ABSTRACT
Sucrose non-fermenting-1-related protein kinase 1 (SnRK1) plays an important role in plant carbohydrate metabolism and starch biosynthesis. The regulatory role of GmSnRK1 from soybean in regulating carbohydrate metabolism and starch accumulation has not been reported. In the present study, a gene encoding the SnRK1 protein, named GmSnRK1, was successfully isolated from soybean, and the functions of this gene were studied. Subcellular localisation analysis in onion epidermal cells indicated that GmSnRK1 protein was localised to the nucleus. The GmSnRK1 gene was introduced into Arabidopsis to obtain transgenic plants. Its overexpression significantly increased the starch content, as well as the sucrose, glucose and fructose content in the transgenic plants compared to the wild-type (WT). Real-time quantitative polymerase chain reaction (PCR) analysis showed that overexpression of GmSnRK1 up-regulated the genes involved in starch biosynthesis, including sucrose synthase (AtSUS), phosphoglucomutase (AtPGM), ADP-glucose pyrophosphorylase (AtAGPase), granule-bound starch synthases (AtGBSS I and AtGBSS II), soluble starch synthases (AtSSS I, AtSSS II, AtSSS III and AtSSS IV) and starch branching enzymes (AtSBE I and AtSBE II) genes. In contrast, the expression of sucrose phosphate synthase (SPS) gene was decreased in the transgenic plants. Meanwhile, the enzyme activity levels of the five starch biosynthetic enzymes (SUS, AGPase, GBSS, SSS and SBE) exhibited higher activities, while the SPS activity was decreased in the transgenic plants compared to WT. These results suggest that the manipulation of GmSnRK1 expression might be used for improving the starch content in engineered plants for biofuel production.
Introduction
Biofuel, which can decrease environmental damage by reducing the extraction and use of fossil fuels, is more and more important with the development of society. Breeding of non-food energy crops for economically viable production as environmentally friendly biofuels is on the way [Citation1,Citation2]. Since the conversion of starch into fermentable sugars is relatively easy, starch has been taken as a major feedstock for first-generation biofuel production [Citation1,Citation3]. Therefore, elucidating how carbohydrates are metabolised in plants, could greatly facilitate the development of crops by means of enhancing starch synthesis, and the improvement of biofuel production efficiency [Citation1].
There are several reports on increasing starch content by genetic engineering in plants. Constitutive expression of the Arabidopsis AtAATP1 increased the starch content in potato tubers [Citation4]. Heterologous expression of MdSnRK1 from apple increased the starch content in transgenic tomato plants [Citation5]. The IbSnRK1/StSnRK1-expressing tobacco plants exhibited increased starch accumulation [Citation6,Citation7]. Overexpression of NtTrxF led to a striking increase of starch accumulation in the leaves of tobacco [Citation1]. The StTrxF/SlTrxF-expressing Arabidopsis plants had higher starch content [Citation8,Citation9]. Wang et al. [Citation10–12] cloned the SlAATP/StAATP/GmAATP gene from tomato/potato/soybean and found that the transgenic Arabidopsis plants exhibited higher starch content. Wang et al. [Citation13] reported that overexpression of IbAATP increased starch content in transgenic sweetpotato.
SnRKs are sucrose non-fermenting-1 (SNF1)-related protein kinases in higher plants [Citation14], which are divided into three subfamilies: SnRK1, SnRK2 and SnRK3 [Citation15]. SnRK1 plays an important role in plant carbohydrate metabolism and starch biosynthesis [Citation5,Citation16,Citation17]. It is required for redox modulation of ADP-glucose pyrophosphorylase (AGPase) activity in response to sucrose [Citation18] and is involved in regulating the expression of genes encoding sucrose synthase (SUS) and AGPase, which are two key enzymes involved in the biosynthetic pathway from sucrose to starch [Citation19,Citation20].
The genes encoding SnRK1 have been cloned from several plant species, such as rye [Citation21], Arabidopsis [Citation22], maize [Citation23], apple [Citation24], sweetpotato [Citation6] and potato [Citation7]. There are a few reports about the function of SnRK1 in regulating carbohydrate metabolism and starch biosynthesis of plants. Antisense inhibition of SnRK1 in developing pollen grains led to an almost complete loss of starch accumulation and viability [Citation25]. In rice, evidence has shown that SnRK1 played an important role in starch accumulation [Citation26]. The expression of SUS and AGPase was up-regulated and their enzyme activities were also increased in the SnRK1-overexpressing potato plants, in which the starch levels in the tubers were increased by up to 30% [Citation20]. Jain et al. [Citation27] found that the expression of a SnRK1 gene coincided with the onset of starch accumulation in sorghum endosperm and maize endosperm. Wang et al. [Citation5,Citation7] and Jiang et al. [Citation6] further demonstrated that overexpression of SnRK1 from apple/sweetpotato/potato increased the soluble sugar and starch content by up-regulating the expression of SUS and AGPase and increasing the activities of SUS and AGPase in transgenic tomato/tobacco plants. It is proposed that SnRK1 channels carbon through the storage pathway to starch [Citation17,Citation20]. Furthermore, it is known that SnRK1 can regulate the carbohydrate metabolism by inactivating sucrose phosphate synthase (SPS) via phosphorylation [Citation17,Citation28]. The activity of SPS was decreased in the transgenic tomato/tobacco plants overexpressing SnRK1 from apple/ sweetpotato/potato [Citation5–7].
Although the function of SnRK1 from other plant species has been well studied, the regulatory role of GmSnRK1 (Genbank accession No. NM_001251194) from soybean in regulating carbohydrate metabolism and starch accumulation has not been reported. In this study, we isolated GmSnRK1 from soybean and estimated its roles in transgenic Arabidopsis. We also developed GmSnRK1-overexpressing Arabidopsis plants and found that overexpression of GmSnRK1 significantly increased sucrose, glucose, fructose and starch content in the leaves of transgenic plants, indicating a great potential of GmSnRK1 in increasing soluble sugar and starch levels of plants in the future.
Materials and methods
Plant materials
Soybean cultivar Williams 82 was employed for GmSnRK1 gene cloning in this study. Arabidopsis [ecotype Columbia-0, wild type (WT)] was used as a model plant to investigate the functions of this gene.
Cloning of the soybean GmSnRK1 gene
Total RNA was extracted from the leaves of Williams 82 with the RNAprep Pure Kit (Tiangen Biotech, Beijing, China). RNA samples were reverse-transcribed according to the instructions of Quantscript Reverse Transcriptase Kit (Tiangen Biotech, Beijing, China). Based on the sequence of GmSnRK1 (Genbank accession No. NM_001251194), we designed gene-specific primers (GC-F/R) for reverse transcription polymerase chain reaction (RT-PCR) (Supplemental Table S1) to obtain its full-length cDNA sequence. PCR was performed with an initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min and a final extension at 72 °C for 10 min. PCR products were separated 1n a 1.0% (w/v) agarose gel. The target DNA band was recovered by gel extraction, then cloned into PMD19-T (TaKaRa, Beijing, China), and finally transformed into competent cells of Escherichia coli strain DH5α. White colonies were checked by PCR and the positive colonies were used for sequencing (Invitrogen, Beijing, China).
Sequence analysis of the GmSnRK1 gene
The open reading frame (ORF) of the cloned GmSnRK1 gene was predicted with ORF Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/). The homology of GmSnRK1 protein was identified using protein BLAST in the National Center for Biotechnology Information (NCBI) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The conserved domain of GmSnRK1 protein was scanned by the InterProScan program (http://www.ebi.ac.uk/Tools/ pfa/iprscan/). The theoretical molecular weight and isoelectronic point (pI) were calculated using ProtParam tool (http://web.expasy.org/protparam/). The nuclear localisation signal (NLS) of the GmSnRK1 protein was predicted by the cNLS Mapper program (http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi). For multiple sequence alignment analysis, the amino acid sequences of GmSnRK1 and other SnRK1 homologues from different plant species retrieved from NCBI were aligned using the DNAMAN software (Lynnon Biosoft, Quebec, Canada). Phylogenetic analysis was conducted with the MEGA4 software (http://www.megasoftware.net/).
Subcellular localisation of GmSnRK1
The subcellular localisation of GmSnRK1 in onion (Allium cepa) epidermal cells was analysed as described by Wang et al. [Citation29]. The ORF of GmSnRK1 was cloned and then inserted into the pMDC83 expressing vector containing the green fluorescent protein (GFP) gene at Spe I and Asc I restriction sites under the control of the CaMV35S promoter and NOS (nopaline synthase) terminator (Table S1). Both the fusion construct (35S-GmSnRK1::GFP) and the control vector (35S::GFP) were transformed into living onion epidermal cells by particle bombardment with a GeneGun (Biorad HeliosTM), according to the instruction manual (helium pressure 260 psi). After incubation on MS (Murashige and Skoog) [Citation30] medium (pH 5.8) solidified with 3% agar at 28 °C for 24 h, the onion cells were observed with bright field and fluorescence using confocal microscopy (Nikon Inc., Melville, NY).
Generation of transgenic Arabidopsis plants
The coding region of GmSnRK1 was amplified using a pair of specific primers with terminal BamH I and Sac I restriction sites, and then inserted into the same enzyme sites in pCAMBIA1301 to create the plant expression vector pCAMBIA1301-GmSnRK1, under the control of the cauliflower mosaic virus (CaMV) 35S promoter and the nopaline synthase (NOS) terminator. This vector also contained β-glucuronidase (gusA) and hygromycin resistance (hpt II) genes driven by the CaMV 35S promoter. Both pCAMBIA1301-GmSnRK1 and the control vector (VC) pCAMBIA1301 were transformed into the Agrobacterium tumefaciens strain LBA4404 cells by the electroporation method for Arabidopsis transformation [Citation31]. Transgenic plants were produced according to methods described previously [Citation32]. Transformants were selected based on their resistance to hygromycin (Hyg). Putative transformant seeds were germinated on agar-solidified MS medium containing 25 mg/L Hyg. Positive transgenic seedlings were grown in pots containing a mixture of soil, vermiculite and humus (1:1:1, v/v/v) for T2 and T3 seed selection. The incubation and growth conditions of Arabidopsis were the same as described previously [Citation32].
Molecular confirmation of transgenic plants
The presence of GmSnRK1 in hygromycin-resistant plants was assessed by PCR analysis using specific primers (Table S1) to amplify fragments of the hpt II coding sequence according to the method of Wang et al. [Citation29]. DNA was first extracted from Arabidopsis leaves, according to the instructions of EasyPure Plant Genomic DNA Kit (Transgen, Beijing, China). PCR amplifications were performed with an initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and final extension at 72 °C for 10 min. The PCR products were separated by electrophoresis in a 1.0% (w/v) agarose gel.
Analyses of sucrose, glucose, fructose and starch content
The content of sucrose, glucose and fructose were measured using the anthrone method, according to the method of Zhang et al. [Citation33]. Starch extraction and quantification were performed as described previously [Citation34]. Seeds were grown on MS medium for 2 weeks and transferred to pots containing a mixture of soil, vermiculite and humus (1:1:1, v/v/v). Plants were grown in growth chamber for 4 weeks at 22 °C under standard long day conditions (14 h light and 10 h dark). Leaves of four-week-old plants were harvested to determine sucrose, glucose, fructose and starch content in light at 10–11 am All treatments were performed in triplicate.
Southern blot analysis
Genomic DNA was extracted from the leaves of transgenic, VC and WT plants by the cetyltrimethylammonium bromide (CTAB) method [Citation35]. Approximately 20 μg genomic DNA of each sample was digested by EcoR I. The restriction fragments were size-fractionated by 1.0% (w/v) agarose gel electrophoresis and transferred to a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech, UK). A 532 bp GmSnRK1 fragment coding sequence generated with specific primers (Table S1) was used as the probe. The labelling of probe, prehybridisation, hybridisation and detection were performed by the protocol of DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics GmbH, Germany).
Expression analysis of the related genes
The expression of GmSnRK1 and starch biosynthesis related genes was analysed by real-time quantitative PCR (qRT-PCR). Transgenic, VC and WT plants were grown in pots for 4 weeks under normal condition. Total RNA was extracted from the leaves of these plants, respectively, using the RNAprep Pure Plant Kit (Tiangen Biotech, Beijing, China). RNA samples were reverse-transcribed using Quantscript Reverse Transcriptase Kit (Tiangen Biotech, Beijing, China). The cDNA solution was used as templates for PCR amplification with gene-specific primers (Table S1). The Arabidopsis Atactin gene (Genbank accession No. NM112764) was used as an internal control [Citation36] (Table S1).
PCR amplifications were conducted by ABI PRISM 7500 (Software for 7500 and 7500 Fast Real-Time PCR Systems, V2.0.1, USA) using SYBR Green PCR Master Mix (Tiangen Biotech, Beijing, China). The amplifications were performed with an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Quantification of gene expression was done with the comparative CT method [Citation37]. All experiments were repeated three times and each data point represents the average of three experiments.
SUS, SPS, AGPase, GBSS, SSS and SBE activity analysis
The activity of SUS, SPS, AGPase, GBSS, SSS and SBE in the leaves of four-week-old transgenic, VC and WT plants was performed according to the methods described by Wang et al. [Citation5] and Nakamura et al. [Citation38].
Statistical analysis
All experiments were repeated three times and the data are presented as mean values with standard error (±SEM). Where applicable, data were analysed by Student's t-test in a two-tailed analysis. Values of P < 0.05 or P < 0.01 were considered to indicate statistically significant differences.
Results and discussion
Cloning and sequence analysis of GmSnRK1
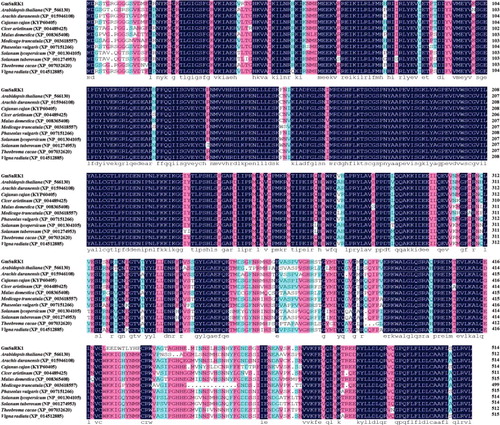
The GmSnRK1 gene, containing a 1545 bp ORF and encoding a polypeptide of 514 amino acids, was cloned by RT-PCR. The molecular weight of the protein was 58.95 kDa and the theoretical isoelectric point (pI) was 6.88. Sequence analysis via the InterProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan/) showed that the GmSnRK1 protein contained the Serine/Threonine-protein kinase catalytic domain (S-TKc) and the Serine/Threonine-protein kinase active site at amino acid residues 139–151, which are the features of plant SnRK1 proteins. A putative nuclear localisation signal sequence was also identified at amino acid residues 223–235.
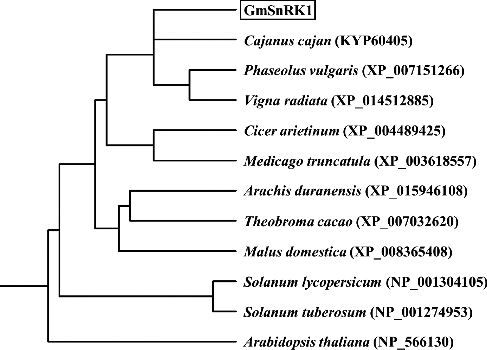
A BLAST search showed that the amino-acid sequence of GmSnRK1 showed a high amino-acid identity with predicted protein products of Cajanus cajan (KYP60405, 93.00%), Vigna radiata (XP_014512885, 92.43%), Phaseolus vulgaris (XP_007151266, 92.23%), Arachis duranensis (XP_015946108, 90.10%), Cicer arietinum (XP_004489425, 88.74%), Nicotiana tomentosiformis (XP_009621306, 86.58%), Malus domestica (XP_008365408, 85.47%), Theobroma cacao (XP_007032620, 85.47%), Solanum lycopersicum (NP_001304105, 82.95%), Arabidopsis thaliana (NP_566130, 82.33%) and Solanum tuberosum (NP_001274953, 81.78%) (). Phylogenetic analysis revealed that GmSnRK1 had a close relationship with the predicted protein product of Cajanus cajan (KYP60405) ().
Nuclear localisation of GmSnRK1
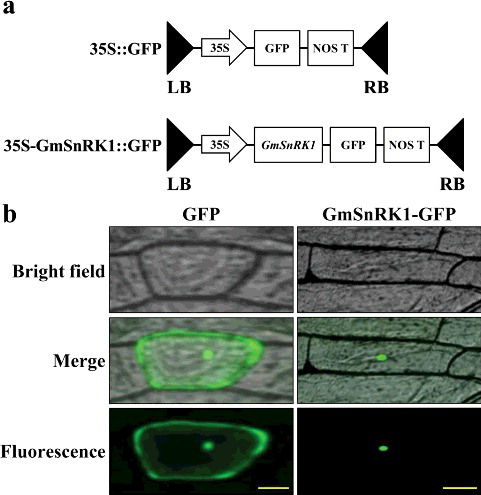
To investigate the subcellular localisation of GmSnRK1, an Agrobacterium-mediated transformation system was used for a transient assay. Two constructs (35S::GFP and 35S-GmSnRK1::GFP) (a) were individually introduced into onion epidermal cells. As shown in b, the GFP fluorescence of GmSnRK1-GFP was exclusively located in the nuclei of the cells, whereas the GFP control was distributed throughout the whole onion cells. These results indicate that GmSnRK1 is a nuclear-localised protein. This localisation may aid in the determination of both the function and the molecular mechanism underlying the function of this protein. Vincent et al. [Citation39] found that SnRK1 was localised to the nucleus and identified a novel signaling pathway that controlled this nuclear localisation in response to glucose phosphorylation in yeast.
Figure 3. Subcellular localisation of the GmSnRK1 protein in onion epidermal cells. (a) Schematic diagram of the 35S::GFP vector construct and 35S-GmSnRK1::GFP vector construct. (b) Transformed cells of the 35S::GFP and 35S-GmSnRK1::GFP constructs cultured in MS medium at 28 °C for 24 h and observed under a microscope.
Increased sucrose, glucose, fructose and starch content in Arabidopsis overexpressing GmSnRK1
The SnRK1 protein belongs to a subfamily of Serine/Threonine kinases [Citation40] and its name is derived from sucrose nonfermenting-1 (SNF1), its homologue in yeast [Citation20,Citation41]. SnRK1 is reportedly involved in plant carbohydrate metabolism and starch biosynthesis in plants. The study of Kanegae et al. [Citation26] provided evidence that SnRK1 had a role in starch accumulation in rice. Antisense inhibition of SnRK1 in developing pollen grains resulted in an almost complete loss of starch accumulation and viability [Citation25]. Overexpression of SnRK1 from apple/sweetpotato/potato increased the soluble sugar and starch content in transgenic tomato/tobacco plants [Citation5–7].
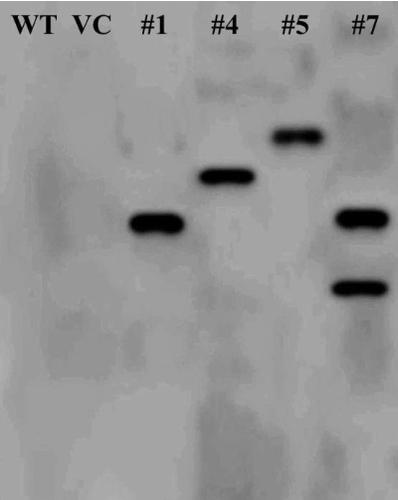
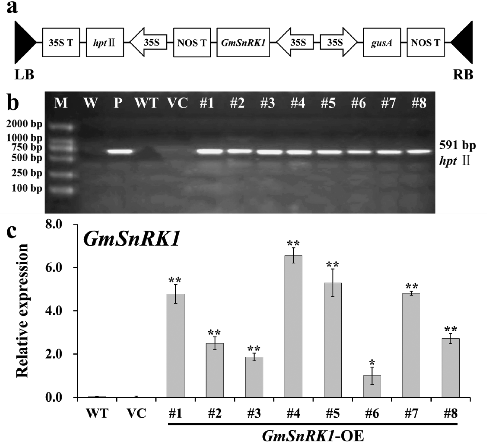
In this work, the ORF of GmSnRK1 was ectopically expressed in Arabidopsis (Col-0, WT) using the binary vector pCAMBIA1301-GmSnRK1 (a). Eight independent transgenic lines overexpressing GmSnRK1 (T1 generation) were obtained by Hyg-resistance selection, named #1–#8, respectively, and their progenies (T3 generation) were generated. The PCR analyses of their genomic DNA confirmed the successful integration of the transgene (b). qRT-PCR analysis showed that the highest expression levels of GmSnRK1 were observed in transgenic lines #1, #4, #5 and #7, while no transgene expression was observed in VC and WT (c). Therefore, transgenic lines #1, #4, #5 and #7 were selected for further analysis.
Figure 4. Molecular confirmation of transgenic plants. (a) Schematic diagram of the T-DNA region of binary plasmid pCAMBIA1301-GmSnRK1. LB, left border; RB, right border; hpt II, hygromycin phosphotransferase II gene; GmSnRK1, soybean sucrose non-fermenting-1 related protein kinase 1 gene; gusA, β-glucuronidase gene; 35S, cauliflower mosaic virus (CaMV) 35S promoter; 35S T, CaMV 35S terminator; NOS T, nopaline synthase terminator. (b) PCR analysis of GmSnRK1 overexpressing Arabidopsis plants. Lane M: DL2000 DNA marker; Lane W: water as negative control; Lane P: plasmid pCAMBIA1301-GmSnRK1 as positive control; Lane WT: wild type; VC, control vector; Lanes #1–#8: different transgenic lines. (c) Expression levels of GmSnRK1 in different transgenic lines. The Arabidopsis actin gene was used as an internal control. Data are presented as means ± SE (n = 3). * P < 0.05 and ** P < 0.01, significant differences compared to WT (Student's t-test).
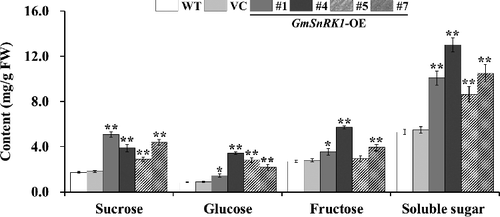
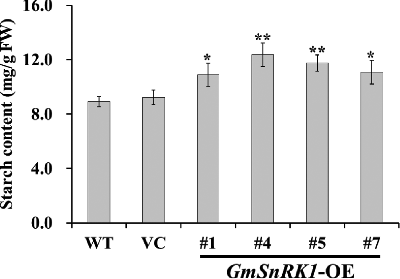
Two-week-old WT, VC and transgenic plants (lines #1, #4, #5 and #7) were grown in pots under normal condition for 4 weeks. The sucrose, glucose, fructose and starch content in the leaves of these plants were quantified. The results showed that the plants overexpressing GmSnRK1 had significantly higher sucrose, glucose, fructose and soluble sugar and starch content, which were increased by 65%–192%, 68%–297%, 9%–112% and 63%–145%, respectively, compared to WT ( and ), whereas no significant difference was observed between VC and WT plants ( and ).
Southern blot analysis of Arabidopsis overexpressing GmSnRK1
The transgene integration patterns of the four transgenic plants (lines #1, #4, #5 and #7) with higher starch content were analysed by Southern blot. The genomic DNA of the transgenic plants, VC and WT was digested with EcoRⅠ, which has a unique cleavage site in the T-DNA region in the vector, and was hybridised with the GmSnRK1 gene probe. The transgenic plants displayed different patterns and the copy number of integrated GmSnRK1 gene varied from 1 to 2 (). No hybridising band was observed in WT and VC as expected (). A clear relationship between the starch accumulation and the copy number was also not found, similar to the results reported by Wang et al. [Citation10], in which the copy number of the integrated SlAATP gene ranged from 1 to 2 in transgenic plants exhibiting higher starch content.
Gene expression and enzyme activity assay in Arabidopsis overexpressing GmSnRK1
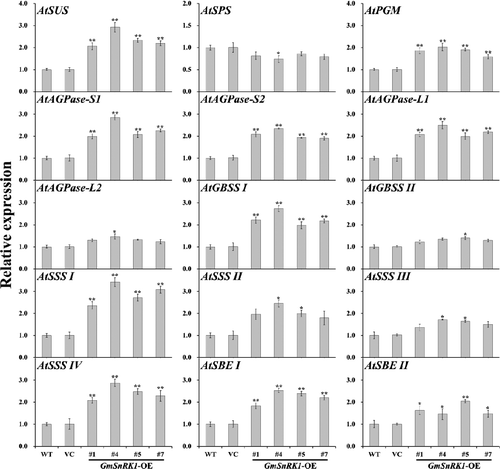
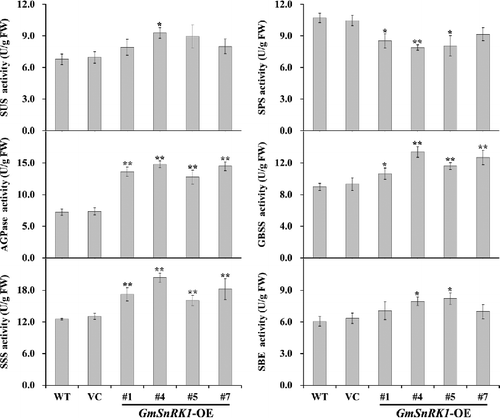
To dissect how the expression of GmSnRK1 increased the starch content in the transgenic plants, the transcript levels of 15 starch biosynthetic genes in WT, VC and transgenic plants (lines #1, #4, #5 and #7) were examined by qRT-PCR (). Meanwhile, the activities of SUS, SPS, AGPase, GBSS, SSS and SBE involved in starch biosynthesis were also investigated in the leaves of WT, VC and transgenic plants (lines #1, #4, #5 and #7) ().
Figure 9. SUS, SPS, AGPase, GBSS, SSS and SBE enzyme activity in the leaves of WT and transgenic plants.
SUS, a key enzyme involved in the biosynthetic pathway from sucrose to starch, catalyses the reversible conversion of sucrose and UDP to UDP-glucose (UDPG) and fructose [Citation6,Citation42,Citation43]. The activity of SUS was closely correlated with sink strength and starch accumulation [Citation6,Citation44]. SPS catalyses the conversion of UDPG and fructose into sucrose [Citation17,Citation28]. In plants, plastidial phosphoglucomutase (PGM), which catalyses glucose-6-phosphate (G-6-P) to glucose-1-phosphate (G-1-P), provides the substrate G-1-P for the committed pathway of starch synthesis [Citation45]. AGPase is a major regulatory enzyme of starch biosynthesis which converts G-1-P to ADP-glucose (ADPG) [Citation18,Citation46]. It is reported that AGPase is subjected to post-translational redox regulation, which provides a mechanism to adjust the rate of starch synthesis in potato tubers to sucrose supply [Citation18].
Purcell et al. [Citation19] provided clear evidence that SnRK1 is involved in the control of sucrose synthase gene in potato, and for the first time confirmed that SnRK1 was involved in the regulation of several aspects of carbon metabolism in plants through the transcriptional regulation of SUS. SnRK1 can regulate carbohydrate metabolism by phosphorylating and inactivating SPS [Citation17,Citation28]. McKibbin et al. [Citation20] reported that the expression of SUS and AGPase was up-regulated and their enzyme activities were also increased in the SnRK1-overexpressing potato plants, in which starch levels in the tubers were increased by up to 30%. Wang et al. [Citation5,Citation7] and Jiang et al. [Citation6] demonstrated that overexpression of SnRK1 from apple/sweetpotato/potato up-regulated the expression of SUS and AGPase, and increased the activities of SUS and AGPase and decreased the activity of SPS, leading to increased soluble sugar and starch content in transgenic tomato/tobacco plants. In our work, the expression of AtSUS, AtPGM, AGPase small subunit (AtAGPase-S1 and AtAGPase-S2) and AGPase large subunit (AtAGPase-L1 and AtAGPase-L2) was up-regulated, while the expression of AtSPS was slightly inhibited in transgenic plants (). Consistently, the enzymatic activity of SUS and AGPase were also significantly increased, and the SPS activity was also decreased in the GmSnRK1-overexpressing Arabidopsis plants ().
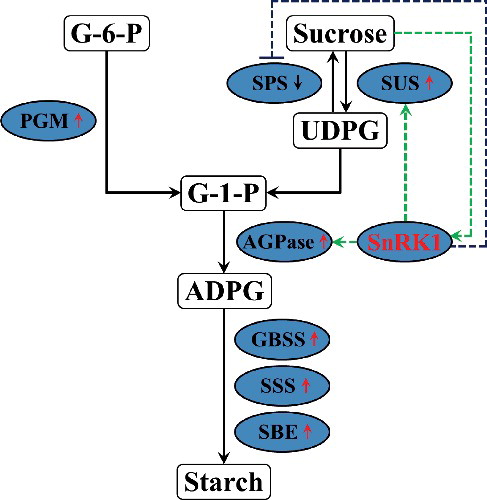
The higher level of starch content is related to the increased expression of starch biosynthesis genes [Citation6,Citation7,Citation47]. Starch synthase can be grouped into five types, granule-bound starch synthase (GBSS) and four types of soluble starch synthases (SSS): SSS I, SSS II, SSS III and SSS IV [Citation47–49]. Up-regulation of these genes could increase starch accumulation in plants [Citation6,Citation7,Citation49–52]. In our study, systematic up-regulation of these genes, granule-bound starch synthase (AtGBSS I and AtGBSS II), soluble starch synthases (AtSSS I, AtSSS II, AtSSS III and AtSSS IV) and starch branching enzyme (AtSBE I and AtSBE II) involved in the starch biosynthesis pathway, was observed in transgenic plants (). Consistent with this phenomenon, the activities of the major enzymes (GBSS, SSS and SBE) were also increased in transgenic plants (). Thus, it is thought that overexpression of GmSnRK1 up-regulates the expression of AtSUS and AtAGPase and increases the activities of SUS and AGPase, which further increase the expression of the genes and the activity of the major enzymes involved in starch biosynthesis, leading to increased starch accumulation in transgenic plants ().
In addition, the availability of sufficient substrate (sucrose) is a very important factor in the starch biosynthesis pathway. Genes encoding SUS and AGPase in potato tubers are inducible by sucrose [Citation6,Citation53,Citation54]. The present results indicated that sucrose levels and SUS and AGPase activities were significantly increased in the transgenic tobacco plants ( and ). Our work found that the high level of sucrose can induce the expression of SUS and AGPase, which further modulated the starch metabolism in transgenic plants ().
All of the results suggest that SnRK1 channels carbon through the storage pathway to starch by regulating the activities of SUS, SPS and AGPase. Our works support the hypothesis proposed by Halford and Hey [Citation17] and Jiang et al. [Citation6] that SnRK1 is activated by sucrose, and then SnRK1 increases the flux through the starch biosynthesis pathway by up-regulating SUS and AGPase and down-regulating SPS (). These results suggest that the potential application of GmSnRK1 expression might serve to improve the starch content in engineered plants for biofuel production.
Conclusions
Taken together, the GmSnRK1 gene was successfully isolated from soybean. Overexpression of GmSnRK1 was found to significantly increase the soluble sugar and starch content in transgenic Arabidopsis plants. Our results suggest that GmSnRK1 plays a crucial role in starch metabolism, and has great potential in the engineering of alternative energy crop plants with improved starch accumulation.
Supplemental_Data.pdf
Download PDF (484.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sanz-Barrio R, Corral-Martinez P, Ancin M, et al. Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol J. 2013;11:618–627.
- Wang FB, Guo XT, Qiao XQ, et al. The maize plastidic thioredoxin F-type gene ZmTrxF increases starch accumulation in transgenic Arabidopsis. Sci Hortic. 2016;210:205–212.
- Smith AM. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J. 2008;54:546–558.
- Tjaden J, Möhlmann T, Kampfenkel K, et al. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J. 1998;16:531–540.
- Wang X, Peng F, Li M, et al. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J Plant Physiol. 2012;169:1173–1182.
- Jiang T, Zhai H, Wang FB, et al. Cloning and characterization of a carbohydrate metabolism-associated gene IbSnRK1 from sweetpotato. Sci Hortic. 2013;158:22–32.
- Wang FB, Ye YX, Chen XH, et al. A sucrose non-fermenting-1-related protein kinase 1 gene from potato, StSnRK1, regulates carbohydrate metabolism in transgenic tobacco. Physiol Mol Biol Plants. 2017;23:933–943.
- Wang FB, Kong WL, Niu Y, et al. StTrxF, a potato plastidic thioredoxin F-type protein gene, is involved in starch accumulation in transgenic Arabidopsis thaliana. Biotechnol Biotecnol Equip. 2017;31(3):486–492.
- Wang FB, Kong WL, Fu YR, et al. Constitutive expression of SlTrxF increases starch content in transgenic Arabidopsis. Biologia Plantarum. 2017;61(3):494–500.
- Wang FB, Ye YX, Niu Y, et al. A tomato plastidic ATP/ADP transporter gene SlAATP increases starch content in transgenic Arabidopsis. Physiol Mol Biol Plants. 2016;22:497–506.
- Wang FB, Fu LF, Kong WL, et al. Constitutive expression of StAATP, a potato plastidic ATP/ADP transporter gene, increases starch content in transgenic Arabidopsis. Biotechnol Biotecnol Equip. 2017;31(2):250–258.
- Wang FB, Chen XH, Zhang F, et al. A soybean plastidic ATP/ADP transporter gene GmAATP increases starch content in transgenic Arabidopsis. Plant Biotechnol Rep. 2017;11:135–146.
- Wang YN, Li Y, Zhang H, et al. A plastidic ATP/ADP transporter gene, IbAATP, increases starch and amylose content and alters starch structure in transgenic sweetpotato. J Integr Agr. 2016;15:1968–1982.
- Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol.1998;37:735–748.
- Hrabak EM, Chan CWM, Gribskov M, et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003;132:666–680.
- Polge C, Thomas M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci. 2007;12:20–28.
- Halford NG, Hey SJ. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J. 2009;419:247–259.
- Tiessen A, Prescha K, Branscheid A, et al. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox acti-vation of ADP-glucose pyrophosphorylase in potato tubers. Plant J. 2003;35:490–500.
- Purcell PC, Smith AM, Halford NG. Antisense expression of a sucrose non-fermenting-1-related protein kinase sequence in potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J. 1998;14:195–202.
- McKibbin RS, Muttucumaru N, Paul MJ, et al. Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnol J. 2006;4:409–418.
- Alderson A, Sabelli P, Dickinson J, et al. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. PNAS. 1991;88:8602–8605.
- Kleinow T, Bhalerao R, Breuer F, et al. Functional identification of an Arabidopsis Snf4 ortholog by screening for heterologous multicopy suppressors of snf4 deficiency in yeast. Plant J. 2000;23:115–122.
- Lumbreras V, Albà MM, Kleinow T, et al. Domain fusion between SNF1-related kinase subunits during plant evolution. EMBO Rep. 2001;2:55–60.
- Li GJ, Peng FT, Zhang L, et al. Cloning and characterization of a SnRK1-encoding gene from Malus hupehensis Rehd. and heterologous expression in tomato. Mol Biol Rep. 2010;37:947–954.
- Zhang Y, Shewry PR, Jones H, et al. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J. 2001;28:431–442.
- Kanegae H, Miyoshi K, Hirose T, et al. Expressions of rice sucrose non-fermenting-1 related protein kinase 1 genes are differently regulated during the caryopsis development. Plant Physiol Bioch. 2005;43:669–679.
- Jain M, Li QB, Chourey PS. Cloning and expression analyses of sucrose nonfermenting-1-related kinase 1 (SnRK1b) gene during development of sorghum and maize endosperm and its implicated role in sugar-to-starch metabolic transition. Physiol Plantarum. 2008;134:161–173.
- Sugden C, Donaghy PG, Halford NG, et al. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol. 1999;120:257–274.
- Wang FB, Tong WJ, Zhu H, et al. A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta. 2016;243:783–797.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum. 1962;15:473–497.
- Lou XM, Yao QH, Zhang Z, et al. Expression of human hepatitis B virus large surface antigen gene in transgenic tomato. Clin Vaccine Immunol. 2007;14:464–469.
- Zhang X, Henriques R, Lin SS. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1:641–646.
- Zhang YJ. Assays of glucose, fructose, sucrose and starch in fruits and vegetables with the anthrone method. Chinese J Anal Chem. 1977;5:167–171.
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nat Protoc. 2006;1:342–1345.
- Rogers SO, Bendich AJ. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol. 1985;5:69–76.
- Li X, Ma H, Huang H, et al. Natural anthocyanins from phytoresources and their chemical researches. Nat Prod Res. 2013;27:456–469.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108.
- Nakamura Y, Yuki K, Park SY, et al. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989;30:833–839.
- Vincent O, Townley R, Kuchin S, et al. Subcellular localization of the Snf1 kinase is regulated by specific β subunits and a novel glucose signaling mechanism. Gene Dev. 2001;15:1104–1114.
- Jossier M, Bouly JP, Meimoun P, et al. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009;59:316–328.
- Celenza J, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180.
- Lee SM, Lee YH, Kim H, et al. Characterization of the potato upreg1gene, encoding a mutated ADP-glucose pyrophosphorylase large subunit, in transformed rice. Plant Cell Tiss Org. 2010;102:171–179.
- Ovono PO, Kevers C, Dommes J. Effects of reducing sugar concentration on in vitro tuber formation and sprouting in yam (Dioscorea cayenensis-D. rotundata complex). Plant Cell Tiss Org. 2009;99:55–59.
- Zrenne rR, Salanoubat M, Willmitzer L, et al. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995;7:97–107.
- Harrison CJ, Mould RM, Leech MJ, et al. The rug3 locus of pea encodes plastidial phosphoglucomutase. Plant Physiol. 2000;122:1187–1192.
- Geigenberger P. Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol. 2011;155:1566–1577.
- Delvallé D, Dumez S, Wattebled F, et al. Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J. 2005;43:398–412.
- Fujita N, Yoshida M, Asakura N, et al. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006;140:1070–1084.
- Szydlowski N, Ragel P, Raynaud S, et al. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthase. Plant Cell. 2009;21:2443–2457.
- Burton RA, Jenner H, Carrangis L, et al. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J. 2002;31:97–112.
- Bustos R, Fahy B, Hylton CM, et al. Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. PNAS. 2004;101:2215–2220.
- Roldan I, Wattebled F, Lucas MM, et al. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504.
- Müller-Röber BT, Koßmann J, Hannah LC, et al. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990;224:136–146.
- Fu H, Park WD. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1369–1385.