Abstract
Antioxidative activity of two in vitro cultivated Hypericum species – H. rumeliacum Boiss. and H. tetrapterum Fr. – was estimated after cryopreservation. Both species were successfully regenerated after a cryopreservation procedure performed by the vitrification method. H. tetrapterum did not manifest any significant oxidative stress-induced changes caused by low-temperature treatment. Conversely, a decrease in green pigments' content of H. rumeliacum was measured, particularly pronounced in chlorophyll b, which was accompanied by an increase of carotenoids in the regenerated plants.
A strong increase of malone dialdehyde and H2O2 levels in H. rumeliacum tissues was detected. Superoxide dismutase activity was enhanced by 170%, as well as the catalase activity, which was 220% above the control. The same trend was observed in H. tetrapterum, although less pronounced – 143% increase of superoxide dismutase and 112% of catalase.
Cryopreservation did not influence the phenol content in the examined plants, but it led to an increase of flavonoid content, especially in H. tetrapterum, by 237%. Total antioxidant activity in regenerated H. tetrapterum varied around the control level, but it was increased in H. rumeliacum. The free proline content in H. tetrapterum remained almost unaffected after freezing, as opposed to H. rumeliacum, where a strong increase of proline content (208% above the control) occurred. An electrolyte leakage from the cells of H. rumeliacum regenerated after cryopreservation was also registered, albeit not significant.
Introduction
Plant species of the genus Hypericum are among the most important medicinal plants.[Citation1,2] Hypericum rumeliacum is a rare species, characteristic for the Balkan flora, with conservational importance for Bulgaria. A number of studies report on its phytochemical composition and valuable pharmacological properties.[Citation3–5] In this regard, the development and improvement of protocols for cryopreservation and in vitro cultivation are one of the most important tasks designed to preserve and propagate these valuable and often endangered species, such as members of genus Hypericum.
Among various environmental stresses, low temperature is one of the most important factors limiting the productivity and distribution of plants and furthermore, it is also responsible for the production of ROS (reactive oxygen species) in the plant cell.[Citation6,7] It is established that cryopreservation also causes ROS-mediated oxidative stress, which could occur in every step of cryopreservation protocol associated with dehydration.[Citation8,9] Low-temperature-induced oxidative stress increases lipid peroxidation, and alters the enzyme activities and plant physiological processes.[Citation10] At a physiological level, photosynthesis is strongly affected by exposure to cold [Citation11] and photosynthetic pigments are highly sensitive to the deleterious effect of low temperatures. Freezing destroys chlorophyll biosynthesis, inhibits thylakoid electron transport and reduces photosynthesis.[Citation12] Low temperature causes a significant reduction in chlorophyll a and chlorophyll b, as well as significant changes in carotenoid composition.[Citation13]
Low temperatures affect substantially enzyme activity in plants.[Citation14] The activity of some antioxidant enzymes is partially correlated with the plant sensitivity to cold and, thus, the antioxidant enzymes possess a significant importance in the chilling tolerance.[Citation15] It was found that the extreme low temperatures could induce an increase of SOD (superoxide dismutase) activity and a decrease in CAT (catalase) activity.[Citation16]
High proline accumulation, in the plant cells under stress conditions, could significantly increase the capacity for ROS detoxifying and for lowering the oxidative damage. The similar effect could be observed in exogenous proline treated cells (as a part of the cryopreservation protocol). There are many studies where cellular membranes have been shown as the primary site of freezing injury in plants.[Citation17–20] The increased membrane permeability enhances ion leakage, allows the entrance of undesirable ions into the cell and hampers the osmosis and diffusion, etc.
According to our previous results, in vitro cultivated H. rumeliacum gave relatively low rates of plant survival but it is important to note that it produced high levels of phenolic and flavonoid compounds comparable to those in intact plants.[Citation21] It is well established that the phenolic compounds are mainly responsible for the unique medicinal characteristics of the Hypericum species and they are among the main reasons for the enhanced scientific interest in recent years.[Citation1,2,Citation5] As we have previously observed, the levels of total polyphenolic and flavonoid compounds are commensurable for both species – H. rumeliacum and H. tetrapterum – but total hypericin is much higher in H. rumeliacum.[Citation22] Since phenolic and flаvonoid compounds were in commenrurable levels for these two species, the high levels of total hypericins in H. rumeliacum in vitro made this species a very intriguing object for further investigation. This gave us a reason to continue the research on both experimental plants and their metabolic activities.
The aim of the present work was to evaluate the antioxidative status of H. rumeliacum and H. tetrapterum regenerated after cryopreservation.
Material and methods
The experimental plants, H. rumeliacum Boiss. and H. tetrapterum Fr. (voucher specimen deposited at the Herbaruim of the Institute of Botany, Bulgarian Academy of Sciences, Sofia-SOM 163 524), were cultivated on MS (Murashige and Skoog) medium at a temperature of 25 °C, 16/8 h photoperiod and light intensity of 0.000060 mol m−2 s−1 with a 45-day period of regular subculture. Cryopreservation was performed by the method of vitrification; shoot tips were pre-cultivated with 0.076 μmol/L АВА (abscisic acid) for 10 days and afterwards equilibrated for 90 min in the ice at 0 °С. Furthermore, the regeneration was performed on the MS medium containing 0.5 mg l−1 BA (benzyladenine).
Chlorophyll and carotenoid contents were determined after 80% acetone extraction according to Arnon et al.[Citation23] Endogenous hydrogen peroxide (H2O2) was determined spectrophotometrically at 390 nm after the reaction of the plant extract with 1 mol/L KI. Values were calculated using a standard curve.[Citation24] Malone dialdehyde (MDA) content was measured according to Dhindsa et al.[Citation25] Concentration of MDA was calculated by means of an extinction coefficient of 15,5000 L mol−1 cm−1. SOD activity was measured after Beauchamp and Fridovich.[Citation26] CAT activity was determined according to Aebi.[Citation27] Total phenolic content was determined according to Singleton et al.,[Citation28] total flavonoid content – according to Chang et al.[Citation29] and total antioxidant activity – according to Prieto et al.[Citation30] Proline content was measured as it was described by Bates et al.[Citation31]
Leaf membrane damage was estimated by recording the electrolyte leakage. Plant material was washed with deionized water and placed in tubes with 30 ml of deionized water and incubated for 24 h at 25 °C in a dark environment. The electrical conductivity of the solution was measured by HI 255 Combined Meter (Hanna Instruments). Samples were autoclaved at 1 atm for 30 min and the conductivity was measured again. The results were presented as % (it was accepted that the conductivity after autoclaving was 100%).
All experiments were repeated three times under the same conditions. The data were averaged of triplicate measurements. Тhe significance of differences between control and each treatment was determined using Student's t-test, p ≤ 0.05.
Results and discussion
In general, H. rumeliacum and H. tetrapterum were successfully regenerated after cryopreservation. We did not observe any visible differences between control and regenerated H. tetrapterum plants. A higher tolerance for low temperature was demonstrated, while regenerated H. rumeliacum plants showed slower growth rates, shorter stems, fewer leaves and poorly developed root system.
Photosynthetic pigments in H. tetrapterum did not change significantly after cryopreservation which confirmed the notion for that species as more tolerant to a freezing procedure (). A considerable decrease of green pigments’ content in H. rumeliacum (especially of chlorophyll b) was measured, accompanied with an increase of carotenoids. An 18% decrease of chlorophyll a in H. rumeliacum was measured after regeneration. Chlorophyll b was more significantly influenced by low temperature – its content was 26% decreased in H. rumeliacum tissues and 8% decreased in H. tetrapterum (). Similar results were obtained by Rahnavard et al.[Citation32] who observed that the higher altitude in the mountains combined with low temperature, provoked reduced chlorophyll biosynthesis in the low-tolerant Hypericum species, especially in H. rumeliacum.
Table 1. Influence of cryopreservation on the content of plastid pigments (mg/g FW, % of control) in the tissues of H. rumeliacum and H. tetrapterum.
Carotenoid content of H. tetrapterum was only slightly changed (5% increased) after cryopreservation. On the contrary, carotenoids in H. rumeliacum were 31% increased, which indicated a possible protective role against oxidative stress (). It is well documented that carotenoids may act as antioxidants, which functions include membrane protection against free radicals’ damage and their abundance increases at low temperatures.[Citation33,34] It has been demonstrated that growth at low temperature considerably modifies the pigment composition and the large reduction in contents of chlorophylls accompanied by the accumulation of large amounts of the de-epoxidized xanthophylls occurred.[Citation35]
Membranes are a primary site of cold-induced injury. It was previously found that there is a slight increase of MDA and Н2О2 levels after cryopreservation,[Citation21,Citation36] which may be indicative for the overcoming of oxidative stress and the recovery of the physiological status of regenerated H. rumeliacum. An increased accumulation of MDA and ROS occurred in regenerated Hypericum plants after cryopreservation, but the biosynthetic capacity of the regenerated plants was not impaired by the freezing procedure. Moreover, the cryopreserved plants showed higher phenolic and flavonoid contents and possess increased antioxidant capacity than those of the unfrozen controls.[Citation9]
After cryopreservation, both experimental species showed enhanced values of MDA and H2O2, most considerable in H. rumeliacum (). Insignificant levels of MDA in the tissues of H. tetrapterum in the control and regenerated plants were observed. On the contrary, MDA content in the regenerated H. rumeliacum was two times higher than that in the control plants, which could be regarded as evidence of increased lipid peroxidation caused by the cryopreservation.
Table 2. Influence of cryopreservation on the malone dialdehyde (MDA) content (mM/g FW, % of control) and Н2О2 content (μM/g FW, % of control) in H. rumeliacum and H. tetrapterum.
It is well known that Н2О2 overproduction induced by ROS and other stress conditions leads to disturbance in plant metabolism. We found that H2O2 content was affected less in comparison with MDA (). The levels of hydrogen peroxide in cryopreserved H. rumeliacum plants were highly increased (156% compared to the control). In the tissues of H. tetrapterum that increase was less pronounced – 12% above the control. These results confirmed the view of H. rumeliacum as a more sensitive species to the cryopreservation protocol that was used.
The reduced activity of antioxidant enzymes in extreme cold is a common effect which accelerates the accumulation of ROS in higher amount. Yang et al.[Citation37] have found that chilling stress reduced the activities of antioxidant enzymes viz. SOD, POD, CAT and APX in Cucumis sativus. Meanwhile, it was shown[Citation37,38] that the enhanced activities of SOD, CAT, APX and POX in some plants reflected in a better tolerance to chilling. It has been also found [Citation39] that there is an increased CAT and peroxidize activity in Hypericum plants growing at a higher altitude. Thus, activated protein synthesis and increased antioxidative enzymes’ activity play a significant role in the protection against stress conditions in the high mountains.
We observed that cryopreservation induced an increase in SOD and CAT activities in both experimental species. According to our results, the activity of SOD in H. rumeliacum tissues was highly increased – 170% above the control variant. That increase was less expressed in H. tetrapterum plants – 143% as compared to the control (). That rise in the activity of SOD could be considered as a consequence of the enhanced content of its substrate – superoxide anion.
Table 3. Changes of superoxide dismutase (SOD) activity (U mg−1 prot., % of control) and catalase (CAT) activity (ΔE min−1 mg−1 prot, % of control) in H. rumeliacum and H. tetrapterum after cryopreservation.
CAT activity in H. rumeliacum cells showed a strong increase after cryopreservation (220%), while the enzyme activity was only slightly increased in H. tetrapterum plants regenerated after cryopreservation (112%), (). The significant increase of CAT activity in cryopreserved H. rumeliacum plants could be used as evidence for ROS formation and development of oxidative stress. In the scientific literature there are many controversial publications about the effects of low temperature on CAT activity. CAT is generally considered to be more labile enzyme at low temperatures as compared to SOD. It is possible that the enzyme is damaged by OH. or by the excess of excitation energy which is not transformed via photosynthesis and further initiates photo-oxidative damage which in turn, affected negatively CAT.[Citation40,41] It has been shown, however, that low-temperature treatments led to a significant increase in total CAT activity and resulted in isoenzyme pattern shifts.[Citation42] It was therefore not surprising for us to measure the increase in the activity of CAT in Hypericum.
It is considered that natural antioxidants with their multifunctional activities may serve as a good alternative of synthetic compounds in preventing the oxidative damage.[Citation43] Those activities depend on a number of parameters including the experimental conditions. It was found that the broad profile of phenolic compounds determine their key role in detoxifying of free radicals in Hypericum species.[Citation44] Rahnavard et al.[Citation32] observed Hypericum species growing at the highest altitude and lowest temperature respectively, contain the highest quantity of hypericin and total phenolic content. On the contrary, the highest flavonoid content was measured in the ecotypes growing at the lowest altitude.
Cryopreservation did not affect negatively the phenolic and flavonoid biosynthesis in both experimental plants. Phenolic biosynthesis was not influenced by the cryopreservation procedure in both experimental plants ((A)) and the values varied around the control levels. It is important to emphasize that H. rumeliacum showed significantly higher biochemical capacity for synthesis of phenolic compounds as compared to H. tetrapterum.
Figure 1. Influence of cryopreservation on the secondary metabolites’ accumulation in the tissues of H. rumeliacum and H. tetrapterum; A – changes in total phenolic content; B – changes in total flavonoid content; C – changes in total antioxidant activity.
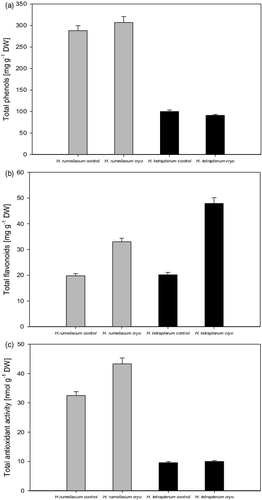
Flavonoids accumulate in leaves and stems in response to low temperatures. Recently, it has been reported that cold stress increases flavonoid biosynthesis.[Citation45] Besides, part of the valuable properties of Hypericum species was attributed mostly to flavonoid compounds. Both experimental species showed an increased flavonoid biosynthesis which is an unexpected beneficial effect caused by cryopreservation ((B)). Flavonoid content was particularly increased in H. tetrapterum tissues – 237% as compared to the control. It was observed that total antioxidant activity in the cryopreserved H. tetrapterum varied around control values while in the regenerated H. rumeliacum plants that parameter was increased (33% above the control) and that indicated the presence of oxidative stress and lower tolerance towards cryopreservation ((C)).
In response to cold and other osmotic stresses, plants accumulate a range of compatible solutes including proline.[Citation14,Citation46] It is well known that accumulation of proteins, carbohydrates and proline plays a major role in the plants’ survival at high-altitude conditions.[Citation39] Proline is successfully applied in the process of cryopreservation of plant cells due to its osmo-protective properties, as well as its importance as a regulator of cellular ROS balance.[Citation47]
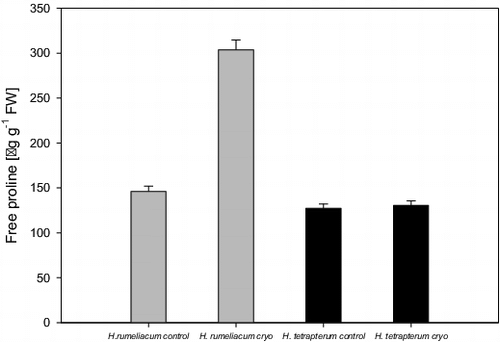
Proline content did not increase in the regenerated H. tetrapterum plants (). In the regenerated H. rumeliacum the concentration of free proline was increased twice compared to control values, which could be a clear indication for the presence of oxidative stress and low tolerance of that species to freezing.
Figure 2. Changes of free proline content in H. rumeliacum and H. tetrapterum under cryopreservation.
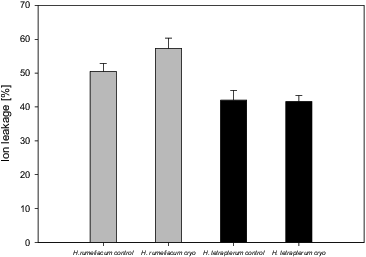
Ion leakage could be used as an indicator for the level of membranes’ injuries caused by different stress factors. As the low temperature disrupts the integrity of the cell membranes, it is possible that the inhibition of membrane associated reactions under low temperatures is attributed to increased membrane permeability.[Citation19] We observed an enhanced ion leakage (57.3%) from the cells of H. rumeliacum, which was an acceptable reason to assume that some membrane damages took place as a consequence of the processes of dehydration and freezing occurring during cryopreservation. No significant differences between the control and regenerated H. tetrapterum plants () were found. These results were in agreement with changes that were measured in MDA, H2O2 and the carotenoid content ( and ) and, thus, the idea that membranes of cryopreserved H. rumeliacum plants were affected in some extent by low temperature (increased ion leakage) was confirmed.
Conclusion
The results that were obtained gave us a good reason to suggest that H. tetrapterum is highly tolerant towards low temperature and cryopreservation. No disturbances in growth, biochemical and cellular processes were registered, nor were there any indications of oxidative stress. On the contrary, H. rumeliacum was less tolerant to cryopreservation. It showed a reduced pigment synthesis, high levels of MDA and H2O2 in the tissues, increased activities of SOD and CAT and some evidence for oxidative damage of the cell membranes. Taking into account that H. rumeliacum is an endemic species, very rich in hypericin, it is necessary to optimize the cryopreservation protocol aiming to reduce the oxidative stress symptoms in the regenerated plants and, thus, to ensure better survival and conservation.
Additional information
Funding
References
- Rainha N, Lima E, Baptista J, Rodrigues C. Antioxidant properties, total phenolic, total carotenoid and chlorophyll content of anatomical parts of Hypericum foliosum. J Med Plant Res. 2011;5(10):1930–1940.
- Robson N. Hypericum botany. In: Ernst E, editor. Hypericum: the genus Hypericum. New York: Taylor & Francis; 2003. p. 1–22.
- Danova K, Urbanová M, Skyba M, Čellárová E, Kapchina V. Evaluation of some physiological markers in Balkan endemic Hypericum rumeliacum Boiss. regenerated after cryopreservation. Poster session presented at: 1st International Symposium on Cryopreservation in Horticultural Species; 5–8 April 20095–8; Leuven, Belgium.
- Galati EM, Contartese G, Miceli N, Taviano MF, Sdrafkakis V, Couladis M, Tzakou O, Lanuzza F. Antiinflammatory and antioxidant activity of Hypericum rumeliacum Boiss. subsp. apollinis (Boiss. & Heldr.) Robson & Strid methanol extract. Phytother Res. 2008; 22:766–771.
- Kitanov G. Hypericin and pseudohypericin in some Hypericum species. Biochem Syst Ecol. 2001;29:171–178.
- Theocharis A, Clement C, Barka E. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235:1091–2005.
- Guo Y, Zhou H, Zhang L. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hortic. 2006;108:260–267.
- Hendry GA. Oxygen, free radical processes and seed longevity. Seed Sci Res. 1993;3:141–153.
- Yordanova ZP, Dimitrova MA, Cellarova E, Kapchina-Toteva VM. Physiological evaluation of Hypericum rumeliacum Boiss. plants regenerated after cryopreservation. In: Proceedings of the final meeting ‘Cryopreservation of crop species in Europe’; 8–11 February 2011; Agrocampus Ouest INHP, Angers, France.
- Chen K, Arora R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach (Spinacia oleracea). Plant Sci. 2011;180:212–220.
- Partelli F, Vieira H, Viana A, Batista-Santos P, Rodrigues A, Leitão A, Ramalho J. Cold impact on photosynthetic related parameters in coffee genotypes. Pesqui Agropecu Bras. 2009;44:1404–1415.
- Samuelson G, Lonneborg A, Gustafsson P, Oquist G. The susceptibility of photosynthesis to photoinhibition and the capacity of recovery in high and low light grown cyanobacterium, Anacystis nidulans. Plant Physiol. 1987;83:438–441.
- Aghaee A, Moradi F, Zare-maivan H, Zarinkamar F, Irandoost H, Sharifi P. Physiological responses of two rice (Oryza sativa L.) genotypes to chilling stress at seedling stage. Afr J Biotechnol. 2011;10:7617–7621.
- Ruelland E, Zachowski A. How plants sense temperature. Environ Exp Bot. 2010;69:225–232.
- Takác T, Luxová M, Gašparíková, O. Cold induced changes in antioxidant enzymes activity in roots and leaves of two maize cultivars. Biologia (Bratisl). 2003;58:875–880.
- Chaneva GT. Changes in the antioxidative defense system of Plectonema caused by extreme light and temperature. In: Proceedings of the International Conference on Development of the Economics and Society on the Base of Knowledge; 4–5 Jun 2009; Stara Zagora, Bulgaria. Vol. 3; p. 62–66.
- Hasanuzzaman M, Nahar K, Fujita M. Extreme temperatures, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C, editors. Abiotic stress – plant responses and application in agriculture. Rijeka: In Tech; 2013. p. 169–205.
- Matteucci M, D’Angeli S, Errico S, Lamanna R, Perrotta G, Altamura M. Cold affects the transcription of fatty acid desaturases and oil quality in the fruit of Olea europaea L. genotypes with different cold hardiness. J Exp Bot. 2011;62:3403–3420.
- Thomashow M. So What's New in the Field of Plant Cold Acclimation? Lots! Plant Physiol. 2001;125:89–93.
- Uemura M, Steponkus P. Cold acclimation in plants: relationship between the lipid composition and the cryostability of the plasma membrane. J Plant Res. 1999;11:245–254.
- Danova K. Production of polyphenolic compounds in shoot cultures of Hypericum species characteristic for the Balkan flora. Bot Serb. 2010;34(1):29–36.
- Danova K, Kapchina-Toteva V. Cryopreservation – a new method for conservation of Hypericum rumeliacum Boiss. In: Proceedings of the International Conference on Development of the Economics and Society on the Base of Knowledge; 4–5 June; Stara Zagora, Bulgaria. Vol. 3. p. 90–95.
- Arnon D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15.
- Jessup W, Dean RT, Gebicki JM. Iodometric determination of hydroperoxides in lipids and proteins. Methods Enzymol. 1994;233: 289–303.
- Dhindsa RS, Plunb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101.
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assay and on assay applicable acrylamide gels. Anal Biochem. 1971;44:276–287.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Singleton V, Orthofer R, Lamuela-Raventys R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178.
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341.
- Bates L, Waldren R, Teare I. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207.
- Rahnavard A, Daneshian J, Heravan E, Valadabadi S, Golein B. Investigation of the most important secondary metabolites of St. John's wort (Hypericum perforatum L.) in Caspian climate. J Food Agric Environ. 2012;10(2):375–381.
- Bulda O, Rassadina V, Alekseichuk H, Laman N. Spectrophotometric measurement of carotenes, xantophylls and chlorophylls in extracts from plant seeds. Russian J Plant Physiol. 2008;55(4):544–551.
- Ivanov A, Sane P, Krol M, Gray G, Balseris A, Savitch L, Oquist G, Huner N. Acclimation to temperature and irradiance modulates PSII charge recombination. FEBS Lett. 2006;580:2797–2802.
- Haldimann P. Low growth temperature-induced changes to pigment composition and photosynthesis in Zea mays genotypes differing in chilling sensitivity. Plant Cell Environ. 1998;21:200–208.
- Danova K, Cellarova E, Mackova A, Daxnerova Z, Kapchina-Toteva V. In vitro culture of Hypericum rumeliacum Boiss. and production of phenols and flavonoids. In Vitro Cell Dev Biol Plant. 2010;46:422–429.
- Yang H, Wu F, Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127:1237–1242.
- Zhao D, Shen L, Fan B, Liu K, Yu M, Zheng Y, Ding Y, Sheng J. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J Food Sci. 2009;74:348–352.
- Chkhubianishvili E, Kacharava N, Badridze G, Chanishvili S, Kurdadze T. Bull. Activity of peroxidase, catalase and content of total proteins in leaves of some herbaceous plants of high mountains of the Caucasus. Georgian Natl Acad Sci. 2011;5(2):96–100.
- Hertwig B, Streb P, Feierabend J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992;100:1547–1553.
- MacRae E, Ferguson I. Changes in catalase activity and hydrogen peroxide concentration in plants in response to low temperature. Physiol Plant. 1985;65(1):51–56.
- Scandalios J, Guan L, Polidoros A. Catalases in plants: gene structure, properties, regulation and expression. In: Scandalios JG, editor. Oxidative stress and the molecular biology of antioxidant defenses. Plainview (NY): Cold Spring Harbor Laboratory Press; 1997. p. 343–406.
- Wang R, Li R, Sun Z, Ren Y, Yue W. Anti-freezing proteins and plant responses to low temperature stress. Chinese J Appl Ecol. 2006;17:551–556.
- Silva B, Malva J, Dias A. St. John’Wort (Hypericum perforatum) extracts and isolated phenolic compounds are effective antioxidants in several in vitro models of oxidative stress. Food Chem. 2008;110(3):611–619.
- Crifo T, Puglisi I, Petrone G, Recupero G, Lo Piero A. Expression analysis in response to low temperature stress in blood oranges: implication of the flavonoid biosynthetic pathway. Gene. 2011;478:1–9.
- Patton A, Cunningham S, Volenec J, Reicher Z. Differences in freeze tolerance of zoysiagrasses: II. Carbohydrates and proline accumulation. Madison (WI): Crop Science Society of America; 2007. p. 2170–2181.
- Panis B, Lambardi M. Status of cryopreservation technologies in plants (crops and forest trees). In: Ruane J, Sunnino A, editors. The role of biotechnology in exploring and protecting agricultural genetic resources. Proceedings of the International Workshop, 2005 March 5–7; Turin: FAO; 2006.