ABSTRACT
The aim of this study is to investigate the effect of doxycycline collagen sponge on bisphosphonate-related osteonecrosis of the jaw (BRONJ) and the level of serum biomarkers as an indicator of osteonecrosis. Twenty-four rats were divided into four groups. Animals in the control group were injected with saline and animals in Groups I, II and III were injected with zoledronate three times a week for eight weeks. After eight weeks, the following procedures were performed in each group. In Group I: extraction of maxillary first molar, in Group II: extraction of maxillary first molar and mucoperiosteal coverage was performed and in Group III: extraction of maxillary first molar and mucoperiosteal coverage with doxycycline collagen sponges was performed. At the end of 16 weeks, all animals were sacrificed. Serum collagen type I C-telopeptide (CTx), tartrate-resistant acid phosphatase (TRACP 5b) and alkaline phosphatase (ALP) levels’ analysis, clinical examination, histological and histomorphometrical analysis were performed. As a result no significant difference in CTx, TRACP 5b and ALP levels was observed between groups. Complete mucosal healing was observed in all animals in the control group and 66.7% of animals in Group III. The necrotic bone area in Group III was significantly lower than the other groups (p < 0.01). Statistically significant difference was observed between groups in terms of detached osteoclast number (p < 0.01). In conclusion, local application of doxycycline could have a positive effect in reducing the risk of BRONJ in rats.
KEYWORDS:
Introduction
Bisphosphonates (BPs) are synthetic analogues of inorganic pyrophosphate that have strong properties for the suppression of osteoclast-mediated bone resorption.[Citation1] The most serious clinical complication of BPs is known as osteonecrosis of the jaw (ONJ) bones. Since association of BP and ONJ was first described in 2003; the pathogenesis of ONJ is not yet fully understood.[Citation1]The multifactorial pathogenesis is related to many local and/or general factors, including disruption of the normal bone turnover cycle, compromised angiogenesis, inhibition of oral cell wound healing, genetic polymorphisms and microbial biofilm.[Citation2–4]
Biochemical monitoring of bone metabolism depends upon measurement of enzymes, proteins and degradation products; produced during bone remodelling. Various biochemical markers are preferred to allow a specific and sensitive assessment of fracture risk, monitoring of BP treatment, risk determination of skeletal-related events and determination of the presence of bone metastases.[Citation5,Citation6] There is insufficient knowledge about serum bone markers to measure bone remodelling and possible ONJ after tooth extraction. It has been well recognized that the main effect of BP is reduced bone turnover, which may be evaluated by assessing bone resorption or formation of biomarkers in the serum.[Citation5] The most preferred predictor for osteoclast-dependent bone resorption is C-terminal telopeptide cross-link of type I collagen (CTx). Marx et al. [Citation6] stated that fasting CTx values of <100 pg/mL are associated with a high risk, 100–150 pg/mL with a moderate risk, and >150 pg/mL with a minimal risk of BRONJ after dental surgery and recommended that dental surgery should not be performed until CTx-level is 150 pg/mL. Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) has also been a promising marker in the fields of bone disease. Low diurnal variability, stable storage, being not affected by feeding and determination of renal or hepatic failure are reported advantages of TRACP 5b as a serum biomarker.[Citation7] Serum level of TRACP 5b has been claimed to reflect the regulation of bone remodelling.[Citation8] Bone alkaline phosphatase (ALP) is an enzyme that is produced by osteoblasts. It has a long half-life and is unaffected by renal clearance. Therefore, its serum level serves as a useful tool for assessing the level of bone formation.[Citation8]
BRONJ lesions are difficult to treat and there is consensus on the importance of prevention.[Citation6] Conservative methods are recommended in the treatment of BRONJ. Some cofactors such as tooth extractions, oral surgery and administered therapy may be related to the development of these lesions.[Citation9] Tooth extraction can induce bacterial invasion to the expose bone, and infection has a crucial role in maintaining these types of the lesion.[Citation10] Recently, it has been shown that immediate mucoperiostal coverage of the extraction socket can reduce the risk of ONJ and have good results in the early stage of ONJ.[Citation11] Doxycycline is a broad-spectrum antibiotic, active against gram-positive and gram-negative organisms. Systemic doxycycline treatment is among recommended antibiotics in stages I and II BRONJ.[Citation12] Local administration of doxycycline is generally used in periodontal surgery, and also reported that local usage results with higher concentrations in crevicular fluid than oral administration.[Citation13] Mucoperiostal coverage with locally applied doxycycline sponge after tooth extraction might be effective in the prevention of BRONJ.
The main purposes of this study were to evaluate the preventive effect of doxycycline collagen sponge on BRONJ, and to investigate and compare the utility of serum biomarkers as an indicator of ONJ.
Materials and methods
The experimental procedures were approved by the Yeditepe University Animal Care and Ethics Committee (protocol number 380/2014 YÜDHEK, Istanbul, Turkey). Twelve-week-old female Wistar rats with a mean weight of 225 ± 25 g were used in this study. All animals were housed at a standard temperature, humidity and with a 12-hour light/dark cycle. All rats were maintained free access to food and water. Animals were kept in the study environment for 1-week acclimation period. Twenty-four rats were randomly divided into four groups. Zoledronate (ZA) injection protocol was performed according to Senel et al.[Citation14] Animals in the control group were administered intraperitoneally 0.1 mg/kg sterile saline injection three times a week for eight weeks. Animals in Groups I, II and III were administered intraperitonally 0.1 mg/kg ZA (Zolenat 4 mg; Mustafa Nevzat, Istanbul, Turkey) injection three times a week for eight weeks.
All-surgical procedures were performed under general anaesthesia by combination with ketamine (Ketasol %10, Richter Pharma Ag, Wels, Austria) (20 mg/kg) and xylazine (10 mg/kg) (Rompun %2, Bayer AG, Istanbul, Turkey). After eight weeks, under general anaesthesia, upper left first molar of all animals were extracted with similar technique, using sharpened dental explorer and dental forceps. In Groups II and III, buccal mucoperiostal sliding flap was preferred to maintain primary closure in the extraction socket. Two vertical incisions were made on the distal and mesial side of the socket and mucoperiostal flap was reflected. Primary closure was achieved without tension by releasing an incision in the periosteum and the flap was then repositioned carefully and sutured. No antibiotic and anti-inflammatory drug was prescribed. Different treatment protocols were applied according to the groups. In the control group and Group I, no treatment was applied after tooth extraction. In Group II, following tooth extraction, the sockets were covered with a mucoperiosteal sliding flap. In Group III, following tooth extraction, the same size of doxycycline collagen sponge (2 mm × 2 mm) (The Research Development National Institute for Textile and Leather, Bucharest, Romania), containing 1 mg/cm2 doxycycline hyclate that releases 0.05 mg doxycycline per day, was applied in extraction socket and the wounds were covered with a mucoperiosteal sliding flap.
At the end of a total of 16 weekly experimental periods, all animals were sacrificed with the permission of the ethical committee for the animal sacrifice (protocol number 380/2014 YÜDHEK, Istanbul, Turkey). Blood samples were collected for serum CTx, TRACP 5b and ALP levels’ analysis. Clinical findings were assessed and histological and histomorphometrical analysis was performed.
Biochemical markers assessment
After sacrifice, blood samples were obtained and centrifuged for serum separation (3000 × g, 10 minutes). All samples were stored at −80 °C until biochemical assessment. Serum CTx, TRACP 5b and ALP levels were analysed according to manufacturer's instructions by using enzyme-linked immunosorbent assay (ELISA) (Shanghai Yehua Biological Technology Co., Shanghai, China). According to clinical examination, all rats were divided into the non-ONJ and ONJ groups and the differences of serum biomarkers levels between non-ONJ and ONJ groups were assessed.
Clinical findings assessment
All animals were inspected intraorally and clinical findings were recorded at the end of the study. Clinical observation was classified as follows: (1) grade 0: complete mucosal healing, (2) grade 1: infection with swelling, hyperemia, (3) grade 2: necrotic bone and pus. Clinical inspection was made blindly by one of the authors.Citation[27]
Histological and histomorphometrical analysis
Bone samples were fixed in 10% neutral buffered formalin for three days, decalcified in 10% ethylenediaminetetraacetic acid for four weeks and processed for paraffin embedding. Haematoxylin and eosin (H + E), toluidine blue (TB), Mallory triple, Hemalen (HL) and periodic acid schiff (PAS) staining were performed for histological and histomorphometrical examinations. Two experienced observers concurrently blinded to the experimental course independently evaluated the sections. Total alveolar bone area around extraction socket, necrotic bone area and new bone area were measured by Argenit Kameram©2008, (2.11.5.1-SW02030010893) software system.
Necrotic bone was defined with empty lacunae and inflammatory cells around the bone trabeculae on H + E stained sections. TB histostaining contributed demonstrating the vital activity of alveolar bone. Cuboidal and dense stained osteoblasts reflected osteoid formation. In addition, basophilic reversal lines were considered to identify the bone remodelling. The presence of actinomyces was assessed using PAS staining. The severity of inflammation was scored on a scale based on the density and distribution of inflammatory cells and foamy histiocytes on PAS stained sections (0 = normal; 1 = mild; 2 = moderate; 3 = severe). The cells with multinuclei (3–8) and eosinophilic cytoplasm around the alveolar bone were considered as osteoclasts on Mallory triple sections. On the other hand, large cells with eight or more nuclei were appointed to be giant type osteoclasts. Detached osteoclast was defined by the presence of other cells between the osteoclast and bone surface. Apoptotic cells were detected by condensate nuclei. The number of osteoclasts attached or detached to the bone, the number of giant type and apoptotic osteoclasts were counted.
The histologic slides were examined by Olympus BX50 light microscopy (Olympus Corporation, Tokyo, Japan) under different magnifications.
Statistical analysis
IBM SPSS (Statistical Package for Social Sciences) Statistics 22 program was used for the statistical analyses. The suitability of normal distribution of parameters was assessed by Kolmogorov–Smirnov test and it was determined that the parameters were not distributed normally, so non-parametric tests were preferred instead. The Kruskal–Wallis test was used for comparing different parameters between the groups. The Mann–Whitney U-test was used for post hoc determinations. The chi-square test was used to compare qualitative data. Spearman's rho correlation analysis was used for the correlation between parameters that were not normally distributed. The significance level was set at p < 0.05.
Results and discussion
BRONJ pathophysiology has not yet been identified and animal studies have a crucial role for investigation of potential mechanism, as well as prevention of the disease and application of different new treatment protocols. Intravenous BPs, especially ZA, have been known as one of the most effective drugs, which are associated with the formation of BRONJ. In many experimental studies, ZA has been frequently chosen because of its high relative potency.[Citation14–17] Therefore, in this study, BRONJ lesions were developed with ZA alone and dose frequency and duration were calculated as described by Senel et al.[Citation14] Small animal models have been widely preferred in case of BRONJ because they are cost-effective and easy to manipulate. Because of rapid bone healing and the size limitation in the rat model, Barba-Recreo et al. [Citation18] have proposed that these type of models are suitable for the early progress of the pathogenesis and initial treatment modalities. Rat model was selected in this study to investigate the preventive effect of locally applied doxycycline sponge on early BRONJ treatment after tooth extraction.
Clinical studies have suggested that some cofactors have a critical role in the development of BRONJ.[Citation9,Citation10] Although it can develop spontaneously, most of the studies reported that surgical procedures are the main comorbidity factor.[Citation9,Citation10,Citation12] The frequency of ONJ caused by tooth extraction in patients who received IV BPs was determined as 40%–80%.[Citation19] The experiment was designed in order to mimic extraction socket healing in oncology patients receiving IV BPs. Therefore, tooth extraction was preferred as a trigger factor in order to facilitate the formation of ONJ in rats receiving IV BPs in this study.
Diagnostic criteria of BRONJ have been defined as necrotic jaw bone exposure for at least eight weeks and history of BPs therapy without radiation therapy on the craniofacial region (American Association of Oral and Maxillofacial Surgeons ([AAOMS], 2009). Ersan et al. [Citation17] stated that tomography might be a reliable method to detect ONJ in clinical practice because bone biopsies are contraindicated in patients receiving BPs. In this study, the clinical evidence of mucosal healing or delayed healing was observed by inspection and histopathological examination.
None of the animals in all groups were lost during the experimental period (16 weeks). All rats recovered rapidly after general anaesthesia and all-surgical procedures were well tolerated.
Comparison of serum CTx, TRACP 5b and ALP levels between the groups are shown in . No significant difference was observed between all groups in terms of biochemical markers (p > 0.05). When all serum biomarkers levels were assessed according to the presence of necrosis, all biochemical marker levels in non-ONJ group were higher than the ONJ group. However, no significant difference was observed between the non-ONJ and ONJ group, which may be explained by the small sample size in this study ().
Table 1. Comparison of CTx, TRACP 5b and ALP levels between the groups.
Table 2. Evaluation of serum biomarkers levels between non-ONJ and ONJ groups.
Biochemical markers have been proposed to determine the bony changes in the skeleton and are mainly used as research tools to investigate the pathogenesis and new treatment protocols of bone diseases.[Citation5] It was stated that there was no evidence to support the use of currently proposed markers.[Citation20] Marx et al. [Citation6] have stated that CTx is an important marker for estimating the risk of BRONJ before the surgical procedures. Serum CTx value is a reliable marker of osteoclastic activity, which is used to measure the bone turnover and resorption.[Citation6] The authors conducted a retrospective study of 30 patients and recommended that dental surgical procedures should be postponed until CTx is ≥150 pg/mL.[Citation6] There is no consensus about the efficiency of CTx value to predict the development of BRONJ. Kunchur et al. [Citation21] proposed that when the CTx value is less than 150 and 200 pg/mL, the patient should be associated with a high risk and they concluded that this value is not predictive of the development of BRONJ for patients receiving BPs. Kwon et al. [Citation22] evaluated CTx-level in 18 patients who received oral BPs and demonstrated an association between low serum CTx values and severity of BRONJ lesions. Moreover, serum CTx measurement can be affected by some variables including fasting, age, alcohol consumption, smoking ovulation, gender, some drugs (corticosteroids), disease (diabetes), exercise and circadian rhythms.[Citation6] Previous studies proposed CTx-level only reflected the level of bone resorption, however, bone-remodelling balance is thought to be more important in the pathogenesis of BRONJ.[Citation23,Citation24]
TRACP 5b is known as an osteoclast-specific molecule and it has been suggested to be effective in the reflection of bone remodelling.[Citation25] Kim et al. [Citation8] evaluated several bone turnover markers in an animal study, it was demonstrated that lower serum TRACP 5b levels were associated with ONJ. However, Kuroshima et al. [Citation26] have demonstrated that serum TRACP 5b levels do not reflect the suppression of the bone resorption status and suggested that this marker is not useful to estimate the efficiency of antiresorptive therapy.[Citation26] Although it has been accepted that BRONJ development is associated with dose-dependent accumulation, various results in BP studies under similar conditions may be explained by different pharmacological responses between individuals.
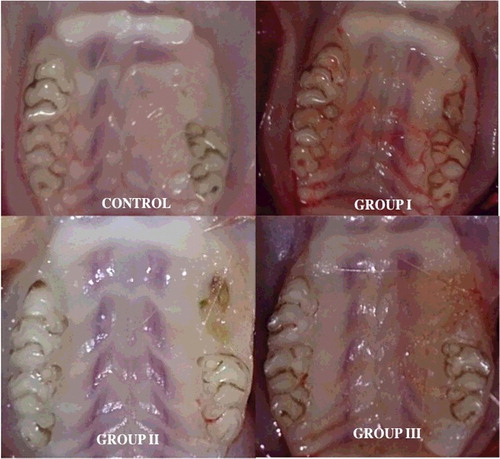
All animals in the control group showed mucosal healing uneventfully. The majority of animals in Group III showed complete mucosal healing (grade 0: 66.7%) (). ONJ occurrence in the Group I was significantly higher compared to other groups (p < 0.05). The incidence of complete mucosal healing in Groups II and III was 50% and 66.7%, respectively (). A statistically significant difference was found between the groups for clinical findings (p: 0.002; p< 0.05). The incidence of grade 0 in Group I (0%) was significantly lower compared to the control group (100%) and Group III (66.7%) (p < 0.01; p < 0.05). The incidence of grades 1 and 2 was significantly higher in Group I than in the control group and Group III (p < 0.05). Abtahi et al. [Citation11] performed a study to evaluate the efficiency of immediate mucosal coverage after tooth extraction on the prevention of osteonecrosis of the jaws in rats receiving BPs. All rats in mucosal coverage and control groups were observed to have intact overlying mucosa after tooth extraction. It is generally recognized that BRONJ pathogenesis is multifactorial.[Citation27] Mostly accepted theories are as follows: high bone turnover of the jaws, the inhibition of angiogenesis, osteoclastic apoptosis and easy bacterial contamination of jaw bone are among the most accepted theories.[Citation9,Citation12,Citation20]
Table 3. Evaluation of clinical findings among the groups.
Figure 1. Clinical appearance of extraction sites: control group; complete mucosal healing, Group I; osteonecrosis with swelling and hyperaemia, Group II; osteonecrosis, Group III; complete mucosal healing.
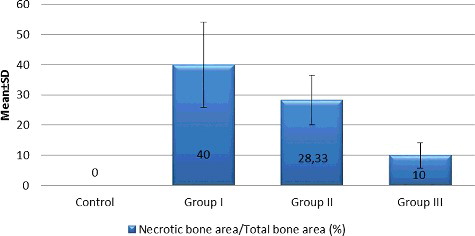
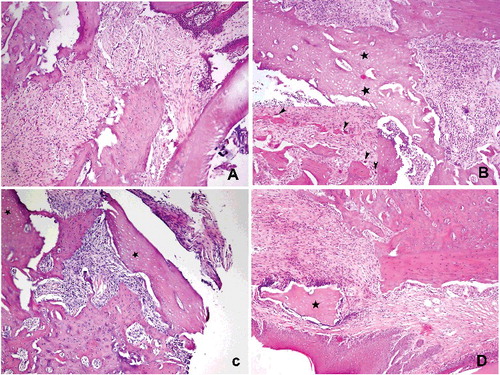
Osteoclastic activity in the jaw bone-containing BPs is suppressed and tissue healing would be altered.[Citation28] The effects of BPs on jaw bones following tooth extraction are as follows: local inflammation occurs and BPs attached to the bone are locally released into the matrix. These released BPs are accumulated by osteoclasts to convert them into an apoptotic stage. Normal tissue healing is interrupted and bone necrosis occurs. It has been reported that BPs induce osteoclastic apoptosis and cause them to detach from the bone surface.[Citation29] Several histopathological reports have been demonstrated that there was an increase in the number of giant, detached and apoptotic osteoclasts in the bone affected by BPs.[Citation17,Citation26,Citation30] In this study, histological analysis showed that no osteonecrosis was observed in the control group, while all animals in Groups I, II and III had osteonecrosis (). Histomorphometric examination revealed that the mean percentage of necrotic bone area in Group III was statistically lower than in Groups I and II (10%, 40% and 28.33%, respectively; p < 0.01) ().
Figure 2. Histopathological view showing the normal and necrotic bone areas on the jaws: (A) control, normal bone cellularity, (B and C) Groups I and II, large necrotic areas (stars) bone, apoptotic osteoclasts with condensate nuclei (arrowhead), (D) Group III, small amounts of necrotic bone, control like histological features (HE, 100×).
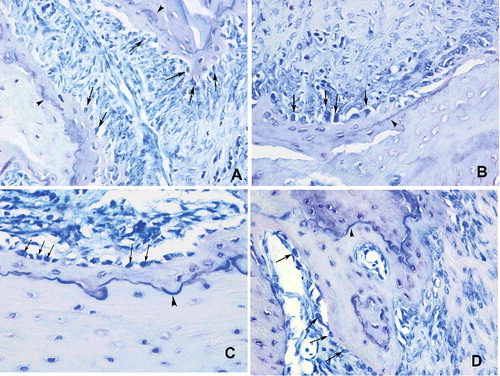
Moderate osteoblastic activity was observed in the control group. Mild to moderate osteoblastic activities along with small amounts of reversal lines were defined in Groups I and II, whereas osteoblastic activity was markedly increased in Group III ().
Figure 4. Histopathological view showing the osteoblastic activity: cuboidal and dense stained osteoblasts (arrow) reflected osteoid formation. Basophilic reversal lines (arrowhead) were considered to identify the bone remodelling (A) control, moderate osteoblastic activity, (B and C) Groups I and II, mild to moderate osteoblastic activities along with small amounts of reversal lines, (D) Group III, an increased osteoblastic activity (Toluidin blue 400x).
Both bone tissue exposed to the oral environment and the slow resorption of the alveolar bone after tooth extraction might cause bacterial colonization, especially by actinomyces species, which has a crucial role in the development of BRONJ.[Citation31,Citation32] Histological studies have shown that microbial infection may be effective in the formation of BRONJ and actinomyces is a specific finding.[Citation33] Although actinomyces colonization in BRONJ lesions has been reported to vary between 39% and 100%, the certain effect in BRONJ pathogenesis is unknown.[Citation34] Pushalkar et al. [Citation35] found that different bacterial species, specifically, Dialister, Prevotella and Atopobium were detected in soft tissue and necrotic bone and concluded that while BP suppresses bone remodelling, these types of bacteria cause inflammatory response which could cause further disruption in this process. In this study, no actinomyces colonization was observed in any group and other types of bacteria might be effective in Groups I, II and III which exhibited ONJ. It has been reported that local inflammation and apoptosis cause to re-releasing of BPs absorbed by osteoclasts into the bone matrix.[Citation36] This is known as ‘secondary accumulation’ and this process results in indirect suppression of osteoclastic bone resorption activity.[Citation37] Therefore, inflammation causes a decrease in the pH level and a rise in BPs level in to the bone matrix, which results in an increase in cellular toxicity.[Citation16] Dayisoylu et al. [Citation16] investigated the preventive effect of locally applied sodium bicarbonate on BPs-related BRONJ and noted that the number of osteoblasts and osteoclasts increase following tooth extraction, however, local inflammation has a negative effect on these cells. In addition, this response induces BRONJ formation by increasing the number of fibroblasts and decreasing osteoclastic activity.[Citation16] Histological features of inflammation were similar between the control and Group III, and between Groups I and II (). There was a statistically significant difference between the groups for severity of inflammation (). In this study, the severity of inflammation in Groups I and II was statistically higher than those in the control group and Group III (p < 0.01). The number of osteoclasts attached to the bone in the control group and Groups II and III was higher than Group I, but no significant difference was determined. Osteoblastic activity in all groups was increased because of apposition and bone healing following tooth extraction. The similar severity of inflammation in the control group and Group III (doxycycline group) shows that local application of doxycycline could have a positive effect on reducing the severity of inflammation. This result supports the idea that inflammation might serve as a trigger factor in BRONJ formation.
Figure 5. Histopathological view showing the severity of inflammation among the groups: (A) control, normal appearance, (B and C) Groups I and II, focal inflammation areas, neutrophils and foamy histiocytes surrounding the extraction sites, severe inflammation, (D) Group III, similar with control group, mild inflammation (PAS + HL 200×).
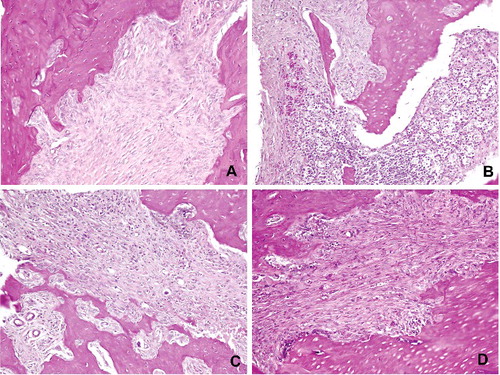
Table 4. Evaluation of severity of inflammation among the groups.
Table 5. Evaluation of osteoclast number among the groups.
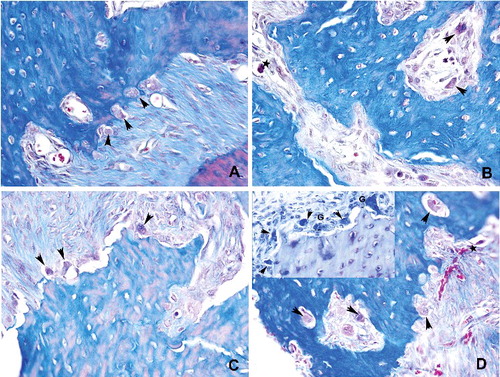
Detached and apoptotic osteoclasts were marked in Group I and an increase in the giant type osteoclast formation was characteristics of Groups II and III (). No significant difference was observed between the groups for the number of osteoclast attached to the bone (p > 0.05). A statistically significant difference in detached osteoclast number was found between the groups (p < 0.01) (). Detached osteoclast number in the control group was statistically lower than Groups I, II and III (p < 0.05; p < 0.01). There was a statistically significant difference between the groups for giant type osteoclast number (p < 0.01). A lower number of giant type osteoclast was observed in the control group compared to Groups I, II and III (p < 0.01). In addition, giant type osteoclast number in Group III was statistically higher than Groups I and II (p < 0.05; p < 0.01). A statistically significant difference in apoptotic osteoclast number was found between the groups (p < 0.01). A lower number of apoptotic osteoclast was observed in the control group compared to Groups I, II and III (p < 0.05; p < 0.01). Apoptotic osteoclast number in Group III was significantly lower than Groups I and II (p < 0.05; p < 0.01). In addition, no significant difference was observed between Groups I and II for apoptotic osteoclast number (p > 0.05).
Figure 6. Histopathological view showing the osteoclast density: (A) control, osteoclasts (arrowhead) were shown associated with bone surface in their active pole in the control group. (B) Detached (arrowhead) and apoptotic (star) osteoclasts were marked in Group I. (C and D) Osteoclast number with normal appearance was increased in Groups II and III. Giant type osteoclasts were characteristics of that groups (Mallory Triple 400x)
Both bone tissue exposed to the oral environment and the slow resorption of the alveolar bone after tooth extraction might cause bacterial colonization, especially by actinomyces species, which has a crucial role in the development of BRONJ.[Citation31,Citation32] Histological studies have shown that microbial infection may be effective in the formation of BRONJ and actinomyces is a specific finding.[Citation33] Although actinomyces colonization in BRONJ lesions has been reported to vary between 39% and 100%, the certain effect in BRONJ pathogenesis is unknown.[Citation34] Pushalkar et al. [Citation35] found that different bacterial species, specifically, Dialister, Prevotella and Atopobium were detected in soft tissue and necrotic bone and concluded that while BP suppresses bone remodelling, these types of bacteria cause inflammatory response which could cause further disruption in this process. In this study, no actinomyces colonization was observed in any group and other types of bacteria might be effective in Groups I, II and III which exhibited ONJ.
Non-surgical conservative treatment methods such as oral antimicrobial rinses and systemic antibiotic therapy (penicillin, metronidazole, quinolones, clindamycin, doxycycline and erythromycin) were suggested for stages I and II of BRONJ.[Citation12] However, it has also been stated that systemic antibiotics were not effective to restrict bacterial colonization or to achieve mucosal healing of lesions after the onset of BRONJ.[Citation38] Periodontal studies have indicated that controlled local antimicrobial drug delivery systems are successful in maintaining a higher concentration and a longer effective period than systemically delivered methods. Locally applied doxycycline was shown to have a wide spectrum effect on periodontal pathogens and to eliminate actinomyces species successfully.[39] Antimicrobial effect of doxycycline may cause a decrease in the inflammation that occurs after tooth extraction. The decline in the level of inflammation in Group III (doxycycline group) may induce some positive effects on bone healing, resulting in a decrease cellular toxicity. Therefore, in the light of this study, application of immediate mucosal coverage with doxycycline collagen sponge after tooth extraction may be described as a preventive method in patients receiving IV BPs.
Conclusion
The results of this study demonstrated that immediate mucosal coverage with doxycycline sponge after tooth extraction could reduce the risk of ONJ in rats receiving BPs. Serum CTx, TRACP 5b and ALP levels were not used as possible predictive biomarkers for BRONJ. Further animal and clinical studies are needed to investigate these biomarker levels in different time interval and the effectiveness of this treatment protocol.
Disclosure statement
The authors stated that they have no conflict of interest.
References
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117.
- Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759.
- Sharma D, Ivanovski S, Slevin M, et al. Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc Cell. 2013;5:1.
- La Ferla F, Paolicchi E, Crea F, et al. An aromatase polymorphism (g.132810C>T) predicts risk of bisphosphonate-related osteonecrosis of the jaw. Biomark Med. 2012;6:201–209.
- Coleman R, Costa L, Saad F, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80:411–432.
- Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65:2397–2410.
- Hannon RA, Clowes JA, Eagleton AC, et al. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004;34:187–194.
- Kim JW, Cha IH, Kim SJ, et al. Biomarkers for bisphosphonate-related osteonecrosis of the jaw. Clin Implant Dent Relat Res. 2016;18:281–291.
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491.
- López-Jornet P, Camacho-Alonso F, Martínez-Canovas A, et al. Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: a study of the changes in the jaws. J Oral Maxillofac Surg. 2011;69:2488–2493.
- Abtahi J, Agholme F, Aspenberg P. Prevention of osteonecrosis of the jaw by mucoperiosteal coverage in a rat model. Int J Oral Maxillofac Surg. 2013;42:632–636.
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67:2–12.
- Stoller NH, Johnson LR, Trapnell S, et al. The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol. 1998;69:1085–1091. Erratum in: J Periodontol. 1999;70:238.
- Senel FC, Kadioglu Duman M, Muci E, et al. Jaw bone changes in rats after treatment with zoledronate and pamidronate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2010;109:385–391.
- Dayisoylu EH, Şenel FÇ, Üngör C, et al. The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int J Oral Maxillofac Surg. 2013;42:1475–1480.
- Dayisoylu EH, Üngör C, Tosun E, et al. Does an alkaline environment prevent the development of bisphosphonate-related osteonecrosis of the jaw? An experimental study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:329–334.
- Ersan N, van Ruijven LJ, Bronckers AL, et al. Teriparatide and the treatment of bisphosphonate-related osteonecrosis of the jaw: a rat model. Dentomaxillofac Radiol. 2014;43:20130144.
- Barba-Recreo P, Del Castillo Pardo de Vera JL, García-Arranz M,et al. Zoledronic acid-related osteonecrosis of the jaws. Experimental model with dental extractions in rats. J Craniomaxillofac Surg. 2014;42:744–750.
- Sarkarat F, Kalantar Motamedi MH, Jahanbani J, et al. Platelet-rich plasma in treatment of zoledronic acid-induced bisphosphonate-related osteonecrosis of the jaws. Trauma Mon. 2014;19:e17196.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956.
- Kunchur R, Need A, Hughes T, et al. Clinical investigation of C-terminal cross-linking telopeptide test in prevention and management of bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:1167–1173.
- Kwon YD, Kim DY, Ohe JY, et al. Correlation between serum C-terminal cross-linking telopeptide of type I collagen and staging of oral bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:2644–2648.
- Glover SJ, Garnero P, Naylor K, et al. Establishing a reference range for bone turnover markers in young, healthy women. Bone. 2008;42:623–630.
- Kim JW, Kong KA, Kim SJ, et al. Prospective biomarker evaluation in patients with osteonecrosis of the jaw who received bisphosphonates. Bone. 2013;57:201–205.
- Rissanen JP, Suominen MI, Peng Z, et al. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–115.
- Kuroshima S, Go VA, Yamashita J. Increased numbers of nonattached osteoclasts after long-term zoledronic acid therapy in mice. Endocrinology. 2012;153:17–28.
- Çankaya M, Cizmeci Şenel F, Kadioglu Duman M, et al. The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int J Oral Maxillofac Surg. 2013;42:1134–1139.
- Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664.
- Rogers MJ. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif Tissue Int. 2004;75:451–461.
- Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62.
- Sedghizadeh PP, Kumar SK, Gorur A, et al. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66:767–775.
- Tsurushima H, Kokuryo S, Sakaguchi O, et al. Bacterial promotion of bisphosphonate-induced osteonecrosis in Wistar rats. Int J Oral Maxillofac Surg. 2013;42:1481–1487.
- Kaplan I, Anavi K, Anavi Y, et al. The clinical spectrum of Actinomyces-associated lesions of the oral mucosa and jaw bones: correlations with histomorphometric analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:738–746.
- Hinson AM, Smith CW, Siegel ER, et al. Is bisphosphonate-related osteonecrosis of the jaw an infection? A histological and microbiological ten-year summary. Int J Dent. 2014;2014:452737.
- Pushalkar S, Li X, Kurago Z, et al. Oral microbiota and host innate immune response in bisphosphonate-related osteonecrosis of the jaw. Int J Oral Sci. 2014;6:219–226.
- Otto S, Hafner S, Mast G, et al. Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg. 2010;68:1158–1161.
- Kimachi K, Kajiya H, Nakayama S, et al. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:297–308.
- Ji X, Pushalkar S, Li Y, et al. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 2012;18:85–95.
- Walker CB, Pappas JD, Tyler KZ, et al. Antibiotic susceptibilities of periodontal bacteria: in vitro susceptibilities to eight antimicrobial agents. J Periodontol. 1985;56:67–74.