Abstract
Drought seriously threatens tomato production worldwide. Despite much research on exogenous salicylic acid (SA) and Ca2+ improving plant resistance to biotic and abiotic stress, the molecular mechanisms of exogenous SA- and Ca2+-mediated drought resistance response in tomato remain unclear. In this study, we analyzed SA- and Ca2+ -induced differentially expressed transcripts under drought in tomato plants using cDNA-amplified fragment length polymorphism (cDNA-AFLP). In total, 34 transcript derived fragments (TDFs) were differentially expressed. The functions identified through NCBI BLAST alignment mainly involved signal transduction, amino acid metabolism, transcription factors, transfer transport and stress response. The quantitative real-time polymerase chain reaction results of 12 TDFs associated with drought response were consistent with the patterns of changes observed with cDNA-AFLP analysis. These differentially expressed transcripts may be used for functional verification, transgenic research and breeding of drought-resistant tomato varieties.
Introduction
Plants are vulnerable to stresses from the external environment, mainly including drought, heat, cold, salinity and toxic metals [Citation1]. In particular, crop yields and agricultural economies worldwide have suffered enormous losses due to these abiotic stresses [Citation2]. However, drought stress has become a top priority in plant research due to global climate change [Citation3]. Tomato is one of the most important horticultural crops and the second most important vegetable worldwide [Citation4]. Almost all varieties of cultivated tomato are sensitive to drought to varying degrees during the entire growth period, especially during the germination and seedling stages. Therefore, drought resistance is a target trait in tomato breeding programs.
Plant hormones play crucial roles in the resistance response against abiotic and biotic stresses. Naturally occurring salicylic acid (SA) regulates plant growth and participates in plant signal transduction in response to biotic and abiotic stresses [Citation5,Citation6]. Exogenous application of SA can affect many physiological processes, such as seed germination, photosynthesis, reactive oxygen species metabolism, osmotic adjustment, plant growth and fruit yield. Several studies have investigated whether SA can mitigate the adverse effects of water stress on barley, rice and safflower and have assessed its role in the adaptation of cell processes associated with plant tolerance [Citation7–9].
Ca2+ participates in plant signaling networks as an important secondary messenger [Citation10]. At present, several calcium signaling elements have been identified, including calcium signaling receptors such as calmodulin (CaM) and calcium-dependent protein kinases (CDPKs), intracellular calcium transport channels and calcium extravasation channels. Calcium can increase the thickness of the cell wall, promote the synthesis of defensive hormones, and alter oxygen activity. Exogenous applications of calcium induce resistance to drought, cold, heat, and salt stresses as well as modulate stress-induced reactive oxygen species (ROS) metabolism in plants, such as tobacco, bermudagrass and soybean [Citation11–14].
Screening for differentially expressed genes (DEGs) may uncover the relevant molecular mechanism. cDNA-amplified fragment length polymorphism (cDNA-AFLP) analysis is a quick and effective method to identify both known and unknown transcripts [Citation15]. This technique ligates adaptor molecules to digest double-stranded cDNA to greatly reduce the number of false positives. The cDNA-AFLP method has been used to confirm drought-related genes and to analyze differentially expressed transcripts in many plant species [Citation16–18]. Here, we analyzed SA- and Ca2+-induced differentially expressed transcripts under drought in tomato using cDNA-AFLP analysis. Our findings provide an insight into genes associated with the response to drought stress. Our study may provide a basis for cloning drought-related genes, which will be useful for understanding the response mechanism of exogenous SA and Ca2+ to enhance drought resistance in tomato and for breeding drought-resistant tomato varieties.
Materials and methods
Plant materials and drought treatment
The tomato cultivar Dongnong 11537 (provided by the Tomato Institute, Northeast Agricultural University) was used in this study. Tomato seeds were sown in pots filled with soil. Based on our previous research on the optimal spray concentration [Citation19], 0.3 mmol·L−1 SA and 10 mmol·L−1 CaCl2 were used in this study. Seeds were divided into the following six groups: CK1 (control group with normal watering), CK2 (control group with drought treatment only), CKS (control group with exogenous SA only), CKC (control group with exogenous CaCl2 only), S0.3 (exogenous SA group with drought) and C10 (exogenous CaCl2 group with drought). At the four-five leaf stage, the seedlings were sprayed for three consecutive days according to the corresponding conditions. Subsequently, the S0.3, C10 and CK2 groups were deprived of water continuously, and the CK1, CKS and CKC groups were maintained with full irrigation. Leaf samples were collected from each group at 0 h, 12 h, 24 h, 36 h, 48 h, 3 d and 5 d from the beginning of drought stress. Each group had 60 plants, and the experiments were repeated three times.
cDNA-AFLP analysis
Total RNA was extracted for each group using the TRIzol method [Citation20]. Double strand cDNA was synthesized using a PrimeScriptTM Double Strand cDNA Synthesis Kit (TaKaRa, Dalian). EcoR I and Mse I restriction enzymes were used to completely digest the double-stranded cDNA, and then the adaptors were used to ligate the fragments for subsequent amplification (Supplementary information Table S1). Preamplification polymerase chain reaction (PCR) was performed using the following program: 94 °C for 5 min; 24 cycles of 94 °C or 30 s, 56 °C for 1 min, and 72 °C for 1 min; and 72 °C for 10 min (ThermoFisher, USA). Subsequently, further selective PCR amplification was performed using the following program: 94 °C for 3 min; 12 cycles of 94 °C for 30 s, 65 °C for 30 s (−0.7 °C per cycle), and 72 °C for 1 min; and 25 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 1 min. The PCR products were separated by electrophoresis using 6% polyacrylamide gel [Citation21].
Sequence analysis and functional annotation
The target bands were selected from the differential bands in the cDNA-AFLP reaction, excised from the polyacrylamide gel and used as templates for re-amplification. The PCR products were purified with a PCR purification kit (Tiangen, China), cloned into the pMD18-T vector (Takara, Dalian) and sequenced. The homologous genes of each transcript derived fragment (TDF) sequence were searched in National Center for Biotechnology Information (NCBI) and SOL Genomics Network (SGN) databases after removing the vector sequence.
Validation of identified TDFs by quantitative real-time PCR (qRT-PCR)
Twelve TDFs were validated using qRT-PCR to verify the expression profiles obtained by cDNA-AFLP and to obtain a more detailed description of the differential expression patterns of the target TDFs. qRT-PCR was performed using AceQ® qPCR SYBR® Green Master Mix (Vazyme, USA) on a qTOWER3G Detection System (Analytik Jena, Germany). Each sample was replicated three times, and data were analyzed using the 2−△△CT method [Citation22]. The following PCR protocol was used: 95 °C for 10 min; and 40 cycles of 95 °C for 5 s, 59 °C for 15 s and 72 °C for 30 s. Subsequently, melting curve analysis was performed to detect primer dimerization and other artifacts of amplification. The actin gene (NM_001321306.1) was used as a reference control for normalization (Supplementary Information Table S2).
Results and discussion
Phenotypic changes of tomato under drought
To visually determine the optimal exogenous chemical concentration, we photographed plants in each group on the third day of drought stress. As shown in , there was no difference among plants in the CK1, S0.3 and C10 groups, which showed normal growth. However, CK2 plants showed wilting and leaf curling. Thus, these findings indicated that appropriate concentrations of SA and CaCl2 effectively improve the drought resistance of tomato by exogenous application.
cDNA-AFLP analysis
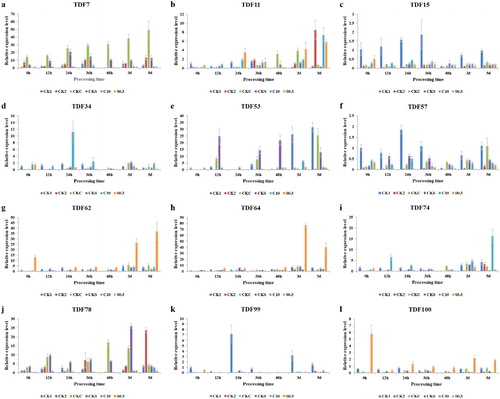
To identify DEGs induced by drought stress mediated by exogenous SA and CaCl2 in tomato, we analyzed the six groups via cDNA-AFLP analysis with 256 primer combinations. In total, 6650 clear bands were obtained. Of the 6650 TDFs, 1436 (approximately 21.6%) were differentially expressed and were involved in seven expression patterns (). Finally, 34 of these TDFs were sequenced successfully (). The sequenced TDFs were annotated by blasting against the SGN and NCBI databases.
Figure 2. Seven differential expression patterns were used to analyze the drought resistance response induced by exogenous SA and CaCl2 in tomato. (A) Expressed in all groups; (B) induced by combination of drought and CaCl2 (expressed in C10 only); (C) induced by combination of drought and SA (expressed in S0.3 only); (D) repressed by drought stress (expressed in CK1, CKC and CKS); (E) repressed by combination of drought and exogenous substances (expressed in CK1, CK2, CKC and CKS); (F) repressed by drought or exogenous substances (expressed in CK1 only); and (G) induced by drought stress (expressed in CK2, C10 and S0.3).
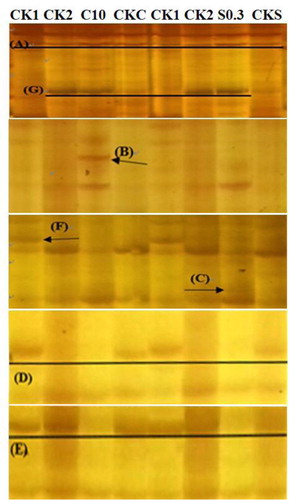
Table 1. Homology analysis of SA- and Ca2+-induced differentially expressed transcripts under drought in tomato.
Validation of expression patterns by qRT-PCR analysis
To verify the results of cDNA-AFLP analysis, 12 sequenced TDFs were selected for qRT-PCR using 3 biological replicates. These TDFs were selected based on significantly different expression patterns identified by cDNA-AFLP analysis and homology to genes known to have a role in stress response, signal transduction and photosynthesis. The expression patterns of the 12 TDFs are shown in . The same expression patterns were found for each TDF with qRT-PCR analysis and cDNA-AFLP analysis. As shown in , the expression of TDFs induced by CaCl2 and drought (TDF34 and TDF74) peaked at different time points with increases of 11.2-fold at 24 h and 16.2-fold at 5 d, respectively. This result suggested that TDF34 may be induced at the early stage of drought stress and that TDF74 may play an important role at the late stage of drought stress. TDF62, TDF64 and TDF100 were all induced by SA and drought, and their expression levels were significantly increased, with maximum increases of 36.9-fold, 76.9-fold and 5.8-fold, respectively. Among them, TDF62 and TDF64 had higher expression levels at 3 and 5 d. These results were highly consistent with the cDNA-AFLP analysis, indicating the reliability of the cDNA-AFLP results. Moreover, these TDFs may play crucial roles in the drought resistance response induced by exogenous SA and CaCl2 in tomato.
Sequence analysis and functional annotation
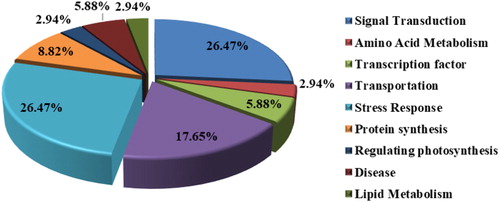
According to the annotation results, the functional classification of 34 TDFs mainly involved stress response, signal transmission and transportation (). In particular, the number of TDFs involved in stress response accounted for 26.47%. These TDFs may play an important role in the drought resistance mechanism of tomato.
The basic-leucine zipper (bZIP) family of transcription factors (TFs) is known to play a role in abiotic stress responses [Citation23]. TDF16 was identified as transcription factor POSF21 belonging to the bZIP class. Moreover, previous studies have suggested that POSF21 plays a positive role in the ABA signaling pathway and that POSF21 expression is positively modulated by ABA and cold stress treatment [Citation24]. TDF16 was induced by SA under drought stress, and the relationship and interaction among POSF21, SA and ABA under abiotic stresses deserves further study. Heat shock proteins (HSPs) are the key components that act as molecular chaperones responsible for protein translocation and degradation under stress conditions and in many normal cellular processes [Citation25,Citation26]. In the present study, TDF64 (HSP83-like) was induced by SA under drought stress, and the expression level peaked at 3 d under drought stress (). The molecular chaperone protein, HSP83, which is a homologue of HSP90, protects organisms and cells against thermal damage [Citation27,Citation28]. In the present study, however, transcriptional reprogramming analysis revealed that HSP also plays a critical role in the defense mechanism induced by exogenous SA during drought stress in tomato. In addition, the FLS2 gene (TDF100), encoding pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), was induced in response to drought stress (). Similarly, previous studies have shown that FLS2 is upregulated in grapevines under drought stress [Citation29]. However, the results of the present study help us to understand the response mechanism by which exogenous SA enhances drought resistance in tomato and suggest defense-related genes against drought stress for tomato breeding programs in the future.
Amino acids can improve plant resistance by regulating osmotic pressure, participating in ion transport and removing oxidative damage under various stresses. The transmembrane transport of these amino acids is critically linked to the specific amino acid transporter family protein [Citation30,Citation31]. In the present study, TDF34 was identified as a transmembrane amino acid transporter family protein, and the expression level of TDF34 was the highest at 24 h under drought stress (). Similar results have been observed in Populus simonii, in which amino acid transporter genes may contribute to drought stress tolerance [Citation32]. These results suggest that transmembrane amino acid transporter family proteins may play a critical role in the drought resistance response induced by exogenous Ca2+. In the present study, TDF74 was identified as the ATP-binding cassette (ABC) transporter G family member 11-like, and its expression level peaked on 5 d under drought stress (). ABC transporters are important in plant growth, development and environmental stress responses [Citation33,Citation34]. Matsuda et al. [Citation35] and Li et al. [Citation36] reported similar findings wherein the ABC transporter responded to drought and salt stress, respectively. MAG2 provides tolerance to common osmotic stresses [Citation37]. In this study, TDF98 was identified as the RINT1-like protein, MAG2, and was induced by exogenous Ca2+ under drought stress. However, these results suggest that MAG2 involves the response to drought resistance induced by exogenous Ca2+.
In this study, TDF11 was identified as 4-hydroxyphenylpyruvate dioxygenase-like (HPPD) and was induced under drought stress. In particular, the expression level of HPPD under exogenous SA or Ca2+ and drought stress treatment was significantly higher than that under drought stress alone (). The increase in HPPD expression might improve the resistance to drought in the plant. HPPD is associated with the biosynthesis of plastoquinones and tocopherols, and it participates in tyrosine catabolism [Citation38]. Similarly, Xu et al. [Citation39] have reported that HPPD is detected only under drought in Hippophae rhamnoides L. Previous studies have suggested that HPPD is important against different stresses as transcriptional regulators of tocopherol production [Citation40]. These results indicate that the expression of HPPD may play a critical role in drought defense. In the present study, TDF29 was identified as Auxin Regulated Gene involved in Organ Size (ARGOS) and was induced by drought, especially by drought and exogenous SA or Ca2+, and ARGOS was indicated to be involved in the ethylene signaling pathway, leading to enhanced drought tolerance. Moreover, ARGOS has been reported to improve stress resistance in tobacco, maize, wheat and Arabidopsis [Citation41–44].
Conclusions
In this study, we successfully analyzed the gene expression patterns associated with the exogenous SA- and Ca2+-induced defense response against drought stress in tomato. Several DEGs, including HSP, FLS2, transcription factor POSF21 and amino acid transporters, were determined to be related to drought resistance. These results facilitate our understanding of the potential mechanism by which exogenous SA and Ca2+ enhance drought resistance in tomato. More importantly, our study can provide a basis for cloning drought-related genes and breeding drought-resistant tomato varieties in the future.
Supplemental Material
Download PDF (89.2 KB)Acknowledgements
The authors thank the Key Laboratory of Cold Biology in Heilongjiang University, Key Laboratory of Hereditary Improvement and Facility for Cultivation of Horticultural Crops in the North of Heilongjiang Province.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Tamirisa S, Vudem DR, Khareedu VR. Overexpression of pigeonpea stress-induced cold and drought regulatory gene (CcCDR) confers drought, salt, and cold tolerance in Arabidopsis. J Exp Bot. 2014;65(17):4769–4781.
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14.
- Wu W, Ma BL, Whalen JK. Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: perspectives for stress adaptation from root system architecture. Adv Agronomy. 2018;151:87–157.
- Bhattarai K, Louws FJ, Williamson JD, et al. Diversity analysis of tomato genotypes based on morphological traits with commercial breeding significance for fresh market production in eastern USA. Aust J Crop Sci. 2016;10(8):1098–1103.
- Kang G, Li G, Guo T. Molecular mechanism of salicylic acid-induced abiotic stress tolerance in higher plants. Acta Physiol Plant. 2014;36(9):2287–2297.
- Sha HJ, Liu HL, Hu BW, et al. Effect of salicylic acid on the dry matter and nitrogen accumulation, partitioning and translocation in two contrasting rice genotypes under salt stress. Pak J Bot. 2019;51(5):1541–1550.
- Fayez KA, Bazaid SA. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agric Sci. 2014;13(1):45–55.
- Farooq M, Wahid A, Lee DJ, et al. Drought stress: comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J Agronomy Crop Sci. 2010; 196(5):336–345.
- Chavoushi M, Najafi F, Salimi A, et al. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind Crops Prod. 2019; 134:168–176.
- Cacho M, Domínguez AT, Elena-Rosselló JA. Role of polyamines in regulating silymarin production in Silybum marianum (L.) Gaertn (Asteraceae) cell cultures under conditions of calcium deficiency. J Plant Physiol. 2013;170(15):1344–1348.
- Hu W, Tian SB, Di Q, et al. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress.Photosynthetica. 2018; 56(4):1204–1211.
- Shi H, Ye T, Zhong B, et al. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J Integr Plant Biol. 2014;56(11):1064–1079.
- An B, Li B, Qin G, et al. Exogenous calcium improves viability of biocontrol yeasts under heat stress by reducing ROS accumulation and oxidative damage of cellular protein. Curr Microbiol. 2012;65(2):122–127.
- Wang X, Komatsu S. Proteomic analysis of calcium effects on soybean root tip under flooding and drought stresses. Plant Cell Physiol. 2017; 58(8):1405–1420.
- Song Y, Wang Z, Bo W, et al. Transcriptional profiling by cDNA-AFLP analysis showed differential transcript abundance in response to water stress in Populus hopeiensis. BMC Genomics. 2012;13(1):286.
- Alimohammadi A, Shiran B, Martínez-Gómez P, et al. Identification of water-deficit resistance genes in wild almond Prunus scoparia using cDNA-AFLP. Sci Hortic. 2013;159:19–28.
- Bidabadi MS, Shiran B, Fallahi H, et al. Identification of differential expressed transcripts of almond (Prunus dulcis ‘Sefied’) in response to water-deficit stress by cDNA-AFLP. J For Res. 2015; 20(4):403–410.
- Archangi A, Heidari B, Mohammadi-Nejad G. Association between seed yield-related traits and cDNA-AFLP markers in cumin (Cuminum cyminum) under drought and irrigation regimes. Ind Crops Prod. 2019;133:276–283.
- Zhang D, Xu X. Effect of salicylic acid and calcium chloride on physiological characteristics of tomato seed-lings under drought stress. Zhejiang Agric J. 2016;28(10):1687–1694.
- Hummon AB, Lim SR, Difilippantonio MJ, et al Isolation and solubilization of proteins after TRIzol extraction of RNA and DNA from patient material following prolonged storage. Biotechniques. 2007;42(4):467–472.
- Bassam BJ, Caetano-Anollés G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196(1):80–83.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Cao L, Lu X, Zhang P, et al. Systematic analysis of differentially expressed maize ZmbZIP genes between drought and rewatering transcriptome reveals bZIP family members involved in abiotic stress responses. Int J Mol Sci. 2019; 20(17):4103.
- Zhong B. Function analysis of PYR/PYL interacting protein POSF21 in Arabidopsis. Chinese Academy of Sciences (Wuhan Botanical Garden); 2016.
- Haider MS, Kurjogi MM, Khalil-Ur-Rehman M, et al. Grapevine immune signaling network in response to drought stress as revealed by transcriptomic analysis. Plant Physiol Biochem. 2017;121:187–195.
- Park CJ, Seo YS. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J. 2015;31(4):323–333.
- Xu J, Shu J, Zhang Q. Expression of the Tribolium castaneum (Coleoptera: Tenebrionidae) hsp83 gene and its relation to oogenesis during ovarian maturation. J Genet Genomics. 2010; 37(8):513–522.
- Dong H, Xu B, Ji K. Comparative transcriptome analysis of genes involved in response to thermal stress and leaf colour change of Acer palmatum. Sci Hortic. 2019;255:77–85.
- Haider MS, Zhang C, Kurjogi MM, et al. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci Rep. 2017;7(1):1–15.
- Popova OV, Dietz KJ, Golldack D. Salt-dependent expression of a nitrate transporter and two amino acid transporter genes in Mesembryanthemum crystallinum. Plant Mol Biol. 2003;52(3):569–578.
- Aranjuelo I, Molero G, Erice G, et al. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J Exp Bot. 2011;62(1):111–123.
- Chen J, Song Y, Zhang H, et al. Genome-wide analysis of gene expression in response to drought stress in Populus simonii. Plant Mol Biol Rep. 2013;31(4):946–962.
- Matsuda S, Funabiki A, Furukawa K, et al. Genome-wide analysis and expression profiling of half-size ABC protein subgroup G in rice in response to abiotic stress and phytohormone treatments. Mol Genet Genomics. 2012;287(10):819–835.
- Nguyen VNT, Moon S, Jung K-H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J Plant Physiol. 2014;171(14):1276–1288.
- Matsuda S, Takano S, Sato M, et al. Rice stomatal closure requires guard cell plasma membrane ATP-binding cassette transporter RCN1/OsABCG5. Mol Plant. 2016;9(3):417–427.
- Li W, Zhang C, Lu Q, et al. The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J Plant Physiol. 2011;168(15):1743–1752.
- Zhao P, Lu J. MAIGO2 is involved in gibberellic acid, sugar, and heat shock responses during germination and seedling development in Arabidopsis. Acta Physiol Plant. 2014;36(2):315–321.
- Chaudhary N, Khurana P. Cloning, functional characterisation and transgenic manipulation of vitamin E biosynthesis genes of wheat. Funct Plant Biol. 2013;40(11):1129–1136.
- Xu G, Li C, Yao Y. Proteomics analysis of drought stress-responsive proteins in Hippophae rhamnoides L. Plant Mol Biol Rep. 2009;27(2):153–161.
- Ji CY, Kim YH, Kim HS, et al. Molecular characterization of tocopherol biosynthetic genes in sweetpotato that respond to stress and activate the tocopherol production in tobacco. Plant Physiol Biochem. 2016;106:118–128.
- Kuluev B, Mikhaylova E, Ermoshin A, et al. The ARGOS-LIKE genes of Arabidopsis and tobacco as targets for improving plant productivity and stress tolerance. J Plant Physiol. 2019;242:153033.
- Shi J, Gao H, Wang H, et al ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J. 2017;15(2):207–216.
- Zhao Y, Tian X, Li Y, et al. Molecular and functional characterization of wheat ARGOS genes influencing plant growth and stress tolerance. Front Plant Sci. 2017;8:170.
- Shi J, Habben JE, Archibald RL, et al. Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol. 2015;169(1):266–282.