importance. However, further studies will be needed to fully understand the agronanotechnological potentialities of AgNPs from Yucca schidigera.
1. Introduction
Strawberries (Fragaria × ananassa Duch.) is the most economically important crop in the global alimentary industry, represent one of the most attractive fruit flavors and is used in many manufactured food products [Citation1]. However, the presence of fungal phytopathogens during the developments of strawberry is very important because these organisms can produced wilt or root rot disease that cause substantial losses to strawberry growers [Citation2]. In recent years, the soil-borne pathogenic fungi such as Fusarium solani and Macrophomina phaseolina have appeared in strawberry crops in different countries (e.g., Spain, Iran, and Pakistan) as the causal agent of crown and root rot and charcoal rot, respectively [Citation3–5]. These pathogens cause root rot, damping-off symptoms, and reduction in size of leaves and fruits, which affect the yield and quality [Citation3,Citation4]. Diverse studies reported the use of different control measures for the control of both diseases in strawberry; these measures include the application of chemical or biological control [Citation6,Citation7]. Currently environmental hazards caused by use of fungicides and the unpredictable results of biological control have been widely discussed [Citation8]. Therefore, new biotechnological alternatives are necessary to reducing the use of chemicals in agriculture. Today, green nanotechnology has great importance due to the presence of different modes of inhibitory action against various pathogens such as fungi and bacterial species [Citation9,Citation10]. In recent years, the use of plants as source of bioactive compounds for reduction of silver nanoparticles has drawn attention, due to the elimination of harmful reagents and effective synthesis of expected products through an economical method. Yucca schidigera is an herbaceous plant of the lily family, native to the deserts of Baja California, Mexico. Actually, Y. schidigera has been used as a major commercial source steroidal saponins [Citation11]. Y. shidigera has been the focus of scientific interest mainly because of their significant content of saponins and phenolic compounds that contribute to their antioxidant and antimicrobial activity [Citation11,Citation12], making this plant an interesting alternative for possible nano-biotechnological applications. Hence, this study was conducted to investigate the synthesis and characterization of AgNPs using the aqueous extract of Y. schidigera. In the present study, their antimicrobial activity of biosynthesized AgNPs was evaluated against fungal strains (F. solani and M. phaseolina) to reveal the agronanotechnological potentialities of Y. schidigera.
2. Material and methods
2.1. Biosynthesis of silver nanoparticles (AgNP) from Y. schidigera
Samples of leaves from Y. schidigera were collected at Ensenada, Baja California, Mexico. The Y. schidigera extract solution was prepared using 30 g of leaves that had been rinsed with deionized water and cut into small pieces. Then the chopped Y. schidigera leaves were mixed with 300 mL of deionized water and heated at 60 °C for 30 min. The samples were then cooled at room temperature and centrifuged at 4000 rpm for 20 min to remove particulate matter and to get clear solutions which were store under refrigeration at 4 °C until use. For AgNPs synthesis, 10 mL of aqueous leaf extract were mixed with 40 mL of AgN03 solution 1 mM in a 100 mL Erlenmeyer flask and heated at 60 °C for 15 min. Finally, a brown solution was formed, which stands as a preliminary identification of the formation of AgNPs (). The AgNPs were purified by centrifugation at 10,000 rpm for 15 min to remove excess silver ions and transferred to freeze dryer (powder obtain was used in antifungal assays).
2.2. Characterization of AgNPs
The formation of AgNPs from Y. schidigera was register trough spectral analysis (UV–visible), between wavelengths of 400 and 500 nm in a spectrophotometer (DR6000™ UV VIS, Rockford, IL, USA), having a resolution of 1 nm and using double distilled water as a blank reference.
2.3. Zeta potential and dynamic light scattering
The hydrodynamic sizes and the zeta potential of biosynthesized AgNPs in solution were analyzed using a Nanotrac Wave instrument (Microtrac). Three milliliter of sample was transferred in the clear disposable zeta cell for the measurement of zeta potential. Measurements were made by means of dynamic light scattering (DLS) in the range of 0.1–1000 µm at 25 °C, using laser wavelength of 780 nm and a scattering angle of 90°. The DLS data were analyzed by the Microtrac FLEX operating software (Montgomeryville, PA, USA).
2.4. Effect of AgNPs against phytopathogenic fungi
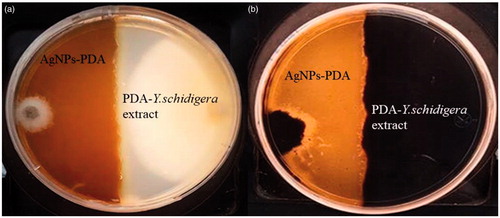
Antifungal activity of AgNPs was performed by dual culture technique in individual culture plates. The plates were divided into two quadrants; the first quadrant was prepared with 15 ml of PDA-AgNPs and the second quadrants were only prepared with PDA medium and Y. schidigera extract solution. After an agar disc (6 mm) was taken from 4-day-old PDA culture plates of each fungus (F. solani and M. phaseolina) and placed at the periphery of the PDA-AgNPS plates. Another agar disc of the same size of each fungus was also placed at the periphery but on the opposing end of the same Petri dish (only PDA). The plates were incubated at 27 °C for 9 days and the zone of inhibition was recorded.
2.5. Statistical analysis
Data were processed by analysis of variance with p <.05, with Tukey test, using the Statistical Analysis System version 6.12 (SAS Institute, 1997).
3. Results
3.1. Biosynthesis of AgNPs – Y. schidigera
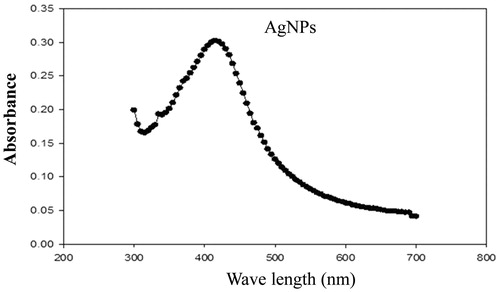
The UV–visible spectroscopy is an important preliminary technique to ascertain the formation and stability of metal nanoparticles in aqueous suspension. In this sense, after adding the leaf extract to the 10 mM AgNO3 solution, the aqueous solutions changed from yellow to reddish brown (). Later thus formation of AgNPs was followed and characterized by UV–visible spectroscopy. As shown in , the surface plasmon resonance of the AgNPs was centered at approximately 460 nm.
3.2. Zeta potential and DLS
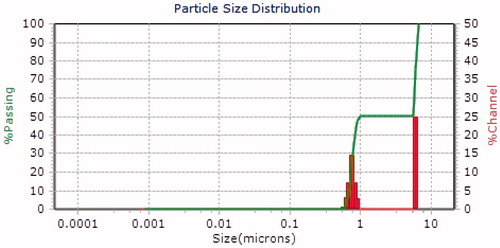
The average hydrodynamic size and zeta potential of the AgNPs was determined by the DLS as shown in and . The sample was a mixture of AgNPs of different sizes, DLS intensity analysis gave two broad peak and was weighted toward the larger particles (z-average size of 6.0 µm and 748 nm). The nanoparticle size is larger as presented by the DLS; this could only be explained based on the hydrodynamic radius which is not a true size as a result of the hydration layer around the particles as well as the presence of capping and stabilizing agents [Citation13]. The zeta potential of the biosynthesized AgNPs was found as a sharp peak at 195.1 mV with polydispersity index of 13.81 while the mean particle size was 729 nm ().
Table 1. Zeta-potential analysis of silver nanoparticles synthesized from Y. shidigera.
3.3. Antifungal activity of synthesized silver nanoparticles
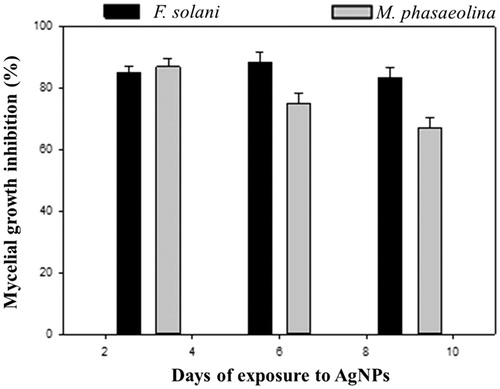
In the present study, the results of dual plate assays demonstrated that AgNPs from Y. schidigera had antagonistic effect on F. solani and M. phaseolina after 9 days incubation compared to control (). These results revealed that AgNPs showed broad spectrum antagonism (p ≤ .05) against F. solani (83.05%) and M. phaseolina (67.05%) when compared to the control after nine days of incubation ().
4. Discussion
In the present study, the biosynthesis of silver nanoparticles from Y. schidigera showed reddish brown color in aqueous solution as a result of bio-reduction mechanism of metal nanoparticle in plant extracts. In this sense, our result suggested that the biomolecules as saponins and phenolics present in Y. schidigera extract solution may be responsible for the reduction of AgNO3 and stabilization of AgNPs. In this context, Rejinolda et al. [Citation14] reported that the presence of saponins in Sapindus emarginatus extract may play key role in reduction and stability of AgNPs. Therefore, the saponins and phenolics present in extract of Y. schidigera could act as reducing and stabilizing agents for AgNPs production [Citation11]. The antifungal activity of silver nanoparticles from Y. schidigera can be identified by inhibition zone formation when compared to the phyto compound of Y. shidigera plant extracts alone which does not show any inhibition zone (). Similar results were reported by Medda et al. [Citation15] and Ouda [Citation16] who observed that used of phytonanoparticles and silver/copper nanoparticles, respectively, against different plant pathogens caused damage on fungal hyphae and conidia. The mechanisms of AgNPs from Y.shidigera inhibition of F. solani and M. phaseolina are not yet fully explored.
Boxi et al. [Citation17] reported that the fungicidal effect of the nanoparticles (not phytonanoparticles) for Venturia inaequalis and F. solani is results of the presence of Ag that acts in the formation of stable Ag-S and disulfide bonds in cellular protein, which leads to cell damage. However, Mahdizadeh et al. [Citation18] observed differences between M. phaseolina and Rhizoctonia isolate in response to nanoparticles. In this sense, Villamizar-Gallardo et al. [Citation19] found that F. solani shows tolerance to AgNPs as a result of their complex multicellular organization. However, in the present study the tolerance to Ag-nanoparticles of Y. schidigera was not register by F. solani or M. phaseolina this could be result of different mechanisms that can occur simultaneously at the surface of AgNPs that potentiates its effect against the phytopathogenic fungus. Recent studies showed that nanoparticle size, shape, and core composition are strong determinants of the inhibitory action of AgNPs on fungal phytopathogens [Citation20,Citation21]. For example, Kotzybik et al. [Citation22] described a change in antifungal activity according to the size of nanoparticles. In this case, the particles of smaller sizes had in general and over the whole experimental setups a more pronounced influence on the inhibition of fugal in comparison to particles exhibiting larger sizes. However, the obtained zeta potential (194.1 mV) suggested that the surface of the nanoparticles is positive charged indicating less stability and thus tendency to agglomerate and form large particles. Additionally, DLS analyses of AgNPs from Y. schidigera showed a major particle size distribution peak at 748 nm and 6.0 µm, which represented the existence of interaction between Ag and biomolecules present in this plant which resulted in aggregation of AgNPs. According to Gosens et al. [Citation23] and Müller et al. [Citation24], the agglomeration of nanoparticles is due to adhesion of particles to each other by weak forces leading to (sub) micron-sized entities.
Therefore, the agglomeration observed in Ag-nanoparticles from Y. schidigera raise questions how to address the biotechnological applications of this nanoparticles when they are no longer in the nano range, but are present as larger entities and inhibit the fungal growth. The above is very important because several authors mention that AgNPs with a high surface to volume ratio favors an increase in the production of reactive oxygen species (ROS) which includes free radical [Citation22,Citation25,Citation26]. The free radical-induced cell membrane damage has been observed to be caused by both silver and gold nanoparticles [Citation26]. Recently Ishida et al. [Citation27] explain that membrane disruption by nanoparticles is likely due to the production of ROS, including free radicals that cause membrane lipid peroxidation. This membrane disruption allows the passage of nanoparticles into the cytoplasm, which causes subsequent damage of DNA followed by destruction of microorganisms [Citation26]. Additionally, ROS produced by Ag-nanoparticles are capable of inhibiting lactate dehydrogenase one of the crucial enzymes in cellular respiration in microorganisms [Citation26]. Although we did not evaluate this aspect, it is possible that inhibition of growth fungal could be results of the production of free radicals by AgNPs from Y. shidigera. Finally, further studies are required to confirm their potential of AgNPs from Y. shidigera in the control of the F. solani and M. phaseolina under field conditions.
Acknowledgments
The authors would like to thank Secretaria de Fomento Agropecuario de Baja California, Mexico (SEFOA) for their persistent encouragement and supports.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Liu L, Ji M, Chen M, et al. The flavor and nutritional characteristic of four strawberry varieties cultured in soilless system. Food Sci Nutr. 2016;6:858–868.
- Narro-Sanchez J, Davalos-Gonzalez PA, Velasquez-Valle R, et al. Main strawberry diseases in Irapuato, Guanajuato, and Zamora, Michoacan, Mexico. Acta Hortic. 2006;708:167–171.
- Sharifi K, Mahdavi M. First report of strawberry crown and root rot caused by Macrophomina phaseolina in Iran. Iran J Plant Pathol. 2011;47:161.
- Pastrana AM, Capote N, De los Santos B, et al. First report of Fusarium solani causing crown and root rot on strawberry crops in southwestern Spain. Plant Dis. 2014;98:161.
- Mehmood N, Riaz A, Jabeen N, et al. First report of Fusarium solani causing fruit rot of strawberry in Pakistan. Plant Dis. 2017;9:1681.
- Nam MH, Park MS, Kim HG, et al. Biological control of strawberry Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae using Bacillus velezensis BS87 and RK1 formulation. J Microbiol Biotechnol. 2009;19:520–524.
- Pastrana A, Basallote-Ureba M, Aguado A, et al. Biological control of strawberry soil-borne pathogens Macrophomina phaseolina and Fusarium solani, using Trichoderma asperellum and Bacillus spp. Phytopathol Mediterr. 2016;55:109–120.
- Adesina MF, Lembke A, Costa R, et al. Screening of bacterial isolates from various European soils for in vitro antagonistic activity towards Rhizoctonia solani and Fusarium oxysporum: site‐dependent composition and diversity revealed. Soil Biol Biochem. 2007;39:2818–2828.
- Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol. 2005;71:7589–7593.
- Lamsal K, Kim S-W, Jung JH, et al. Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology. 2011;39:26–32.
- Piacente S, Pizza C, Oleszek W. Saponins and phenolics of Yucca schidigera Roezl: chemistry and bioactivity. Phytochem Rev. 2005;4:177–190.
- Miyakoshi M, Tamura Y, Masuda H, et al. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J Nat Prod. 2000;63:332–338.
- Ezealisiji KM, Noundou XS, Ukwueze SE. Green synthesis and characterization of monodispersed silver nanoparticles using root bark aqueous extract of Annona muricata Linn and their antimicrobial activity. Appl Nanosci. 2017;7:905--911.
- Rejinolda NS, Muthunarayanan M, Muthuchelian K, et al. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr Polym. 2011;84:407–416.
- Medda S, Hajra A, Dey U. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci. 2015;5:875–880.
- Ouda SM. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternate and Botrytis cinerea. Res J Microbiol. 2014;9:34–42.
- Boxi SS, Mukherjee K, Parja S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology. 2016;8:085103.
- Mahdizadeh V, Safaie N, Khelghatibana F. Evaluation of antifungal activity of silver nanoparticles against some phytopathogenic fungi and Trichoderma harzianum. J Crops Prot. 2015;4:291–300.
- Villamizar-Gallardo R, Cruz OJF, Ortiz-Rodriguez OR. Efeito fungicida de nanopartículas de prata em fungos toxigênicos em cacaueiro. Pesq Agropec Bras. 2016;51:1929–1936.
- Shafaghat A. Synthesis and characterization of silver nanoparticles by phytosynthesis method and their biological activity. Synth React Inorg Met-Org Nano-Met Chem. 2015;45:381–387.
- Kim SW, Kim KS, Lamsal K, et al. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J Microbiol Biotechnol. 2009;19:760–764.
- Kotzybik K, Gräf V, Kugler L, et al. Influence of different nanomaterials on growth and mycotoxin production of Penicillium verrucosum. PLoS One. 2016;11:e0150855.
- Gosens I, Post JA, de la Fonteyne LJ, et al. Impact of agglomeration state of nano and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol. 2010;7:37.
- Müller KH, Motskin M, Philpott AJ, et al. The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles in human monocyte-derived macrophages. Biomaterials. 2014;35:1074–1088.
- Ogar A, Tylko G, Turnau K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci Total Environ. 2015;521–522:305–314.
- Dakal TC, Kumar A, Majumdar RS, et al. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831.
- Ishida K, Cipriano TF, Rocha GM, et al. Silver nanoparticle production by the fungus Fusarium oxysporum: nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem Inst Oswaldo Cruz. 2014;109:220–228.