ABSTRACT
Some wild strains of Saccharomyces cerevisiae form a pellicle on the surface of contaminated wines during the post-fermentation period of wine making. In this study, we found that both pellicle formation and FLO11 expression by a wild pellicle-forming strain of S. cerevisiae isolated from contaminated wine were repressed in a glucose-containing medium. Substitution of the promoter region of FLO11 in the cells with a constitutive promoter caused derepression of pellicle formation in the glucose-containing medium and wine. These findings indicate that glucose repression of the expression of the FLO11 gene, but not the glucose repression of the expression of other glucose-repressed genes, is responsible for the glucose-dependent regulation of pellicle formation by the wild pellicle-forming yeast strain. Furthermore, we found that the wild pellicle-forming strain had the same deletion of the 111-bp repression sequence in the FLO11 promoter as the flor strains commercially used for making sherry-like wines. Based on the results obtained in this study, a new method that would prevent deleterious pellicle formation in wineries is discussed.
Introduction
Some wild strains of Saccharomyces cerevisiae form a pellicle on the surface of contaminated wines during the post-fermentation period of wine making. In several European countries, pellicle-forming S. cerevisiae strains are traditionally used for the biological ageing of wines, called ‘flor ageing’, to produce specific types of wines, such as Fino Sherry in Spain (Jerez area), Vernaccia di Oristano in Italy (Sardinia), Szamorodni in Hungary (Tokaj Hegyalja) and Vin Jaune in France (Jura) [Citation1]. The flor-ageing process involves formation of a pellicle, or ‘flor’, on the surface of the wine after the alcoholic fermentation process is completed. The S. cerevisiae cells present in the flor perform a special task: these cells metabolize alcohol and remaining carbohydrates oxidatively while generating many aromatic compounds, such as acetaldehyde, sotolon and solerone, which give the resulting wines their unique flavours [Citation1–4]. This biological ageing process, in which the flor-forming yeasts produce sherry-like wines, is also used in other areas of the world such as South Africa, Armenia, California and southern Australia [Citation5].
In Japan, however, this ageing process using flor-forming yeasts is not practiced in wine making. Instead, many wineries in Japan have suffered losses from pellicle formation, in stored wines, by wild pellicle-forming S. cerevisiae strains that are resistant to high concentrations of ethanol and sulphite; the contaminating wild yeast cells present in the pellicle alter the quality of the wines via oxidative metabolism, resulting in inferior products [Citation6]. These pellicle-forming, spoilage strains of S. cerevisiae remain in the non-pellicle stage during the fermentation period of wine making, but then reach the pellicle stage during the post-fermentation period [Citation6]. Laboratory experiments reveal that pellicle formation is induced in a medium that contains ethanol (assimilation of which requires oxygen) as the sole carbon source, but not in a medium that contains glucose as the sole carbon source [Citation6]. Recently, Zara et al. [Citation7] demonstrated that pellicle formation is also induced in media that contain either glycerol or ethyl acetate as the sole carbon source; both of these compounds are non-fermentable carbon sources. This carbon source-dependent regulation of pellicle formation can be viewed as an adaptive mechanism that allows yeast cells to access oxygen and assimilate non-fermentable carbon sources, such as ethanol, in an aerobic environment [Citation8].
To prevent the formation of deleterious pellicle in wineries, Hara et al. [Citation9] previously developed a fermentation method using a killer yeast strain of S. cerevisiae that had the ability to kill wild pellicle-forming strains of S. cerevisiae. However, this method is not suitable for general use for wine making because of the limited availability of wine yeast strains. Therefore, a new method that would prevent the formation of deleterious pellicle in wines is highly desired. Improved understanding of the mechanism by which these pellicle-forming strains switch from their non-pellicle-forming stage to the pellicle-forming stage in response to available carbon sources would provide useful insights that might help in developing a new method for preventing the formation of deleterious pellicle.
The main gene involved in pellicle formation is FLO11, which encodes a glycosylphosphatidylinositol-anchored cell surface glycoprotein with a serine- and threonine-rich central region [Citation5]. Fidalgo et al. [Citation10] have demonstrated that FLO11 is essential for pellicle formation by a flor yeast strain of S. cerevisiae that was isolated in Spain from the flor of a sherry wine. Subsequently, Nakagawa et al. [Citation11] reported that FLO11 is also essential for pellicle formation by wild pellicle-forming strains of S. cerevisiae isolated from the pellicles of contaminated wines in Japan. However, it is unclear whether FLO11 is involved in carbon source-dependent regulation of pellicle formation. Kuchin et al. [Citation12] reported that the expression of FLO11 in a haploid laboratory strain with a Σ1278b background was repressed in the presence of high-concentration (2% w/v) glucose; this repression was mediated via the main glucose repression pathway and involved the Snf1p kinase and the transcriptional repressors Nrg1p and Nrg2p. However, Van de Velde and Thevelein [Citation13] reported that the expression of FLO11 in a diploid laboratory strain with a Σ1278b background was not only partially repressed via the main glucose repression pathway, but was also activated via the Ras/cAMP/PKA pathway following the addition of 100 mmol/L (1.8% w/v) glucose. On the other hand, in a flor strain of S. cerevisiae (isolated from the flor of a sherry-like wine in Italy), FLO11 was highly expressed when either glucose or ethanol was used as the sole carbon source [Citation14]. Furthermore, despite the presence of 40 g/L sugar, formation of a flor of S. cerevisiae was induced on the surface of Tokaji Szamorodni wine produced in Hungary [Citation5,Citation15]. Taken together, the above-mentioned findings indicate that the effect of glucose on FLO11 expression and pellicle formation needs to be clarified in pellicle-forming strains, as was pointed out earlier by Alexandre [Citation5].
In the present study, we aimed to clarify the effect of glucose on FLO11 expression and also to determine the role of transcriptional regulation of FLO11 on pellicle formation by a wild pellicle-forming yeast strain isolated from contaminated wine. As we report here, glucose represses the transcription of the FLO11 gene in the wild pellicle-forming yeast strain, and glucose repression of FLO11 gene expression is responsible for the glucose-dependent regulation of pellicle formation by this strain. A new method that would prevent deleterious pellicle formation in wineries is also discussed.
Materials and methods
S. cerevisiae strains and media
S. cerevisiae strain YFY-6, which was previously isolated from the pellicle of a contaminated wine in Yamanashi prefecture, Japan [Citation11], and the commercial wine yeast strain S. cerevisiae W3 (NBRC 10661; NITE Biological Resource Center, Tokyo, Japan) were used. S. cerevisiae strains were cultivated in the following media: synthetic dextrose (SD) medium [Citation16] containing 2% glucose as the sole carbon source; yeast extract-polypeptone-dextrose (YPD) medium (1% yeast extract, 2% polypeptone, 2% glucose); flor medium containing 0.67% yeast nitrogen base without amino acids and 3% (v/v) ethanol as the sole carbon source and in which the pH was adjusted to 3.5 with hydrochloric acid [Citation11] and glucose medium, the composition of which was the same as that of the flor medium except that 10% (w/v) glucose was used as the sole carbon source in place of ethanol. Solid media were prepared using 2% agar.
Genetic and biochemical methods
Genetic manipulation of S. cerevisiae cells was performed as described previously [Citation16]. The assay to assess the yeast pellicle-forming ability was performed as described previously [Citation11]. S. cerevisiae cells were transformed using a Frozen-EZ Yeast Transformation II Kit (Zymo Research, Irvine, CA, USA). Yeast colony polymerase chain reaction (PCR) was performed using KOD-FX (Toyobo Co., Ltd., Osaka, Japan), in accordance with the manufacturer's instructions. The glucose concentration in the red wine was determined by the mutarotase–glucose oxidase (GOD) method using a Glucose C2 kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in accordance with the manufacturer's instructions.
Real-time reverse transcription (RT)-PCR to measure expression levels of FLO11
Yeast cells were pre-cultivated overnight at 30 °C in 10 mL of SD medium with vigorous shaking. Cells from 1 mL of each overnight culture were collected by centrifugation (7000× g), washed once with sterile distilled water and then re-suspended in 10 mL of flor or glucose medium in 18 mm × 180 mm test tubes. Total RNA was isolated from cells that were statically cultivated for three days at 30 °C using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA); RNA was then incubated with the RNase-Free DNase Set (Qiagen) and reverse transcribed using a PrimeScript RT reagent Kit (Takara Bio Inc., Shiga, Japan) in accordance with the manufacturer's instructions. Quantitative real-time PCR analysis was performed in a total volume of 25 μL using a Thermal Cycler Dice Real Time System TP860 (Takara Bio) and SYBR Premix Ex Taq II (Takara Bio). The relative gene expression level was determined by the ΔΔCt method. The FLO11 mRNA expression level was normalized to the ACT1 mRNA expression level. The specific primers used for the PCR amplification of FLO11 were 5'-CTCCCGTTGTCACATCTCCATC-3' (forward primer) and 5′-GCAGCACCTTGGTAAGTACTCG-3' (reverse primer), whereas the primers used for the PCR amplification of ACT1 were 5′-TCCAGCCTTCTACGTTTCCATC-3' (forward primer) and 5'-CGACGTGAGTAACACCATCACC-3′ (reverse primer).
Analysis of the 111-bp repression sequence in the FLO11 promoter
Previous work showed that deletion of a 111-bp repression sequence in the FLO11 promoter resulted in increased FLO11 gene expression [Citation10]. In the present study, the presence/absence of this 111-bp repression sequence was assessed as follows. The promoter region of FLO11 was amplified by PCR using the chromosomal DNA of YFY-6 and W3 strains as a template and oligonucleotides 5'-CAGCCCCAGAGTATGTTCTCACAG-3' (Flo11promFw) [Citation1] and 5'-AATCACCTTCTAAACGCTCGGA-3' (Flo11promRv) [Citation1] as the forward and reverse primers, respectively. The PCR product was cloned into a pMD20-T vector (Takara Bio) using a Mighty TA-cloning Reagent Set for PrimeSTAR (Takara Bio) in accordance with the manufacturer's instructions. The insert was sequenced using oligonucleotides Flo11promFw and Flo11promRv as sequencing primers with the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and Applied Biosystems 3730xl DNA Analyzer (Thermo Fisher Scientific). The sequences of these fragments from the YFY-6 and W3 strains are available in GenBank under the accession numbers LC133257 and LC133258, respectively.
Substitution of the promoter region of FLO11 in YFY-6 cells
Substitution of the promoter region of FLO11 in YFY-6 cells with the constitutive promoter of the TDH3 gene (TDH3p), which encodes for glyceraldehyde-3-phosphate dehydrogenase [Citation17], was performed as follows. To construct a promoter substitution cassette that contained TDH3p and the dominant drug resistance module kanMX4 [Citation18], we first amplified TDH3p by PCR using the chromosomal DNA of BY4741 [Citation19] as a template and oligonucleotides 5'-CTCGAATTCATAAAAAACACGCTTTTTCAGTTCG-3′ (EcoRI_TDH3-698) and 5′-CTCGATATCTTTGTTTGTTTATGTGTGTTTATTCG-3′ (EcoRV_TDH3-1c) as the forward and reverse primers, respectively. The PCR product was doubly digested with EcoRI and EcoRV and cloned into the EcoRI-EcoRV digested plasmid pFA6a-kanMX4 [Citation18] to obtain the resulting plasmid pYNB110 (pFA6a-kanMX4-TDH3p). To use this kanMX4-TDH3p cassette for the promoter substitution of FLO11, we used fusion PCR [Citation20]. The upstream region of FLO11 was amplified by PCR using the chromosomal DNA of BY4741 as a template and oligonucleotides 5'-ACAATTGGAAGCGCAGAGCTT-3′ (FLO11-650) and 5'-CAATTTGTACTGGTAAGTCTATG-3' (FLO11-151c) as the forward and reverse primers, respectively. The kanMX4-TDH3p cassette flanked by a 45-bp sequence corresponding to the 5′ end of the FLO11 open reading frame was amplified by PCR using pYNB110 as the template and oligonucleotides 5'-CATAGACTTACCAGTACAAATTGCGTACGCTGCAGGTCGAC-3′ (FLO11-173_U2) and 5′-TAGAAGCGAAAGGACCAAATAAGCGAGTAGAAATGGTCTTTGCATTTTGTTTGTTTATGTGTGTTTATTCG-3′ (FLO11+45c_TDH3-1c) as the forward and reverse primers, respectively. The underlined sequence in the FLO11-173_U2 oligonucleotide was complementary to the sequence of the oligonucleotide FLO11-151c, thus permitting fragment fusion. The fusion PCR product was used for the transformation of YFY-6 to obtain the kanMX4-TDH3p-FLO11 strain of YFY-6 (designated as YFY-6[TDH3p-FLO11]).
Results and discussion
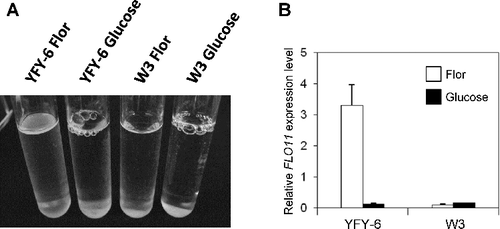
Effect of glucose on pellicle formation and FLO11 expression of a wild pellicle-forming strain of S. cerevisiae isolated from contaminated wine
In this study, we used the pellicle-forming S. cerevisiae strain YFY-6, which was previously isolated from the pellicle of a contaminated wine in Yamanashi prefecture, Japan [Citation11]. Specifically, we sought to examine the effect of glucose on FLO11 expression and also to determine the role of transcriptional regulation of FLO11 on the pellicle formation by wild pellicle-forming strains, a process known to cause deterioration of wine quality. Although the commercial wine yeast strain W3 failed to form any pellicle in the flor medium, the YFY-6 strain did form a pellicle in this medium ((A)). In contrast, neither YFY-6 nor W3 formed any pellicle in the glucose medium ((A)). These results suggested that pellicle formation by YFY-6 is repressed by glucose. To investigate whether the expression of FLO11 is also repressed by glucose in the wild pellicle-forming yeast strain, we measured the mRNA expression levels of FLO11 in YFY-6 and W3 strains cultivated in the flor and glucose media by using the real-time RT-PCR technique. As shown in (B), the expression level of FLO11 transcripts in YFY-6 cells decreased 27-fold when these cells were cultivated in the glucose medium as compared to the level in cells cultivated in the flor medium. In contrast, the expression level of FLO11 transcripts in W3 cells grown in the flor medium was very low and almost the same as that in cells grown in the glucose medium ((B)). These findings suggested that the expression of FLO11 is repressed by glucose in the YFY-6 strain isolated from contaminated wine. The level of FLO11 transcripts found in the W3 cells cultivated in the flor medium was, in fact, as low as 1/34 of the level found in the YFY-6 cells cultivated in the same medium, suggesting that the derepression mechanism for FLO11 expression was not functional in W3 cells.
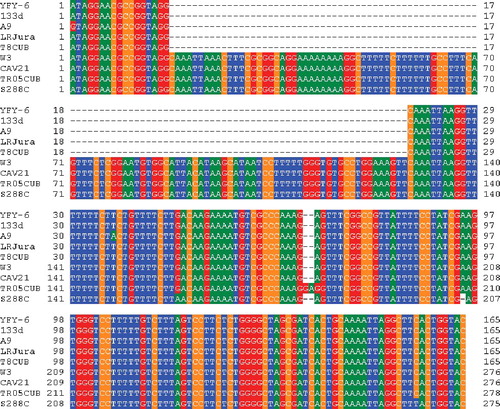
The 111-bp repression sequence in the FLO11 promoter is absent in a wild pellicle-forming strain of S. cerevisiae isolated from contaminated wine
Previously, Fidalgo et al. [Citation10] found that a flor yeast strain of S. cerevisiae for sherry wine making in Spain had a 111-bp deletion within a repression region of the FLO11 promoter, and that this deletion significantly increased FLO11 gene expression. Since the 111-bp sequence involved one of the two direct repeats of the nucleotide sequence CAAATTAA, an intra-molecular recombination event was speculated to be responsible for the 111-bp deletion. Therefore, the 111-bp deletion in the FLO11 promoter was proposed as one of the mechanisms for adaptive evolution of the flor yeast strain [Citation10]. Interestingly, the 111-bp deletion also was observed in other S. cerevisiae flor yeast strains for sherry-like wine making in Italy, France and Hungary [Citation1,Citation21]. In order to examine whether the 111-bp sequence is present in YFY-6 and W3 strains, the corresponding region of the FLO11 promoter was amplified by PCR and sequenced. The YFY-6 strain did not have the 111-bp sequence in the FLO11 promoter, as is the case with commercially used flor-forming yeast strains 133d [Citation10] in Spain, A9 [Citation21] in Italy, LRJura [Citation1] in France and T8CUB [Citation1] in Hungary (). On the other hand, the W3 strain had the same 111-bp sequence in the FLO11 promoter as the non-pellicle-forming wine yeast strains CAV21 [Citation1] in France and TR05CUB [Citation1] in Hungary (). These results demonstrated that the wild pellicle-forming strain of S. cerevisiae isolated from contaminated wine has the same deletion in the FLO11 promoter as the flor strains commercially used for making sherry-like wines, and suggested that the 111-bp sequence is responsible for the low-level expression of FLO11 in the non-pellicle-forming W3 strain. The finding that the same 111-bp deletion was observed not only in commercially used flor-forming yeast strains in Europe, but also in a wild pellicle-forming strain isolated from contaminated wine in Japan, which is geographically distant from Europe, supports the importance of the 111-bp deletion for the acquisition of pellicle-forming ability.
Glucose repression of FLO11 gene expression is responsible for the glucose-dependent regulation of pellicle formation in the wild pellicle-forming yeast strain
Although glucose (or fructose) is the preferred carbon source for S. cerevisiae cells, these cells are also able to utilize a wide range of other carbon sources [Citation22]. When glucose (or fructose) is present, the enzymes required for the utilization of alternative carbon sources are synthesized at low rates or not at all [Citation23]. This phenomenon is known as ‘glucose repression’ or ‘carbon catabolite repression’ [Citation22–24]. The mechanism of glucose repression involves not only the repression of transcription when glucose levels are high, but also the release of glucose repression and subsequent activation of expression when glucose is limiting [Citation22]. In S. cerevisiae, glucose repression has been reported to act when glucose concentration in the medium exceeds 18 mmol/L (0.32% w/v) and to be released when glucose concentration in the medium is 14 mmol/L (0.25% w/v) or lower [Citation25]. A large number of S. cerevisiae genes, including the genes involved in the utilization of alternate carbon sources, gluconeogenesis, respiration and peroxisomal functions, are subject to glucose repression [Citation22]. To clarify whether glucose repression of the expression of the FLO11 gene, but not the glucose repression of the expression of other glucose-repressed genes, is responsible for the glucose-dependent regulation of pellicle formation, we substituted the promoter region of FLO11 in YFY-6 cells with the constitutive promoter of the TDH3 gene (TDH3p), which encodes glyceraldehyde-3-phosphate dehydrogenase [Citation17], yielding strain YFY-6[TDH3p-FLO11]. The YFY-6 and YFY-6[TDH3p-FLO11] strains then were assessed for their ability to form pellicle in glucose medium. As shown, YFY-6[TDH3p-FLO11], but not YFY-6, formed a well-defined pellicle in the glucose medium ((A)). To determine whether YFY-6[TDH3p-FLO11] could also form a pellicle in wine containing glucose, YFY-6 and YFY-6[TDH3p-FLO11] strains were assessed for their ability to form pellicle in red wine made from Muscat Bailey A grapes, a wine that naturally contains 0.01% (w/v) glucose, in the absence and presence of 10% (w/v) added glucose. As shown, both the YFY-6 and YFY-6[TDH3p-FLO11] strains formed well-defined pellicles in the red wine in the absence of any added glucose ((B)). Since glucose repression is reported to be released when the glucose concentration in the medium is 14 mmol/L (0.25% w/v) or lower [Citation25], glucose repression of FLO11 gene expression in the YFY-6 strain appears to be released in the red wine in the absence of added glucose. However, YFY-6 did not form a pellicle in red wine supplemented with 10% glucose ((B)). In contrast, YFY-6[TDH3p-FLO11] formed a well-defined pellicle in the red wine with 10% added glucose ((B)). Taken together, these findings indicated that glucose repression of the expression of FLO11 gene, but not the glucose repression of the expression of other glucose-repressed genes, is responsible for the glucose-dependent regulation of pellicle formation in the wild pellicle-forming yeast strain.
Figure 3. Pellicle-forming abilities of YFY-6 and YFY-6[TDH3p-FLO11] strains in glucose medium (A) and wine (B).
![Figure 3. Pellicle-forming abilities of YFY-6 and YFY-6[TDH3p-FLO11] strains in glucose medium (A) and wine (B).](/cms/asset/6b44b159-9a16-438b-b6e1-fa39290ade5d/tbeq_a_1246203_f0003_oc.jpg)
In the absence of any fermentable carbon sources, such as glucose, the pellicle-forming cells on the surface of a liquid medium have a great advantage in assimilating non-fermentable carbon sources, such as ethanol, which require oxygen for their assimilation, because these surface-dwelling cells have easy access to oxygen [Citation8]. In contrast, the pellicle-forming cells are at a disadvantage in assimilating fermentable carbon sources, such as glucose, as fast as the other competing microorganisms, because these surface-dwelling cells have a smaller area for making contact with the medium than the surface-to-medium contact area of the non-pellicle-forming cells, which are submerged in the medium. Therefore, it would be important for the wild pellicle-forming strains of S. cerevisiae to repress their ability to form pellicle in glucose-containing media so that these cells can assimilate glucose as fast as the other competing microorganisms. In the present study, we found that glucose repressed the transcription of the FLO11 gene in a wild pellicle-forming yeast strain isolated from contaminated wine, and that the glucose repression of FLO11 gene expression is responsible for the glucose-dependent regulation of pellicle formation by this strain. Interestingly, the flor strains of S. cerevisiae, which are commercially used for making sherry-like wines, can form pellicle when the sugar present in the grape is depleted; the sole exception is the flor strain used for making Tokaji Szamorodni wine in Hungary, which is a strain that can form pellicle even in the presence of sugar (40 g/L) [Citation5,Citation15]. The effect of glucose on FLO11 expression and the importance of glucose-mediated transcriptional regulation of FLO11 in flor formation are, however, not well studied for the commercially used flor-forming yeast strains. Therefore, it would be intriguing to examine whether flor formation by commercial flor strains of S. cerevisiae is also regulated by glucose repression of FLO11 gene expression.
Conclusions
In conclusion, we have shown here for the first time (to our knowledge) that the transcription of the FLO11 gene in a pellicle-forming S. cerevisiae strain is repressed by glucose, and that the glucose repression of FLO11 gene expression is important for the inhibition of pellicle formation. Based on the results obtained in this study, we expect that the glucose repression mechanism regulating FLO11 gene expression could serve as an effective target for the development of a new method that would prevent deleterious pellicle formation in wineries. For example, it may be possible to develop a designer strain of yeast or lactic acid bacteria, normally found in wines, to secrete an inhibitor of the release of glucose repression of FLO11 gene expression.
Acknowledgments
We thank Mr Shuhei Mochiduki of our laboratory for his technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Legras JL, Erny C, Charpentier C. Population structure and comparative genome hybridization of European flor yeast reveal a unique group of Saccharomyces cerevisiae strains with few gene duplications in their genome. PLoS One. 2014; 9:e108089.
- Thuy PT, Elisabeth G, Pascal S, et al. Optimal conditions for the formation of sotolon from α-ketobutyric acid in the French "Vin Jaune". J Agric Food Chem. 1995;43:2616–2619.
- Cortes MB, Moreno J, Zea L, et al. Changes in aroma compounds of sherry wines during their biological aging carried out by Saccharomyces cerevisiae races bayanus and capensis. J Agric Food Chem. 1998;46:2389–2394.
- Collin S, Nizet S, Claeys Bouuaert T, et al. Main odorants in Jura flor-sherry wines. Relative contributions of sotolon, abhexon, and theaspirane-derived compounds. J Agric Food Chem. 2012;60:380–387.
- Alexandre H. Flor yeasts of Saccharomyces cerevisiae – their ecology, genetics and metabolism. Int J Food Microbiol. 2013;167:269–275.
- Iimura Y, Hara S, Otsuka K. Cell surface hydrophobicity as a pellicle formation factor in film strain of Saccharomyces. Agric Biol Chem. 1980;44:1215–1222.
- Zara S, Gross MK, Zara G, et al. Ethanol-independent biofilm formation by a flor wine yeast strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2010;76:4089–4091.
- Zara S, Antonio Farris G, Budroni M, et al. HSP12 is essential for biofilm formation by a Sardinian wine strain of S. cerevisiae. Yeast. 2002;19:269–276.
- Hara S, Iimura Y, Otsuka K. Breeding of useful killer wine yeasts. Am J Enol Vitic. 1980;31:28–33.
- Fidalgo M, Barrales RR, Ibeas JI, et al. Adaptive evolution by mutations in the FLO11 gene. Proc Natl Acad Sci USA. 2006;103:11228–11233.
- Nakagawa Y, Toda Y, Yamamura H, et al. FLO11 is essential for pellicle formation by wild pellicle-forming yeasts isolated from contaminated wines. J Biosci Bioeng. 2011;111:7–9.
- Kuchin S, Vyas VK, Carlson M. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol Cell Biol. 2002;22:3994–4000.
- Van de Velde S, Thevelein JM. Cyclic AMP-protein kinase A and Snf1 signaling mechanisms underlie the superior potency of sucrose for induction of filamentation in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:286–293.
- Ishigami M, Nakagawa Y, Hayakawa M, et al. FLO11 is the primary factor in flor formation caused by cell surface hydrophobicity in wild-type flor yeast. Biosci Biotechnol Biochem. 2006;70:660–666.
- Kovacs M, Stuparevic I, Mrsa V, et al. Characterization of Ccw7p cell wall proteins and the encoding genes of Saccharomyces cerevisiae wine yeast strains: relevance for flor formation. FEMS Yeast Res. 2008;8:1115–1126.
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1990.
- Kuroda S, Otaka S, Fujisawa Y. Fermentable and nonfermentable carbon sources sustain constitutive levels of expression of yeast triosephosphate dehydrogenase 3 gene from distinct promoter elements. J Biol Chem. 1994;269:6153–6162.
- Wach A, Brachat A, Pohlmann R, et al. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808.
- Brachmann CB, Davies A, Cost GJ, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132.
- Amberg DC, Botstein D, Beasley EM. Precise gene disruption in Saccharomyces cerevisiae by double fusion polymerase chain reaction. Yeast. 1995;11:1275–1280.
- Zara G, Zara S, Pinna C, et al. FLO11 gene length and transcriptional level affect biofilm-forming ability of wild flor strains of Saccharomyces cerevisiae. Microbiology. 2009;155:3838–3846.
- Carlson M. Glucose repression in yeast. Curr Opin Microbiol. 1999;2:202–207.
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361.
- Schuller HJ. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr Genet. 2003;43:139–160.
- Meijer MM, Boonstra J, Verkleij AJ, et al. Glucose repression in Saccharomyces cerevisiae is related to the glucose concentration rather than the glucose flux. J Biol Chem. 1998;273:24102–24107.