?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Caralluma flava is a perennial, succulent edible plant distributed in the hillsides of the United Arab Emirates. In order to validate its use in the traditional system as an edible and medicinal source, we evaluated the antioxidant and in vitro free-radical-scavenging properties of its ethanol extract. The antioxidant activity of the extract was evaluated in vitro by 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS+), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), hydroxyl radical and nitric oxide radical scavenging assays. The antioxidant enzyme activities (superoxide dismutase, catalase and glutathione peroxidase) and non-enzymatic antioxidant contents (total reduced glutathione, and Vitamin E and Vitamin C) in the extracts were also determined. The total phenolics content of the plant extract was 10.14 mg/g and the flavonoid content was 4.13 mg/g. The results showed a positive linear correlation between these phytochemicals and the free-radical-scavenging activities. The C. flava extract exhibited significant radical-scavenging properties in the ABTS(+•), DPPH(•), hydroxyl radical and nitric oxide radical assays. The study revealed that C. flava could be considered an excellent source of enzymatic and non-enzymatic antioxidants. The results indicate that the plant extract has potent free-radical-scavenging activity and ability to prevent radical chain reactions. Based on the results obtained, C. flava seems to be a very promising species for further investigation in order to identify the compounds responsible for its biological activity.
Introduction
In recent years, due to the increased demand of finding alternative medicines, there has been an urge to evaluate the existing traditional plants for their medicinal potentials. Many plants are potential sources of natural antioxidants and phytochemicals which can be utilized in the pharmaceutical industry [Citation1]. The pharmaceutical industry views plant wealth as a source of income. Throughout the world, the demand for medicinal plants is increasing due to easy availability, less side-effects and sometimes their being the only source of health care [Citation2]. Thus, the research on antioxidant products is a significant topic in the medical field as well as in the food industry.
Free radicals are the major cause of aging as well as of various chronic and degenerative diseases, including heart disease and even cancer [Citation3,Citation4]. There are many free radicals formed in the human body, like superoxide radical, hydroxyl radical, singlet oxygen etc., and all these derivatives are collectively termed reactive oxygen species (ROS) [Citation5].
Antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and peroxidases (GPx), catalytically remove free radicals and other ROS. Decreased antioxidant enzyme activities have been associated with various pathologies. For example, the decreased capacity of a variety of tumours for detoxifying H2O2 is linked to a decreased CAT level [Citation6]. GPx competes with CAT for H2O2 as substrate and is the major source of protection against low levels of oxidative stress [Citation7].
The non-enzymatic antioxidants (low-molecular-weight compounds) derived from the diet include α-tocopherol (vitamin E), ascorbic acid (vitamin C), carotenoids and plant phenols. Low-molecular mass antioxidant compounds synthesized in vivo include serotonin, bilirubin, α-ketoacids, uric acid, sex hormones, coenzyme Q, lipoic acid etc. [Citation8].
The medicinal plant research in the United Arab Emirates is blooming as an array of potential plants are present in the desert flora, and most of them have been used widely in the traditional medicinal system. There is a very rich tradition of herbal medicines among the Arab, [Citation9] which blended with Greek practices to become what is popularly known as the Unani (Greco-Arab medicine) system [Citation2]. There are many traditional systems of medicine used in the Arabian Peninsula [Citation9]. Use of herbal plants in these medical systems is a common practice among the ‘bedu’ peoples of the United Arab Emirates since time immemorial [Citation10,Citation11]. Approximately 678 terrestrial plant species grow in the United Arab Emirates. These plants have adaptations to survive in extreme arid environments. In a study by Sakkir et al. [Citation2], a total of 132 plants species were found to possess medicinal properties. They belong to 114 genera and 49 families. Research to unveil the antioxidant potential of the United Arab Emirates plants has been of major interest in recent years [Citation12–18]. According to Sultan et al. [Citation19], there is a severe shortage of plant material in the traditional system of medicine, while the market demand of almost all cases of medicinal plants is ever increasing.
Plants provide a lot of antioxidant compounds like flavonoids, which are used widely in the pharmaceutical industry [Citation20,Citation21]. In this aspect, it seems important to test the traditional medicinal plants for the antioxidant potential of their extracts. For example, there are previous reports revealing prebiotics and antioxidants from wild flowers from Bulgaria [Citation22]. Use of wild edible plants as a source of natural antioxidants is important, as many synthetic antioxidants have side effects and pose potential threats to the health [Citation23]. Some plant products have been used in traditional medicine to treat chronic diseases and also as food plants. Many plants that widely grow in the United Arab Emirates are used both in folk medicine and as food, and their biological and antioxidant properties are not thoroughly evaluated [Citation17].
Caralluma flava is a flowering plant belonging to family Apocynaceae. It is reported that more than 260 species of genus Caralluma are found in tropical Asia and Mediterranean regions and out of these, some species have been used as traditional and modern dietary ingredients as well as in traditional medicine for the treatment of many diseases [Citation24,Citation25]. Out of these species, Caralluma flava N.E. Brown is a perennial plant, mainly found on hillsides and wadis in the United Arab Emirates [Citation26]. C. flava is used for edible purposes as well as in the traditional medicinal system of the United Arab Emirates [Citation26]. The active ingredient in this plant are pregnane glycosides and triterpenes [Citation27]. However, the radical-scavenging abilities and antioxidant enzymes of this species are not yet explored. Therefore, the aim of this study was to determine the total phenolic and flavonoid contents in the ethanol extract from aerial parts of C. flava and to evaluate the antioxidant power using different in vitro free radical scavenging assays together with various enzyme activities.
Materials and methods
The fresh whole plants of C. flava used in this study were collected from Hillside of Al-Fujairah Emirate, United Arab Emirates. The plants were identified and authenticated at United Arab Emirates University (UAEU) and the Environment Agency (EAD), Abu Dhabi.
Plant sample extraction
The plant samples were carefully washed under running tap water, dried with a soft cloth and the fresh flesh was then cut into small pieces. The fresh samples were homogenized in a pre-chilled mortar and pestle with 80% ethanol at room temperature and were centrifuged at 10,000 r/min at 4 °C for 15 min (PrO-Analytical, Centurion Scientific, UK). The supernatant thus obtained was used within 4 h for various enzymatic and non-enzymatic antioxidants assays. The residue was re-extracted twice with 80% ethanol and supernatants were pooled, put into evaporating dishes and evaporated to dryness at room temperature. The residue was dissolved in 5 mL of distilled water and stored at 4–8 °C in a refrigerator for further analysis of in vitro free-radical-scavenging activity.
Determination of phenolic and flavonoid contents
The total phenolic content was determined by using the Folin–Ciocalteu method with gallic acid as standard [Citation28]. Different concentrations of gallic acid solution (5 mg/100 mL) were used to plot the calibration curve. The total flavonoid content in the plant was determined by the method of Zhishen et al. [Citation29] and was expressed in micrograms of gallic acid equivalent (GAE).
Antioxidant enzyme activity assays
SOD activity (EC: 1.15.1.1) was determined by measuring the inhibition rate of nitroblue tetrazolium (NBT) at 560 nm [Citation30]. CAT (EC: 1.11.1.6) activity was assayed by the method of Sinha [Citation31]. The activity of glutathione peroxidase (EC: 1.11.1.9) was measured according to the method of Rotruck et al. [Citation32].
Determination of reduced glutathione (GSH), vitamin c and vitamin e
The amount of reduced glutathione in the plant extract was determined by the method of Boyne and Ellman [Citation33]. Vitamin levels in the plant extract were determined spectrophotometrically by the method of Rutkowski and Grzegorczyk [Citation34].
Antioxidant assays
2,2'-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS•+) decolorization assay [Citation35], 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) radical scavenging activity [Citation36], hydroxyl radical scavenging activity [Citation37] and nitric oxide radical scavenging assay [Citation38] were performed. The inhibition was calculated using the following formula:
Where A0 is the absorbance of the control, A1 is the absorbance of the extract/standard, respectively. The percent inhibition values were plotted versus the concentration curve and the concentration of the sample required to achieve 50% inhibition was determined and expressed as the half maximal inhibitory concentration (IC50). A lower the IC50 value indicates high antioxidant capacity.
Statistical analysis
Data are presented as means with standard deviation (±SD). Statistical analysis was performed using SPSS version 13.0 and Excel 2007. Each experiment was repeated three times.
Results and discussion
Different species of Caralluma have been used in the traditional system as food as well as for treating various health conditions. According to the scientific evaluation performed by Adnan et al. [Citation24], Caralluma was confirmed as one of the most widely used genera in some parts of the world. There are many reports regarding the antioxidant activities of various species of Caralluma, but little has been reported regarding Caralluma flava. In the present study, an attempt was made to evaluate the antioxidant potential of ethanol extracts from C. flava aerial parts.
Total phenol and flavonoid contents
Phenolic compounds may contribute directly to the antioxidative action. The concentration of total phenol contents (TPC) in the C. flava extract was 10.14 mg/g. The total flavonoid content was 4.13 mg/g in the plant extract. The total phenolic and flavonoid contents of the extract of C. flava were significantly higher when compared to the gallic acid standard.
The antioxidant activity was proportional to the amount of total phenolics present in the extracts. Through this type of studies, certain extracts and their components can be recommended for use an additive in food preparations and nutraceuticals [Citation39]. Al-Attabi et al. [Citation40] reported that the ethanol extract and ethyl acetate fraction from C. arabica have strong antioxidant activity. This antioxidant activity was correlated with the high level of phenolic content in different plant parts. Similarly, the plant C. tuberculata also contains potential secondary metabolite molecules [Citation41]. Recently, Gujjala et al. [Citation42] showed an appreciable amount of polyphenolic compounds in C. fimbriata followed by saponins and flavonoids.
Free-radical-scavenging activities
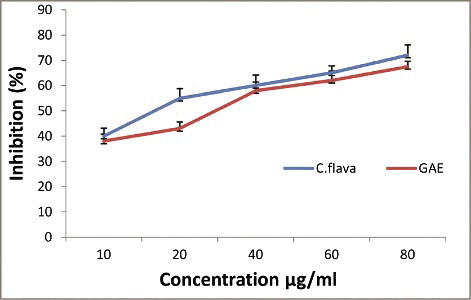
The ABTS radical scavenging activity of C. flava compared with gallic acid is shown in . Various concentrations of C. flava extract and gallic acid were observed to scavenge the ABTS radical in a dose-dependent manner. The C. flava extract exhibited potent scavenging effects against ABTS with an IC50 value almost equivalent to that of standard gallic acid. The percentage of inhibition was 86% and 59% for the extract and gallic acid, respectively, at a concentration of 80 µg/mL. The mean values across the concentration range indicate that the ethanol extract of C. flava was highly potent in neutralizing ABTS cation radicals and its activity was comparable to that of gallic acid.
Figure 1. ABTS free radical scavenging activity of Caralluma flava ethanol extract in comparison with gallic acid.
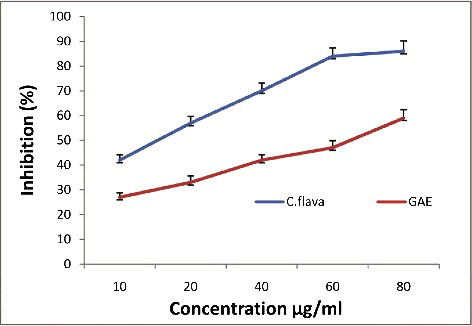
shows the DPPH radical scavenging activity of the C. flava extract and gallic acid. From the dose-dependent response curve, the ethanol extracts were shown to have high DPPH radical scavenging activity. At a concentration of 80 μg/mL, the activity of the ethanol extract reached 72%, which was comparable to that of the standard gallic acid (67.5%). These results are in agreement with previous findings that C. wissmannii O. Schwartz. showed significant antioxidant activity with the DPPH radical method [Citation43]. The DPPH assay usually involves a hydrogen atom transfer reaction [Citation44]. The DPPH method has been considered an indication of the capacity of plant extracts to scavenge free radicals, and will refer to hydrogen atom or electron donation ability, independently of any enzymatic activity [Citation45].
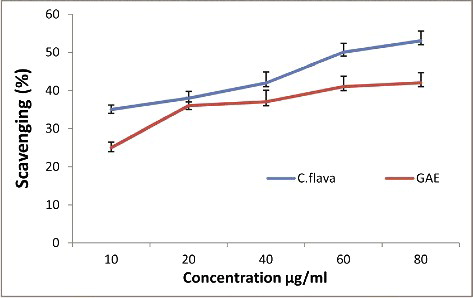
shows the hydroxyl radical scavenging activity of C. flava using various concentrations in comparison with the standard gallic acid. The plant extract showed a maximum inhibition of 53% at 80 µg. The concentration needed for 50% inhibition was 60 µg GAE. The radical scavenging capacity may be attributed to phenolic compounds in the ethanolic extract of C. flava with the ability to accept electrons, which can combine with free radicals competitively to decrease the hydroxyl radical concentration. The results of a recent study by Al-Attabi et al. [Citation40] revealed that C. arabica has good antioxidant power and thereby it is concluded that the intake of this plant, as an antioxidant-rich food, may reduce health risks and cellular oxidative stress.
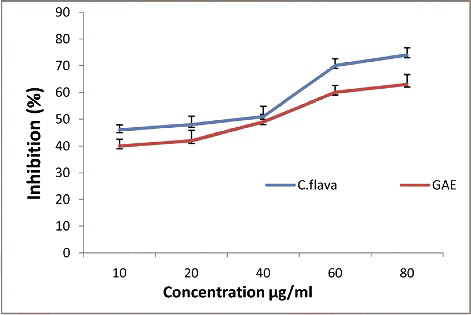
The results from the nitric oxide radical scavenging assay of the C. flava extract and gallic acid are illustrated in . Various concentrations of C. flava extract and gallic acid were shown to dose dependently scavenge the nitric oxide radical. The ethanolic extract of the plant showed moderate nitric oxide scavenging activity at a concentration of 60 µg when compared to gallic acid as a standard. Rehman et al [Citation46] reported the antioxidant properties of different extracts of C. tuberculate. Jerome et al. [Citation47] reported the phytochemical constituents in C. umbellate, also suggesting that this genus has high pharmacological potential.
Antioxidant enzyme activities
The specific SOD and CAT activities in the C. flava extract were 14.2 ± 1.18 units/mg protein and 27.2 ± 1.51 µmole of H2O2 consumed/min/mg protein, respectively. The GPx activity in the C. flava extract was found to be 34.81 ± 2.14. There are reports on the presence of peroxidase activity in C. umbellate [Citation48]. In a study by Shanmugam et al. [Citation49], the ethanolic extract from C. umbellate was reported to enhance the activities of antioxidant enzymes. The antioxidant activity of extracts from different species of Caralluma has been studied, e.g. C. adscendens, C. stalagmifera and C. stalagmifera [Citation50]. Similarly, a phytochemical assay was performed from four species, C. lasiantha, C. umbellata, C. attenuata and C. diffusa, and it showed significant metabolite contents [Citation51].
Non-enzymatic antioxidant contents
The total reduced glutathione was 38.88 ± 2.11 µg/mg and that of Vitamin C and Vitamin E, 2.58 ± 0.64 and 5.33 ± 2.96 µg/mg, respectively. A recent review explained the use of C. tuberculate in traditional medicine mainly in diabetes, rheumatism, leprosy, peptic ulcer, inflammation, jaundice, dysentery, constipation, stomach pain, hepatitis B and C [Citation52]. The plant C. diffusa contains a high amount of total phenolics, which have antioxidant potential [Citation53]. In another report, Poodineh et al. [Citation54] confirmed the antihyperglycemic and antioxidant activities of C. tuberculata in STZ-induced diabetic rats. Kumar et al. [Citation55] reported the potential of C. attenuate in the treatment of diabetes. Similarly, C. fimbriata may be beneficial for the suppression of high-fat diet-induced insulin resistance and oxidative stress [Citation56].
Overall, the results from this study suggest that Caralluma flava may be considered a potential source of antioxidants for the nutraceutical industry. Further studies on the structural elucidation of potential compounds are necessary towards better exploitation of the traditional uses of C. flava. Furthermore, the in vivo antioxidant activity of this extract also has to be assessed in view of its potential clinical use.
Conclusions
The results from this study showed that C. flava plant extract has antioxidant potential. It could, thus, be recommended for utilization in the formulation of novel drugs in the pharmaceutical industry. Further studies underway in our lab focus on the isolation and purification of specific compounds responsible for the antioxidant potential of the C. flava ethanol extract.
Acknowledgments
The authors gratefully acknowledge the Environment Agency, Abudhabi, for scientific collaboration and assistance provided for collection and authentication of plant samples. Special thanks to Mr. Saeed Ahmed Mohamed A. Abdouli, student of MS Horticulture, Department of Aridland Agriculture, UAEU for sample collections.
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Bitis L, Sen A, Ozsoy N, et al. Flavonoids and biological activities of various extracts from Rosa sempervirens leaves. Biotechnol Biotechnol Equip. 2017;31(2):299–303.
- Sakkir S, Kabshawi M, Mehairbi M. Medicinal plants diversity and their conservation status in the United Arab Emirates (UAE). J Med Plants Res. 2012;6(7):1304–1322.
- Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis. 2007;115(2):81–103.
- Cheng HY, Lin TC, Yu KH, et al. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharmaceut Bull. 2003;26:1331–1335.
- Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev. 1997;23:209–240.
- Andre C, Kim SW, Yu XH, et al. Fusing catalase to an alkane-producing enzyme maintains enzymatic activity by converting the inhibitory byproduct H2O2 to the cosubstrate O2. Proc National Acad Sci. 2013;110(8):3191–3196.
- Antunes F, Han D, Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H2O2 detoxification in in vivo conditions. Free Rad Biol Med. 2002;33(9):1260–1267.
- Podda M, Grundmann-Kollmann M. Low molecular weight antioxidants and their role in skin ageing. Clin Exp Dermatol. 2001;26(7):578–582.
- Ghazanfar SA, Al-Al-Sabahi AM. Medicinal plants of northern and central Oman (Arabia). Econ Bot. 1993;47(1):89–98.
- Gallacher DJ, Hill JP. Effects of camel versus oryx and gazelle grazing on the plant ecology of the Dubai desert conservation reserve. In: Reclaiming the desert: towards a sustainable environment in arid lands: proceedings of the Third Joint UAE-Japan Symposium on Sustainable GCC Environment and Water Resources (EWR2006); 2006 Jan 30–Feb 1; Abu Dhabi, UAE. Vol. 3. London: CRC Press; 2006. p. 85.
- Al Ameri SA, Al Shaibani FY, Cheruth AJ, et al. Comparative phytochemical analysis of Moringa oleifera and Moringa peregrina. Pharmacologyonline. 2014;3:216–221.
- Cybulska I, Brudecki G, Alassali A, et al. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emir J Food Agric. 2014;26(12):1046–1056.
- Nessa F, Khan SA. Evaluation of antioxidant and xanthine oxidase inhibitory activity of different solvent extracts of leaves of Citrullus colocynthis. Pharmacog Res. 2014;6(3):218–226.
- Maqsood S, Benjakul S, Abushelaibi A, et al. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Comp Rev Food Sci Food Safe. 2014;13(6):1125–1140.
- Cheruth AJ, Kurup SS, Subramaniam S. Variations in hormones and antioxidant status in relation to flowering in early, mid, and late varieties of date palm (Phoenix dactylifera) of United Arab Emirates. The Sci World J. 2015 [cited 2017 Jun 16];2015:846104. doi:10.1155/2015/846104
- Cheruth AJ, Al Naqbi KM, El-Kaabi AA, et al. In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of Calligonum comosum L'Her. Orient Pharm Exp Med. 2016;16(3):209–215.
- Cheruth AJ, Al Baloushi SA, Karthishwaran K, et al. Medicinally active principles analysis of Tephrosia apollinea (Delile) DC. growing in the United Arab Emirates. BMC Res Notes. 2017 [cited 2017 Jun 16];10(1):61. doi:10.1186/s13104-017-2388-0
- Ksiksi T, Rasheed PABT, Ppoyil S, et al. Immature leaves of Acridocarpus orientalis A. Juss. exhibit high antioxidant and anti-LOX properties. Curr Bioactive Comp. 2017;13(2):144–151.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016 [cited 2017 Jun 16];57(1):7. doi:10.1186/s40529-016-0121-2
- Devasagayam TP, Tilak JC, Boloor KK, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52(10):794–804.
- de Oliveira-Júnior RG, Ferraz CA, Souza GR, et al. Phytochemical analysis and evaluation of antioxidant and photoprotective activities of extracts from flowers of Bromelia laciniosa (Bromeliaceae). Biotechnol Biotechnol Equip. 2017;31(3):600–605.
- Petkova N, Mihaylova D. Flower heads of Onopordum tauricum Willd. and Carduus acanthoides L. – source of prebiotics and antioxidants. Emir J Food Agric. 2016;28(10):732–736.
- Silva MM, Lidon FC. An overview on applications and side effects of antioxidant food additives. Emir J Food Agric. 2016;28(12):823–833.
- Adnan M, Jan S, Mussarat S, et al. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J Pharm Pharmacol. 2014;66(10):1351–1368.
- Dutt HC, Singh S, Avula B, et al. Pharmacological review of Caralluma R. Br. with special reference to appetite suppression and anti-obesity. J Med Food. 2012;15(2):108–119.
- Jongbloed M, Feulner G, Böer B, et al. The comprehensive guide to the wild flowers of the United Arab Emirates. Dubai: Emirates Printing Press, Environmental Research and Wildlife Development Agency; 2003.
- Marwah RG, Fatope MO, Al Mahrooqi R, et al. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007;101(2):465–470.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16(3):144–158.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559.
- Das K, Samanta L, Chainy GB. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys. 2000;37:201–204.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394.
- Rotruck JT, Pope AL, Ganther HE, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590.
- Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–653.
- Rutkowski M, Grzegorczyk K. Modifications of spectrophotometric methods for antioxidative vitamins determination convenient in analytic practice. Acta Sci Polon Technol Aliment. 2007;6(3):17–28.
- Wolfenden BS, Willson RL. Radical-cations as reference chromogens in kinetic studies of one- electron transfer reactions; pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate). J Chem Soc Perkin Trans. 1982;2:805–812.
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–328.
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts is a feasible source of hydroxy radicals in vivo. Biochem J. 1982;205(2):461–463.
- Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138.
- Maheshu V, Priyadarsini DT, Sasikumar JM. Antioxidant capacity and amino acid analysis of Caralluma adscendens (Roxb.) Haw var. fimbriata (wall.) Grav. & Mayur. aerial parts. J Food Sci Technol. 2014;51(10):2415–2424.
- Al-Attabi Z, AlMamri R, Aslam KA. Antioxidant potential properties of three wild omani plants against hydrogen peroxide-induced oxidative stress. Clin Nutr. 2015;3(2):16–22.
- Rauf A, Jan MR, Rehman WU, et al. Phytochemical, phytotoxic and antioxidant profile of Caralluma tuberculata NE Brown. Wudpecker J Pharm Pharmacol. 2013;2(2):21–25.
- Gujjala S, Putakala M, Nukala S, et al. Renoprotective effect of Caralluma fimbriata against high-fat diet-induced oxidative stress in Wistar rats. J Food Drug Anal. 2016;24(3):586–593.
- Al-Naqeb G. Antioxidant and antibacterial activities of some Yemeni medicinal plants. Internat J Herb Med. 2015;3(3 Part A):6–11.
- Li W, Hosseinian FS, Tsopmo A, et al. Evaluation of antioxidant capacity and aroma quality of breast milk. Nutrition. 2009;25(1):105–114.
- Mileva MM, Kusovski VK, Krastev DS, et al. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int J Curr Microbiol App Sci. 2014;3(7):11–20.
- Rehman R, Chaudhary M, Khawar K, et al. In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia. 2014;69(3):341–349.
- Jerome JJ, Kamaraj M, Nandagopalan V, et al. Study of phytochemical constituents in Caralluma umbellata by GG-MS Analysis. Internat J Pharm Sci Invent. 2013;2(4):37–41.
- Achar RR, Venkatesh BK, Sharanappa P, et al. Evidence for peroxidase activity in Caralluma umbellata. App Biochem Biotechnol. 2014;173(8):1955–1962.
- Shanmugam G, Ayyavu M, Dowlathabad MR, et al. Hepatoprotective effect of Caralluma umbellata against acetaminophen induced oxidative stress and liver damage in rat. J Pharm Res. 2013;6:342–345.
- Vajha M, Chillara SR. Evaluation of cellular antioxidant activity of selected species of Caralluma and Boucerosia on cell lines. Internat J App Sci Biotechnol. 2014;2(1):83–87.
- Patel K. Pharmacognostic evaluation of selected species of Caralluma genus. J Phytopharmacol. 2015;4(1):34–40.
- Bibi Y, Tabassum S, Zahara K, et al. Ethnomedicinal and pharmacological properties of Caralluma tuberculata NE Brown-A review. Pure App Biol. 2015;4(4):503–510.
- Chandran R, Sajeesh T, Parimelazhagan T. Total phenolic content, anti-radical property and HPLC profiles of Caralluma diffusa (Wight) NE Br. J Biol Act Prod Nat. 2014;4(3):188–195.
- Poodineh J, Khazaei FA, Nakhaee A. Antioxidant activities of Caralluma tuberculata on streptozotocin-induced diabetic rats. Drug Devel Res. 2015;76(1):40–47.
- Kumar P, Sharma A, Varshney P, et al. Antidiabetogenic and antioxidant effects of Caralluma attenuata extract on streptozotocin induced diabetes in rats. J Pharm Res. 2013;7(3):257–262.
- Sudhakara G, Mallaiah P, Sreenivasulu N, et al. Beneficial effects of hydro-alcoholic extract of Caralluma fimbriata against high-fat diet-induced insulin resistance and oxidative stress in Wistar male rats. J Physiol Biochem. 2014;70(2):311–320.