Abstract
To improve our understanding of the relationship between soil higher fungi (belonging to Ascomycota and Basidiomycota) and Abies koreana, we surveyed A. koreana soil fungal communities in a forest in Mt. Halla, Jeju Island, Korea by next-generation sequencing (Illumina Miseq). To confirm the soil higher fungal communities, we collected two types of soils from a defined plot: soils with dead (AKDTs) and living A. koreana (AKLTs), respectively. Soil fungi were classified into 2 phyla, 19 classes, 64 orders, 133 families, 195 genera, and 229 OTUs (895,705 sequence reads). Nonmetric multidimensional scaling (NMDS) showed significantly different soil higher fungal communities between AKDTs and AKLTs (p < .05). In addition, the saprophyte composition was significantly affected by A. koreana status (p < .05). The proportion of the mycorrhizal Clavulina spp. was different between soils with AKDTs and AKLTs, suggesting that Clavulina spp. may be a crucial soil fungal species influencing A. koreana. This study will lead to a better understanding of the ecological status of A. koreana in Mt. Halla. In addition, this study could be useful for the conservation and management of A. koreana habitats.
1. Introduction
Soil fungi are an important component of forest ecosystems because they are the dominant degraders of organic matter and mediators of carbon cycling [Citation1]. Among the soil fungi, mycorrhizal fungi have an important association with plants. Mycorrhizal fungi are seven types: arbuscular, ecto-, ectendo-, arbutoid, monotropoid, ericoid, and orchidaceous mycorrhizae; however, arbuscular mycorrhizae, and ectomycorrhizae are the most abundant and widely distributed [Citation2,Citation3]. However, ectomycorrhizal (EcM) fungi are associated with only 3% of vascular plant, such as Abies, Betula, Fagus, Picea, Pinus, Pseudotsuga, Quercus, and Salix [Citation3]. These fungi are members of the phyla Ascomycota and Basidiomycota (higher fungi), but most EcM fungi belong to the class Basidiomycetes, which includes best-known EcM genera Amanita, Cortinarius, Lactarius, Russula, and Suillus [Citation4].
Abies koreana Wilson is endemic to Korea and distributed throughout the southern part of Korea and Jeju Island, which has altitude ranges of 1000–1900 m [Citation5]. On Jeju Island, there was a decline in A. koreana from withering death in the 1980s, and since then, the distribution area has continued to decline [Citation6]. Although the cause of this decline is still unclear, it may be a result of complex interactions among multiple environmental factors affected by climate change and global warming [Citation5–7]. However, most research on these interactions has focused on the abiotic environmental factors, such as soil properties, soil moisture stress, temperature, typhoon, and drought in Korea [Citation8–12]. In contrast, relationship studies between A. koreana and soil fungal communities are limited.
According to Sim et al. [Citation13] and Lee and Eom [Citation14], A. koreana is associated with approximately 15 EcM fungi: Cenococcum geophilum, Clavulina cinerea, Coelogyne cristata, Cortinarius camphoratus, Cortinarius patibilis, Inocybe transitoria, Russula brevipes, Russula chloroides, Russula favrei, Rhodiola rosea, Sebacina dimitica, Tomentella stuposa, Tomentella sublilacina, and two unknown species that belong to the order Helotiales, closely related the ericoid mycorrhiza genus Rhizoscyphus found in Mt. Halla. However, their investigations were based on morphotyping EcM root tips, and fungal barcoding for regional sequencing of the representative morphotypes was limited by the number of collected root-tip samples. Therefore, further classification from morphotyping is difficult to discern. High-throughput metagenomic technology is an alternative approach of surveying fungal diversity and investigating the EcM fungi related to A. koreana. High-throughput metagenomic technologies, such as next-generation sequencing (NGS), have been widely used in recent studies to analyze microbial diversity in various environments [Citation15–22]. Generally, fungal hyphae are typically more persistent in soil than bacteria, although the hyphae of some fungal species experience a high turnover [Citation17,Citation18,Citation23,Citation24]. Therefore, the NGS technique could be a useful tool to evaluate the soil fungal diversity in the environment.
The objective of this study was to understand the soil-fungal communities associated with dead and living A. koreana in Mt. Halla by NGS. Especially, we focused on soil higher fungi belonging to Ascomycota and Basidiomycota, because these phyla contain the EcM fungi. Furthermore, we discuss the correlation between soil higher fungal communities and the decline in A. koreana in Mt. Halla.
2. Materials and methods
2.1. Study site and soil-sampling
The study site is located in Mt. Halla, Jeju Island, Korea (33°21′30.1ʺN, 126°30′24.9ʺE; elevation 1657 m; approximately 100 × 100 m plot), in a Korean fir (A. koreana) forest accounting for approximately 90% of its tree layer. The study was conducted on November 27 2018. The parent soil material in this site is almost volcanic ash soil. The mean annual precipitation is 1500–1600 mm and the annual temperature ranges from 15 to 16 °C annually in Jeju Island.
We randomly selected five dead and living A. koreana in a defined plot with each tree at intervals of > 10 m (). After removing the leaf litter, soil samples were collected from around the selected trees (< 1 m; depth 0–10 cm). Ten soil-samples (from dead trees: AKDT01–05; from living trees: AKLT01–05) were collected; they were sieved through a 2-mm mesh to remove rocks and root fragments.
2.2. DNA extraction, amplification, sequencing, and taxonomical assignment
The total DNA was extracted from 0.5 g of soil using a PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA). Subsequently, the primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify the ITS2 region of fungal ribosomal DNA (located in the internal transcribed spacer (ITS) of rDNA region) [Citation25]. Polymerase chain reaction (PCR) was performed under the following conditions: initial denaturation at 94 °C for 5 min; 35 cycles of denaturation at 94 °C for 50 s, annealing at 58 °C for 50 s, elongation at 72 °C for 50 s; and a final extension at 72 °C for 10 min. Sequencing was performed by Macrogen Ltd. (Seoul, Korea) and the amplicons were sequenced using the Illumina Mi-Seq platform (Illumina Inc., San Diego, CA) in a 2 × 300 flow cell.
Raw fastq files were filtered using QIIME version 1.8.0 [Citation26] and chimeric sequences were filtered using Usearch version 5.2.236 in QIIME [Citation27]. The remaining sequences were then clustered into operational taxonomical units (OTUs) at a 97% similarity threshold using Uparse version 7.1 [Citation28].
Reads with sequence match of E value < 0.01 were treated as fungal ITS2 region sequences, and less than 1% of all sequences were non-ITS sequences. Taxonomic assignment of the sequenced reads was conducted using the National Center for Biotechnology Information (NCBI) Taxonomy Database (http://www.ncbi.nlm.nih.gov/taxonomy); for each sequence, the five most similar sequences were identified based on the bit score and E-value in the Basic Local Alignment Search Tool (BLAST) program. Needleman–Wunsch global alignment algorithm was used to determine the optimal alignment of two sequences along their entire length. Pairwise global alignment was performed with the selected candidate hits to find the best-aligned hit. The taxonomy of the sequence with the highest similarity was assigned to the sequence read. Similarly, taxonomy was assigned down to the following taxonomical hierarchy; species. Sequences identified as non-fungal were discarded.
2.3. Statistical analyses
Relationships among fungal communities in each sampling site were visualized using nonmetric multidimensional scaling (NMDS) with the Bray–Curtis dissimilarity matrix using the metaMDS function in the vegan package in R version 3.2.3 [Citation29]. We used Wilcoxon test and permutational multivariate analysis of variance (PERMANOVA) to confirm significant correlations between soil fungal community composition and A. koreana status (living and dead). The ecotype of each OTUs was manually and carefully determined as mycorrhizal, saprophytic, and parasitic. If the OTUs were not classified up to the genus level or uncertain, their ecology was considered unknown.
3. Results
3.1. General soil fungal composition at the phylum level
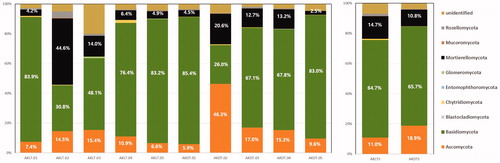
Good’s coverage estimator for all soil samples was >99%, which indicated that the number of sequences was adequate to reveal the fungal diversity in each sample. To characterize fungal communities in A. koreana forest soil, we sequenced 1,125,088 fungal amplicons (excluding short and poor-quality reads) from 10 soil-samples using the Illumina Mi-seq technique (). The overall fungal community was dominated by phylum-level sequences assigned to Basidiomycota (65.2%), Ascomycota (15.0%), Mortierellomycota (12.7%), and unidentified (5.4%) (). The composition rate of soil-fungi was not significantly different between AKLTs and AKDTs; however, the detected sequence reads of Basidiomycota was nearly 4.3 times higher than that of Ascomycota in both soil types (; ).
Table 1. Soil fungal sequence reads from each soil-sampling site organized by phylum-level.
3.2. Soil higher fungal communities in Abies koreana forests in Mt. Halla
Among the representative fugal amplicons, soil higher fungi (Ascomycota and Basidiomycota) were clustered into 229 OTUs that comprised 2 phyla, 19 classes, 64 orders, 133 families, and 195 genera (Supplementary Table S1 and ).
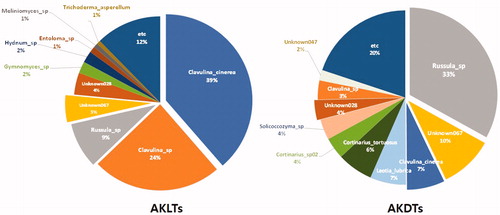
Among the 173 OTUs in the AKDTs, Russula sp. (158,308 reads; 33%), Unknown067 belonging to the family Thelephoraceae (47,133 reads; 10%), Clavulina cinerea (33,484 reads; 7%), Leotia lubrica (31,643 reads; 7%), and Cortinarius tortuosus (31,552 reads; 6%) were the most common species. In the AKLTs soils, among the 161 OTUs, Clavulina cinerea (161,044 reads; 39%), Clavulina sp. (100,915 reads; 24%), Russula sp. (35,820 reads; 9%), and Unknown067 belonging to the family Thelephoraceae (21,089 reads; 5%) were the most common (). We found that 105 OTUs were commonly detected between the two soil types, which were mainly C. cinerea, Clavulina sp., Russula sp., Unknown067, and Unknown028 that belonged to the order Helotiales (Supplementary Table S1 and ).
3.3. Ecotype of soil higher fungi in Mt. Halla A. koreana Forest
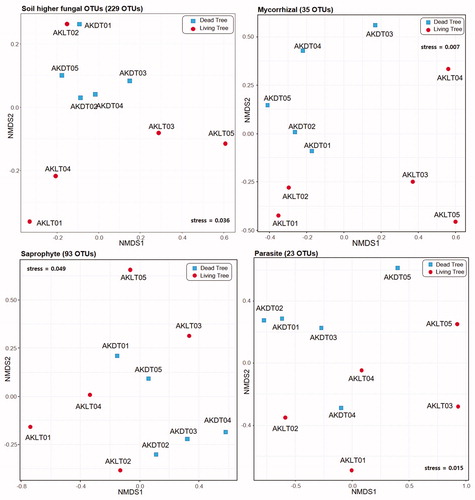
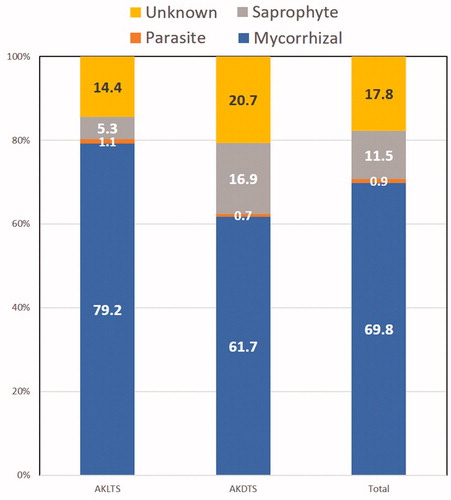
Among the 229 soil higher fungal OTUs in our collected soil samples, we identified 69.8% mycorrhizal, 11.5% saprophytic, 0.9% parasitic, and 17.8% unknown ecotypes (). The results of Wilcoxon test revealed that soil higher fungal OTUs (p = .032) and mycorrhizal groups (p = .036) were significantly affected by the status of A. koreana (). However, the saprophyte and parasite groups were not significantly affected (p < .05) by A. koreana status ().
Figure 5. Comparison between OTUs of dead (AKDTs) and living (AKLTs) A. koreana. Venn diagrams show overlapping and non-overlapping OTUs from these sampling groups. Bold letters indicate ectomycorrhizal fungi.
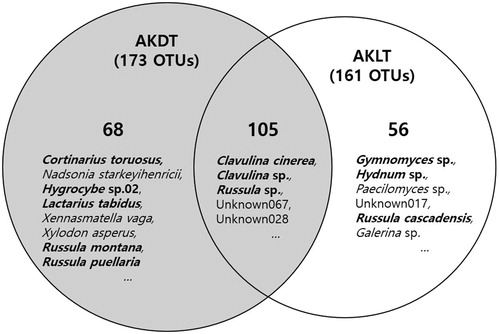
Table 2. Wilcoxon test results of two independent groups (living/dead) by ecotypes (mycorrhizal, saprophyte, and parasite).
The ecotype composition rate was significantly different between the saprophyte group of AKLTs and AKDTs (p = .005; and Supplementary Table S2). As shown in , the saprophyte composition rate in AKDTs (16.9%; 70 OTUs) was approximately 3.2 times higher than that in AKLTs (5.3%; 60 OTUs). On the contrary, the composition rates of the mycorrhizal and parasite groups were not significantly different between AKLTs and AKDTs. However, the mycorrhizal composition rate in AKLTs (79.2%; 23 OTUs) was approximately 1.3 times higher than that in AKDTs (61.7%; 29 OTUs). The parasite composition (including plant, insect, and nematode pathogens and one human pathogen – which was a Rhinocladiella sp. and only detected in AKDT02) in AKLTs (1.1%; 20 OTUs) was approximately 1.6 times higher than that in AKDTs (0.7%; 12 OTUs). Among the parasites, Tolypocladium sp. 02 (insect pathogen; 2118 reads detected from all soil-samples) was dominant, followed by Cadophora sp. (plant pathogen; 2097 reads), and Pochonia cordycepisociata (nematode pathogen; 870 reads).
Table 3. PERMANOVA statistics for soil higher fungal community composition using Bray–Curtis dissimilarities with 999 permutations.
4. Discussion
The NMDS showed significantly different total soil higher fungal communities between AKDTs and AKLTs, except in AKLT02 (). In the AKLT02 sample, the fungal community tended to be close to that in AKDTs in the NMDS analysis (), suggesting that the status of A. koreana in this site was dead or dying. The detected plant pathogens (Cadophora sp., Mycosphaerella sp., Peltaster sp., Pseudocercosporella sp., Ramularia sp., Sphaerulina sp., Thelonectria rubi, and T. veuillotiana; Supplementary Table S1) were mainly in the AKLT samples. Among the plant pathogens, Cadophora sp. was highly detected in AKLT02 (1025 reads; Supplementary Table S1). Most Cadophora spp. cause brown stem rot in soybean [Citation30], wood decay in kiwifruit and grapevine [Citation31,Citation32], and soft rot in the wooden structure in polar regions [Citation33]. Therefore, Cadophora sp. may accelerate the decline in A. koreana in Mt. Halla. Because Cadophora genus is not reported in Korea, taxonomical and pathogenetic studies are needed for the conservation of A. koreana.
Figure 6. Composition rate of soil higher fungi based on sequence reads of ecotype (unknown, saprophyte, parasite and mycorrhizal).
In the AKLT02 sample, Mortierellomycota was the dominant the phylum (44.6%, mostly belonging to the genus Mortierella; Supplementary Table S3). This phylum was recently established by Tedersoo et al. [Citation34] and contained 1 class, 1 order, 1 family, and 6 genera. The species of this phylum live mostly as saprotrophs in soil, on decaying leaves, and other organic material. It is uncertain why there was a high composition of Mortierellomycota members in the AKLT02 sample, although we speculate that AKLT02 may be almost dead or dying even if it looked alive.
In the AKDT02 sample, the proportion of Ascomycota was higher than that of the other phyla. Among Ascomycota members, Leotia lubrica of the order Helotiales (37.2%, 303,471 reads) was the dominant species (, Supplementary Table S1, and ). In addition, this species was at a very low proportion in AKLTs. Generally, L. lubrica is saprotrophic and globally distributed. Several ecologists suggest that L. lubrica is an EcM fungus of some plant species, such as Comarostaphylis arbutoides, Nothofagus menziesii, Polygonum sp., and Quercus rotundifolia, based on morphological, anatomical, and molecular methods using plant root tips [Citation35–38]. However, this study was conducted on soil samples, and therefore we cannot ensure that L. lubrica is a mycorrhizal fungus of A. koreana. In addition, our soil samples were collected from dead A. koreana, and although L. lubrica has a dual mycorrhizal and saprotrophic lifestyle, further investigations are needed to determine if this is the case for the relationship between L. lubrica and A. koreana. Future investigations on fungal diversity using A. koreana root tips and NGS will provide more accurate evidence of these relationships.
Interestingly, C. cinerea and Clavulina sp., which are EcM fungi, comprised 10% of samples in AKDTs (C. cinerea 7% and Clavulina sp. 3%), but the proportion in AKLTs was 63% (C. cinerea 39% and Clavulina sp. 24%; ). The proportion of Clavulina spp. in AKLTs was approximately six times higher than that in AKDTs. This result indicates that Clavulina might be the most important bio-influencing factor to live A. koreana (). Lee and Eom [Citation14] reported that C. cinerea was a dominant species in A. koreana in Mt. Halla based on root tip sampling and sequencing of its representative morphotypes. Similarly, Argüelles-Moyao et al. [Citation39] showed that Clavulina-Membranomyces lineage (especially, C. cf. cinerea and Membranomyces sp.) is the most dominant and important EcM fungi in A. religiose in the temperate forests in Mexico.
In contrast to Clavulina spp., the reads of Russula sp. in AKDTs (158,308 reads) were 4.4 times higher than those in AKLTs (35,820 reads; and ). This means that living A. koreana may not be significantly affected by Russula sp., although this species is an EcM fungus that is well known to be associated with several Abies species [Citation40–43]. In addition, we detected the following 12 mycorrhizal species: Cotinarius tortuosus, Co. acutovelatus, Co. hinnuleus, Co. orixanthus, Elaphomyces sp., Lactarius tabidus, Lactarius sp., Hygrocybe sp. 01 and Hygrocybe sp. 02, R. montana, R. puellaris, and Helvella sp. only in AKDTs (Supplementary Table S1). This distribution could be the reason that the composition of Clavulina spp. changes in response to other EcM species by microbial primary succession for plant succession [Citation42]. Because fungal diversity is affected by soil and plant properties, the soil and plant vegetation in our study sites could shift due to climate change and global warming.
EcM fungi make a significant contribution to forest ecosystems by increasing their network among trees through which nutrients can be transported. In addition, EcM fungi improve the growth of host plants in the seedling stage [Citation3]. The establishment of many pioneer plants in barren tips and other waste lands is facilitated by EcM fungi. This plant-fungi association has been successfully applied to the reforestation of tropical forests by inoculating mycorrhizae on nursery seedlings [Citation43,Citation44]. In forest nursery management, it is well known that pine seedlings cannot be directly replanted from the nursery to another location once they start to expand new lateral roots in the spring, although it is easy to replant them in winter [Citation45]. This effect can be attributed to the disruption of mycorrhizal association with newly developed lateral roots that develop in the spring. Therefore, we propose that two Clavulina spp. (C. cinerea and Clavulina sp.) that were detected in this study should be used for the inoculation of A. koreana seedlings. This procedure would equip plants with a set of EcM fungi that are already adapted to local soils and climates, able to produce abundant mycorrhizae, and have the capacity to connect the seedlings with nursery plants through mycorrhizal networks. To achieve this goal, research should focus on the production of inocula that can be applied on a large scale (spores or mycelia), as well as on the capacity to establish ectomycorrhizae in co-inoculation experiments under greenhouse and field conditions.
5. Conclusions
Here, we investigated the importance of soil fungi in not only ecosystem dynamics but also forest conservation. An understanding of soil fungal diversity is required for biodiversity conservation in forest and forest management policies in Korea. To the best of our knowledge, this is the first study to use Illumina Miseq to directly sequence fungal communities from soil samples in an Abies koreana forest. This study will help expand our understanding of the relationship between A. koreana and soil fungal communities, and how to develop conservation strategies for A. koreana that grow in Mt. Halla. Although the overall A. koreana forest health condition was not established in this study, these basic data, such mycorrhizal fungal and plant pathogen composition, can be used as evaluation indices of forest health conditions.
Supplemental Material
Download MS Excel (65.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rinnan R, Bååth E. Differential utilization of carbon substrates by bacteria and fungi in Tundra soil. Appl Environ Microbiol. 2009;75(11):3611–3620.
- Allen MF, Swenson W, Querejeta JI, et al. Ecology of mycorrhizae: a conceptual framework for complex interactions among plants and fungi. Annu Rev Phytopathol. 2003;41:271–303.
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. 3rd ed. New York (NY): Academic Press.
- Hacskaylo E. Mycorrhiza: the ultimate in reciprocal parasitism? BioScience. 1972;22(10):577–582.
- Kwak M, Hong JK, Park JH, Lee BY, et al. Genetic assessment of Abies koreana (Pinaceae), the endangered Korean fir, and conservation implications. Conserv Genet. 2017;18(5):1165–1176.
- Kim NS, Lee HC. A study on changes and distributions of Korean fir in sub-alpine zone. J Korean Environ Restor Technol. 2013;16(5):49–57.
- Ahn US, Kim DS, Yun YS, et al. The inference about the cause of death of Korean Fir in Mt. Halla through the analysis of spatial dying pattern – Proposing the possibility of excess soil moisture by climate changes. Kor J Agri for Met. 2018;21:1–28.
- Kang SJ. Regeneration process of subalpine coniferous forest in Mt. Jiri. J Ecol Environ. 1984;7:185–193.
- Park WK, Seo JW. A dentroclimatic analysis on Abies koreana in Cheonwang-bong area of Mt. Chiri Korea. Kor J Quat Res. 1999;13:25–33.
- Lee CS, Cho HJ. Structure and dynamics of Abies koreana Wilson community in Mt. Gaya. Kor J Ecol. 1993;16:75–91.
- Koo KA, Park WK, Kong WS. Dendrochronological analysis of Abies koreana W. at Mt. Halla, Korea: effects of climate change on the growths. Kor J Ecol. 2001;24:281–288.
- Koh JG, Kim DS, Kim JG, et al. Growth dynamics of Korean fir in Mt. Halla. Hallasan Res Rep. 2015;14:9–25.
- Sim MY, Eo JK, Eom AH. Diversity of Ectomycorrhizal fungi of Abies koreana at Mt. Halla. Kor J Mycol. 2009;37(2):134–138.
- Lee JE, Eom AH. Ectomycorrhizal fungal diversity on Abies koreana and Taxus cuspidata at two altitudes in Mt. Halla. Kor J Mycol. 2019;47:199–208.
- Jumpponen A, Jones KL, Mattox JD, et al. Massively parallel 454-sequencing of fungal communities in Quercus spp. ectomycorrhizas indicates seasonal dynamics in urban and rural sites. Mol Ecol. 2010;19:41–53.
- Lim YW, Kim BK, Kim C, et al. Assessment of soil fungal communities using pyrosequencing. J Microbiol. 2010;48(3):284–289.
- Kim CS, Nam JW, Jo JW, et al. Studies on seasonal dynamics of soil-higher fungal communities in Mongolian oak-dominant Gwangneung forest in Korea. J Microbiol. 2016;54(1):14–22.
- Kim CS, Han SK, Nam JW, et al. Fungal communities in a Korean red pine stand, Gwangneung forest. Kor J Asia Pac Biodivers. 2017;10(4):559–572.
- Buée M, Courty PE, Mignot D, et al. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal community. Soil Biol Biochem. 2007;39(8):1947–1955.
- Petrosino JF, Highlander S, Luna RA, et al. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55(5):856–866.
- Nagano Y, Nagahama T, Hatada Y, et al. Fungal diversity in deep-sea sediments – the presence of novel fungal groups. Fungal Ecol. 2010;3(4):316–325.
- Voříšková J, Brabcová V, Cajthaml T, et al. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014;201(1):269–278.
- Martin JP, Haider K. Biodegradation of C-labeled model and cornstalk lignins, phenols, model phenolase humic polymers, and fungal melanins as influenced by a readily available carbon source and soil. Appl Environ Microbiol. 1979;38(2):283–289.
- Amelung W, Lobe I, Du Preez CC. Du Preez CC. Fate of microbial residues in sandy soils of the South African highveld as influenced by prolonged arable cropping. Eur J Soil Sci. 2002;53(1):29–35.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and application. New York (NY): Academic Press; 1990. p. 315–322.
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336.
- Kõljalg U, Nilsson RH, Abarenkov K, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2013;22(21):5271–5277.
- Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998.
- Oksanen J, Blanchet FG, Friendly M, et al. 2019. Vegan: community ecology package. Available from: https://cran.r-project.org/web/packages/vegan/vegan.pdf(internet)
- Allington WB, Chamberlain DW. Brown stem rot of soybean. Phytopathology. 1948;38:793–802.
- Di Marco S, Calzarano F, Osti F, et al. Pathogenicity of fungi associated with a decay of kiwifruit. Austral Plant Pathol. 2004;33(3):337–342.
- Travadon R, Lawrence DP, Rooney-Latham S, et al. Cadophora species associated with wood-decay of grapevine in North America. Fungal Biol. 2015;119(1):53–66.
- Blanchett RA, Held BW, Jurgens JA, et al. Wood-destroying soft rot fungi in the historic expedition huts of Antarctica. Appl Environ Microbiol. 2004;70(3):1328–1335.
- Tedersoo L, Sánchez-Ramírez S, Kõljalg U, et al. High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018;90:135–159.
- Branco S, Ree RH. Serpentine soils do not limit mycorrhizal fungal diversity. PLoS One. 2010;5(7):e11757.
- Gao Q, Yang ZL. Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the eastern Himalaya. Mycorrhiza. 2010;20(4):281–287.
- Orlovich DA, Draffin SJ, Daly RA, et al. Piracy in the high trees: ectomycorrhizal fungi from an aerial ‘canopy soil’ microhabitat. Mycologia. 2013;105(1):52–60.
- Kühdorf K, Münzenberger B, Begerow D, et al. Leotia cf. lubrica forms arbutoid mycorrhiza with Comarostaphylis arbutoides (Ericaceae). Mycorhiza. 2015;25(2):109–120.
- Argüelles-Moyao A, Garibay-Orijel R, Márquez-Valdelamar LM, et al. Clavulina-Membranomyces is the most important lineage within the highly diverse ectomycorrhizal fungal community of Abies religiosa. Mycorrhiza. 2017;27(1):53–65.
- Unuk T, Martinović Finžgar D, Šibanc N, et al. Root-associated fungal communities from two phenologically contrasting silver fir (Abies alba Mill.) groups of trees. Front Plant Sci. 2019;10:214.
- Rudawska M, Pietras M, Smutek I, et al. Ectomycorrhizal fungal assemblages of Abies alba Mill. outside its native range in Poland. Mycorrhiza. 2016;26(1):57–65.
- Tian J, Qiao Y, Wu B, et al. Ecological succession pattern of fungal community in soil along a retreating glacier. Front Microbiol. 2017;8:1028.
- Lakhanpal TN. Ectomycorrhiza-an overview. In: Mukerji, KG, Chamola BP, Singh J, editors. Mycorrhizal biology. New York (NY): Kluwer Academic/Plenum; 2000. p.101–118.
- Ważny R, Kowalski S. Ectomycorrhizal fungal communities of silver-fir seedlings regenerating in fir stands and larch forecrips. Trees. 2017;31(3):929–939.
- Reverchon F, Ortega-Larrocea MP, Pérez-Moreno J, et al. Changes in community structure of ectomycorrhizal fungi associated with Pinus montezumae across a volcanic soil chronosequence at Sierra Chichinautzin, Mexico. Can J for Res. 2010;40(6):1165–1174.