ABSTRACT
Sucrose synthase (SUS, EC 2.4.1.13) is a key enzyme involved in sucrose metabolism. In this study, amplification by polymerase chain reaction confirmed that there are at least seven SUS genes (BeSUS1-7) in the Bambusa emeiensis genome. The expression patterns of the BeSUSs genes differed from each other in various bamboo tissues. Among these BeSUSs, BeSUS2 was expressed predominately in the root and BeSUS5 was most abundant in developing shoots, indicating that these two isozymes might play more important physiological roles in root and shoot than the other BeSUSs genes, respectively. In addition, BeSUSs response to abscisic acid (ABA), methyl jasmonate (MeJA) and 2,6-dichlorobenzonitrile treatment (2,6-DCB, an inhibitor of cellulose synthesis) was also investigated in leaves. The results showed that the expression levels of the BeSUS1, 5 and 7 genes were not only conspicuously induced by ABA treatment but also up-regulated by exogenous application of MeJA, indicating that these three isozymes are most likely involved in ABA and MeJA stress responses and are important in meeting the increased glycolytic demand that occurs during these stresses. Moreover, after 2,6-DCB treatment, the cellulose content of leaves was decreased and the transcripts of BeSUS2, 3 and 7 were markedly decreased, while those of BeSUS 5 and 6 were conspicuously induced. The results suggest that the possible roles of BeSUSs genes in the pathway of sucrose metabolism, bamboo resistance to abiotic stresses and the regulation of cellulose biosynthesis may be divergent.
Introduction
Sucrose, as a major photoassimilate, is exported from source tissues to sink tissues in many plants. Usually the utilization of sucrose requires its cleavage by either sucrose synthase (SUS, EC 2.4.1.13) or invertase (Inv) in the plant cell. SUS is a key enzyme in sucrose metabolism of plants that converts sucrose and uridine diphosphate (UDP) into fructose and UDP-glucose [Citation1]. SUS has been well characterized in various plants and plays an important role in the regulation of carbon partitioning into various pathways, such as starch biosynthesis [Citation2,Citation3], cellulose synthesis and secondary cell-wall formation [Citation1,Citation4–8]. Moreover, SUS activity is also implicated in many other important metabolic processes, such as nitrogen fixation [Citation9] and response to some abiotic stresses [Citation10]. In bamboo, SUS has shown its potential roles in providing substrates for the polysaccharide biosynthesis or energy production necessary to support rapid plant growth [Citation11]. Furthermore, previous studies showed that bamboo shoots are rich in SUS [Citation12] and it has been observed during bamboo fibre elongation [Citation13], indicating that SUS might be a key enzyme in manipulating the bamboo growth.
The identification of the SUS genes is the starting point towards revealing their physiological roles. Presently, a number of SUS gene families have been identified in many plant species [Citation14–19]. These studies have demonstrated that SUS genes occur as isoforms and are encoded by a small multi-gene family. For instance, the SUS family has six members in Arabidopsis thaliana and is classified into three groups [Citation14]. Similarly, in Lotus japonicas [Citation20], rice (Oryza sativa) [Citation21] and rubber tree (Hevea brasiliensis) [Citation22], at least six SUS genes are known to exist. In addition, seven SUS genes are found in cotton (Gossypium arboreum) [Citation18] and poplar (Populus trichocarpa) [Citation23]. Members of the SUS gene family are divergent in function and show differential expression. For example, in cotton, except GaSUS2 and GaSUS7, most GaSUSs are differentially expressed in a wide range of tissues, and show development-dependent expression profiles in cotton fibre cells [Citation18]. The Nttab0259170 and Ntab0259180 are expressed ubiquitously in tobacco, while Ntab0288750, Ntab0234340 and Ntab0784850 are mainly expressed in buds and sepals, suggesting probable tissue-specific functions for these three genes [Citation24]. In all cases, each member of the SUS gene families has a particular function in a given tissue or organ of the species [Citation10].
Unlike bamboo species with scattered distribution, such as Dendrocalamus latiflorus and Phyllostachys edulis, Bambusa emeiensis is an important sympodial-tufted bamboo species widely cultivated in south-western China as a good-quality wood for pulping. The regulation of cellulose biosynthesis in bamboo is very important for bamboo fibre quality improvement. Although SUS has shown its potential roles in cellulose biosynthesis and fibre growth through the analysis of SUS enzyme activities or their transcripts levels in various tissues and organs, the possible roles of B. emeiensis SUS genes are still unclear.
Although four SUS genes (BoSuSy1-4) were isolated from Bambusa oldhamii shoots by RT-PCR [Citation11], it was predicted that there would be more SUS genes in the bamboo genome due to the number of SUS genes reported in other plants. In the present work, we succeeded in identifying seven SUS genes from a shoot cDNA library of B. emeiensis. The analysis in this study mainly focused on sequence characterization, evolutionary relationship and gene expression pattern of the B. emeiensis SUS gene family in different tissues and in response to treatment with abscisic acid (ABA), methyl jasmonate (MeJA), 2,6-dichlorobenzonitrile (2,6-DCB, a specific inhibitor of cellulose synthesis in plants). The roles of the B. emeiensis SUSs in cellulose biosynthesis metabolism of bamboo are also discussed. The results might provide a foundation for further studies to gain a systematic and comprehensive understanding of the SUS genes in regulating the bamboo plant growth.
Materials and methods
Plant materials
Bamboo (Bambusa emeiensis, 2 years old) shoots (30 cm and 100 cm), stems, leaves and roots used in this research were collected from the Botanical Resource Center of Southwest University of Science and Technology, Mianyang, Sichuan, China. After removing the sheaths, the shoots, stems and roots were cut into smaller pieces and frozen in liquid nitrogen immediately. The leaves, including unexpanded leaves and mature leaves, were also frozen in liquid nitrogen.
Isolation of RNA
The TRIzol® Reagent (TIANGEN BIOTECH, Beijing, China) was used to extract total RNA from various tissues. For reverse transcription polymerase chain reaction (RT-PCR), 1 μg of total RNA was reverse transcribed in a total volume of 20 μL, using PrimeScript® RT reagent Kit (Perfect DNA) (Takara, Dalian, China) according to the manufacturer's instructions. The quality of the RNA was analysed by 1% agarose gel electrophores and was checked spectrophotometrically.
Cloning of SUS genes in Bambusa emeiensis
Primers used for cloning and quantitative real-time PCR (qRT-PCR) amplification of the B. emeiensis sucrose synthase (BeSUS) cDNA (Supplementary Table S1) were designed based on the mRNA sequences of rice SUS genes. The full-length cDNAs of the BeSUS genes were obtained and cloned into the pMD19-T cloning vector (TaKaRa Biotechnology, Dalian, China) and were then transformed into Escherichia coli cells (DH5α) for massive sequencing (BGI Technology, Shenzhen, China).
Sequence alignment and phylogenetic analysis
The amino acid sequences were analysed by DNAMAN and MEGA version 4.0. Amino acid sequence alignment was carried out with DNAMAN and Clustal X. The characteristic sucrose synthase domain and a glycosyl transferase domain were identified by SMART (http://smart.embl-heidelberg.de/). The phylogenetic tree of SUS genes was constructed with MEGA 4.0 using the neighbour-joining method.
qRT-PCR
qRT-PCR assay was performed in a 20 μL reaction volume containing 9 μL of 2.5 × Real Master Mix/20 × SYBR solution (TaKaRa, Dalian, China), 8 μL of free water, 1 μL of each primer and 1.0 μL of cDNA, and run on a iQ5 Multicolor Real-Time PCR detection system. Relative quantitation for expression level of each BeSUS gene was standardized to the expression level of the bamboo constitutive Tubulin gene, calculated by the 2−ΔΔCT method, and the gene-specific primers are displayed in Table S1.
ABA, MeJA and 2,6-DCB treatment, determination of cellulose content and Fourier transform infrared (FTIR) analysis
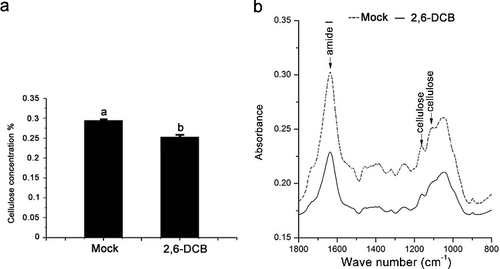
One-year-old B. emeiensis plants were grown in a greenhouse under natural light and irrigated manually every other day before ABA, MeJA and 2,6-DCB treatment. The aerial parts of the plants were sprayed with ABA (10 mmol/L) and MeJA (0.1 mmol/L, dissolved in 0.0025% ethanol), and the leaves were collected at 2, 6 and 24 h for qRT-PCR analyses. Plants treated with water and 0.0025% ethanol without MeJA were used as control samples, respectively. Additionally, the aerial parts of the plants were sprayed with 2,6-DCB (1 mmol/L, dissolved in dimethylsulphoxide (DMSO) and alcohol) or an equal amount of DMSO and alcohol (mock treatment). To perform cellulose content and FTIR analysis, leaves (unexpanded leaves and mature leaves from the same node) were collected at 6 days after 2,6-DCB treatment and then the tissues were mixed. A part of the sample was frozen using liquid nitrogen and stored at −80 °C for gene tissue expression analysis, and the other samples were used to determine the cellulose content and perform FTIR analysis. FibertecTM M6 1020/1021 (FOSS, Denmark) was used to determine the cellulose content, according to the instructions.
The FTIR analysis was performed according to Cao et al. [Citation25]. FTIR spectra were acquired in the range of 4000–400 cm−1 (Perkin Elmer, Spectrum One). An area of 100×100 μm was selected for FTIR analysis, and the acquisition parameters were 4 cm+ resolution. The experiment was performed three times with control and 2,6-DCB-treated plants.
Statistical analysis
The experiments were repeated three times. Data are mean values from three independent experiments, with standard error of the means. Statistical analysis was performed using SPSS 13.0. For analyses of cellulose content and gene expression, the LSD (Least significant difference) test was used at p = 0.05. The differences were considered statistically significant when p < 0.05 and highly significant when p < 0.01.
Results and discussion
Identification of seven SUS genes in B. emeiensis
To detect potential SUS homologs in B. emeiensis, using the mRNA sequences of rice SUS genes as queries, seven homologous SUS genes were isolated from B. emeiensis by RT-PCR. They were denoted as BeSUS1–7. Molecular analysis of the full-length deduced polypeptides indicated that the proteins of these bamboo SUS genes contain 612 – 849 deduced amino acids and have molecular weight of 69.29–96.69 kDa. Moreover, their predicted isoelectric points (pI) ranged from 5.62 to 7.78, which were approximately equivalent to those of the SUS subunits identified from other plant species ().
Table 1. Physical and chemical properties of the protein encoded by SUS genes from Bambusa emeiensis, Bambusa oldhamii and Oryza sativa.
Additionally, multiple sequence alignments showed that these BeSUSs shared high similarities in the amino-acid sequences (Supplementary Figure S1). BeSUS1, BeSUS3 and BeSUS5 exhibited a high degree of similarity with each other; especially BeSUS1 and BeSUS5 showed 94.15% similarity at the nucleotide level and 99.14% similarity at the amino-acid level. Similarly, BeSUS2 and BeSUS4 shared high sequence similarity (72.15% at the nucleotide level and 69.06% at the amino-acid level) (Supplementary Table S2). The other two paralogues, BeSUS6 and BeSUS7, had relatively lower levels of similarity when compared to BeSUS1 to 5, or compared with each other ().
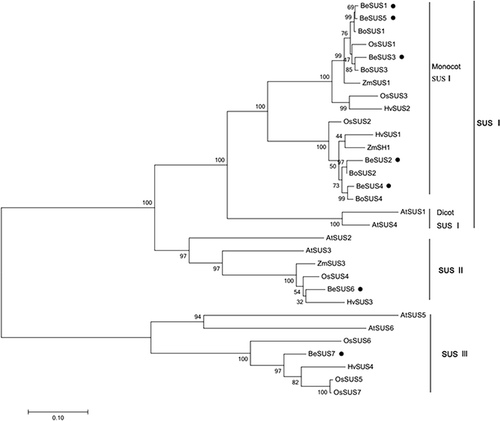
Phylogenetic analysis of BeSUS genes and other plant SUS homologs
To determine the evolutionary relationships among these BeSUSs, a phylogenetic analysis was performed. The phylogenetic tree was constructed by the neighbour-joining method implemented in MEGA-4. As shown in , these SUS genes could be classified into three distinct clades. Consistent with several previous studies, our results here designated these major clades as follows: SUS I, SUS II and SUS III, respectively [Citation18,Citation23]. The SUS I clade could be subdivided into two groups, one dicot SUS I group and one monocot SUS I group. The BeSUS1–5 genes fell into the monocot SUS I group, while BeSUS6 and 7 belonged to the SUS II and SUS III group, respectively. Additionally, there were no bamboo genes in the dicot SUS I group. Within the monocot SUS I clade, BeSUS1, BeSUS3 and BeSUS5 grouped more closely in the phylogenetic tree. Moreover, the three paralogues were also closely related to the SUS1 sequences of maize, rice and green bamboo. Meanwhile, BeSUS2 and BeSUS4 also shared high sequence similarities with other genes from maize (ZmSh1), rice (OsSUS2) and green bamboo (BoSUS2 and 4) (). Although these bamboo SUS paralogues shared high sequence similarities, the phylogenetic results showed that diversification has occurred within this family, likely indicating that the evolutionary histories of these BeSUS genes could be discrete and the members of this family had diverse roles in different metabolic processes.
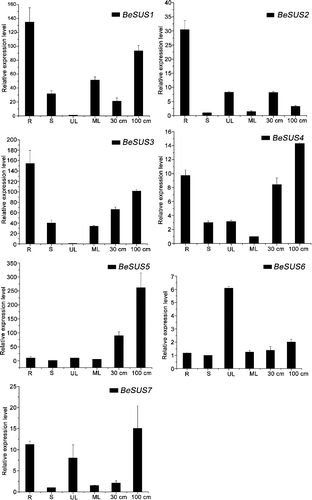
Expression analysis of the BeSUS genes in B. emeiensis tissues
In order to predict the potential physiological functions of each member of the BeSUS family, systematic analysis of the expression patterns of these BeSUS genes in different tissues was performed by qRT-PCR (). The results showed that the transcripts of these BeSUSs were presented at different expression levels in all tissues examined and showed distinct but partially overlapping expression patterns. In detail, BeSUS1, BeSUS3 and 4 were expressed in all the examined tissues, but the transcripts were much abundant in the root. In contrast, BeSUS2 and BeSUS5 showed distinct tissue-specific patterns, their transcripts were expressed predominantly in the root and developing shoot, respectively. BeSUS6 and BeSUS7 had lower expression levels than the other BeSUSs genes. The highest transcript level of BeSUS6 was detected in unexpanded leaves, while BeSUS7 was predominantly expressed in the root, shoot and unexpanded leaves (). In our results, most BeSUS transcripts appeared to be slightly expressed in mature leaves (source tissues), but highly expressed in the root and shoot (sink tissues). These results are in agreement with previous studies supporting the general understanding that SUS are expressed mainly in sink tissues [Citation24]. For example, Hirose et al. [Citation21] reported that all six SUS genes of rice were much more highly expressed in elongating flag leaves (sink tissues) than in fully expanded flag leaves (source tissues).
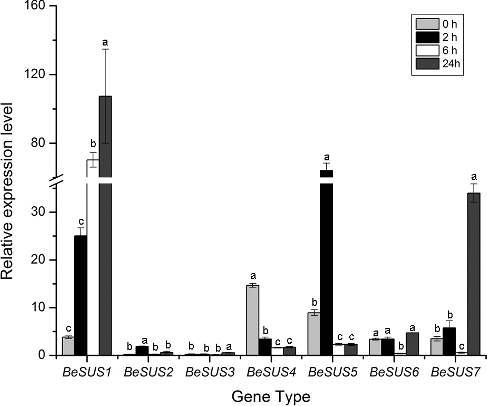
Expression of BeSUS genes in response to ABA, MeJA and 2,6-DCB treatment, and changes of cellulose content after 2,6-DCB treatment
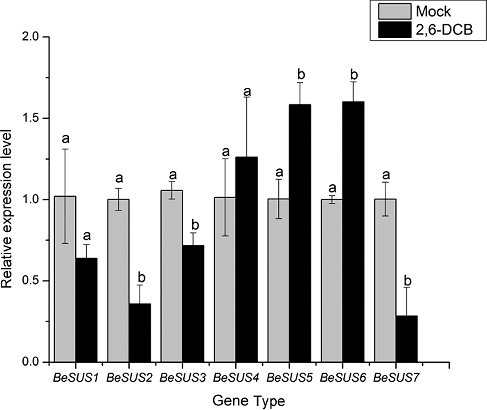
It has been well recognized that ABA is a stress hormone which is triggered by drought, cold and dehydration stress [Citation26]. Similarly, jasmonic acid (JA) or its methyl ester jasmonate (MeJA) plays a critical role in the response to wounding and environmental stresses. Exogenous MeJA could cause the accumulation of some defense enzymes in plants [Citation27]. Previous studies have revealed that SUS have functions in the responses to many environmental abiotic stresses, such as cold, drought, high temperature or changes in nitrogen fixation [Citation28] and so on. Moreover, some current researches have shown that SUS play roles in the regulation of plant morphology [Citation29] and sugar signalling pathway [Citation30]. However, the responses of these SUS genes to hormones, especially ABA and MeJA, to the best of our knowledge, have not been investigated in bamboo thus far. As shown in , after ABA treatment, BeSUS2 and 3 had low expression levels throughout the period, while the expression level of BeSUS6 had no significant difference during each period. Additionally, the BeSUS1 and 7 were conspicuously induced after 24 h, and the expression of BeSUS5 was upregulated within 2 h, and then its expression level decreased after ABA treatment. Similarly, MeJA treatment also induced these genes. BeSUS1 was induced within 2 h, while BeSUS5 and 7 were upregulated after 24 h ().
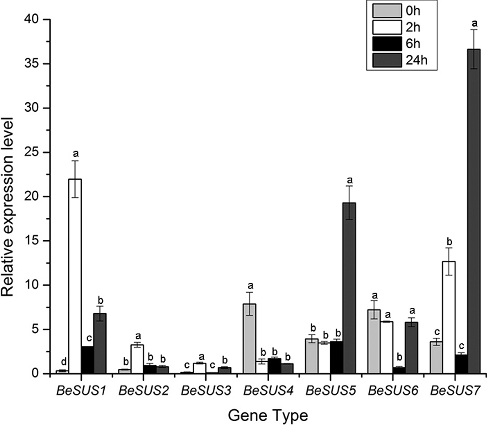
Previous reports have demonstrated that 2,6-DCB in an effective inhibitor of cellulose synthesis and could decrease cellulose accumulation in many plants [Citation31–34]. DeBolt et al. [Citation35] showed that 2,6-DCB rapidly inhibited the motility of YFP-CESA6 complexes and caused hyper-accumulation of CESA complexes. We treated one-year-old B. emeiensis plants by 2,6-DCB, collected the leaves and then determined the cellulose content. The results showed that the cellulose content was markedly reduced compared with that in the control plants ((a)). FTIR analysis showed that the FTIR spectrum of B. emeiensis plants in the presence of 2,6-DCB changed in absorbance intensity and locations of specific peaks ((b)). As SUS activity has been shown to be involved in cellulose synthesis and callose synthesis [Citation1], we investigated whether the transcript levels of BeSUSs genes were affected by 2,6-DCB treatment. As shown in , in the presence of 2,6-DCB, the transcript levels of BeSUS5 and 6 were significantly induced, whereas the levels of BeSUS2, 3 and 7 transcripts were decreased. This observation suggests that the roles of BeSUSs in response to 2,6-DCB stress or in bamboo cellulose biosynthesis might be divergent.
Conclusions
The present study is the first to explore the sequence characteristics and expression pattern of the SUS gene family in bamboo Bambusa emeiensis. The obtained results indicated that there are at least seven SUS genes in bamboo. To the best of our knowledge, this study is the first to investigate the possible roles of BeSUSs in response to hormones and 2,6-DCB. The results provide an underlying foundation for further research into the potential physiological roles of BeSUS genes involved in hormone stresses and cellulose biosynthesis during bamboo development.
Supplemental_Data.pdf
Download PDF (730.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amor Y, Haigler CH, Johnson S, et al. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357.
- Barratt DH, Barber L, Kruger NJ, et al. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. 2001;127:655–664.
- Baroja-Fernandez E, Jose MF, Li J, et al. Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proc Natl Acad Sci USA. 2012;109:321–326.
- Haigler CH, Ivanova-Datcheva M, Hogan PS, et al. Carbon partitioning to cellulose synthesis. Plant Mol Biol. 2001;47:29–51.
- Albrecht G, Mustroph A. Localization of sucrose synthase in wheat roots: increased in situ activity of sucrose synthase correlates with cell wall thickening by cellulose deposition under hypoxia. Planta. 2003;217:252–260.
- Persia D, Cai G, Del CC, et al. Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol. 2008;147:1603–1618.
- Coleman H, Yan J, Mansfield S. Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc Natl Acad Sci USA. 2009;106:13118–13123.
- Zhang M, Song X, Sun X, et al. The relationship between cellulose content and the contents of sugars and minerals during fiber development in colored cotton cultivars. Cellulose. 2012;19:2003–2014.
- Baier M, Keck M, Goedde V, et al. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant Physiol. 2010;152:1000–1014.
- Islam MZ, Hu X, Long FJ, et al. Genome-wide identification and expression profile analysis of citrus sucrose synthase genes: investigation of possible roles in the regulation of sugar accumulation. PloS One. 2014 [cited 2017 Jul 3];9(11):e113623. DOI:10.1371/journal.pone.0113623.
- Chiu WB, Lin CH, Chang CJ, et al. Molecular characterization and expression of four cDNAs encoding sucrose synthase from green bamboo Bambusa oldhamii. New Phytol. 2006;170:53–63.
- Su JC. Purification and characterization of sucrose synthetase from the shoot of bamboo Leleba oldhami. Plant Physiol. 1977;60:17–21.
- Rai V, Ghosh JS, Pal A, et al. Identification of genes involved in bamboo fiber development. Gene. 2011;478:19–27.
- Baud S, Vaultier MN, Rochat C. Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot. 2004;55:397–409.
- Duncan KA, Hardin SC, Huber SC. The three maize sucrose synthase isoforms differ in distribution, localization, and phosphorylation. Plant Cell Physiol. 2006;47:959–971.
- Alonso-Simón A, Neumetzler L, Garcia-Angulo R, et al. Plasticity of xyloglucan composition in bean (Phaseolus vulgaris)-cultured cells during habituation and dehabituation to lethal concentrations of dichlobenil. Mol Plant. 2010;3:603–609.
- Cho JI, Kim HB, Kim CY, et al. Identification and characterization of the duplicate rice sucrose synthase genes OsSUS5 and OsSUS7 which are associated with the plasma membrane. Mol Cells. 2011;31:553–556.
- Chen A, He S, Li F, et al. Analyses of the sucrose synthase gene family in cotton: structure, phylogeny and expression patterns. BMC Plant Biol. 2012 [cited 2017 Jul 3];12:85. DOI:10.1186/1471-2229-12-85
- Zhang CH, Yu ML, Ma RJ, et al. Structure, expression profile, and evolution of the sucrose synthase gene family in peach (Prunus persica). Acta Physiol Plant. 2015 [cited 2017 Jul 3];37:81. DOI:10.1007/s1173
- Horst I, Welham T, Kelly S, et al. TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol. 2007;144:806–820.
- Hirose T, Scofield GN, Terao T. An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci. 2008;174:534–543.
- Xiao X, Tang C, Fang Y, et al. Structure and expression profile of the sucrose synthase gene family in the rubber tree: indicative of roles in stress response and sucrose utilization in the laticifers. Febs J. 2014;281:291–305.
- Zhang D, Xu B, Yang X, et al. The sucrose synthase gene family in Populus: structure, expression, and evolution. Tree Genet Genomes. 2011;7:443–456.
- Wang Z, Wei P, Wu M, et al. Analysis of the sucrose synthase gene family in tobacco: structure, phylogeny, and expression patterns. Planta. 2015;242:153–166.
- Cao Y, Tang X, Giovannoni J, et al. Functional characterization of a tomato COBRA-like gene functioning in fruit development and ripening. BMC Plant Biol. 2012 [cited 2017 Jul 3];12:211. DOI:10.1186/1471-2229-12-211.
- Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(suppl.):S15–S45.
- Koo AJ, Gao X, Jones AD, et al. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009;59:974–986.
- Zhu XD, Wang MQ, Li XP, et al. Genome-wide analysis of the sucrose synthase gene family in grape (Vitis vinifera): structure, evolution, and expression profiles. Genes. 2017 [cited 2017 Jul 3];8(4):111. DOI:10.3390/genes8040111
- Shlomo G, Nitsan L, Ofer S, et al. Suppression of sucrose synthase affects auxin signaling and leaf morphology in tomato. PloS One. 2017 [cited 2017 Jul 3];12(8): e0182334. DOI:10.1371/journal.pone.0182334
- Quynh AN, Sheng L, Seung GW, et al. Pronounced phenotypic changes in transgenic tobacco plants overexpressing sucrose synthase may reveal a novel sugar signaling pathway. Front Plant Sci. 2016 [cited 2017 Jul 3];6:1216. DOI:10.3389/fpls.2015.01216
- Delmer DP, Read SM, Cooper G. Identification of a receptor protein in cotton fibers for the herbicide 2,6-dichlorobenzonitrile. Plant Physiol. 1987;84:415–420.
- Shedletzky E, Shmuel M, Trainin T, et al. Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2, 6-dichlorobenzonitrile: a comparison between two dicotyledonous plants and a graminaceous monocot. Plant Physiol. 1992;100:120–130.
- Himmelspach R, Williamson RE, Wasteneys GO. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J. 2003;36:565–575.
- Peng L, Zhang L, Cheng X, et al. Disruption of cellulose synthesis by 2, 6-dichlorobenzonitrile affects the structure of the cytoskeleton and cell wall construction in Arabidopsis. Plant Biol. 2013;15:405–414.
- DeBolt S, Gutierrez R, Ehrhardt DW, et al. Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2,6-dichlorobenzonitrile treatment. Plant Physiol. 2007;145:334–338.