?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Verticillium wilt is a severe disease caused by Verticillium dahliae, which afflicts many crops, particularly cotton in the Xinjiang province of China. Chemical fungicides are harmful to the environment, and biological control agents against the fungal pathogens of plants provide an alternative to chemicals. Biological control agents include antagonistic bacteria or metabolic products. We investigated the inhibitory effects of two plant-growth-promoting rhizobacterial (PGPR) strains, namely Bacillus tequilensis C-9 and Sphingobacterium A1, against V. dahliae in vitro and in the field. We used the mixed fermentation of two bacteria to inhibit V. dahliae. Antimicrobial assays were performed and showed that spore production and germination, and the virulence protein of V. dahliae were reduced after treatment with cell-free mixed fermentation broth. In field studies, there was improvement in the disease severity, along with declined incidence and index after the treatment in the bud and bell stages. To explore the molecular mechanisms of this inhibition, we studied six metabolism-related genes by real-time quantitative polymerase chain reaction (qRT-PCR). The results showed that in response to mixed fermentation broth, some genes related to growth and development were significantly upregulated, explaining the structural changes observed in V. dahliae. Some genes that regulate oxidative stress were also dramatically induced, indicating that mixed bacterial fermentation had a significant impact on V. dahliae. These results support the idea that the cell-free mixed fermentation broth of Bacillus tequilensis C-9 and Sphingobacterium A1, when applied for biocontrols, can inhibit V. dahliae in vitro and in vivo.
Introduction
Verticillium wilt afflicts hundreds of dicotyledonous plant species worldwide, including field crops, vegetables and fruits [Citation1, Citation2]. The disease is caused by Verticillium dahliae, which is devastating to the cotton industry [Citation3]. A major challenge in the control of V. dahliae are genetic mutations that promote variability and adaptability to new environments, chemicals and hosts [Citation4]. V. dahliae control necessitates the use of coordinated disease management strategies.
The use of microorganisms to control pests or plant pathogens is considered a reliable and promising alternative to pesticide use in the field. This method is highly appropriate for controlling fungal growth and decreasing the levels of agrochemical residues during crop cultivation [Citation5–7]. Interest in the utilization of non-pathogenic plant-growth-promoting rhizobacteria (PGPR) in the search of biological control agents is increasing, as these microorganisms are well adapted to their host and can colonize an ecological niche similar to that of phytopathogens [Citation8]. PGPR also promote plant health by suppressing phytopathogens using various mechanisms, including competition, the production of antibacterial substances, antagonism and induced systemic resistance [Citation9]. They are additionally considered good producers of secondary metabolites that are required for survival in the face of host defense responses due to their antifungal and antibacterial activity, which can inhibit the growth of pathogenic microorganisms [Citation8, Citation10].
Genomic studies have shown that a large number of metabolism-related genes are not expressed by bacteria or fungi under standard laboratory growth conditions [Citation11]. Some gene clusters of interest responsible for expressing the metabolites that increase competitiveness in natural environments may remain silent under unnatural conditions [Citation12, Citation13]. Some reports show that mixing microorganism cultures can stimulate the production of secondary metabolites and increase the biological activity of microbial extracts [Citation14–17]. Mixed cultures can induce biocontrol and secondary metabolite synthesis under laboratory conditions. Studies on the biocontrol of cotton Verticillium wilt have only been performed under monoculture conditions [Citation18]. To our knowledge, improving the biocontrol of V. dahliae using mixed cultures has not been reported so far. The biocontrol of pathogenic fungi can occur via a variety of mechanisms. Systemic resistance in plants can be stimulated to enhance latent defenses leading to the activation of multiple defense-related compounds/enzymes at sites distant from the areas of pathogen attack [Citation19]. Fungal signaling pathways, particularly high osmolality glycerol (HOG) and cell wall integrity (CWI) pathways against V. dahliae can also be activated [Citation20].
The aims of this study were to: (1) investigate the antagonistic ability of bacterial co-cultures against V.dahliae and to evaluate the potential of selected optimal strains to inhibit V. dahliae in vitro and in vivo; (2) to infer the intrinsic mechanisms of the inhibitory effects of the co-culture against V.dahliae. To investigate the biocontrol potential of Bacillus tequilensis C-9 and Sphingobacterium A1, we performed co-culture experiments to obtain mixed bacterial cultures. We assessed the suppressive effects of this cell-free mixed fermentation broth on V.dahliae. Based on our results, we hypothesize that compounds in the mixed fermentation filtrate enhance the defense against ecological phytopathogenic fungi and the PGPR–plant interactions.
Materials and methods
Microorganisms and culture conditions
This study is based on the use of two microorganisms, Sphingobacterium A1 and Bacillus tequilensis C-9. Sphingobacterium A1 has ACC deaminase activity and was isolated from the Xinjiang featured Resina ferulae rhizosphere. Bacillus tequilensis C-9 produces proteases and was obtained from the soil of cotton roots. In preliminary experiments (data not shown), both strains were selected through in vitro screening to study their combined efficiency to inhibit V. dahliae. These two strains were selected for this study, due to their higher antimicrobial activity against V. dahliae. V. dahliae Vd278 was isolated and maintained in the Key Laboratory of Agricultural Biotechnology, Shihezi University, China.
Establishment of mixed culture systems
To obtain 10−10 CFU/mL pure culture bacterium mother liquor, Sphingobacterium A1 and Bacillus tequilensis C-9 were cultured individually in 50 mL of PDB (potato dextrose broth) medium containing 20 g sucrose, 200 g potato extract in 1 L distilled water at 28 °C, 160 rpm, for 3 days. Mixed culture systems were initiated by inoculating the two mother liquors in different proportions (the ratios of the two bacteria were as follows A1:C-9 = 10:0; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9; 0:10) in a single flask of optimized PDB medium.
Assessment of antimicrobial activity against V. dahliae
To obtain mixed cell-free fermentation broth, we centrifuged the mixed liquid cultures at 4 °C, 6000 rpm(TG16-WS Tabletop high speed centrifuge)for 20 min. Supernatants were passed through 0.22-μm microporous membrane filters.
Production of V. dahliae conidia
Sterile fermentation broth was mixed with 100 mL PDA (potato dextrose agar) culture medium at a ratio of 1:10, 1:50 and 1:100 and poured into five Petri dishes (20 mL per plate). Control groups were mixed with equal amounts of sterile water and PDA. V. dahliae (6 mm diameter) were placed onto the mixed culture medium and were incubated at 25 °C for 7 days in the dark. Sterile water (5 mL) was added to each treated dish, and spores on the colonies were washed by force shock. The number of V. dahliae was counted using blood count plates on three occasions. According to the following formula, the inhibition rates of sterile fermentation broth on the spores of V. dahliae were calculated as follows:
where ⊿1(%) is the inhibition rate; NSC: is the number of spores in the control group; NSS is the number of spores in the sample group.
Germination of V. dahliae spores
Sterile fermentation broth was mixed with PDA culture medium at a ratio of 1:10 and poured into Petri dishes. Control groups were mixed with equal amounts of sterile water and PDA. The Vd278 agar was perforated with a hole punch (diameter ∼6 mm) and the agar plug was inserted into an inverted Petri dish in the dark at 25 °C for 8 days. Vd278 was placed in PDB culture medium and was incubated for 8 days for the production of spore suspensions. Suspensions (100 μL) containing 10−7 V. dahliae spores per milliliter were spotted onto plates and allowed to grow for 3 days at 25 °C in the dark. Once the germination rates of the control group exceeded 80%, all germination rates were recorded and the inhibition rates were calculated using the following formula:
where ⊿2(%) is germination rate, ⊿3(%) is inhibition rate, TN is the total number of spores spots on the plate; GNC is the number of spores germinated in the control group.
RNA extraction
Total fungal RNA was extracted 8 days after inoculation using commercial RNA extraction kits (BIOMIGA company EZgeneTM Fungal RNA Kit (R6618)). We selected marginal Verticillium hyphae from an 8-day culture plate to which sterile fermentation broth was added and plates were incubated at 160 rpm in PDB medium for 8 days at 25 °C, filtered through eight layers of a sterile gauze, and the mycelia were collected. Hyphae were washed with sterile water three times and samples were inoculated in PDB containing mixed cell-free culture medium (final concentrations diluted 10-fold). PDB lacking the mixed culture medium was used as a control. After incubation for 0.5 h, 1 h and 2 h, respectively, mycelium was collected by centrifugation at 5000 rpm for 10 min and the RNA of V. dahliae was extracted using BIOMIGA Fungal RNA Extraction Kits.
Quantitative real-time polymerase chain reaction (PCR) analysis
Primers complementary to the conserved gene regions were designed based on the complete sequence of the target gene available at the NCBI (National Center for Biotechnology Information) database. The primers are listed in . Extracted RNA was reverse transcribed into cDNA according to the instructions of the TransScript II first-stand cDNA Synthesis SuperMix kit. The obtained cDNA was used as a template. The gene for β-tubulin of V. dahliae was used as an internal reference gene. SYBR GreenIMaster Mix Kits were used on a light Cycler® 480II PCR instrument (Roche). The reaction conditions were: 95 °C for 2 min; 95 °C for 15 s; 48 °C for 15 s; and 72 °C for 20 s for 30 cycles. Each sample was run in triplicate. Data were analyzed using the 2-ΔΔCt method. Transcript levels were calculated using the standard curve method from duplicate data and normalized to β-tubulin.
Table 1. Primers used in this study.
Field study
The field resistance of cotton plants to verticillium wilt was assessed with and without application of a pre-prepared fungicide obtained by fermentation using a mixed bacterial culture (effective viable levels of over 8 × 10−10cfu/g) of Sphingobacterium A1 and B. tequilensis C-9, and the addition of appropriate carriers and auxiliaries. According to verticillium wilt incidence in previous years (2016–2018), the experiments were set up in two different areas of the experimental field: a mild (light) disease area with low incidence of verticillium wilt (<20%) and a severe disease area with high incidence (> 20%).
Two treatments for each variety of cotton, Xinluzao 7 (X7) and Xinluzao 33(X33), were set up: control (CK) and treatment (TR) groups. Control and experimental groups were set up in both the mild and severe disease areas. The diseased nursery soil contained cotton verticillium wilt pathogens. In the control groups (CK), bacterial agents were not used, so the cotton seeds were treated with water. In the treatment groups (TR), the developed fungicide was dripped into the soil (two drip irrigations with water for the whole experiment) at 20 g/m2. Three repetition zones were set for each treatment group, with 60 cotton plants in each plot, 10 plants per plot. Each plot consisted of 2 rows of plants, each 5 m in length and spaced at 0.5 m.
The field trials were managed according to conventional water and fertilizer measures. From May to September 2017, experiments were performed in 177 mission field in Shihezi City, Xinjiang (E86.04, N44.30). The cultivated field has been planted with cotton for 27 years in which the occurrence of verticillium wilt was serious.
Assessment of biocontrol efficacy
To evaluate the biocontrol efficacy of the fungicides, the effects on disease prevention and growth promotion were investigated during the budding (early July) and boll stages (mid-August). Ten cotton plants were randomly selected in z-shapes in each treatment group. Plant height was measured and the number of buds and bolls was scored. Sixty cotton plants in each plot were selected as statistical data for the survey.
The verticillium wilt disease status was classified into 5 grades: Grade 0: asymptomatic; Grade 1: yellowing or withering leaves fewer than 25%; Grade 2: yellowing or withering leaves 25–50%; Grade 3: yellowing or withered leaves ranging from 50% to 75%; Grade 4: yellowish withered leaves more than 75%.
Disease incidence rates (DI %) were calculated as disease severity (DS, Biocontrol efficacy, BE, %) according to the formula: DI = 100 × Σ (disease grade × number of plants at disease grade/highest disease grade × total number of plants).
Statistical analysis
Analysis of variance (ANOVA) was used to compare percentage values subjected to angular transformation for DI and DS at both evaluation times. Significant differences amongst the treatments were evaluated using post-hoc Tukey tests with STATGRAPHICS PLUS software (STATPOINT TECHNOLOGIES, INC., Virginia). The level of significance was set at p ≤ 0.05.
Results and discussion
Effects of mixed culture on the spore production of V. dahliae
The fungistatic effects of the mixed fermentation filtrates on V. dahliae were higher than those of either monoculture cell-free broth. Among the tested combinations, the most pronounced fungistatic effect was observed when A1: c-9 = 1:9. Therefore, a 1:9 ratio of the two cultures was used in the subsequent experiments. The sterile fermentation filtrate of mixed bacterial cultures significantly inhibited the spore production and growth of V. dahliae. When diluted 1:10, the mixed culture inhibited the spore production by 97.8%. Spores remained inhibited at dilutions of 1:100 ().
Table 2. Inhibitory effects of cell-free mixed culture filtrate on conidia production of V. dahliae.
Effects of the mixed culture on the spore germination of V. dahliae
The sterile fermentation filtrate of the mixed bacterial cultures inhibited the germination of V. dahliae spores. In control treatments, the spore germination rate was 63.33%, which declined to 0 in 1:10, 1:50 and 1:100 dilutions of mixed bacterial culture filtrates. The inhibition of spore germination was 100% when the pure culture fermentation broth of the C-9 strain was diluted 1:10 and 1:50. The inhibitory rates declined to 14.8% when the C-9 broth was diluted 1:100, which was less potent than the mixed broth ().
Table 3. Inhibitory effects of the mixed-culture filtrate on the germination of V. dahliae spores.
Effects of the mixed culture on the expression of genes encoding virulence proteins of V. dahliae
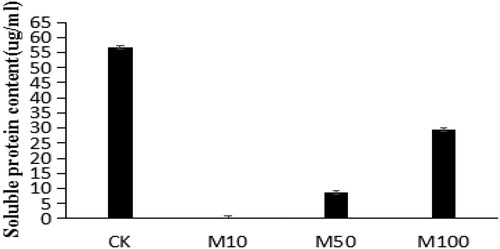
As shown in , the addition of the mixed fermentation broth to the PDB medium decreased the levels of secreted proteins from V. dahliae compared to CK groups (protein content in CK: 56.73 μg/mL vs. M10 treatment: 0.53 μg/mL, p = 0.0031). Following M100 treatment, the protein content was 29.5 μg/mL (52% of the control), indicating that the mixed culture was effective at high dilutions (p = 0.017). Taken together, these data demonstrate that the mixed sterile fermentation broth reduces toxin secretion and inhibits the growth of V. dahliae.
Figure 1. Effects of mixed-culture filtrates on Verticillium dahliae protein secretion.
Note: The fermentation broth obtained by the mixed culture of A1 and C-9 was filtered through a microporous filtration membrane. After mixing the filtrate with PDA medium, the protein content of the inhibited Verticillium culture was measured in the plate.
Effects of the mixed fermentation liquid on the development of mycelia
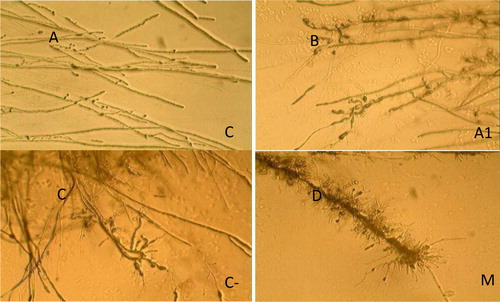
As shown in , the mycelium at the edge of the inhibition rings showed deformation in response to the mixed culture broth. Malformation, abnormal bending and swelling were observed (). For the single-strain (monoculture) fermentation broth, the mycelial of V. dahliae showed abnormal mycelial growth, but less deformation compared to that treated with the mixed broth (). It is possible that volatile substances produced in the mixed culture inhibit the growth of the hyphae. Studies have shown that the volatile organic compounds and soluble substances produced by antagonistic bacteria have significant inhibitory effects on the growth and nucleate activity of Verticillium hyphae. These include alcohols, several of which reported antifungal activity including 3-methyl-1-butanol [Citation22]. Interestingly, only distinct Verticillium species were shown to produce alcohols [Citation23].
Figure 2. Effects of the mixed-culture filtrate against Verticillium dahliae mycelia observed under a microscope.
Note: A.CK: Morphology of hyphae uninhibited by fermentation broth; B.A1: Morphology of hyphae inhibited by A1 monoculture fermentation filtrate; C.C-9: Morphology of hyphae inhibited by C-9 monoculture fermentation filtrate; D.M: Morphology of hyphae inhibited by the mixed-culture fermentation filtrate of A-1 and C-9.
Effects of the mixed bacteria fermentation broth on gene expression in V. dahliae
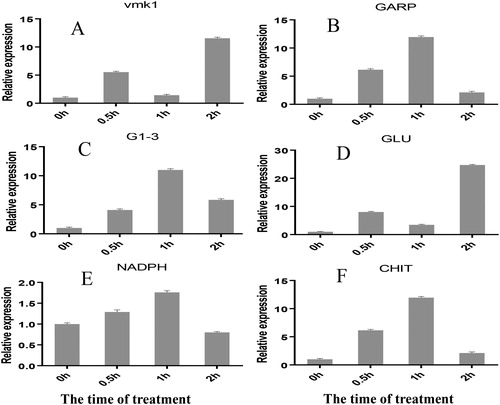
As shown in , following treatment with the mixed culture medium, the expression levels of VAMK1 and GARP genes, which are related to the development of V. dahliae, were upregulated 11- and 5- fold at 2 h, respectively (). After 30 min of treatment, the expression of glycerol-3-phosphate dehydrogenase was significantly induced, and peaked at 1 h, indicating that treatment with the mixed culture medium induced osmotic stress (). The expression of glutathione reductase increased 24-fold, and NADPH oxidase levels were 1.76-fold higher than those in the control samples at 1 h, suggesting that treatment with the mixed culture filtrate evoked production of reactive oxygen species (ROS) and oxidative stress (). Chitinase was significantly induced after 30 min and 1 h of treatment, indicating that the mixed culture medium induced the synthesis of cell wall hydrolyzing enzymes in the CWI pathway, leading to Verticillium fungus cell death ().
Effects of the microbial agents on cotton growth
The structure of microsclerotia, which form a dormant structure under adverse conditions, allow them to survive in the soil for prolonged time periods. Due to these characteristics, the control of verticillium wilt is extremely difficult. Microbial pesticides (i.e. microorganisms that are used for biocontrol) or biochemical pesticides (i.e. metabolites with antimicrobial activities) have attracted intense research interest. However, their application in China can be problematic due to the lack of dosage information, and the instability of the physicochemical components of these agents.
shows the growth status of two cotton varieties in response to mixed culture treatment: X7:XinLuzao7 (susceptible) and X33:XinLuzao (disease-resistant). The plant height of Xinluzao 7 TR following treatment was 4.68 ± 2.95 cm higher than that of Xinluzao 7 CK, and Xinluza 33 TR was 8.22 ± 3.72 cm higher than Xinluza 33 CK. Both fields showed higher growth in TR compared to CK groups, indicating that the treatment promoted cotton plant growth. The number of buds on both varieties of cotton plants increased compared to the CK group following the application of microbial agents, suggestive of enhanced branch growth.
Table 4. Application of microbial agents promotes cotton growth.
We further investigated the number of bolls in each treatment group. There were more bolls than in the CK group. Once the cotton had matured, the lint weight of each treatment plot was measured. The results showed that the treatment with the microbial agents was associated with an increase in the production of Xinluzao 7 and Xinluzao 33 cotton by 11.76% and 14.58%, respectively. Although differences in yields amongst different fields and cultivars were observed, the trends remained consistent and the yield in the treatment area remained higher than that in the control area (p = 0.027). Thus, the use of our microbial gents in the field effectively increases cotton yields.
Reduction of incidence rate and disease index of verticillium wilt in cotton
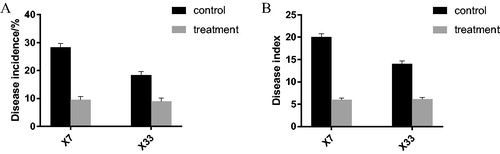
We investigated the disease prevention potential during the budding stage and the bolling stage. We studied 240 cotton plants in 4 plots across two parcels: a severely affected one and a mildly affected one. At the beginning of July (budding stage), all diseased plants were distributed in the severely affected parcel, but no diseased plants were found in the mildly affected parcel. The incidence and disease index of Xin Lu Zao 7 (CK) in the severely affected parcel were 28.3% and 20, respectively, whilst Xin Lu Zao 7 (TR) had disease rates and a disease index of only 9.5% and 6, respectively (). The control effect of the microbial agents on Xin Lu Zao 7 was 70%; the incidence and disease index of Xin Lu Zao No.33 (CK) were 18.3% and 14.03, respectively, whilst Xin Lu Zao No.33 (TR) had a disease rate and a disease index of only 8.9% and 6.13, respectively (). The control effect of the microbial agents on Xin Lu Zao 33 was 56.3%, indicating preventive effects on the outbreak of V. dahliae at the budding stage in severely diseased fields.
Figure 4. Effects of microbial agents on Verticillium wilt disease of cotton in the budding stage of nurseries. X7, Xin Lu Zao 7 (susceptible); X33, Xin Lu Zao 33 (resistant).
shows the disease prevention effects during the second episode in the middle of August (boll period). During the cotton bolling period, the disease incidence and index rates of Xin Lu Zao 7 (CK) were 18.3% and 14.45, respectively; the disease incidence and index rates of Xin Lu Zao 7 (TR) were 6.7% and 3.06, respectively (). The control effect of microbial agent on Xinluzao 7 was 78.8% and the incidence and disease index in Xinluzao 33 (CK) in the mild parcel were 10.6% and 7.78, respectively. However, Xin Lu Zao 33 (TR) showed disease incidence and index rates of only 5.6% and 2.5, respectively, with a control effect of 67.8% ().
Figure 5. Effects of microbial agents on the occurrence of cotton Verticillium wilt in the bolling period in the mildly affected parcel (A, B) and in the severely affected parcel (C, D).
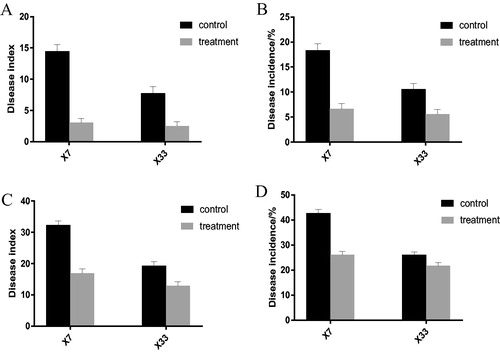
During the bolling period in the severely affected parcel, the incidence and disease index of Xin Lu Zao 7 (CK) were 42.8% and 32.36, respectively. In the severely affected parcel, the incidence and disease index of Xin Lu Zao 7 (TR) were only 26.1% and 16.96, respectively (). The control effect on Xinyuzao 7 was 47.6%. The incidence and disease index in Xin Lu Zao 33 (CK) in the severely affected parcel were 26.1% and 19.31. Xin Lu Zao 33 (TR) showed the incidence of disease and disease index values of 21.7% and 12.92, respectively (). The control effect was 33.1%.
Effects of the microbial agents on the disease status of verticillium wilt in cotton
Following the application of microbial agents, no diseased plants were evident in the first peak (budding stage) in the mildly affected parcel. The application of microbial agents to the severely affected parcel reduced the Grade 4 disease rates from 35% in Xin Lu Zao 7 (CK) to 12% in Xin Lu Zao 7 (TR) (). Grade 4 and Grade 3 in Xin Lu Zao 33 showed a significant reduction in TR plants, particularly Grade 4, which declined from 30% in CK to 6% in TR (). The disease status of the plants was reduced to Grade 1 and Grade 2 for Xin Lu Zao 33 in response to the microbial agents ().
Figure 6. Incidence grades of Verticillium wilt in cotton plants at different stages and areas: bud stage in heavy disease areas (A, B), boll stage in light-disease areas (C, D), ball stage in heavy disease areas (E, F).
Note: X7CK: Xin Lu Zao 7 without treatment; X7TR: Xin Lu Zao 7 with treatment; X33CK: Xin Lu Zao 33 without treatment; X33TR: Xin Lu Zao 33 with treatment. Incidence Grades (1) yellowing or withering leaves less than 25%; (2) yellowing or withering leaves 25–50%; (3) yellowing or withered leaves ranging from 50% to 75%; (4) yellowish withered leaves greater than 75%.
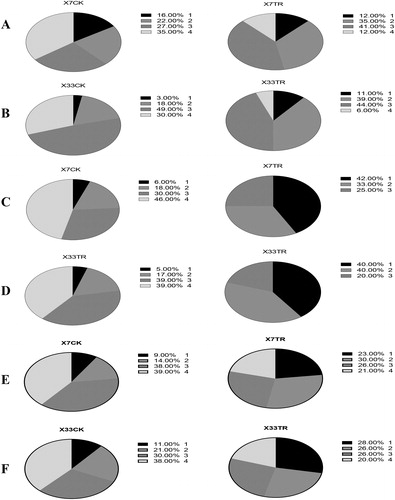
We also investigated the disease conditions during the second peak (boll stage) of cotton. The results showed that, in the mildly affected parcel, the morbidity grades were 4 and 3, accounting for 46% and 30% in the Xin Lu Zao 7 CK group, respectively, whilst Grade 2 and Grade 1 accounted for 18% and 6%, respectively. Grade 4 was not found in Xin Lu Zao 7 TR and Grade 3 was reduced to 25%. The major morbidity grades were mainly Grade 2 and Grade 1, accounting for 33% and 42% respectively (). Xin Lu Zao 33 cotton plants showed similar results to Xin Lu Zao 7 cotton plants (). The two varieties of cotton in the severely affected parcel during the bolling period had serious outbreaks of Verticillium wilt with a high incidence. Xin Lu Zao 7 CK showed Grades 4 and 3, accounting for 39% and 38% of the plants, respectively, which decreased to 21% and 26% in TR (). There was a shift toward grade 1 and 2 disease levels, which increased from 9% and 14% in CK to 23% and 30% in TR (), indicating that the application of microbial agents reduced the incidence of Verticillium wilt, and alleviated the critical illness grade to mild disease ().
The results from this study suggest that the mixed culture of Bacillus tequilensis C-9 and Sphingobacterium A1 strains could be further explored as a potential biocontrol measure against Verticillium wilt of cotton. This disease is a global problem [Citation24], which spread to China in 1935 by the introduction of American SPAR cotton seeds. After the 1990s, the disease had spread to Cotton areas including Xinjiang, especially in 1993, when the affected area was as high as 2.67 million hm2 with a 100 million kilogram cotton loss. In the years that followed, in most of China's cotton growing areas, including Xinjiang province, the damage has continued to grow: annual occurrence area of about 3 million hm2, the annual economic loss amounting to about 200 million yuan [Citation25, Citation26]. These non-specific above-ground symptoms, however, lead to the excess overuse of fertilizers and ineffective treatments with pesticides [Citation27].
A lot of work has been done to prevent Verticillium wilt of cotton. At present, chemical pesticides are mainly used in the prevention and control of Verticillium wilt in China. These highly toxic, highly residual chemicals are difficult to degrade, which brings about serious damage to the ecosystem in the long run. With the enhancement of people's awareness of environmental protection, the development of biological prevention measures are gaining attention. Pollution-free biological pesticides have become a research hotspot [Citation28, Citation29]. Among all the biological pesticides, biocontrol bacteria or their metabolites are used to control plant diseases, and the work principle is to balance the beneficial and harmful microorganisms around host plants, so as to achieve the purpose of disease prevention and yield increase [Citation30–32]. Bacterial species that have been extensively studied as biocontrol agents belong to the genera Bacillus, Burkholderia, Collimonas and Pseudomonas [Citation33–35] and promote plant growth through a variety of mechanisms [Citation36, Citation37].
The occurrence of Verticillium wilt in cotton is particularly serious in Xinjiang in China, where the cotton planting area is large and the duration of continuous cropping is longer. Over 90% of the planting process in Xinjiang cotton fields adopts drip irrigation methods [Citation38]. This imposes a higher requirement for bacterial agents. Water-dispersible fungicides meet this requirement and effectively control the occurrence of V. dahliae, leading to economic benefits.
Based on the results from the present study, we could speculate that the mechanisms underlying the effect of the mixed bacterial culture filtrate probably include induction of oxidative stress through the production of ROS; osmotic stress and CWI pathway.
Cotton may also be protected from various pathogens, pests and abiotic stresses by plant defenses such as salicylic acid (SA), jasmonic acid (JA). gibberellic acid (GA), cytokinin (CK) and brassinosteroids (BR) signaling [Citation39]. Cryptochrome-1 (CRY1), DNA replication licensing factor MCM2 (MCM2) and 5’3’-exoribonuclease 3 (XRN3) also respond to Verticillium wilt infection and play a vital role in plant immunity [Citation40, Citation41]. Cotton plants can directly or indirectly induce defense responses. In this study, treatment with our experimental fungicidal agent containing mixed cell-free culture filtrate induced defense systems in cotton plants and regulated cotton development, thus promoting cotton plant growth. We developed experimental fungicidal agent bacterial cultures in water droplets that inhibited cotton Verticillium wilt in diseased gardens, with increasing cotton yields. At an application rate of 20 g/m2, favorable control effects were observed. This indicated that the microbial agent could effectively inhibit field cotton Verticillium wilt.
According to GB China National Standards, application of the experimental fungicide with drip irrigation reduced disease incidence and morbidity. Investigations on the effect of the biocontrol agent on the beneficial and pathogenic bacterial flora in the field are also underway at our laboratory. We suggest that this represents a potentially effective method to reduce V. dahliae in the soil to achieve disease prevention.
Conclusions
The mixed fermentation filtrate of Bacillus tequilensis C-9 and Sphingobacterium A1 when applied as a biocontrol agent can inhibit V. dahliae in vitro and in vivo. The fungicidal effect was associated with increasing yield and promoting the growth of two different varieties of cotton.
| Abbreviations | ||
| ANOVA | = | analysis of variance |
| BE | = | biocontrol efficacy (%) |
| CFU | = | colony-forming unit |
| DI | = | disease incidence rates (DI %) |
| DS | = | disease status |
| PDA | = | potato dextrose agar |
| PDB | = | potato dextrose broth (liquid medium) |
| PGPR | = | plant-growth-promoting rhizobacteria |
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Woolliams GE. Host range and symptomatology of Verticillium dahliae in economic, weed, and native plants in interior British Columbia. Can J Plant Sci. 1966;46(6):661–669.
- Krikun J, Bernier CC. Infection of several crop species by two isolates of Verticillium dahliae. Can J Plant Pathol. 1987;9(3):241–245.
- Huang JL, Li HL, Yuan HX. Effect of organic amendments on Verticillium wilt of cotton. Crop Prot. 2006a;25(11):1167–1173.
- Klosterman SJ, Atallah ZK, Vallad GE, et al. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol. 2009;47(1):39–62.
- Bleve G, Grieco F, Cozzi G, et al. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int J Food Microbiol. 2006;108(2):204–209.
- Paolo C, Angioni A. Pesticide residues in grapes, wine, and their processing products. J Agric Food Chem. 2000;48(4):967–973.
- Gil-Serna J, Patiño B, Cortés L, et al. Mechanisms involved in reduction of ochratoxin A produced by Aspergillus westerdijkiae using Debaryomyces hansenii CYC. Int J Food Microbiol. 2011;151(1):113–118.
- Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agricultur. Appl Microbiol Biotechnol. 2009;84(1):11–18.
- Gomez LCC, Schiliro E, Valverde CA, et al. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces plant systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol. 2014;5(427):1–14.
- Brader G, Compant S, Mitter B, et al. Metabolic potential of endophytic bacteria. Curr Opin Biotechnol. 2014;27:30–37.
- Scherlach K, Hertweck C. Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem. 2009;7(9):1753–1760.
- Chagas FO, Dias LG, Pupo MT. A mixed culture of endophytic fungi increases production of antifungal polyketides. J Chem Ecol. 2013;39(10):1335–1342.
- Pettit RK. Small-molecule elicitation of microbial secondary metabolites. Microb Biotechnol. 2011;4(4):471–478.
- Thomas D, Heinze S, Schlegel B, et al. Formation of new lipoaminopeptides, acremostatins A, B, and C, by Co-cultivation of Acremonium sp. Tbp-5 and Mycogone rosea DSM 12973. Biosci Biotechnol Biochem. 2002;66(4):883–886.
- Glauser G, Gindro K, Fringeli J, et al. Differential analysis of mycoalexins in confrontation zones of grapevine fungal pathogens by ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry and capillary nuclear magnetic resonance. J Agric Food Chem. 2009;57(4):1127–1134.
- Pettit RK, Repp KK, Hazen KC. Temperature affects the susceptibility of Cryptococcus neoformans biofilms to antifungal agents. Med Mycol. 2010;48(2):421–426.
- Yashavantha Rao HC. A Co-culture of microbial endosymbionts increases the production of antimicrobial polyketide metabolites. EC Microbiol ECO. 2017;01:41–42.
- Kusari S, Hertweck C, Spiteller M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol. 2012;19(7):792–798.
- Vanitha SC, Umesha S. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol Plant. 2011;55(2):317–322.
- Han Q, Wu F, Wang X, et al. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol. 2015; 17(4):1166–1188.
- Liang M. Screening of abnormal nuclear development mutants and identification of related genes in Rotadella dahlia. Nanjing (China): Nanjing Normal University; 2015.
- De Souza JRB, Kupper KC, Augusto F. In vivo investigation of the volatile metabolome of antiphytopathogenic yeast strains against Penicillium digitatum using comprehensive two dimensional gas chromatography and multivariate data analysis. Microchemistry. 2018;141:204–209. [
- Li N, Wang W, Bitas V, et al. Volatile compounds emitted by diverse Verticillium species enhance plant growth by manipulating auxin signaling. MPMI. 2018;31(10):1021–1031.
- Yang Y, Wu ZM, Li KT. The peculiar physiological responses of Rhizoctonia solani under the antagonistic interaction coupled by a novel antifungalmycin N2 from Streptomyces sp. N2. Arch Microbiol. 2019;201(6):787–794.
- Xu L, Zhu L, Zhang X. Research on resistance mechanism of cotton to Verticillium wilt. AAS. 2013;38(9):1553–1560.
- Zhu HQ, Zili F, Yin Z, et al. Pathogenicity differentiation and ISSR fingerprint analysis of cotton Verticillium dahliae in China. Acta Phytopathol Sin. 2012;42(3):225–235.
- Wolfgang A, Taffner J, Guimarães RA, et al. Novel strategies for soil-borne diseases: exploiting the microbiome and volatile-based mechanisms toward controlling meloidogyne-based disease complexes. Front Microbiol. 2019;10:1296.
- Regev U, Gutierrez AP, DeVay JE, et al. Optimal strategies for management of Verticillium wilt. Agric Syst. 1990;33(2):139–152.
- Kefalogianni I, Gkizi D, Pappa E, et a1. Combined use of biocontrol agents and zeolite as a management strategy against Fusarium and Verticillium wilt. BioControl. 2017; 62(2):139–150.
- Yu GY, Sinclair JB, Hartman GL, et al. Production of iturin A by Bacillus amyloliquefaciens, suppressing Rhizoctonia solani. Soil Biol Biochem. 2002;34(7):955–963.
- Das P, Mukherjee S, Sen R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol. 2008;104(6):1675–1684.
- Zhang QH, Yang L, Zhang J, et al. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil. 2015;392(1-2):101–114.
- Garas NA, Acjr W. Differential accumulation and distribution of antifungal sesquiterpenoids in cotton stems inoculated with Verticillium dahliae. Phytopathology. 1986;84(11):4097–4105.
- Liu HY, Wang W, Zhang RF, et al. A survey of cotton wilt in the main cotton areas of Xinjiang. Plant Protect. 2015;41(3):138–142.
- Yin XS, Liu RJ. Research progress on cotton Verticillium wilt. Chin Cotton. 1996;5:2–6.
- Huang WK, Cui JK, Liu SM, et al. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalastrum racemosum, and Paecilomyces lilacinus, as being most effective. Biol Control. 2016; 92:31–37.
- Zhou ZH, Zhang TZ, Pan JJ, et al. Pathogenicity differentiation of Verticillium fungus on cotton varieties. Chin Agric Sci. 2000; 33(2):51–57.
- Li GY, Zhang XQ, Song YP, et al. Study on the occurrence trend and resistance of cotton Verticillium in cotton area of northern Xinjiang. Xinjiang Agric Sci. 2015;01:185–190.
- Ding Y, Sun T, Ao K, et al. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell. 2018;173(6):1454–1467.
- Jingo S, Jiang L, Yang J, et al. Over-expression of Oryza sativa Xrn4 confers plant resistance to virus infection. Gene 2018;639:44–51.
- Xu Y, Zhang Y, Cheng Y, et al. Transcriptome analysis reveals multiple signal network contributing to the Verticillium wilt resistance in eggplant. Sci Hort. 2019;256:108576.