ABSTRACT
The cDNA encoding α-L-arabinofuranosidase was cloned from the edible fungus Auricularia auricula for the first time. The open reading frame of the α-L-arabinofuranosidase gene abf was 1953 bp encoding 650 amino acids, with a predicted protein molecular weight of 71.19 kDa and a theoretical isoelectric point of 5.23. The putative protein was predicted to belong to the glycoside hydrolase family-51. In addition, abf was cloned into the pET-32a vector and then expressed in Escherichia coli BL21. The recombinant protein, with an expected molecular weight, was observed in sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Moreover, the transcription levels of abf in response to different carbon sources were investigated in this study. The results showed that the expression of abf was mostly up-regulated when the mycelia were grown in different carbon sources, and L-arabinose or maltose induction had a significant effect on the expression of abf, which was 5.13- and 4.58-fold higher than that in the untreated control sample, respectively. In addition, the highest transcript levels induced by glucose and sucrose appeared on the third day and the levels were 2.47- and 3.11-fold higher compared to the control. These results laid a foundation for further studies on the α-L-arabinofuranosidase from A. auricula.
Introduction
China is an agricultural country that produces large quantities of hemicellulose waste every year, such as corn cobs, straw, sugarcane bagasse, rice bran, etc. Due to improper handling, this agricultural and forest waste may be a source of environmental pollution. However, it is encouraging that the hemicellulose waste could be digested by fungi.
Auricularia auricula, a white-rot fungus, absorbs its nutrients through degrading lignin, cellulose and hemicellulose. It is one of the most cultivated mushrooms in the world due to its high nutritive, economic and medicinal value [Citation1]. It has potential anti-tumour, anti-inflammatory, hypoglycemic, hypolipidemic and anticoagulant characteristics [Citation2–6]. Furthermore, its extracts have been reported to possess antioxidant and radical scavenging properties [Citation7–9]. Despite these important properties of A. auricula, the cultivation of this black fungus has been poorly explored, especially the degradation mechanism of cellulose or hemicellulose at the molecular level.
Xylan, the major component of plant hemicelluloses, found in the cell walls of monocots and hard woods, represents one of the most abundant biomass resources [Citation10]. Xylan is a heteroglycan with a backbone of β-(1→4)-linked D-xylopyranose residues that can be partially substituted with α-L-arabinofuranose as side chains [Citation11]. The hydrolysis of xylan is a complex process with the participation of a series of enzymes including α-L-arabinofuranosidase (E.C. 3.2.1.55), which hydrolyses arabinose residues in the alpha configuration linked at positions C-2 and/or C-3 of the xylose [Citation12].
α-L-arabinofuranosidases are currently found in glycoside hydrolase (GH) families 2, 3, 10, 43, 51, 54 and 62 on the basis of amino-acid sequence similarities (http://afmb.cnrs-mrs.fr/CAZY/) [Citation13]. The classification is helpful to study the evolutionary relationships, mechanistic information and structural features of these enzymes [Citation14]. In recent years, several α-L-arabinofuranosidases have been characterized in fungi, including Aureobasidium pullulans, Aspergillus niger, Penicillium purpurogenum, Saccharomyces cerevisiae, Rhizomucor pusillus, etc. [Citation10,Citation15–18]. Among the various α-L-arabinofuranosidases, GH family-51 α-L-arabinofuranosidases have been well studied at the structural level. Analysis of these structures has revealed that GH family-51 α-L-arabinofuranosidases are composed of a catalytic domain characterized by a (β/α)8 barrel with two conserved glutamate residues in the catalytic site [Citation19].
In this study, to the best of our knowledge, we described for the first time the structure of a GH family-51 α-L-arabinofuranosidase gene (abf) from A. auricula and its encoded product ABF. Moreover, we also reported the transcript levels of abf in response to different carbon sources.
Materials and methods
Strain and growth conditions
The A. auricula strain DL202 was collected from Quercus mongolica at Liangshui Nature Reserve, Lesser Xing'an Mountains in Yichun city, Heilongjiang Province, China, in August 2008, and was identified based on internal transcribed spacer sequence alignment. The mycelia of A. auricula were maintained on potato dextrose agar plates and kept in the dark at 25 °C for 7 d. Then they were inoculated into liquid potato dextrose (PD) medium and the cultures were allowed to grow in a shaking incubator at 180 r/min and 25 °C.
RNA extraction and cDNA synthesis
The A. auricula mycelia were harvested after 8 d of growth in the liquid PD medium, and frozen at −80 °C until RNA extraction. Total RNA was extracted using TRIzol reagent according to the manufacturer's directions (Invitrogen, Carlsbad, CA, USA); afterwards, the samples were treated with RNase-free DNaseI. The quality and quantity of RNA were assessed by agarose gel electrophoresis and checked by BioPhotometer D30 (Eppendorf, Hamburg, Germany). cDNA synthesis was carried out using PrimeScript™ 1st strand cDNA synthesis kit (TaKaRa, Dalian, China) following the manufacturer's instructions.
Cloning of full-length abf
A putative α-L-arabinofuranosidase gene fragment was found in the A. auricula transcriptome database (unpublished). A pair of degenerate oligonucleotide primers, abf-F1 and abf-R1 (), were designed according to the fragment sequences and then used for the amplification of the cDNA fragment of abf. Using cDNA as a template, the polymerase chain reaction (PCR) amplification procedure was set as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 40 s, and final extension at 72 °C for 10 min (Bio-Rad, Hercules, CA, USA). A 1954 bp DNA fragment was obtained, cloned into the pMD18-T vector (TaKaRa, China) and subjected to nucleotide sequencing (Boshi, Harbin, China). This fragment was subsequently used for designing gene-specific primers (GSP) for the cloning of 3’ and 5’ ends of the abf by RACE (rapid amplification of cDNA ends) PCR.
Table 1. List of primers used in the study.
Next, 3’ and 5’-RACE-Ready cDNA synthesis was carried out using a SMARTer RACE cDNA amplification kit (Clontech, Carlsbad, CA, USA), following the manufacturer's instructions. Afterwards, the cloning of 3’ and 5’ ends of the abf by RACE-PCR was performed using Advantage 2 PCR kit (Clontech, USA), according to the manufacturer's directions. The primers of abf-GSP3 and abf-GSP5 listed in were used for 3’ and 5’ RACE-PCR, respectively. The 3’ RACE-PCR reactions were carried out under the following conditions: 5 min at 94 °C, 35 cycles (30 s at 94 °C; 30 s at 68 °C; 30 s at 72 °C) and 7 min at 72 °C. Meanwhile, 5’ RACE-PCR amplification procedure was set as follows: 5 min at 94 °C, 35 cycles (30 s at 94 °C; 30 s at 65 °C; 30 s at 72 °C) and 7 min at 72 °C. The amplified fragments of both 3’ and 5’ RACE were cloned into pMD18-T vector (TaKaRa, China) and transformed into Escherichia coli DH5α cells (TaKaRa, China). Based on the blue–white screening, positive clones were selected and sequenced. After assembling the sequences of 3’ and 5’ RACE products, a full-length cDNA sequence of abf was obtained.
Sequence analysis
The coding region of abf was obtained using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The molecular weight, theoretical pI, amino-acid composition and hydrophobicity analysis of the predicted protein were estimated using ProtParam (http://www.expasy.ch/tools/protparam.html) [Citation20]. Signal peptide prediction was carried out using SignalP software (http://www.cbs.dtu.dk/services/SignalP/). Structural regions were identified in deduced protein sequence by the SMART tool (http://smart.embl-heidelberg.de/) and NCBI (National Center for Biotechnology Information) Conserved Domains. Also, secondary structure was determined by Predictprotein tool (http://www.predictprotein.ong/) and the three-dimensional structure of ABF was predicted using the Phyre web tool (http://www.sbg.bio.ic.ac.uk/∼phyre/index.cgi).
Phylogenetic analysis
Sequence similarity searches were performed in GenBank through the BLAST (basic local alignment search tool) algorithm (http://www.ncbi.nlm.nih.gov/blast) at NCBI, using our abf sequence as a query. Then similar sequences were downloaded and aligned with the abf sequence using the ClustalX software; subsequently, a neighbour-joining tree was constructed with MEGA 5.0 software [Citation21] and bootstrap analysis with 1000 replicates was also conducted in order to obtain confidence levels for the branches.
Heterologous expression of abf in E. coli
Using cDNA as a template, the entire open reading frame (ORF) containing the start codon and the stop codon regions was amplified by abf-B and abf-H primers, which harbour BamHI and HindIII restriction sites, respectively (). The amplification procedure was as follows: 5 min at 94 °C, 35 cycles (30 s at 94 °C; 30 s at 57 °C; 30 s at 72 °C) and 7 min at 72 °C. Afterwards, the amplified product was double digested with BamHI and HindIII enzymes and subsequently ligated into a pET-32a vector (Novagen, Darmstadt, Germany) which was digested with the same restriction enzymes. The pET-32a-abf construct was transformed into E. coli BL-21 (DE3) cells.
An overnight culture of E. coli BL-21(DE3) cells containing the pET-32a-abf construct was grown in a rotary shaker incubator (180 r/min) at 37 °C in Luria–Bertani (LB) medium. Then, 2% of the overnight culture was inoculated into fresh LB medium to obtain an exponentially growing culture (optical density at 600 nm (OD600) of 0.5–0.8). Afterwards, 1 mmol/L isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the exponentially growing cells. Induced cultures of 1 mL each were harvested at 2 h time span for 10 h. Then 1 mL of harvested culture was centrifuged at 12,000 xg for 10 min and the pellet was re-suspended in 100 μL of 4× protein buffer (Solarbio, Beijing, China). Cells were subsequently disrupted by boiling water bath for 10 min. Finally, the lysate was loaded on an (8%) sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Transcription analysis of abf in A. auricula
In this study, five different carbon sources (glucose, sucrose, maltose, D-xylose and L-arabinose) were separately added into the basal medium in which A. auricula was grown. The induction medium was composed of 200 g potatoes/L, 20 g carbon source/L, 3 g KH2PO4/L, 1.5 g MgSO4/L and 10 mg VB/L.
The seed culture was incubated using a rotary shaker incubator (160 r/min) at 25 °C for 8–10 d in liquid PD medium. The second set of experiments was performed in 500 mL flasks containing 250 mL of induction medium after inoculation with 10% (v/v) of the seed culture. The flasks were then cultured at 160 r/min at 25 °C. Induction experiments were performed in triplicate (three flasks per inducing medium). The mycelia were harvested at 0, 1, 2, 3, 4 and 5 d, respectively, and then frozen at −80 °C until RNA extraction.
Total RNA was extracted using an RNAprep pure Plant Kit (Tiangen, Beijing, China) in accordance with the manufacturer's protocols. Quality and quantity of RNA were assessed as described earlier. Subsequently, total RNA (1 μg) was reverse-transcribed to cDNA using a PrimeScript™ RT reagent Kit (TaKaRa, China), according to the manufacturer's instructions. Finally, the synthesized cDNA (20 μL) was diluted to 200 μL with deionized water and used as the template for the molecular experiments.
Primers abf-qF and abf-qR were used to evaluate the transcript levels of abf, and GAPDH was used as a reference gene (). Then quantitative real-time PCR (qRT-PCR) was performed with the Mx3000P Sequence Detection System (Agilent Technologies, Santa Clara, CA, USA), and the amplification procedure was set as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min. The mycelia that were harvested at 0 d served as the reference sample against which all other genes were compared. Transcript levels were evaluated by qRT-PCR according to the 2−△△CT method described by Livak and Schmittgen [Citation22].
Results and discussion
Cloning and sequence analysis of the abf gene
A 1954 bp fragment was obtained and sequenced based upon our transcriptome data of A. auricula, and the gene fragment was confirmed to be an abf fragment due to its high similarity with known sequences. Then 5’- and 3’-cDNA ends were amplified by the RACE technique, depending on the specific fragment obtained. The 2520 bp full-length cDNA of the abf gene was isolated from A. auricula by RACE, including a 426 bp 5'-untranslated region (UTR), a 141 bp 3' UTR and a 1953 bp ORF encoding 650 amino-acid residues (). The cDNA sequence was deposited in GenBank under the accession number KX272626.
Analysis of the deduced protein sequence
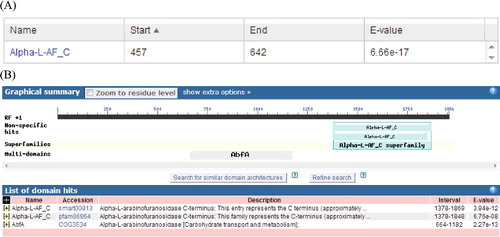
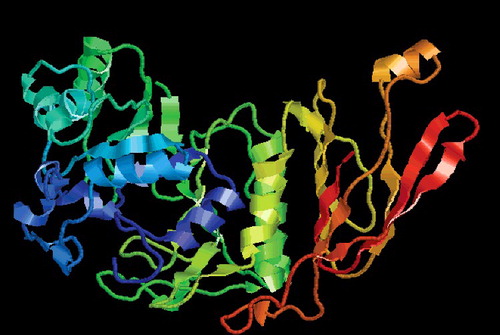
The calculated molecular weight of the deduced protein was 71.19 kDa with a theoretical pI of 5.23 and it was a hydrophobin, according to the ProtParam tool. ABF was identified as a member of the α-L-AF_C Superfamily by the SMART tool ((A)) and NCBI Conserved Domains ((B)). Scanning transmembrane protein topology using the TMHMM tool [Citation23] revealed that there was no transmembrane region in the ABF protein. In addition, ABF has a signal peptide with a length of 15 residues predicted by the Signal 4.0 server [Citation24]. The secondary structure analysis of ABF done using PredictProtein showed that the predicted ABF protein consists of 19.08% alpha-helix, 27.85% beta-sheet and 53.08% loop (coil). Moreover, the three-dimensional structure of ABF was predicted using the Phyre web tool, and the results are shown in . These results further confirmed that the ABF protein from A. auricula was a member of the α-L-AF_C Superfamily.
Phylogenetic analysis
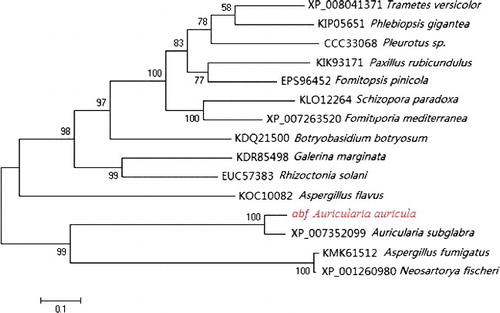
Using the ABF sequence as a query, 14 homologous sequences were selected from the GenBank database through the BLAST algorithm at NCBI, and the information of homologous sequences from other species is listed in . The constructed phylogenetic tree showed that ABF from A. auricula was most similar to ABF from A. subglabra and had a larger evolutional distance from ABF of Trametes versicolor and ABF of Phlebiopsis gigantea (). As a result of this search, ABF from A. auricula can be assigned to GH family-51. To date, several fungi have been reported to express more than one α-L-arabinofuranosidase. The two isoenzymes from A. niger [Citation25], Aspergillus awamori [Citation26] and Penicillium chrysogenum [Citation27], belong to families 51 and 54.
Table 2. Origins of homologous amino-acid sequences from other species.
Bacterial expression of abf
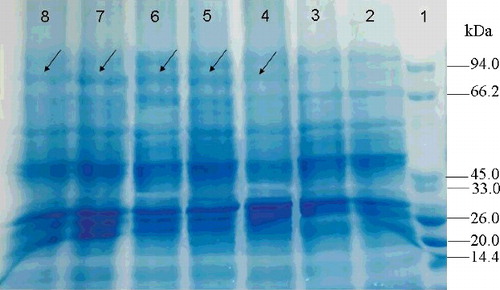
In order to observe the expression of abf in E. coli, the entire protein-coding cDNA of abf was cloned into the expression vector pET-32a. Then the pET-32a-abf construct was transformed into E. coli BL-21 cells. Compared to the control transformant BL21-pET-32a, the recombinant transformant BL21-pET-32a-abf produced a clear protein band with a molecular weight of approximately 71.2 kDa in an 8% SDS-PAGE gel (). The results indicated that the ABF protease had been successfully synthesized in the E. coli cells. The biochemical properties of ABF such as optimal temperature, pH etc. will be investigated in further studies.
Figure 5. Heterologous protein expression in E. coli with IPTG induction (1 mmol/L) at 37 °C for 0, 2, 4, 6, 8 and 10 h. Lane 1, protein molecular weight marker (MW, TransGen, Beijing, China); Lane 2, E. coli harbouring empty vector; Lane 3, E. coli harbouring pET-32a-abf construct induced by IPTG (1 mmol/L) at 0 h; Lane 4, at 2 h; Lane 5, at 4 h; Lane 6, at 6 h; Lane 7, at 8 h; Lane 8, at 10 h.
Transcription of abf in response to different carbon sources
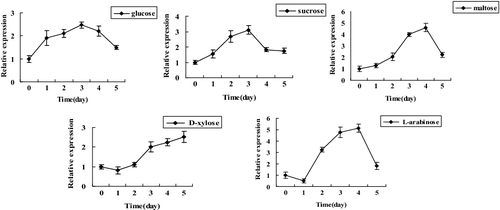
To investigate the transcription of abf in response to different carbon sources (glucose, sucrose, maltose, D-xylose and L-arabinose), qRT-PCR was performed. The real-time PCR results indicated that the transcription of abf was differentially regulated in the presence of different carbon sources (). The transcript peak of abf was obtained in response to glucose, sucrose, maltose and L-arabinose induction within 5 d, except for D-xylose. Among them, the highest transcript levels induced by glucose and sucrose appeared on the third day and were 2.47- and 3.11-fold higher than that in the control, respectively. As for maltose and L-arabinose induction, the highest expression values were observed on the fourth day. In addition, the transcript levels of abf induced by D-xylose were up-regulated at 2–5 d. In summary, the results indicated that L-arabinose or maltose induction had a significant effect on the expression of abf, which was 5.13-and 4.58-fold compared to the control, respectively. However, L-arabinose is much more expensive than maltose in the cultivation of A. auricula. Therefore, we could recommend the use of maltose instead of L-arabinose to increase the expression of abf in A. auricula.
Conclusions
In this work, we successfully cloned the full-length cDNA of abf from A. auricula. The E. coli BL-21 was selected as a host for heterologous expression of abf, and its product, the ABF enzyme, was observed in SDS-PAGE. Moreover, the transcript levels of abf were investigated in fungal growth under different carbon sources. These results provide theoretical basis for abf functional research and may be of significant interest to understand the regulatory role of abf in the degradation mechanism of hemicellulose.
Acknowledgements
The authors are grateful to Professor Xuemei Chen (Department of Botany and Plant Sciences, University of California, Riverside, CA, USA) for her helpful suggestions and critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fan XZ, Zhou Y, Xiao Y, et al. Cloning, expression and phylogenetic analysis of a divergent laccase multigene family in Auricularia auricula-judae. Microbiol Res. 2014;169:453–462.
- Misaki A, Kakuta M, Sasaki T, et al. Studies on interrelation of structure and antitumor effects of polysaccharides: antitumor action of periodate-modified, branched-beta-D-glucan of Auricularia auricula-judae, and other polysaccharides containing-glycosidic linkages. Carbohydr Res. 1981;92:115–129.
- Ukai S, Kiho T, Hara C, et al. Polysaccharides in fungi XIV. Anti-inflammatory effect of the polysaccharides from the fruit bodies of several fungi. J Pharmacobio-Dyn. 1983;6:983–989.
- Yuan Z, He P, Cui J, et al. Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel on genetically diabetic KK-Ay mice. Biosci Biotech Biochem. 1998;62:1898–1903.
- Reza MA, Hossain MA, Damte D, et al. Hypolipidemic and hepatic steatosis preventing activities of the wood ear medicinal mushroom Auricularia auricula-judae (Higher Basidiomycetes) ethanol extract in vivo and in vitro. Int J Med Mushrooms. 2015;17:723–734.
- Yoon SJ, Yu MA, Pyun YR, et al. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb Res. 2003;112:151–158.
- Acharya K, Samui K, Rai M, et al. Antioxidant and nitric oxide synthase activation properties of Auricularia auricula. Indian J Exp Biol. 2004;42:538–540.
- Khaskhelia SG, Zheng W, Sheikh SA, et al. Characterization of Auricularia auricula polysaccharides and its antioxidant properties in fresh and pickled product. Int J Biol Macromol. 2015;81:387–395.
- Zou Y, Zhao Y, Hu WZ. Chemical composition and radical scavenging activity of melanin from Auricularia auricula fruiting bodies. Food Sci Tech. 2015;35:253–258.
- Ohta K, Fujii S, Higashida C. Characterization of a glycoside hydrolase family-51 α-L-arabinofuranosidase gene from Aureobasidium pullulans ATCC 20524 and its encoded product. J Biosci Bioeng. 2013;116:287–292.
- Puls J, Schuseil J. Chemistry of hemicelluloses: relationship between hemicellulose structure and enzymes required for hydrolysis. In: Coughlan MP, Hazlewood GP, editors. Hemicellulose and hemicellulases. London: Portland Press; 1993. p. 1e27.
- Numan MT, Bhosle NB. Alpha-L-Arabinofuranosidases: the potential applications in biotechnology. J Ind Microbiol Biotechnol. 2006;33:247–260.
- Coutinho PM, Henrissat B. Carbohydrate-active enzymes server at http://afmb.cnrs-mrs.fr/_/cazy/CAZY/index.html. 1999.
- Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859.
- Luonteri E, Beldman G, Tenkanen M. Substrate specificities of Aspergillus terreus α-arabinofuranosidases. Carbohydr Polym. 1998;37:131–141.
- Ravanal MC, Eyzaguirre J. Heterologous expression and characterization of α-L-arabinofuranosidase 4 from Penicillium purpurogenum and comparison with the other isoenzymes produced by the fungus. Fungal Biol. 2015;119:641–647.
- Wirajana IN, Kimura T, Sakka K, et al. Secretion of Geobacillus thermoleovorans IT-08 α-L-Arabinofuranosidase (AbfA) in Saccharomyces cerevisiae by fusion with HM-1 signal peptide. Procedia Chem. 2016;18:69–74.
- Rahman SAKM, Kato K, Kawai S, et al. Substrate specificity of the α-L-arabinofuranosidase from Rhizomucor pusillus HHT-1. Carbohydr Res. 2003;338:1469–1476.
- Lee SH, Lee YE. Cloning, expression, and characterization of a thermostable GH51 α-L-arabinofuranosidase from Paenibacillus sp. DG-22. J Microbiol Biotechnol. 2014;24:236–244.
- Gasteiger E, Gattiker A, Hoogland C, et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucl Acids Res. 2003;31:3784–3788.
- Tamura K, Dudley J, Nei M, et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods. 2001;25:402–408.
- Melen K, Krogh A, von Heijne G. Reliability measures for membrane protein topology prediction algorithms. J Mol Biol. 2003;327:735–744.
- Petersen TN, Brunak S, von Heijne G, et al. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786.
- Rombouts FM, Voragen AGJ, Searle-van Leeuwen MF, et al. The arabinanases of Aspergillus niger: purification and characterization of two α-Larabinofuranosidases and an endo-1,5-α-L-arabinanase. Carbohydr Polym. 1988;9:25–47.
- Kaneko S, Arimoto M, Ohba M, et al. Purification and substrate specificities of two α-L-arabinofuranosidases from Aspergillus awamori IFO 4033. Appl Environ Microb. 1998;64:4021–4027.
- Sakamoto T, Kawasaki H. Purification and properties of two type B α-L-arabinofuranosidases produced by Penicillium chrysogenum. Biochim Biophys Acta. 2003;1621:204–210.