 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Entisol soil is hard and compact in nature, rendering it high in bulk density, which influences root penetration adversely and thereby poor plant growth. In this experiment, used seven treatments in different combination in normal soil, were used as growth media for the Terminalia arjuna seedling. T3 (60% entisol) found the best as it gave the highest biomass in the species regardless of arbuscular mycorrhizal fungi (AMF) treatment. AMF treatment enhanced the growth and biomass of plants significantly in all the given treatments. AMF colonization observed a maximum in tertiary roots. T1 (100% entisol soil) exhibited the highest degree of AMF colonization in tertiary roots, resulting in the highest mycorrhiza dependency of plants for this soil. The addition of normal soil to entisol soil was found to decrease the bulk density, resulting in increased root diameter, and T3 plants exhibited the highest biomass and AMF compatibility for T. arjuna species. The T. arjuna plant’s growth and biomass responded positively to AMF in all types of treatments. The plant’s growth and biomass were highest in the T3 treatment, which had a bulk density of 1.50 g/cm3. In this study, we combined the entisol with mycorrhizal inoculation of the nursery growing medium to promote plant growth and biomass, improve the plant’s ability to hold water and absorb nutrients, and lower the entisol’s bulk density. The T. arjuna (Roxb) plant responds very favorably to mycorrhiza inoculation in nursery conditions with the entisol growth medium.
1. Introduction
Entisols are very extensive soils all over the world. They occupy approximately 18% of the Earth’s surface. In India, this soil group is present in 80.1 Mha and covers 24.27% of the total soil area [Citation1]. Entisols are soils distinguished by the absence or near absence of soil-forming horizons (layers). During the monsoon season, entisol soil lands are primarily utilized for grazing, with the growth of some spare grass. This land is dominated in Chhattisgarh (India), characterized by gentle slopes and excessive gravel due to a spherical mass of soil called murrum [Citation2–4]. The entisol soil is generally hard and compact, even forming a laterite pan at a shallow depth below the surface. Soils are deficient in organic matter and nutrients, which makes these lands completely unsuitable for agricultural purposes [Citation5–7]. Entisol soil is poor in productivity and is often barren or suitable only for coarse millets and therefore treated as permanent pastures due to poor water holding capacity, heavy biotic pressure, and low microbial activity [Citation8–11]. Although entisol soil is very compact and thus difficult for root penetration and plant establishment. Therefore, after tedious efforts, only a few forest species survive with poor growth [Citation2,Citation12].
Biofertilizers are more suitable microbial inoculants, defined as preparations containing live or latent cells of efficient strains of nitrogen-fixing, phosphate-solubilizing, or cellulolytic microorganisms.
Arbuscular mycorrhizal fungi (AMF) are widely present in nature and extensively utilized worldwide in agriculture, horticulture, and forestry [Citation13]. AMF form associations with plants called symbiotic associations, which are usually beneficial to both organisms. In exchange for carbohydrates produced by the host through photosynthesis, the fungi help the plant take up water and immobile soil nutrients [Citation14,Citation15]. Endomycorrhizas form associations with most plants (approximately 80% of all plant species) [Citation16]. They occur on bryophytes, pteridophytes, agricultural crops, horticultural trees, and the majority of forest tree species [Citation17]. These fungi require association with plant roots for growth, as they cannot be grown in pure culture. They form branched structures called arbuscules within the host’s root cells, and thus they are known as AM fungi. Fungal root infection is a complex process that includes spore germination, hypha differentiation, appressorium formation, root penetration, intercellular growth, vesicle and arbuscular formation, and nutrient transfer [Citation16, Citation18–20]. As roots develop, the conditions for inoculation by AMF improve, and the carbohydrates are used by AMF for the extension of the hyphal network. AMF may increase plant tolerance to biotic and abiotic stresses [Citation21].
The interactions between soil and plant roots result in a decrease in soil bulk density in compact soil [Citation19,Citation22]. The production of extensive hyphae in AMF makes its surface area much larger. This helps plants grow in harsh conditions like drought stress [Citation23–25] and nutrient deficiency, which is common in entisol soil [Citation26]. The main objectives of the research are to increase the growth and biomass of the Terminalia arjuna plant used by the AMF in the amendment and anamendment of entisol. In this research, mycorrhiza was used as an experiment because mycorrhiza is a symbiotic fungus that sustains the environment and provides various nutrients without having harmful effects on plants.
2. Materials and methods
The researchers conducted the experiment in the Forestry Department Nursery in Guru Ghasidas Vishwavidyalaya Bilaspur, Chhattisgarh, India. The nursery recorded an average monthly minimum temperature of 12.8 °C in December and an average maximum temperature of 42.5 °C in May of 2014–2017. The area is low in altitude (267 m above sea level), with an average annual precipitation of 1259 mm. Most rainfall occurs from July to September. The plants (T. arjuna) were planted in a randomized block design with seven treatments replicated. The AMF composition developed the experimental purposes. The Funneliformis mosseae AMF species used were cultivated for their use in the experiments of the present study. Therefore, the present experiment was conducted to find out the most suitable mixture of entisol soil for plant growth by mixing entisol soil and normal soil in different ratios (T1 = 100% entisol soil, T2: 80% entisol soil + 20% normal soil, T3: 60% entisol soil + 40% normal soil, T4: 50% entisol soil + 50% normal soil, T5: 40% entisol soil + 60% normal soil, T6: 20% entisol soil + 80% normal soil, and T7: 100% normal soil) followed by AMF inoculation. The soil mixtures autoclaved in 2 h at 105 °C to make an AMF − and microbial-free substrate.
Different soil mixtures mixed in a definite proportion were filled in a polythene bag of size 23 × 11 cm. Each poly bag was inoculated with 20 g of mixed AMF (isolated from entisol soil) containing 800 viable spores and 10 g of infected roots. Seeds of T. arjuna were grown separately (single seed in one polybag) in sterilized normal soil and then transferred to bags after one month as per the experimental design. The seed of T. arjuna was collected in nearby Bilaspur city. The experiment consisted of seven treatments with 10 replicates, each having 10 seedlings in a block. We carried out inter-culture operations such as watering and weed cleaning according to the needs of the seedlings. The seedlings of the experiment were uprooted after six months of AMF inoculation, and 10 seedlings (one from each replicate) were selected randomly from each treatment, and the following measurements were considered for recording.
2.1. Soil and root sample collection
The soil and root samples were collected in 3 pits of 30 cm size within the plantation, and samples were collected from 0 to 30 cm depth. Samples collected separately from each tree species were thoroughly mixed, dried, and sieved (2 mm sieve size). We kept the soil and root samples separate without drying and imported them to the department for further analysis of symbiosis and spore population. AMF colonization and the development of mycelia, vesicles, and arbuscules were observed to characterize the colonization expression of AMF.
2.2. Bulk density (Mg m−3)
The bulk density of the soil is a measure of the mass and volume of oven-dried soil samples. Measuring the size of the sampling cylinder or the quantity of sand or water allows for obtaining the volume of a soil sample [Citation27]. Calculate the dry soil ρb using the formula:
where ρb is in g/cm3, Ms is the weight of the saturated soil sample g, and Vs is the volume of the dry soil sample in cm3 [Citation28].
2.3. Seedling growth parameter
At the beginning of experiments, we measured seedling height (cm) using a digital forestry caliper before harvesting, and collar diameter (cm) using a digital caliper at the same time.
2.4. Number of root in plant
The number of plant roots (primary, secondary, and tertiary) was measured during the harvesting of the plant.
Beginning with the seed germination and ending six months later with the seedling plant.
2.5. Dry biomass (g/pl.)
The total oven-dry weight (80 °C during 24 h) of each seedling-sapling part was determined using the following formula [Citation29,Citation30]:
where dw is the total dry weight (kg), sdw is the dry weight of the sample (g), and fw is the total fresh weight (kg).
2.6. AMF spore counting
The 500-ml conical flask was filled with 10 g of soil and 100 ml of water. We stirred the mixture ferociously to dislodge the AMF spores from the soil. After allowing it to settle for 15–45 min, the supernatant was decanted through customary sieves. We took spores under a dissecting microscope using a pipette or needle [Citation31].
2.7. Root colonization (%)
We treated plant root samples with a 10% KOH solution and stained them with trypan blue. The following formula was used to determine the percentages of mycelial, arbuscular, vesicular, and total colonization:
2.8. Mycorrhiza dependency (%)
Mycorrhizal dependency was calculated using the formula below, according to [Citation32]:
2.9. Statistical analysis
For calculation and interpretation of the data, we used the SPSS 16.0 version (SPSS Inc., Chicago, IL) to perform the statistical analyses. Pearson’s correlation coefficients were employed to determine the relationships between fungal colonization parameters and environmental factors. We calculated standard errors of means for all the parameters studied and used the Duncan multiple range (DMR) test to compare means at a 5% p level in one-way ANOVA.
3. Results
Results are summarized in and and . The result reveals that bulk density decreased from 1.70 g/cm3 in T1 (100% entisol soil) to 1.29 g/cm3 in T6 while it was observed at its lowest of 1.16 g/cm in T7 (normal soil) (). Plant height and collar diameter significantly increased and were recorded as highest in T3 (60% entisol and 40% normal soil combination) and lowest in T1 (). AMF inoculation was found to be compatible and beneficial for growth attributes, as it exhibited higher results in mycorrhized plants compared to control plants without inoculation ().
Figure 1. Effect of different ratio of soil substrate on (a) height (cm/plant); (b) collar diameter; and (c) total dry biomass (g plant−1) of Terminalia arjuna seedling inoculated with AMF and its comparison to control plant grown in different ratio of entisol and normal soil. Same letter of different column represent non-significant difference at p < .5% as DMR test and bar on the column ± standard error.
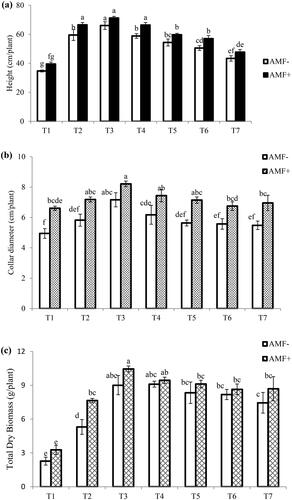
Table 1. Primary root diameter, secondary root diameter, number of first root, number of second root, number of third root, third root colonization, second root colonization, first root colonization of Terminalia arjuna seedling/plant after AMF inoculation with different amended entisol soil and normal soil composition.
Table 2. The impact of different ratio of soil amendments on fresh and dry biomass of Terminalia arjuna seedling with AMF (AMF+) and without inoculation (AMF-) as long as different amended entisol soil and normal soil composition.
The plants of T1 soil rendered the highest number of primary, secondary, and tertiary roots, which followed a declining trend consistently with the addition of normal soil in treatments T2–T7, giving a significant difference between treatments at p < .001 for the species (). Primary root and secondary root diameter were lowest in T1, which increased significantly toward an increasing ratio of normal soil from T1 to T7. This shows that the extra normal soil in entisol soil helps to lower the bulk density of the soil, which makes the collar diameter of the roots bigger than in T1 in this species.
All treatment results show a decrease in the number of primary, secondary, and tertiary roots. The lowest number of roots was primary at 26.33 per plant, secondary at 215 per plant, and tertiary at 255.3 per plant, recorded under T7 soil (). The results were significant for primary and tertiary roots at p < .05, but secondary root development showed a non-significant result. AMF colonization was not significantly different considering the treatment factor, but showed significant differences compared to AMF colonization in primary, secondary, and tertiary roots. AMF colonization in tertiary roots ranged from 58 to 68%, in secondary roots from 32 to 37%, and in primary roots from 9 to 12% (). This is indicative that tertiary roots are a primary site of AMF compared to other roots, which helps in the higher uptake of nutrients and water by forming strong AMF symbioses. The plant species had the most shoot, root, and total biomass of AMF inoculants and non-AMF inoculants in T3, which confirms the best result. On the other hand, the plant species was most dependent on AMF in T1, which shows how important mycorrhiza is for plants that grow in soils with a higher bulk density ().
The highest dry biomass shoot found was 10.39 g and root 9.59 g/plant in T3 inoculated with AMF in T. arjuna, which was 29.71% and 19.72% higher compared to non-inoculated control plants grown in the same ratio of soil (). This treatment rendered the highest total biomass with AMF inoculation (). Plants grown in T1 exhibited the lowest biomass, but the highest mycorrhizal dependency calculated for the plant species in this soil indicates the positive effects of AMF in entisol soil for better symbiosis with hosts, enabling efficient utilization of nutrients and higher resistance mechanisms in plants.
4. Discussion
AMF inoculation in amended soil might enhance the bulk density in entisol and help in plant growth, as in the case of the current investigation and also as described by [Citation33,Citation34]. Moreover, improved growth and biomass due to amendment and AMF were the result of higher nutrient and water uptake by the plants, which can be expected in response to better AMF growth and fungal network [Citation13,Citation35,Citation36]. Colonization with AMF can result in root morphological changes that are well known [Citation37]. Similar to the present findings, Miransari [Citation38] reported that AMF is important for soil aggregation, where direct hyphal involvement is thought to be most pronounced and differences in root architecture also determine the overall influence of root penetration. Some researchers also investigated that the demand for food material AMF from the plants and regular supply of the nutrient of the plant by AMF influence the growth and biomass of the AMF plant [Citation39–41]. Due to environmental factors (temperature, moisture, etc.), tertiary roots expose more than the primary and secondary roots. According to some investigations, whenever roots face any severe condition, plant roots exudate strigolactone hormones, one of which is responsible for the AMF and root association [Citation42,Citation43]. Strigolactone hormones and AMF spores produce myc factors for the association, and this association is structured in the epidermal cell of the root [Citation42]. Mycorrhiza is always associated with the feeder root as compared to the main root [Citation44]. Further accentuated that the AM is especially important in degraded soils for a number of reasons, such as higher availability of nutrients and improving soil structure through extraradical hyphal networks, which release glomalin and help in water infiltration [Citation44–46]. Managing soil could thus be a potential way to optimize the proliferation of indigenous AM fungi. In the present study, fertile normal soil was mixed with entisol soil, resulting in decreased bulk density and an improvement in porosity that resulted in elevated plant biomass compared to entisol without any amendments. Additionally, it has been demonstrated by Ryan et al. [Citation47], Joner and Jakobsen [Citation48], and Debashis and Somdatta [Citation49], that the addition of organic matter can increase soil porosity and decrease mechanical soil resistance to the formation of AMF hyphae. AMF gives better results in adverse conditions as compared to normal soil. It is also concluded that the high bulk density of entisol affects root diameter but favors tertiary roots, which causes more AMF colonization and helps in developing an efficient plant mechanism when soil conditions are adverse in terms of nutrients, water, and physical adherence [Citation50,Citation51]. We can increase the biomass and plant height by using 40% of the normal soil (1.50 g/cm3). According to our experiment, we can conclude that 40% of the normal soil is required to mix in the entisol soil for the better growth of the plant [Citation52]. The high mycorrhiza dependency value suggests that the AMF in MycoSilvi is good for growing high-quality seedlings in the field and in nurseries. It is also resistant to root pathogen attacks, drought, heavy metal stress, and not having enough nutrients in the soil [Citation53]. AMF has been shown to have a high dependence in many tropical tree species, including Acacia nilotica [Citation54], Acacia melanoxylon [Citation55], Acacia mangium [Citation54], Hancornia speciosa [Citation56], Dyera polyphylla, and Aquilaria filarial [Citation57]. Tawaraya [Citation58] found from the same study that the tree species group had the highest mycorrhizal dependence when compared to field crops, wild grasses, and forage crops.
5. Conclusions
AMF was mixed with various proportions of fertilizer, as normal, to test its compatibility with the growth and biomass of the T. arjuna plant. AMF colonized the tertiary roots in the pure entisol to the highest degree, resulting in the highest MD of plants in this soil. When normal soil was added to entisol soil, the bulk density went down. This made the roots bigger and gave the T. arjuna species the most biomass and AMF compatibility. This research finds that pure entisol decreases the root penetration of the plant. The mixture of normal soil and entisol decreases the bulk density of the soil. They increase the number of roots, root diameter, and biomass of the plant. The AMF associations of the entisol increase the nutrient and water uptake in the plant. Entisol is found in large areas of the world, and it decreases the plant growth characteristics that are required in mycorrhiza inoculation for better growth. In the future, this research will be very helpful in developing more quantities of forest seedlings for use in entisol.
Acknowledgements
The authors gratefully acknowledge the contributions of the Department of Forestry, Wildlife, and Environmental Science, Guru Ghasidas University, Bilaspur. They provide the working instruments and the research area for the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be available on request.
Additional information
Funding
References
- Chandrakala M, Lakhsman K, Maske SP. Indian soils: characteristics, distribution, potentials and constraints. Chron Bioresour Manage. 2021;5:121–127.
- Bhardwaj AK, Chandra KK. Biomass and carbon stocks of different tree plantations in entisol soil of Eastern Chhattisgarh India. Curr World Environ. 2016;11(3):819–824. doi: 10.12944/CWE.11.3.17.
- Chandra KK, Bhardwaj AK. Growth, biomass and carbon sequestration by trees in nutrient-deficient Bhata land soil of Bilaspur, Chhattisgarh, India. In: Singh VP, Yadav S, Yadava R, editors. Energy and environment, water science and technology library. Singapore: Springer Nature Singapore Pte Ltd; 2018. p. 39–45.
- Anthony MA, Crowther TW, van der Linde S, et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022;16(5):1327–1336. doi: 10.1038/s41396-021-01159-7.
- Loka SP, Nasution DLS. Revamping of entisol soil physical characteristics with compost treatment. IOP Conf Ser Earth Environ Sci. 2018;122:012090. doi: 10.1088/1755-1315/122/1/012090.
- Al-Shammary AAG, Kouzani AZ, Kaynak A, et al. Soil bulk density estimation methods: a review. Pedosphere. 2018;28(4):581–596. doi: 10.1016/s1002-0160(18)60034-7.
- Moore JAM, Anthony MA, Pec GJ, et al. Fungal community structure and function shifts with atmospheric nitrogen deposition. Glob Chang Biol. 2021;27(7):1349–1364. doi: 10.1111/gcb.15444.
- Planning Commission State Report. Ecological features of Chhattisgarh; 2013. p. 53. Available from: http://planning commission.nic.in
- Bhardwaj AK, Chandra KK. AMF symbiosis in Forest species plantations and its relationship with major soil nutrients in entisol soil of Bilaspur (C.G.). Life Sci Bull. 2017;14:27–32.
- Costa CRGd, Silva Fraga VD, Lambais GR, et al. Chemical and physical quality of the entisol in a natural regeneration area in the semiarid region of Paraiba. J Exp Agric Int. 2019;35:1–7. doi: 10.9734/jeai/2019/v35i230202.
- Javaid A, Khan IH. Mycorrhiza fungi associated with mungbean. Mycopath. 2019;17:45–48.
- Shahid SA, Zaman M, Heng L. Introduction to soil salinity, sodicity and diagnostics techniques. In: Zaman M, Shahid SA, Heng L, editors. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Vienna: International Atomic Energy Agency, Springer; 2018. p. 1–42. doi: 10.1007/978-3-319-96190-3_1.
- Begum N, Qin C, Ahanger MA, et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068.
- Ganugi P, Masoni A, Pietramellara G, et al. A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy. 2019;9(12):840. doi: 10.3390/agronomy9120840.
- Bhardwaj AK, Chandra KK, Kumar R. Water stress changes on AMF colonization, stomatal conductance and photosynthesis of Dalbergia sissoo seedlings grown in entisol soil under nursery condition. Forest Sci Technol. 2023;19(1):47–58. doi: 10.1080/21580103.2023.2167873.
- Bonfante P, Genre A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1(1):48. doi: 10.1038/ncomms1046.
- Smith SE, Read D. Mycorrhizal symbiosis; 2008. p. 637–768. doi: 10.1016/b978-012370526-6.50020-9.
- Harrier LA. The arbuscular mycorrhizal symbiosis: a molecular review of the fungal dimension. J Exp Bot. 2001;52(Spec Issue):469–478. doi: 10.1093/jxb/52.suppl_1.469.
- Kumar R, Bhardwaj AK, Chandra KK, et al. Mycorrhizae: an historical journey of plant association. Chhattis J Sci Technol. 2022;19:437–447.
- Bhardwaj AK, Chandra KK, Kumar R. Mycorrhizal inoculation under water stress conditions and its influence on the benefit of host microbe symbiosis of Terminalia arjuna species. Bull Natl Res Cent. 2023;47(1):13. doi: 10.1186/s42269-023-01048-3.
- Aroca R, Ruiz-Lozano JM, Zamarreno AM, et al. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol. 2013;170(1):47–55. doi: 10.1016/j.jplph.2012.08.020.
- Barea JM, Gryndler M, Lemanceau PH, et al. The rhizosphere of mycorrhizal plants. In: Gianinazzi S, Schuepp H, Barea JM, et al., editors. Mycorrhiza technology in agriculture: from genes to bioproducts. Basel, Switzerland: Birkhauser Verlag; 2002. p. 1–18.
- Chandra KK. Recovery pattern in diversity and species of ground vegetation and AMF in reclaimed coal mine dumps of Korba (India). Expert Opin Environ Biol. 2014;3:1–12.
- Al-Karaki G, McMichael B, Zak J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 2004;14(4):263–269. doi: 10.1007/s00572-003-0265-2.
- Suz LM, Bidartondo MI, van der Linde S, et al. Ectomycorrhizas and tipping points in forest ecosystems. New Phytol. 2021;231(5):1700–1707. doi: 10.1111/nph.17547.
- Bagheri V, Shamshiri MH, Shirani H, et al. Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J Agric Sci Technol. 2012;14:1591–1604. doi: 10.5367/oa.2012.0109.
- Campbell DJ. Determination and use of soil bulk density in relation to soil compaction. Dev Agric Eng. 1994;11:113–139. doi: 10.1016/B978-0-444-88286-8.50014-3.
- Han YZ, Zhang JW, Mattson KG, et al. Sample sizes to control error estimates in determining soil bulk density in California forest soils. Soil Sci Soc Am J. 2016;80(3):756–764. doi: 10.2136/sssaj2015.12.0422.
- Hairiah K, Sitompul SM, Noordwijk MV, et al. Methods for sampling carbon stocks above and below ground, ASB lecture note 4B. Bogor: International Centre for Research in Agroforestry; 2001. p. 23–29.
- Ministry of Forestry Indonesia. Development of allometric equations for estimating Forest carbon stocks based on field measurement (ground based forest carbon accounting). Indonesia: Centre for Standardization and Environment, Ministry of Forestry; 2011.
- Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc. 1963;46(2):235–244. doi: 10.1016/S0007-1536(63)80079-0.
- Graham JH, Syvertsen JP. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol. 1985;101(4):667–676. doi: 10.1111/j.1469-8137.1985.tb02872.x.
- Vaidya GS, Shrestha K, Khadge BR, et al. Study of biodiversity of arbuscular mycorrhizal fungi in addition with different organic matter in different seasons of Kavre District (Central Nepal). Sci World. 1970;5(5):75–80. doi: 10.3126/sw.v5i5.2660.
- Deepika S, Kothamasi D. Soil moisture a regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza. 2015;25(1):67–75. doi: 10.1007/s00572-014-0596-1.
- Xie X, Weng B, Cai B, et al. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth and nutrient uptake of Kandelia obovata seedlings in autoclaved soil. Appl Soil Ecol. 2014;75:162–171. doi: 10.1016/j.apsoil.2013.11.009.
- Wu YH, Wang H, Liu M, et al. Effects of native arbuscular mycorrhizae isolated on root biomass and secondary metabolites of Salvia miltiorrhiza Bge. Front Plant Sci. 2021;12:1–13. doi: 10.3389/fpls.2021.617892.
- Garg N, Chandel S. Arbuscular mycorrhizal networks: process and functions, a review. Agron Sustain Dev. 2010;30:581–599. doi: 10.1051/agro/2009054.
- Miransari M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010;12(4):563–569. doi: 10.1111/j.1438-8677.2009.00308.x.
- Berta G, Fusconi A, Trotta A, et al. Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol. 1990;114(2):207–215. doi: 10.1111/j.1469-8137.1990.tb00392.x.
- Marschner H, Dell B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil. 1994;159(1):89–102. doi: 10.1007/BF00000098.
- Chandrasekaran M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: a meta-analysis. J Fungi. 2022;8(7):660. doi: 10.3390/jof8070660.
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6(10):763–775. doi: 10.1038/nrmicro1987.
- Genre A, Chabaud M, Faccio A, et al. Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell. 2008;20(5):1407–1420. doi: 10.1105/tpc.108.059014.
- Purin S, Rillig MC. The arbuscular mycorrhizal fungal protein glomalin: limitations, progress, and a new hypothesis for its function. Pedobiologia. 2007;51(2):123–130. doi: 10.1016/j.pedobi.2007.03.002.
- Wang F, Sun Y, Shi Z. Arbuscular mycorrhiza enhances biomass production and salt tolerance of sweet sorghum. Microorganisms. 2019;7(9):289. doi: 10.3390/microorganisms7090289.
- Priscila SM, Cristiane FDS, Junior MD, et al. Beneficial services of glomalin and arbuscular mycorrhizal fungi in degraded soils in Brazil. Sci Agric. 2022;79:1–13. doi: 10.1590/1678-992X-2021-0064.
- Ryan MH, Chilvers GA, Dumaresq DC. Colonisation of wheat by VA-mycorrhizal fungi was found to be higher on a farm managed in an organic manner than on a conventional neighbour. Plant Soil. 1994;160(1):33–40. doi: 10.1007/BF00150343.
- Joner EJ, Jakobsen I. Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem. 1995;27(9):1153–1159. doi: 10.1016/0038-0717(95)00047-I.
- Debashis K, Somdatta G. Aspects, problems and utilization of arbuscular mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr Res Microb Sci. 2022;3:1–11. doi: 10.1016/j.crmicr.2022.100107.
- Pauwels R, Graefe J, Bitterlich M. An arbuscular mycorrhizal fungus alters soil water retention and hydraulic conductivity in a soil texture specific way. Mycorrhiza. 2023;33(3):165–179. doi: 10.1007/s00572-023-01106-8.
- Egboka NT, Fagbola O, Nkwopara UN, et al. Density of arbuscular mycorrhizal fungi and nutrient status of soils in selected land use types and soil depths. Sarhad J Agric. 2022;38(2):633–647. doi: 10.17582/journal.sja/2022/38.2.633.647.
- Cao Y, Li N, Lin J, et al. Root system-rhizosphere soil-bulk soil interactions in different Chinese fir clones based on fungi community diversity change. Front Ecol Evol. 2022;10:1028686. doi: 10.3389/fevo.2022.1028686.
- Ghosh S, Verma NK. Growth and mycorrhizal dependency of Acacia mangium Willd. inoculated with three vesicular arbuscular mycorrhizal fungi in lateritic soil. New Forest. 2006;31(1):75–81. doi: 10.1007/s11056-004-4763-7.
- Sharma MP, Bhatia NP, Adholeya A. Mycorrhizal dependency and growth responses of Acacia nilotica and Albizia lebbeck to inoculation by indigenous AM fungi as influenced by available soil P levels in a semi-arid Alfisol wasteland. New For. 2001;21(1):89–104. doi: 10.1023/A:1010636614005.
- Renuka G, Rao MS, Kumar VP, et al. Arbuscular mycorrhizal dependency of Acacia melanoxylon R. Proc Natl Acad Sci India Sect B Biol Sci. 2012;82(3):441–446. doi: 10.1007/s40011-012-0025-1.
- Cardoso Filho JA, Lemos EEP, de Santos TMC, et al. Mycorrhizal dependency of mangaba tree under increasing phosphorus levels Pesqui. Agropec Bras. 2008;43:887–892.
- Turjaman M, Tamai Y, Santoso E, et al. Arbuscular mycorrhizal fungi increased early growth of two nontimber forest product species Dyera polyphylla and Aquilaria filaria under greenhouse conditions. Mycorrhiza. 2006;16(7):459–464. doi: 10.1007/s00572-006-0059-4.
- Tawaraya K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nutr. 2003;49(5):655–668. doi: 10.1080/00380768.2003.10410323.
