Abstract
We previously isolated 18 Saccharomyces cerevisiae strains from coconut toddy in Sri Lanka. All the 18 strains (SLY-1 to SLY-18) could grow aerobically up to 18 mmol L−1 vanillin in yeast extract–peptone–dextrose (YPD) medium, and the SLY-10 strain showed the highest vanillin tolerance (up to 21 mmol L−1). Among these 18 strains, even in the presence of 24 mmol L−1 vanillin, five strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) produced alcohol at 72 h in the range of 11.4–33.1 g L−1 and the SLY-10 strain showed highest alcohol production. The five strains showed the conversion of vanillin to vanillyl alcohol. They also tolerated other strong inhibitors: 4-hydroxybenzoic acid (24 mmol L−1), furfural (30 mmol L−1), 5-hydroxymethyl-2-furaldehyde (5-HMF, 36 mmol L−1) and acetic acid (75 mmol L−1). All these five strains showed no growth and no alcohol production when cultured in the medium with inhibitor mixture of 2.7 mmol L−1 vanillin, 30 mmol L−1 furfural, 30 mmol L−1 5-HMF, 75 mmol L−1 acetic acid and 75 mmol L−1 formic acid but showed significant growth and alcohol production of 16.2–32.7 g L−1 with inhibitor mixture of 30% concentration (0.81 mmol L−1 vanillin, 9 mmol L−1 furfural, 9 mmol L−1 5-HMF, 22.5 mmol L−1 acetic acid and 22.5 mmol L−1 formic acid). The four strains (SLY-3, SLY-4, SLY-8 and SLY-9) were shown to tolerate the inhibitor mixture to a similar extent and better than SLY-10, while SLY-10 was the best to vanillin alone.
Introduction
In the previous study, we isolated 18 strains of Saccharomyces cerevisiae from coconut toddy in Sri Lanka, and five strains among them showed a comparatively higher salt tolerance and thermo-tolerance and produced a substantial amount of alcohol even at 45 °C [Citation1]. These isolates are likely to have a strong tolerance to stresses other than high salt concentration and temperature.
Lignocellulosic biomass from industrial and agricultural residues is considered to be an ideal substrate for the production of bioethanol because it is the most abundant renewable feedstock on the planet and does not compete with foods for human beings, and the use of the biomass supports an environmentally sustainable process [Citation2–5]. However, although lignocellulosic materials contain 70% carbohydrates (cellulose and hemicellulose), several pretreatments are necessary in order to convert carbohydrates to fermentable sugars [Citation6–9]. After the treatment, numerous byproducts are generated: such as phenolic compounds including vanillin and 4-hydroxybenzoic acid (PHBA), furan derivatives, like 5-hydroxymethyl-2-furaldehyde (5-HMF) and furfural and weak acids (acetic acid and formic acid). These byproducts are toxic to yeast cells and eventually affect alcohol production [Citation8,Citation10]. Klinke et al. [Citation11] reported that among those inhibitors, vanillin is found to be the most potent inhibitor, produced by the degradation of lignin from lignocellulosic materials, and it inhibits ethanol production at low concentrations.
To produce alcohol from the hydrolysates of lignocellulosic materials in the presence of such inhibitors, S. cerevisiae strains with high tolerance to those inhibitors are required [Citation12–14]. If the yeast strains isolated by us from Sri Lanka tolerate and produce alcohol with vanillin and other inhibitors, these strains would be beneficial for the bioethanol industry. Thus, we examined the vanillin tolerance of the isolated 18 S. cerevisiae strains at first. We found that all the strains showed high tolerance to vanillin with the SLY-10 strain being the most tolerant. Moreover, several strains also showed high tolerance to the other inhibitors. To test the performance of the yeast strains, the concentrations of each inhibitor were chosen based on knowledge of the inhibitory compounds available in lignocellulosic hydrolysate and their effect [Citation15,Citation16]. Furthermore, we found that these strains produced alcohol with an inhibitor mixture (vanillin, furfural, 5-HMF, acetic acid and formic acid) at different concentrations.
Materials and methods
Pre-cultured yeast preparation
A loopful of cells of each strain from yeast extract–peptone–dextrose (YPD) (1.0 g of yeast extract, 2.0 g of polypeptone, 2.0 g of d-glucose per 100 mL distilled water) medium agar plate was transferred into 5 mL YPD broth and incubated at 30 °C with agitation (200 rpm) for 22–24 h (McFarland units = 16.6 or 1 × 107 CFU mL−1).Cell growth was periodically monitored by measuring culture turbidity (McFarland units).
Measurement of the growth of S. cerevisiae in the presence of vanillin and other inhibitors
The 18 S. cerevisiae strains (SLY-1 to SLY-18) isolated from coconut toddy [Citation1] were used to examine the inhibitor tolerance in the presence of 3–24 mmol L−1 vanillin (Wako, Japan). Selected yeast strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) were used to examine their tolerance in the presence of up to 24 mmol L−1 PHBA (Alfa Aesar, USA), up to 36 mmol L−1 5-HMF (Sigma-Aldrich, USA), up to 30 mmol L−1 furfural (TCI, Tokyo, Japan), up to 75 mmol L−1 acetic acid (Nacalai Tesque, Kyoto, Japan) and up to 75 mmol L−1 formic acid (Cica, Kanto Chemical Co., Inc., Tokyo, Japan). YPD supplemented with each inhibitor individually (vanillin, PHBA, 5-HMF, furfural, acetic acid and formic acid) were prepared by mixing corresponding volumes of inhibitor stock solutions (1 mol L−1) in sterile YPD. Prepared medium with inhibitor solution was subsequently inoculated with pre-cultured yeast (50 µL) and incubated at 30 °C with shaking at 200 rpm. A widely used laboratory yeast strain, S288C (supplied by the Microbiology and fermentation Laboratory, University of the Ryukyus, Okinawa, Japan) and Awamori yeast 101-18 (supplied by the National Research Institute of Brewing, Hiroshima, Japan) strains were used as reference strains. Awamori yeast 101-18 strain is a kind of S. cerevisiae widely used in producing a local distilled alcoholic beverage, named as Awamori, from steamed rice in Okinawa, Japan [Citation17]. Cell growth was monitored by measuring culture turbidity as McFarland units using a densitometer DEN-IB (Waken Btech Co. Ltd., Kyoto, Japan). Three replicate experiments were carried out.
Analysis of each inhibitor compounds in culture supernatants
To determine the remaining vanillin in the culture during the lag or exponential phase of yeast growth, culture supernatants supplemented with 3–21 mmol L−1 vanillin from the five strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) were analyzed by high-performance liquid chromatography (HPLC). The prepared YPD media supplemented with vanillin (100 mL in a 500-mL Erlenmeyer flask) were inoculated with 1 mL of pre-cultured yeast and incubated at 30 °C with shaking (200 rpm) for 4–5 days. Periodically, 1 mL of each culture was taken and centrifuged (3000 rpm for 5 min), and the supernatants were collected. The remaining vanillin concentrations were determined using HPLC (WatersTM LC Module one Plus, Japan) equipped with a Shodex RS pak DE-413L (4.6 mm I.D. × 250 mm) column (Showa Denko k. k, Tokyo, Japan). Prepared samples (50 µL) were separated with a linear gradient (0–100%) of acetonitrile (HPLC grade, Wako, Japan) to the MiliQ water with the flow rate of 1.0 mL min−1. Peak detection was performed using a UV/Vis detector (SPD-10A, Shimadzu, Japan) at 280 nm. Vanillin, vanillyl alcohol and vanillic acid concentrations in the samples were quantified using the corresponding standard curve prepared with the standard solutions. Prior to HPLC analysis, all samples and standards were filtered using a 0.22-µm pore-size syringe filter (Starlab Scientific Co., Ltd., China).
To determine the residual furfural and 5-HMF during the lag, exponential and stationary phases of yeast growth, we analyzed the culture supernatants supplemented with 15–30 mmol L−1 furfural and 30 mmol L−1 5-HMF from five strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) by HPLC. Prepared YPD medium supplemented with furfural or 5-HMF subsequently inoculated by pre-cultured yeast strains were incubated at 30 °C while shaking the cells at 200 rpm. Sampling was done by short intervals, and the culture supernatants were analyzed using HPLC with a linear gradient (5–95%) of acetonitrile (HPLC grade, Wako, Japan) to the 0.01% trifluoroacetic acid (TFA) in MiliQ water with the flow rate of 1.0 mL min−1 [Citation18]. Furfural and 5-HMF were detected at 280 nm.
Alcohol production in the presence of vanillin or other inhibitors
The fermentation abilities of the selected S. cerevisiae strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) in the presence of 2.7–24 mmol L−1 vanillin were examined under static conditions. Pre-culture (5 mL; 1 × 107 CFU mL−1) was centrifuged for 5 min at 3000 rpm (4 °C), and the supernatant was removed. A small amount of the medium with vanillin was added to the harvested yeast cells, and the whole-cell suspension was transferred to 100 mL of the medium supplemented with different concentrations of vanillin. The effect of incubation temperature (30 °C or 40 °C) on fermentation in the presence of vanillin on yeast extract–peptone (YP) medium containing 100 or 160 g L−1 glucose was investigated.
In addition to the vanillin, the alcohol production of all the five strains with other inhibitor alone in YP medium contained 100 g L−1glucose: furfural up to 30 mmol L−1, 5-HMF up to 30 mmol L−1, acetic acid up to 75 mmol L−1 or formic acid up to 75 mmol L−1 was also examined in the same way. All experiments were independently performed three times and expressed as means with standard deviations.
Inhibitor mixture
Inhibitor stock solutions (1 mol L−1) of vanillin, 5-HMF, furfural, acetic acid and formic acid were prepared individually. Then, the medium with inhibitor mixture was prepared by mixing the corresponding volumes of each inhibitor stock solution. The final concentration of formulated inhibitors with 2.7 mmol L−1 (0.41 g L−1) vanillin, 30 mmol L−1 (3.8 g L−1) 5-HMF, 30 mmol L−1 (2.9 g L−1) furfural, 75 mmol L−1 (4.5 g L−1) acetic acid and 75 mmol L−1 (3.5 g L−1) formic acid was expressed as 100% mixture.
Fermentation with inhibitor mixture
To examine yeast fermentation of selected S. cerevisiae strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10) in the presence of inhibitors, we prepared the YP medium containing 100 g L−1 glucose supplemented with the inhibitor mixture to achieve initial concentrations of 100% as mentioned earlier. Then the medium with 100% inhibitor mixture was diluted to achieve 70%, 60%, 40%, 35%, 30% and 20% (vol/vol). Pre-cultured yeast (250 µL; 1 × 107 CFU mL−1) was centrifuged for 5 min at 3000 rpm (4 °C), and the supernatant was removed. The harvested yeast cells were transferred to 5 mL of the medium supplemented with inhibitor mixture and incubated at 30 °C under static conditions. All experiments were independently performed at least three times and expressed as means with standard deviations.
We collected 1 mL of the sample at 12 h intervals and then centrifuged the samples at 6000 rpm for 5 min (4 °C) to obtain the supernatant. Alcohol contents in the collected supernatants were quantified by using the enzyme alcohol dehydrogenase (ADH) extracted from acetic acid bacteria as described in the following section.
Alcohol quantification
Alcohol samples collected at 0 h were diluted 200 times with distilled water, whereas those collected after 12 h of fermentation were diluted 1000 times with distilled water. Diluted samples (100 µL) were mixed with 900 µL of the enzyme reaction buffer composed of potassium ferricyanide (100 mmol L−1) and McIlvaine buffer (500 mmol L−1, pH 6.5), and then 5 µL of the ADH enzyme was added. The reaction mixtures were vortexed and incubated for 5 min at room temperature. After 500 µL of dupanol reagent was added to stop the enzymatic reaction, the reaction mixtures were vortexed and incubated for 20 min. Finally, 3.5 mL of distilled water was added, the reaction mixtures were vortexed, and the absorbance at 660 nm was measured. The alcohol concentrations (g L−1) of samples were calculated along with prepared standard curves (0%, 0.002%, 0.004%, 0.006%, 0.008% and 0.01% ethanol). The ADH enzyme was prepared from the membrane fraction of Gluconobacter oxydans [Citation19].
Results and discussion
Tolerance of the yeast isolates from Sri Lanka against vanillin
In the previous study, we isolated S. cerevisiae strains from the coconut toddy in Sri Lanka [Citation1]. Fitzgerald et al. [Citation20] observed a notable inhibitory effect on the growth of S. cerevisiae in the presence of 5 mmol L−1 vanillin, and when the vanillin concentration was 10 mmol L−1, 85% inhibition of cell growth was observed. However, we found almost no growth inhibition for all the 18 S. cerevisiae isolates at 6 mmol L−1 of vanillin (Supplemental Figure S1), and when the medium was supplemented with 12 mmol L−1 vanillin, a prolonged lag phase was observed, but all strains were still able to grow (Supplemental Figure S1). The SLY-7 strain showed a slightly lower (∼25%) growth compared to that without vanillin (. In the presence of 18 mmol L−1 vanillin, the SLY-10 strain showed significant growth with the shortest lag phase of 36 h. Additionally, six strains (SLY-3, SLY-4, SY-8, SLY-9, SLY-14 and SLY-16) grew after a 48 h lag phase and three strains (SLY-12, SLY-15, and SLY-17) started to grow after a longer (48–60 h) lag phase (. Though Fitzgerald et al. [Citation20] also reported that 20 mmol L−1 vanillin caused complete inhibition of cell growth, contradictory, the SLY-10 strain showed tolerance even in the presence of 21 mmol L−1 vanillin (, Supplemental Figure S1). Several research studies reported that vanillin is the most potent inhibitor of fermentation among the by-products produced during pretreatments of lignocellulosic materials [Citation11,Citation21–23]. However, we found that all the isolated strains of S. cerevisiae except SLY-7 grew (after a specific lengthy lag phase) in the presence of 12 mmol L−1 or 18 mmol L−1 vanillin in YPD medium, whereas the two reference strains (laboratory yeast S288C and Awamori yeast 101-18) did not show any significant growth up to 80 h (Supplemental Figure S1).
Figure 1. Growth of eleven isolated S. cerevisiae strains, SLY-3, SLY-4, SLY-7 to SLY-10, SLY-12 and SLY-14 to SLY-17 in the presence of 12 mmol L−1 (A), 18 mmol L−1 (B) and 21 mmol L−1 (C) vanillin [SLY-3 (closed triangle), SLY-4 (closed square), SLY-7 (plus), SLY-8 (hyphen), SLY-9 (closed circle), SLY-10 (closed diamond), SLY-12 (open triangle), SLY-14 (asterisk), SLY-15 (cross), SLY-16 (open circle), SLY-17 (open square), S288C (closed diamond on dotted line) and 101-18 (closed circle on dotted line)]. Note: Laboratory yeast S288C and Awamori yeast 101-18 strains were used as reference strains. Each experiment was carried out in 5 mL of YPD medium supplemented with different concentrations of vanillin, and all yeast cultures were incubated at 30 °C with shaking at 200 rpm.
![Figure 1. Growth of eleven isolated S. cerevisiae strains, SLY-3, SLY-4, SLY-7 to SLY-10, SLY-12 and SLY-14 to SLY-17 in the presence of 12 mmol L−1 (A), 18 mmol L−1 (B) and 21 mmol L−1 (C) vanillin [SLY-3 (closed triangle), SLY-4 (closed square), SLY-7 (plus), SLY-8 (hyphen), SLY-9 (closed circle), SLY-10 (closed diamond), SLY-12 (open triangle), SLY-14 (asterisk), SLY-15 (cross), SLY-16 (open circle), SLY-17 (open square), S288C (closed diamond on dotted line) and 101-18 (closed circle on dotted line)]. Note: Laboratory yeast S288C and Awamori yeast 101-18 strains were used as reference strains. Each experiment was carried out in 5 mL of YPD medium supplemented with different concentrations of vanillin, and all yeast cultures were incubated at 30 °C with shaking at 200 rpm.](/cms/asset/46675be2-69ea-41d7-8785-11c4361085f0/tbeq_a_1676167_f0001_b.jpg)
Fermentation ability of the five selected strains in the presence of vanillin
Among the seven strains which showed shorter lag phase with 18 mmol L−1 vanillin as mentioned earlier, the five selected S. cerevisiae strains (SLY-3, SLY-4, SLY-8, SLY-9 and SLY-10), which grew better than the other isolated strains in high salt concentration and high temperature and produced a substantial amount of alcohol at high temperatures [Citation1], were used to examine alcohol production in the presence of 9–24 mmol L−1 vanillin at 30 °C.
All the five strains produced substantial amounts of alcohol after 72 h even in the presence of 9 mmol L−1 or 12 mmol L−1 vanillin, in the range of 43.7–48.0 g L−1or 37.8–43.0 g L−1, respectively (). On the other hand, in the presence of 9 mmol L−1, the laboratory yeast S288C and Awamori yeast 101-18 strains produced similar amounts of alcohol, 49.1 g L−1 and 48.4 g L−1, respectively. However, in the presence of 12 mmol L−1 vanillin, they produced lower concentrations of ethanol, 40.2 g L−1and 31.1 g L−1, respectively (). In the presence of 18 mmol L−1 vanillin, all the five strains produced ethanol in the range of 32.9–46.3 g L−1 higher than the two reference strains did; the laboratory yeast S288C and Awamori yeast 101-18 produced ethanol, 24.7 g L−1 and 12.7 g L−1, respectively. Klinke et al. [Citation11] stated that furans and phenolic compounds generally inhibited growth and ethanol production rate but not ethanol yield in S. cerevisiae. Here, a similar phenomenon was observed in the case of SLY-10 up to 18 mmol L−1 () and the other four strains, up to 12 mmol L−1 (data not shown). At higher concentrations of vanillin, this is not the case when the fermentation medium was supplemented with 21 or 24 mmol L−1 vanillin, the fermentation yield of SLY-10 strain (72 h) was reduced by approximately 20% or 28%, respectively, and the other four strains also showed comparatively lower yield above 18 mmol L−1 (, ). Though SLY-3, SLY-4, SLY-8 and SLY-9 strains did not show the growth with vanillin 21 mmol L−1 aerobically (Supplemental Figure S1), they produced alcohol (under static incubation) in the range of 25.2–35.3 g L−1 or 11.4–24.5 g L−1 with 21 and 24 mmol L−1 vanillin, respectively. Interestingly, the SLY-10 strain which showed the significant growth in the presence of 21 mmol L−1 vanillin (), produced the highest alcohol concentration after 72 h (40.4 g L−1 or 33.1 g L−1) among the five strains in the presence of 21 mmol L−1, or 24 mmol L−1 vanillin, respectively ().
Figure 2. Alcohol production by the SLY-10 strain in the presence of 0 mmol L−1 (open circle), 9 mmol L−1 (hyphen), 12 mmol L−1 (closed triangle), 18 mmol L−1 (closed square), 21 mmol L−1 (closed circle) or 24 mmol L−1 (closed diamond) vanillin in YP batch fermented media containing 100 g L−1 glucose. Note: Fermentation was carried out at 30 °C under static incubation condition. Data obtained at 24, 48, 60 and 72 h were shown as mean ± standard deviation of three replicates.
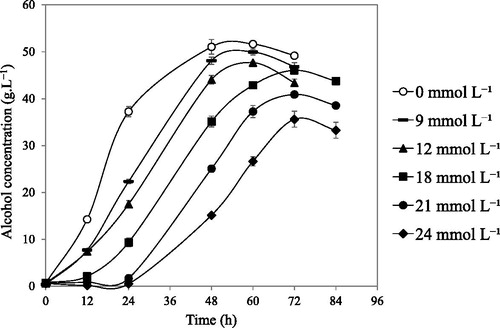
Table 1. Ethanol yield at 72 h and production rate by the selected yeast strains in the presence of a different concentration of vanillin in YP medium with 100 g L−1 glucose.
Reduction of vanillin to vanillyl alcohol
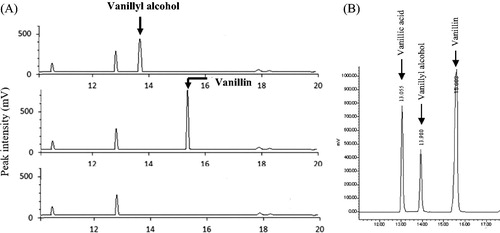
Vanillin could affect essential metabolic processes either by directly inhibiting the oxidoreductase enzymes or indirectly by inhibiting other processes that supply the reduced co-factors required by the enzymes [Citation20]. To understand the mechanism of tolerance of five strains, which showed the significant growth tolerance to vanillin at higher concentrations, the vanillin concentration remaining in the culture supernatant was examined by HPLC. The growth of the SLY-10 strain cultured in YPD supplemented with 3 mmol L−1 of vanillin was not inhibited at all and vanillin could no longer be detected in the culture supernatant obtained after 24 h, but another peak corresponding to vanillyl alcohol [retention time (RT) 13.9 min as shown in ] was observed (. The reduction of the aldehyde into a less toxic corresponding alcohol is a common detoxification strategy, which is mainly catalyzed by alcohol dehydrogenases [Citation16,Citation19], and our strains also reduced vanillin to vanillyl alcohol.
Figure 3. (A) HPLC chromatograms showing the bioconversion of vanillin to vanillyl alcohol. Samples were the control YPD (showed in the bottom) and the supernatant from the SLY-10 strain grown in the presence of 3 mmol L−1 vanillin for 0 h (showed in the middle) or 24 h (showed at the top) incubation period in YPD. (B) HPLC chromatograms detected at 280 nm for standard solutions of vanillic acid, vanillyl alcohol and vanillin. Note: The cells were cultured with shaking at 200 rpm at 30 °C.
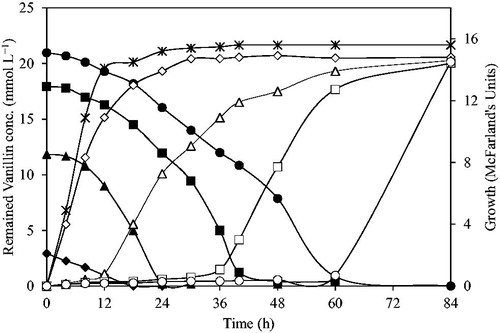
Moreover, we found that when the initial vanillin concentrations of the media were 12 mmol L−1 and 18 mmol L−1, the SLY-10 strain started to grow after the vanillin concentrations were reduced to 9 mmol L−1 and 5 mmol L−1, respectively (). When the initial vanillin concentration was 21 mmol L−1, the SLY-10 strain started to grow after the vanillin concentration was almost completely reduced to zero () and the peak for the corresponding vanillyl alcohol (14.6 mmol L−1) appeared (data not shown). Also, we found that the SLY-10 strain reduces vanillin to vanillyl alcohol during its lag phase, and after the vanillin concentration is lowered to non-toxic levels, the yeast cells start to grow. Previous studies reported that the yeasts S. cerevisiae NCYC 956 strain [Citation20] and NAN-27 strain and it’s all mutants [Citation24] showed tolerance to vanillin up to 20 mmol L−1 and 8.7 mmol L−1, respectively, and S. cerevisiae both NAN-27 and 1278b strains [Citation25] were also able to convert vanillin into vanillyl alcohol. The S. cerevisiae NCYC 956 strain showed the bioconversion of vanillin into not only vanillyl alcohol but also vanillic acid [Citation20]. Here, all the five strains we isolated seemed to convert vanillin only to vanillyl alcohol, but not to vanillic acid. Also, we observed that vanillyl alcohol itself was not toxic to the growth of S. cerevisiae (data not shown), but it seemed to affect the toxicity of vanillin. As SLY-10 strain, the other four strains (SLY-3, SLY-4, SLY-8 and SLY-9) showed the growth by reducing vanillin to vanillyl alcohol during the lag phase where they showed very slow growth (data not shown). In addition, to convert vanillin to vanillyl alcohol or vanillic acid, it is suggested that ergosterol biosynthesis is another key to various inhibitor tolerances [Citation26]. Though many researchers indicated that yeast possessing high ergosterol showed higher vanillin tolerance [Citation8], another study reported that ergosterol biosynthesis and effects of vanillin are unrelated [Citation27]. Therefore, further investigations are required for understanding the effects of vanillin on yeast growth and mechanism of vanillin tolerance in our strains.
Figure 4. Growth of the SLY-10 strain in the presence of 3 mmol L−1 (open diamond), 12 mmol L−1 (open triangle), 18 mmol L−1 (open square) or 21 mmol L−1 (open circle) vanillin in YPD and without vanillin (asterisk). Note: Corresponding filled symbols represented the remaining vanillin concentrations in each culture supernatant. Cultures were incubated at 30 °C with shaking at 200 rpm.
Effects of vanillin under stress conditions
We observed fermentation in the presence of 12 or 18 mmol L−1 vanillin with 160 g L−1 glucose and/or at 40 °C. Previously, we found that reduced alcohol production and productivity of the SLY-10 strain at high temperature were partially recovered with a higher concentration of glucose [Citation1]. This enhancement was also seen at 30 °C at a lower extent (), indicating that a high glucose concentration of 160 g L−1 is not a stress for the SLY-10 strain. Here, we reported that the SLY-10 strain produced a substantial amount of alcohol (31.1 g L−1) at 40 °C in medium supplemented with 18 mmol L−1 vanillin and 160 g L−1 glucose, much higher than it was produced with 100 g L−1 glucose (14.3 g L−1). This amount of alcohol was much higher than that produced by the two reference strains, laboratory yeast S288C and Awamori yeast 101-18 strains (). Though Krouwel and Barber [Citation28] stated that natural yeast fermentation ability could be lost in the temperature range from 35 °C to 45 °C, we previously found that the SLY-10 strain produced 46.3 g L−1 alcohol with 160 g L−1 glucose at 40 °C [Citation1], and even in the presence of vanillin, it also produced considerable amounts of alcohol at 40 °C ().
Table 2. Comparison of ethanol concentrations produced at 72 h by the SLY-10 strain with two reference strains in the presence of combined stresses (vanillin, incubation temperature and high concentration of glucose).
Tolerance to inhibitors other than vanillin
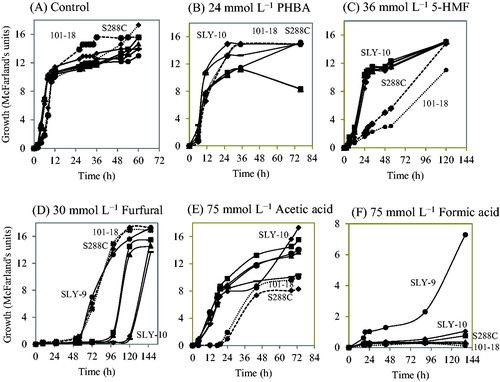
Besides vanillin, we also investigated the tolerance of the SLY-10 strain and the other four strains (SLY-3, SLY-4, SLY-8 and SLY-9) to other strong inhibitors such as PHBA, furfural, 5-HMF, acetic acid and formic acid, which are profoundly appeared in the hydrolysis of lignocellulosic materials. The concentrations of each inhibitor were chosen based on the knowledge of the inhibitory compounds available in lignocellulosic hydrolysate and their inhibitory effect in order to assay the performance of the yeast strains [Citation15,Citation16]. All the five strains tolerated high concentrations of PHBA (24 mmol L−1), 5-HMF (36 mmol L−1) and acetic acid (75 mmol L−1) as shown in , respectively. In the presence of formic acid (75 mmol L−1), most of the strains along with two reference strains showed very poor tolerance. However, SLY-9 showed significant growth (. PHBA and vanillin are reported to be the most versatile phenolic compounds which cause loss of yeast membrane integrity [Citation29], and PHBA has been used as a model compound to study the influence of phenolic compounds on fermentation [Citation30]. In the presence of furfural (30 mmol L−1), the SLY-9 strain and two reference strains, laboratory yeast S288C and Awamori yeast 101-18, showed high tolerance compared to the other four strains which showed growth after a prolonged lag phase as shown in .
Figure 5. Growth of the selected five strains (SLY-3 (closed triangle), SLY-4 (closed square), SLY-8 (hyphen), SLY-9 (closed circle) and SLY-10 (closed diamond) in the presence of each inhibitor: (A) control (without any inhibitor), (B) 24 mmol L−1 PHBA, (C) 36 mmol L−1 5-HMF, (D) 30 mmol L−1 furfural, (E) 75 mmol L−1 acetic acid and (F) 75 mmol L−1 formic acid. Note: Laboratory yeast strain S288C (closed diamond on the dotted line) and Awamori yeast strain 101-18 (closed circle on the dotted line) were used as reference strains. All the experiments were carried out at 30 °C with shaking at 200 rpm.
In contrast, all the five strains showed tolerance to furan inhibitors, 5-HMF at 36 mmol L−1 () as described earlier. Previous studies reported that, at nearly the same concentrations, furfural was shown to be more inhibitory to cell growth than 5-HMF was [Citation31,Citation32], and similar observations were found through our study as well. Inhibitors like 5-HMF and furfural are formed by degrading pentoses and hexoses, respectively, during the pre-treatment of lignocellulosic hydrolysates [Citation30,Citation33]. It has been reported that 5-HMF and furfural induce accumulation of reactive oxygen species in yeast cells [Citation34,Citation35]. The ability to tolerate furfural is directly related to the ability to convert furfural to furfuryl alcohol, a less potent inhibitory compound [Citation36–39]. We assume that all the five strains also did this conversion and HPLC data revealed that when the initial furfural concentration was significantly reduced to low concentrations, then the yeast cells started to grow (data not shown).
On the other hand, these five strains showed comparatively high tolerance in the presence of 5-HMF at 36 mmol L−1, as shown in , without its reduction (data not shown). It was reported that the formation of processing bodies (P-bodies) and stress granules (SGs) at higher than 2 mmol L−1 vanillin [Citation27], 15 mmol L−1 furfural or 40 mmol L−1 5-HMF [Citation40] are responsible for translation regulation [Citation41,Citation42] and can stimulate yeast tolerance. We should check the formation of P-bodies and SGs in our strains in future studies. Since the most common concentrations of furfural and 5-HMF in bioethanol fermentation are 20–40 mmol L−1 and 50 mmol L−1, respectively [Citation11,Citation34], our five naturally isolated strains which showed a distinct inhibitor tolerance in the presence of 30 mmol L−1 furfural and 36 mmol L−1 5-HMF () would be beneficial for the bioethanol industry.
Fermentation with individual inhibitors
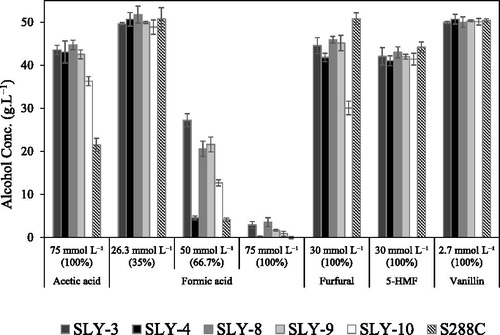
In addition to vanillin, we examined the ability of alcohol production of all five yeast strains (SLY-3, SLY-4, SLY-8, SY-9 and SLY-10) with high concentrations of each inhibitor in YPD: furfural (30 mmol L−1), 5-HMF (30 mmol L−1) or acetic acid (75 mmol L−1) which showed only slight inhibition on growth. In the presence of each inhibitor alone in YP medium with 100 g L−1 glucose, furfural (30 mmol L−1), 5-HMF (30 mmol L−1) or acetic acid (75 mmol L−1), all the five strains produced a substantial amount of alcohol as shown in . Among toxic byproducts from the pretreatment of lignocellulosic biomass for saccharification, the most common weak acids in lignocellulosic hydrolysates are acetic acid and formic acid, formed by the de-acetylation of hemicellulose and by furan degradation, respectively [Citation43]. It is reported that uncoupling of proton and intracellular anion accumulation are the two mechanisms to explain the inhibitory effect of weak acids [Citation44]. Several studies reported that yeast growth and alcohol production were inhibited by acetic acid at low concentration [Citation45,Citation46]. However, all the five strains examined here grew well and produced substantial amounts of alcohol, 36.5–43.6 g L−1, even in high concentrations of acetic acid (75 mmol L−1) ( and . In contrast, both their growth and alcohol yield (0.2–2.9 g L−1) ( and ) were inhibited by 75 mmol L−1 formic acid, and similar results were observed by Guo and Olsson [Citation47]. Because formic acid (75 mmol L−1) severely inhibited growth and fermentation, as shown in and , respectively, we examined alcohol production by lowering the concentration of formic acid (26.3 mmol L−1 or 50 mmol L−1). At 26.3 mmol L−1 formic acid, all five strains successfully produced similar alcohol amounts to those without formic acid, but at 50 mmol L−1, reduced amounts of alcohol were produced 4.6–27.2 g L−1 ().
Figure 6. Ethanol fermentation (72 h) by the selected five yeast strains in the presence of each inhibitor in the YP medium with 100 g L−1 glucose: vanillin (2.7 mmol L−1, 100% inhibitor mixture), furfural (30 mmol L−1, 100% inhibitor mixture), 5-HMF (30 mmol L−1, 100% inhibitor mixture), acetic acid (75 mmol L−1, 100% inhibitor mixture) or formic acid (26.3 mmol L−1, 50 mmol L−1 and 75 mmol L−1 in 35%, 66.7% and 100% inhibitor mixtures, respectively). Note: Cultures were incubated at 30 °C under static condition. Obtained data were shown as means ± standard deviation of three replicates.
Fermentation ability with a mixture of inhibitors
To examine whether the five strains possess the fermentation ability in inhibitor mixture found in the hydrolysate of lignocellulosic materials, these strains were incubated in the YP medium contained 100 g L−1 glucose with inhibitor mixture containing 2.7 mmol L−1 vanillin, 30 mmol L−1 furfural, 30 mmol L−1 5-HMF, 75 mmol L−1 acetic acid and 75 mmol L−1 formic acid. These concentrations of inhibitors were expressed as 100% inhibitor mixture.
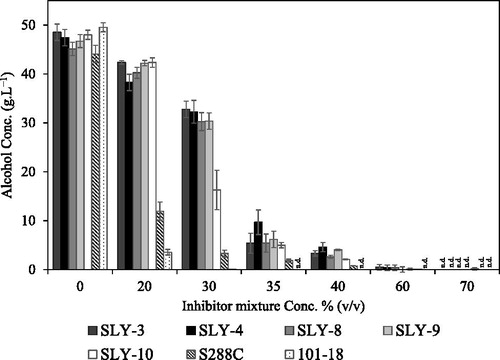
None of the strains showed alcohol production in 100% inhibitor mixture. We examined fermentation in a diluted inhibitor mixture, 20–70% (vol/vol) because all the five strains produced alcohol with formic acid at 50 mmol L−1 corresponding to 66.7% inhibitor mixture and no inhibition at 26.3 mmol L−1 corresponding to 35% inhibitor mixture (). Though fermentation was significantly inhibited by 35% inhibitor mixture, the five strains showed alcohol production in the range of 16.2–32.7 g L−1 in the presence of inhibitor mixture of 30% (vol/vol), while two reference strains produced only less than 5 g L−1 (). Furthermore, these strains still produced alcohol in the range of 4.9–9.6 g L−1and 2.1–4.6 g L−1 with 35% and 40% inhibitor mixtures, respectively. However, the five strains showed significantly high tolerance to 30% inhibitor mixture, especially four strains (SLY-3, SLY-4, SLY-8 and SLY-9), while SLY-10 did less extent (). Iwaki et al. [Citation40] reported that combining of furfural (15 mmol L−1) and 5-HMF (40 mmol L−1) significantly decreased the polysome fraction, leading to definite repression of translation activity. Even though we found that combined inhibitors synergistically reduced the alcohol fermentation, the four strains (SLY-3, SLY-4, SLY-8 and SLY-9) tolerated the inhibitor mixture better than the SLY-10 strain did, which showed the highest tolerance to vanillin (). Although several studies were conducted, the cellular stress response by combining inhibitors is not fully explained yet. Therefore, future works are needed to elucidate the underlying inhibitory mechanisms and the metabolic pathway adjustments to achieve tolerance against inhibiting compounds. All five strains produced considerable amounts of alcohol with each inhibitor alone and still produced alcohol with inhibitor mixture contained relatively high concentrations of inhibitors.
Figure 7. Ethanol concentrations produced by the five selected strains from left to right: SLY-3 (dark gray), SLY-4 (black), SLY-8 (gray), SLY-9 (light gray), SLY-10 (white) and two reference strains; S288C (hatched), and 101-18 (dotted) in the YP medium with 100 g L−1 glucose-containing different concentrations of inhibitor mixture. Note: The fermentation was carried out for 72 h at 30 °C under static incubation conditions. Data obtained with 0%, 20%, 30%, 35% and 40% inhibitor mixtures were shown as means ± standard deviation of three replicates. n.d., not detected.
Conclusion
Throughout this work, we obtained five yeast strains which tolerated vanillin and other strong inhibitors profoundly represented in lignocellulosic materials. All the five strains showed tolerance to high concentrations of vanillin by converting toxic vanillin into vanillyl alcohol. Interestingly, the SLY-10 strain showed much higher alcohol production in the presence of vanillin (18 mmol L−1) at 40 °C with 160 g L−1 glucose than with 100 g L−1 glucose. All the five strains produced considerable amounts of alcohol with each inhibitor alone and still produced alcohol with inhibitor mixture containing relatively high concentrations of inhibitors. Therefore, we suggest that these strains examined in this study might be not only useful for the bioethanol industry using lignocellulosic biomass but also useful for study of response by combining inhibitors.
Supplemental Material
Download PDF (500.7 KB)Acknowledgement
Authors would like to thank Oriental Yeast Co. Ltd. (Tokyo, Japan) for providing the Yeast extract.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Additional information
Funding
References
- Ahangangoda AMS, Yoshida S, Toyama H. Thermo-and salt-tolerant Saccharomyces cerevisiae strains isolated from fermenting coconut toddy from Sri Lanka. Biotechnol Biotechnol Equip. 2019;33:1–8.
- Gutiérrez-Rivera B, Waliszewski-Kubiak K, Carvajal-Zarrabal O. Conversion efficiency of glucose/xylose mixtures for ethanol production using Saccharomyces cerevisiae ITV01 and Pichia stipitis NRRL Y-7124. J Chem Technol Biotechnol. 2012;87(2):263–270.
- Narayanan V, Schelin J, Gorwa-Grauslund M, et al. Increased lignocellulosic inhibitor tolerance of Saccharomyces cerevisiae cell populations in early stationary phase. Biotechnol Biofuels. 2017;10(1):114.
- Ishola MM, Isroi, Taherzadeh MJ. Effect of fungal and phosphoric acid pretreatment on ethanol production from oil palm empty fruit bunches (OPEFB). Bioresour Technol. 2014;165:9–12.
- Tesfaw A, Assefa F. Current trends in bioethanol production by Saccharomyces cerevisiae: substrate, inhibitor reduction, growth variables, coculture, and immobilization. Int Sch Res Notices. 2014;2014:532852 [11 pages].
- Zaldivar J, Nielsen J, Olsson L. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol. 2001;56(1–2):17–34.
- Gray KA, Zhao L, Emptage M. Bioethanol. Curr Opin Chem Biol. 2006;10(2):141–146.
- Endo A, Nakamura T, Shima J. Involvement of ergosterol in tolerance to vanillin, a potential inhibitor of bioethanol fermentation, in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2009;299(1):95–99.
- McMillan DJ. Bioethanol production: status and prospects. Renew Energy. 1997;10(2–3):295–302.
- Olsson L, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol. 1996;18(5):312–331.
- Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66(1):10–26.
- Theivendirarajah K, Chrystopher RK. Microflora and microbial activity in palmyrah (Borassus flabellifer) palm wine in Sri Lanka. Mircen J. 1987;3(1):23–31.
- Bettiga M, Bengtsson O, Hahn-Hägerdal B, et al. Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microb Cell Fact. 2009;8(1):40.
- Knox AM, Du Preez JC, Kilian SG. Starch fermentation characteristics of Saccharomyces cerevisiae strains transformed with amylase genes from Lipomyces kononenkoae and Saccharomycopsis fibuligera. Enzyme Microb Technol. 2004;34(5):453–460.
- Larsson S, Palmqvist E, Hahn-Hägerdal B, et al. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol. 1999;24(3–4):151–159.
- Larsson S, Quintana-Sáinz A, Reimann A, et al. Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2000;84–86(1–9):617–632.
- Takagi H, Hashida K, Watanabe D, et al. Isolation and characterization of awamori yeast mutants with l-leucine accumulation that overproduce isoamyl alcohol. J Biosci Bioeng. 2015;119(2):140–147.
- Li M, Yang Z, Yang M, et al. Determination of furfural in beer by high-performance liquid chromatography with solid-phase extraction. J Inst Brew. 2009;115(3):226–231.
- Ameyama M. Enzymic microdetermination of d-glucose, d-fructose, d-gluconate, 2-keto-d-gluconate, aldehyde, and alcohol with membrane-bound dehydrogenase; carbohydrate metabolism. In: Wood WA, editor. Methods in enzymology. Vol. 89. Michigan: Elsevier Academic Press; 1982. p. 20–29.
- Fitzgerald DJ, Stratford M, Narbad A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int J Food Microbiol. 2003;86(1–2):113–122.
- Elvis FK, Sanette M, Anton M. Simulated inhibitory effects of typical byproducts of biomass pretreatment process on the viability of Saccharomyces cerevisiae and bioethanol production yield. Afr J Biotechnol. 2015;14(30):2383–2394.
- Moreno AD, Ibarra D, Ballesteros I, et al. Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour Technol. 2013;135:239–245.
- Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II. Inhibitors and mechanisms of inhibition. Bioresour Technol. 2000;74(1):25–33.
- Shen Y, Li H, Wang X, et al. High vanillin tolerance of an evolved Saccharomyces cerevisiae strain owing to its enhanced vanillin reduction and antioxidative capacity. J Ind Microbiol Biotechnol. 2014;41(11):1637–1645.
- De Wulf O, Thonart P. Bioconversion of vanillin to vanillyl alcohol in a two-phase reactor. Appl Biochem Biotechnol. 1989;20–21(1):165–180.
- Endo A, Nakamura T, Ando A, et al. Genome-wide screening of the genes required for tolerance to vanillin, which is a potential inhibitor of bioethanol fermentation, in Saccharomyces cerevisiae. Biotechnol Biofuels. 2008;1(1):3.
- Iwaki A, Ohnuki S, Suga Y, et al. Vanillin inhibits translation and induces messenger ribonucleoprotein (mRNP) granule formation in Saccharomyces cerevisiae: application and validation of high-content, image-based profiling. PLoS One. 2013;8(4):e61748–11. [cited 2018 Feb 02].
- Krouwel PG, Braber L. Ethanol production by yeast at supraoptimal temperatures. Biotechnol Lett. 1979;1(10):403–408.
- Heipieper HJ, Weber FJ, Sikkema J, et al. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12(10):409–415.
- Antal MJ, Mok WSL, Richards GN. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr Res. 1990;199(1):91–109.
- Liu ZL, Slininger PJ, Dien BS, et al. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol. 2004;31(8):345–352.
- Taherzadeh MJ, Gustafsson L, Niklasson C, et al. Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2000;53(6):701–708.
- Liu ZL, Moon J. A novel NADPH-dependent aldehyde reductase gene from Saccharomyces cerevisiae NRRL Y-12632 involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Gene. 2009;446(1):1–10.
- Almedia JRM, Modig T, Petersson A, et al. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharmyces cerevisiae. J Chem Technol Biotechnol. 2007;82:1115–1121.
- Sasano Y, Watanabe D, Ukibe K, et al. Overexpression of the yeast transcription activator Msn2 confers furfural resistance and increases the initial fermentation rate in ethanol production. J Biosci Bioeng. 2012;113(4):451–455.
- Liu ZL, Slininger PJ, Gorsich SW. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl Biochem Biotechnol. 2005;121:451–460.
- Taherzadeh MJ, Gustafsson L, Niklasson C, et al. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J Biosci Bioeng. 1999;87(2):169–174.
- Wang C-F, Zhang X-Y, Xu Z-H, et al. Selective synthesis of furfuryl alcohol from biomass-derived furfural using immobilized yeast cells. Catalysts. 2019;9:70.
- Sárvári Horváth I, Franzén CJ, Taherzadeh MJ, et al. Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl Environ Microbiol. 2003;69:4076–4086.
- Iwaki A, Kawai T, Yamamoto Y, et al. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl Environ Microbiol. 2013;79(5):1661–1667.
- Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008;183(3):441–455.
- Balagopal V, Parker R. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol. 2009;21(3):403–408.
- Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. I. Inhibition and detoxification. Bioresour Technol. 2000;74(1):17–24.
- Russell JB. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol. 1992;73(5):363–370.
- Narendranath NV, Thomas KC, Ingledew WM. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol. 2001;26(3):171–177.
- Guo ZP, Olsson L. Physiological responses to acid stress by Saccharomyces cerevisiae when applying high initial cell density. FEMS Yeast Res. 2016;16:1–11.
- Guo Z, Olsson L. Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res. 2014;14(8):1234–1248.
