Abstract
For the purpose of protecting the rights of Lentinula edodes breeders, we developed a new simple sequence repeat (SSR) marker set consisting only of genetically independent tetranucleotide or longer core motifs. Using available genome sequences for five L. edodes strains, we designed primers for 13 SSR markers that amplified polymorphic sequences in 20 L. edodes cultivars. We evaluated the independence of every possible marker pair based on genotype data. Consequently, eight genetically independent markers were selected. The polymorphic information content values of the markers ranged from 0.269 to 0.764, with an average of 0.409. The markers could distinguish among 20 L. edodes cultivars and produced highly repeatable and reproducible results. The markers developed in this study will enable the precise identification of L. edodes cultivars, and may be useful for protecting breeders’ rights.
1. Introduction
Lentinula edodes (Berk.) Pegler, commonly known as shiitake, is one of the most economically important edible mushrooms. In Japan, L. edodes is cultivated over a wide area and is the third most produced cultivated mushroom. Additionally, 241 L. edodes cultivars have been registered in the Ministry of Agriculture, Forestry and Fisheries (as of July 26 2019), which is the largest number among mushroom species currently sold in Japan. Developing new cultivars is more expensive and time-consuming for L. edodes than for other commercial mushrooms because of its very long cultivation period (about 100 days for sawdust-based cultivation, while at least one year for natural log cultivation). Moreover, the illegal proliferation and distribution of patented L. edodes cultivars by third parties have recently become serious problems. To protect the rights of L. edodes breeders, a tool for identifying cultivars will need to be developed.
Mushroom cultivars have traditionally been identified based on morphological analyses and dual cultures. However, these methods are laborious and depend largely on the experience and subjectivity of the researcher. Additionally, these methods require fresh mycelia, and are inappropriate for inactivated samples such as dry products. In contrast, molecular techniques involving DNA analyses may be used to examine inactivated samples and can be completed relatively quickly. Several molecular techniques and markers have been developed for identifying L. edodes, including the following: amplified fragment length polymorphism analysis [Citation1], DNA sequencing of the intergenic spacer region in nuclear ribosomal DNA [Citation2,Citation3], intersimple sequence repeat markers [Citation4], random amplified polymorphic DNA markers [Citation5], sequence characterized amplified region markers [Citation6], and simple sequence repeat (SSR) markers [Citation7,Citation8]. Among these DNA markers, SSRs are useful for identifying cultivars because of co-dominant inheritance and the abundance of alleles per locus. Conventional approaches for developing SSR markers (e.g., enriched library method) are costly and time-consuming. However, the advent of next-generation sequencing (NGS) technology for whole-genome analyses has enabled the cost-effective development of many SSR markers [Citation9].
Accurately identifying a large number of cultivars requires multiple SSR markers. If these markers are genetically linked, the genotypes between the linked markers are considered to be essentially the same. An easy and simple method for obtaining an independent marker set involves selecting one marker from each linkage group on the linkage map comprising the same number of linkage groups as the number of chromosomes. However, although the number of L. edodes chromosomes has been reported [Citation10,Citation11], there remains some uncertainty regarding the accuracy of this determination. Therefore, markers must be statistically analyzed to confirm they are located on different chromosomes.
Reliable markers are needed to decrease or eliminate genotyping mistakes. Long core motifs, namely tetranucleotides or longer, enable the separation of neighboring alleles better than dinucleotide repeat motifs, which frequently produce stutter peaks, possibly leading to the incorrect interpretation of electropherograms [Citation12,Citation13]. Long core motifs are now broadly used for analyses of humans [Citation14], animals [Citation15], and plants [Citation16–18]. However, an SSR marker set consisting only of long core motifs has not been reported for mushrooms.
In this study, we developed a new set of SSR markers with the aim of efficiently discriminating among cultivars and minimizing genotyping errors. To the best of our knowledge, this article is the first to report a SSR marker set comprising only genetically independent tetranucleotide or longer core motifs for identifying L. edodes cultivars.
2. Materials and methods
2.1. Cultivars
Twenty L. edodes cultivars currently cultivated in Japan were included in this study. Five L. edodes cultivars (MH009092, MH009093, MH009106, MH009107, and MH009108) were obtained from Hokuto Co. (Nagano, Japan) and the others (ComCul_1–15) were purchased from supermarkets in Japan. To investigate whether markers were inherited independently, we prepared the following six monokaryotic populations: 94 strains from MH009107, 93 strains from ComCul_8, 90 strains from ComCul_10, 88 strains from ComCul_12, 96 strains from ComCul_13, and 92 strains from ComCul_14.
2.2. DNA extraction
Strains were grown on potato dextrose agar (Kanto Chemical, Tokyo, Japan) in Petri plates for 2 weeks at 25 °C in darkness. Genomic DNA was extracted with the GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO, USA). The DNA quality was evaluated by agarose gel electrophoresis, whereas the concentrations were determined with Multiskan™ GO (Thermo Fisher Scientific, Vantaa, Finland). All samples were stored at −20 °C until used.
2.3. Development of SSR markers
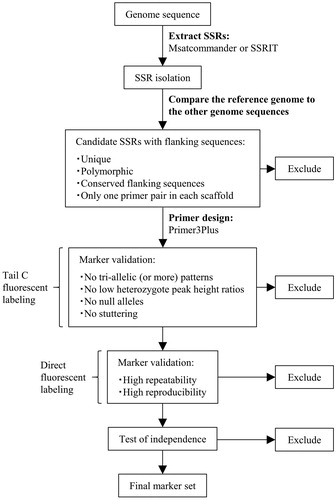
An overview of the SSR marker development strategy used in this study is provided in . The draft genomes for five L. edodes strains were used to develop SSR markers (). The draft genome of L. edodes monokaryon B17 [Citation19] and the mitochondrial genome of Le(Bin) 0899 ss11 v1.0 obtained from the Joint Genome Institute MycoCosm genome portal (https://genome.jgi.doe.gov/Lenedo1/Lenedo1.home.html) were screened with Msatcommander (version 0.8.2) [Citation20] and Simple Sequence Repeat Identification Tool (SSRIT) [Citation21]. The parameters were set to search for tetranucleotide or longer perfect SSR motifs with at least three repeats. The SSR sequences as well as their 200 bp upstream and downstream flanking sequences were extracted from each reference genome. These sequences were then subjected to a local BLAST search of each reference genome with BioEdit (version 7.2.5) [Citation22], and sequences with multiple copies in different genomic regions were eliminated. Moreover, to detect SSR motifs with varying numbers of repeats and the conserved flanking sequences, candidate sequences with a unique genomic location were extracted from other L. edodes draft genomes following a local BLAST search. These sequences were aligned with the default options of MUSCLE [Citation23] in the MEGA6 program [Citation24]. Sequences lacking polymorphisms and conserved flanking sequences were removed. Only conserved nucleotide positions that were identical to the aligned sequences were used to design new primers for amplifying sequences in diverse L. edodes cultivars. Furthermore, to minimize the probability of the linkage of SSR markers, only one primer pair in each scaffold was selected for analyses of independence. Primers were designed with Primer3Plus (https://primer3plus.com/cgi-bin/dev/primer3plus.cgi) according to the following requirements: primer size: 18–23 bp; product size: 100–300 bp; primer melting temperature: 57–63 °C (optimum: 60 °C); and primer GC%: 40–60%.
Table 1. Details regarding the genome sequences used for developing SSR markers.
2.4. Screening of SSR markers
The designed primers were tested regarding their utility for amplifying the expected fragments and polymorphisms with a three-primer polymerase chain reaction (PCR) approach involving templates from 20 L. edodes cultivars. All forward primers included a universal primer sequence (Tail C: 5′-CAGGACCAGGCTACCGTG-3′) [Citation25] at the 5′ end, whereas the reverse primers included a PIG-tail sequence (5′-GTTTCTT-3′) [Citation26] to promote adenylation. Universal forward primers were labeled at the 5′ end with fluorescent dyes (6-FAM, HEX, NED, or PET). A PCR was performed in a total volume of 15.0 μL containing 7.5 μL AmpliTaq Gold 360 MasterMix (Applied Biosystems, Vilnius, Lithuania), 2.25 pmol tailed forward primer, 7.5 pmol PIG-tailed reverse primer, 3 pmol fluorescent-labeled universal primer, and 7.5 ng genomic DNA. The PCR program was as follows: 95 °C for 10 min; 40 cycles of 95 °C for 30 s, 59 °C for 30 s, and 72 °C for 1 min; 72 °C for 7 min. The amplified products were diluted 150-fold with sterile water, after which 1.0 μL diluted sample was mixed with 9.7 μL Hi-Di formamide (Applied Biosystems, Warrington, UK) and 0.3 μL GeneScan 600 LIZ dye Size Standard v2.0 (Applied Biosystems, Foster City, CA, USA). The mixture was denatured for 5 min at 95 °C and then cooled on ice. Capillary electrophoresis was performed on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, Hitachi, Japan). Allele sizes were determined with Peak Scanner™ Software (version 1.0) (Applied Biosystems). Primer pairs associated with low heterozygote peak height ratios, a lack of polymorphism, null alleles, stuttering, and tri-allelic (or more) patterns were discarded.
2.5. Genotyping of SSR markers
The SSR markers detected with the three-primer PCR approach were directly labeled with fluorescent dyes. All forward primers were labeled at the 5′ end with fluorescent dyes (6-FAM, NED, PET, or VIC), and the reverse primers were designed with the “Tail” option. All primer pairs were purchased from Applied Biosystems. A PCR was performed in a final volume of 15.0 μL containing 7.5 μL AmpliTaq Gold 360 MasterMix, 3.75 pmol each primer, and 7.5 ng genomic DNA. The PCR program was as follows: 95 °C for 10 min; 35 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min; 72 °C for 7 min. Allele sizes were determined as described above.
2.6. Validation of the repeatability and reproducibility of markers
We evaluated the repeatability and reproducibility of every marker based on the procedures described in ISO13495 (https://www.iso.org/standard/53822.html). Repeatability was tested with one DNA extract per strain, and the PCR was performed three times per extract. Reproducibility was assessed by performing the amplification with three DNA extracts per strain. The resulting amplicons were analyzed by capillary electrophoresis.
2.7. Analyses of SSR polymorphisms
The number of alleles (NA), the observed/expected heterozygosity (HO and HE, respectively), the polymorphic information content (PIC), and the estimated null allele frequency (F(Null)) at each marker were calculated for 20 L. edodes cultivars with CERVUS (version 3.0) [Citation27].
2.8. Test of marker independence
We assumed that genetically independent markers were those that were on different chromosomes. We evaluated the independence of every possible pair of markers based on the genotype data for the six monokaryotic populations. The p-value was calculated with the Chi-square test of R (version 3.4.1) (https://www.R-project.org/). We determined that combinations with a p-value <0.05 in populations might not be independent.
3. Results
3.1. Development of SSR markers
A total of 13 SSR markers were detected by the three-primer PCR approach (). Of these markers, only LE_19 was derived from mitochondrial DNA. The markers directly labeled with fluorescent dyes were successfully amplified and identified as polymorphic among the 20 L. edodes cultivars. Replicating the amplifications and fragment analyses for these markers confirmed the high repeatability and reproducibility of the allele sizes.
Table 2. Characteristics of 13 SSR markers.
3.2. Analyses of SSR polymorphisms
The results of the statistical analysis of 12 markers (i.e., all except for LE_19) in the 20 cultivars are presented in . The NA per locus ranged from 2 to 6, the HO from 0.300 to 0.900, the HE from 0.328 to 0.815, the PIC value from 0.269 to 0.764, and the F(Null) from −0.188 to 0.099.
3.3. Marker independence
We assessed the independence of every combination of 12 markers, with LE_19 derived from mitochondrial DNA excluded because it was independent of any marker derived from nuclear DNA. The p-values calculated with the Chi-square test are provided in . The LE_01/LE_17, LE_01/LE_23, LE_01/LE_24, LE_17/LE_23, LE_17/LE_24, and LE_20/LE_22 marker combinations had a p-value <0.0001 in two or more populations, whereas the LE_13/LE_20, LE_13/LE_22, and LE_23/LE_24 combinations had a p-value <0.0001 in one population. On the basis of these results, LE_01, 17, 23, and 24 were located on the same chromosome, as were LE_13, 20, and 22. Regarding these two groups of linked markers, we selected LE_13 and LE_17 because they had the highest PIC value within their respective groups. Finally, we selected eight genetically independent markers (LE_06, 10, 12, 13, 15, 17, 18, and 19) that could distinguish among the 20 L. edodes cultivars ().
Table 3. P-values for the Chi-square test of independence between two markers.
Table 4. Genotypes of 20 L. edodes cultivars based on eight SSR markers.
4. Discussion
In this study, we developed a new SSR marker set with genetically independent tetranucleotide to hexanucleotide core motifs for identifying L. edodes cultivars. This set of eight markers could differentiate among 20 L. edodes cultivars, with PIC values ranging from 0.269 to 0.764, with an average of 0.409 ().
Novel SSR markers for L. edodes were previously described by Lee et al. [Citation7] and Moon et al. [Citation8]. Lee et al. [Citation7] reported that the PIC values of 44 SSR markers with dinucleotide to pentanucleotide repeats ranged from 0.10 to 0.89, with an average of 0.511 and Moon et al. [Citation8] determined that the PIC values of 16 SSR markers with dinucleotide and trinucleotide repeats were between 0.07 and 0.89, with an average of 0.612. Our markers with tetranucleotide to hexanucleotide repeats were less useful for identifying individuals compared with the markers mainly comprising dinucleotide repeats. However, the long core motif markers developed in this study generated peak distances of 4 bp or more and did not result in any stuttering. Thus, these markers may be advantageous regarding practicality, thereby simplifying the allele calling for distinguishing among L. edodes cultivars.
Recent studies revealed the construction of de novo L. edodes genome sequences [Citation19,Citation28,Citation29]. However, details regarding genomic structures, such as the number of chromosomes, remained unclear. We examined whether 13 SSR markers developed in the current study were located on different chromosomes based on a progeny test, and eventually selected eight genetically independent markers. Each combination was tested in 1–4 populations, with no inconsistencies among the results. Although, the p-value (0.06) of the LE_01/LE_15 combination was close to the cutoff value (0.05) defined in this study, the independence between LE_15 and LE_17, 23, and 24 confirms the independence between LE_01 and LE_15 (). To reveal more details regarding the relationships among the chromosomal positions of these markers, additional experiments, such as linkage analyses involving NGS technology, are required.
On the basis of a linkage map of L. edodes derived from tetrad analyses, L. edodes likely contains 8–11 chromosomes [Citation11], which is fewer than the number of chromosomes in plants and animals. The small number of chromosomes is a considerable limiting factor on the number of markers that can be developed. Future increases in the number of related cultivars may make it more difficult for researchers to accurately identify cultivars. Although, the number of markers developed in this study is close to the estimated number of chromosomes, additional markers that are more informative will need to be developed.
The marker development strategy applied in this study may be applicable for basidiomycetous mushrooms other than L. edodes. Our SSR markers will enable the precise identification of L. edodes cultivars, and may be useful for protecting the rights of L. edodes breeders.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Terashima K, Matsumoto T. Strain typing of shiitake (Lentinula edodes) cultivars by AFLP analysis, focusing on a heat-dried fruiting body. Mycoscience. 2004;45(1):79–82.
- Babasaki K, Neda H, Murata H. megB1, a novel macroevolutionary genomic marker of the fungal phylum Basidiomycota. Biosci Biotechnol Biochem. 2007;71(8):1927–1939.
- Song XX, Zhao Y, Song CY, et al. Intergenic spacer 1 (IGS1) polymorphism map: a marker for the initial classification of cultivated Lentinula edodes strains in China. J Integr Agr. 2018;17(11):2458–2466.
- Zhang R, Huang C, Zheng S, et al. Strain-typing of Lentinula edodes in China with inter simple sequence repeat markers. Appl Microbiol Biotechnol. 2007;74(1):140–145.
- Zhang Y, Molina FI. Strain typing of Lentinula edodes by random amplified polymorphic DNA assay. FEMS Microbiol Lett. 1995;131(1):17–20.
- Wu X, Li H, Zhao W, et al. SCAR makers and multiplex PCR-based rapid molecular typing of Lentinula edodes strains. Curr Microbiol. 2010;61(5):381–389.
- Lee HY, Moon S, Shim D, et al. Development of 44 novel polymorphic SSR markers for determination of shiitake mushroom (Lentinula edodes) cultivars. Genes. 2017;8(4):109.
- Moon S, Lee HY, Shim D, et al. Development and molecular characterization of novel polymorphic genomic DNA SSR markers in Lentinula edodes. Mycobiology. 2017;45(2):105–109.
- Guichoux E, Lagache L, Wagner S, et al. Current trends in microsatellite genotyping. Mol Ecol Resour. 2011;11(4):591–611.
- Arima T, Morinaga T. Electrophoretic karyotype of Lentinus edodes. Trans Mycol Soc Japan. 1993;34:481–485.
- Miyazaki K, Huang F, Zhang B, et al. Genetic map of a basidiomycete fungus, Lentinula edodes (shiitake mushroom), constructed by tetrad analysis. Breed Sci. 2008;58(1):23–30.
- Cipriani G, Marrazzo MT, Di Gaspero G, et al. A set of microsatellite markers with long core repeat optimized for grape (Vitis spp.) genotyping. BMC Plant Biol. 2008;8(1):127.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5(6):435–445.
- Butler JM. Genetics and genomics of core short tandem repeat loci used in human identity testing. J Forensic Sci. 2006;51(2):253–265.
- Munyard KA, Ledger JM, Lee CY, et al. Characterization and multiplex genotyping of Alpaca tetranucleotide microsatellite markers. Small Ruminant Res. 2009;85(2–3):153–156.
- De la Rosa R, Belaj A, Muñoz-Mérida A, et al. Development of EST-derived SSR markers with long-core repeat in olive and their use for paternity testing. J Am Soc Hortic Sci. 2013;138(4):290–296.
- Faria DA, Mamani EMC, Pappas GJ Jr., et al. Genotyping systems for Eucalyptus based on tetra-, penta-, and hexanucleotide repeat EST microsatellites and their use for individual fingerprinting and assignment tests. Tree Genet Genomes. 2011;7(1):63–77.
- Kishine M, Tsutsumi K, Kitta K. A set of tetra-nucleotide core motif SSR markers for efficient identification of potato (Solanum tuberosum) cultivars. Breed Sci. 2017;67(5):544–547.
- Shim D, Park SG, Kim K, et al. Whole genome de novo sequencing and genome annotation of the world popular cultivated edible mushroom, Lentinula edodes. J Biotechnol. 2016;223:24–25.
- Faircloth BC. MSATCOMMANDER: detection of microsatellite repeat arrays and automated, locus-specific primer design. Mol Ecol Resour. 2008;8(1):92–94.
- Temnykh S, DeClerck G, Lukashova A, et al. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001;11(8):1441–1452.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5(1):113.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729.
- Blacket MJ, Robin C, Good RT, et al. Universal primers for fluorescent labelling of PCR fragments – an efficient and cost-effective approach to genotyping by fluorescence. Mol Ecol Resour. 2012;12(3):456–463.
- Brownstein MJ, Carpten JD, Smith JR. Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20(6):1004–1010.
- Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 2007;16(5):1099–1106.
- Chen L, Gong Y, Cai Y, et al. Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS One. 2016;11(8):e0160336.
- Sakamoto Y, Nakade K, Sato S, et al. Lentinula edodes genome survey and postharvest transcriptome analysis. Appl Environ Microbiol. 2017;83:e02990-16.