Abstract
This mini review presents the standard methods for the analytical identification of virus species. These methods have been trusted and known as accredited methods. The development of new and improved analytical techniques is important to overcome the lack and the drawbacks of the well-known methods. Due to the uncertain and unprecedented time of pandemic, new approaches with high performance in the analysis are needed to retain the required and growing diagnostic tools complementary to real-time polymerase chain reaction (RT-PCR) for COVID-19 diagnosis. The researchers are still developing direct methods for the detection of SARS-CoV-2 in a short turnaround time. The process of analytical validation is an important part of experimentation that is needed for the developed methods to be trusted. This review presents the major standard, commercial and designed methods for detecting SARS-CoV-2 and their analytical performance. Also, chromatographic techniques have been introduced as a rapid examination for SARS-CoV-2 identification, and the newly developed assays have been addressed.
Introduction
The term pandemic has been applied to a few diseases in history and appeared today on the global scene through coronavirus disease (COVID-19) spreading over the world. The people and governments are endeavouring to avoid and suppress the COVID-19 outbreak. At first, the research and diagnosis were slow if compared to this pandemic's rapid flurry. COVID-19 was discovered and diagnosed for the first time in Wuhan city in China in December 2019. The beta coronavirus (βCoV) which causes COVID-19 was identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and detection became easier [Citation1–3]. Most countries, including the United States and Saudi Arabia, confirmed the first cases between January and March 2020 [Citation4]. One of the challenges is that the COVID-19 symptoms only appear within two weeks, and the patients who do not know about their diagnosis will deal with other people and may potentially be infectious. Moreover, there are limited reagents for commercial examination to meet the standard COVID-19 tests' high consumption rates. Therefore, to combat this pandemic, the utmost important step for confronting the propagation and spreading of COVID-19 is the accurate and rapid detection methods of the virus [Citation5]. Numerous diagnostic techniques in clinical, and public health applications were used to detect the coronavirus [Citation6–15]. Direct tests detect the infection immediately by measuring the viral RNA, whereas indirect tests measure the virus antibodies in a host [Citation6]. To make quick and adequate clinical decisions during a pandemic, a diagnostic test method should have adequate sensitivity and accuracy.
The scientists and researchers have presented several efforts to apply and develop accredited methods to accelerate the testing results. Mainly, these methods depend on real-time polymerase chain reaction (RT-PCR). In addition, the researchers spent their efforts to explore different sources that COVID-19 could be tested for, either from the patients or health workers [Citation16,Citation17]. One of these studies examined the pathogens in sewage by investigating the collected samples from wastewater treatment plants as valuable data points measuring viral content, which is recently well recognized as wastewater-based epidemiology (WEB) [Citation18,Citation19]. The application of these different samples has revealed that SARS-CoV-2 presented in patients' stool with an appreciative percentage above 16.5% [Citation20–23]. On the other hand, the chromatographic techniques used through the chromatography principle have been favoured. The scientists applied the chromatographic techniques for separating materials to develop serological tests such as immunochromatographic lateral flow assay (LFA) for COVID-19 detection [Citation24–26].
This mini review presents the principle of the well-established analytical methods used either for research use only (RUO) or for diagnosis, comprising the ePlex® SARS-CoV-2 and RealStar® SARS-CoV-2 RT-PCR (real-time polymerase chain reaction) kits, and compared with each other [Citation6–15]. In addition, the rapidly developed analytical methods for the diagnosis of COVID-19, which are highly sensitive, specific, and accurate, are presented, and the analytical validation is discussed. Furthermore, the different environmental samples, airborne and respiratory specimens that are nasopharyngeal (NP) and bronchoalveolar lavage (BAL) are examined. Finally, chromatographic techniques have been introduced as a rapid examination for COVID-19 diagnosis, and the newly developed methods have been addressed.
Standard and developed methods for COVID-19 diagnosis
Analytical principle of RT-PCR technique
The polymerase chain reaction (PCR) is a simple approach that uses a DNA template to amplify in vitro unique DNA fragments. Traditional methods of replicating a DNA sequence in a living cell into a vector are often very time-consuming and labor-consuming, whereas PCR only takes a few hours to replicate the DNA sequences of interest. Although most biochemical tests work with a large quantity of biological material, for example nucleic acid recognition with radioisotopes, the quantitiy is very small with PCR. Thus, in less time, PCR can perform accurate detection and greater amplification rates than other methods. Reverse transcription PCR integrates two flexible and robust methodologies, reverse transcription and chain reaction of the polymerase, to create and amplify cDNA from RNA or mRNA transcripts. The acceptable reverse transcription PCR demands a significant fidelity of amplification. The RNA must be translated into cDNA first to obtain a thermostable DNA template for the polymerase to use in PCR in the test for RNA (). This procedure is termed reverse transcription, hence the name of reverse transcription PCR.
Figure 1. Diagram of RT-PCR approach (https://worldwide.promega.com. ).
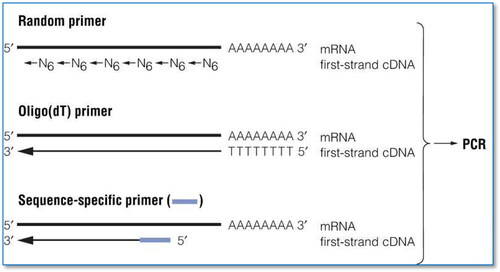
One of the most important prerequisites to obtaining correct findings is understanding the molecular examination's analytical performance for defining the RNA sensitivity detection. In general, the RT-PCR technology amplifies and accumulates the specific target sequences for the detection of RNA. Then, through the fluorescent reporter dye/molecule, the amplification progress will be monitored during PCR reactions, and the amount of the emitted fluorescence will be detected [Citation27]. The detection technique used is TaqMan™ probes, which depends on the examination made of an oligonucleotide sandwiched between the two PCR primers marked with a fluorophore that is linked covalently to a quencher and a reporter. The emitted fluorescence is inhibited so long as the reporter and the quencher are close. Several reporter dyes are successfully used for labelling in TaqMan probes such as FAM, Cy5 and JOE [Citation28–31].
The availability of genetic data allows the development of the primers and probes needed for the progress of SARS-CoV-2-specific testing [Citation32]. RT-PCR depends on the capability to transmit a limited genetic viral quantity in a specimen and is known as the gold standard for SARS-CoV-2 virus recognition. RT-PCR testing for COVID-19 has been used for ocular secretions, saliva, or serum a few reports [Citation33,Citation34].
Recently, the Rutgers Laboratory discovered an RT-PCR assessment that collects saliva samples faster and far less painfully than most other sample collection approaches which reduces the risk to healthcare professionals, and may lead to a greater volume of experiments [Citation35,Citation36]. As the PCR progresses, the DNA amplification in RT-PCR is monitored in real-time by applying a dye or probe labelled with a quencher and a fluorescent substance, such as in the case of TaqMan tests [Citation32]. Normally, RT-PCR is performed as a one- or two-step approach. One-step real-time RT-PCR utilizes a single tube having the required primers to complete the RT-PCR process. Two-step real-time RT-PCR requires two tubes to separate between reverse transcription and amplification processes from the assay, but it provides additional flexibility and greater sensitivity than the one-step approach [Citation37,Citation38]. In general, the one-step technique is the favoured method for SARS-CoV-2 detection. It is reliable to operate. It includes restricted handling of the sample, minimized test time and reduced errors.
A notable example to examine the performance of the standard methods for COVID-19 diagnosis is the method described by Radbel et al. [Citation29]. The analysis of different samples such as OP and saliva testing compared to NP using the well-known RT-PCR method was reported. The contribution of this investigation is the use of phosphate-buffered saline (PBS) as a preservation medium instead of a viral transport medium (VTM) [Citation39,Citation40]. The preparation in 2 mL screw-top vials was contained in a Hanks’Balanced Salt Solution (HBSS), foetal bovine serum with a final percentage of 2%, 0.5 µg/mL of amphoteric B 100 µg/mL of gentamicin. According to the approved protocol by the Rutgers Institute Review Board (protocol number Pro2020000800), the respiratory excretions collected from 16 samples showed COVID-19 positive subjects within four days [Citation39]. The collected specimens were kept in vials containing both preservation media, as well as VTM and PBS. The experiments were divided into two protocols, the first one with the standard preservation medium VTM and the second one with PBS. The accurate evaluation for detection of SARS-CoV-2, quantitative PCR (qPCR), was performed and compared the cycle threshold (Ct) values for three SARS-CoV-2 viral genes, specifically nucleocapsid (N), open reading frame 1ab (ORF1ab) and spike protein (S) genes. For the examination of positive control, bacteriophage MS2 (MS2) spiked into the samples was utilized. The viral RNA extraction was performed as the manufacturer described (PerkinElmer) on a Chemagic 360 instrument. Briefly, 4 µL of poly(A) RNA, 10 µL of proteinase K, 300 µL of lysis buffer, and 8 µL of MS2 were added into each sample. Then, 300 µL of each TaqPathCOVID-19C positive and nCOV negative controls were added, followed by 150 µL of magnetic beads and 900 µL RNA binding buffer. After washing the mixture of beads/RNA with 500 µL of both buffer 3 and buffer 4, respectively, the 50 µL of extracted RNA final volume sample was eluted through the elution buffer.
The amplification of the extracted RNA was performed on the RT-PCR system to detect the viral genes mentioned above. This method received a great attention as the reaction was performed in 20 µL-volumes and ran through the following program: for 2 min the temperature was kept at 2 °C, then the temperature increased sharply to 53 °C for 10 min, after that 95 °C for 2 min, then 95 °C for 3 s, and finally 60 °C for 30 s. The fluorescence signal collected during the final 60 °C step was repeated for a total of 40 cycles. Tests were conducted in triplicate, and the lower limit of detection (LOD) of SARS-CoV-2 is 200 copies/mL. A strong correlation of the values was observed in both the transport media, and confirmed that either the VTM or PBS could be used, providing a new solution for preserving the media and storing the samples with efficacy [Citation40].
Isothermal nucleic acid amplification
For each loop, RT-PCR needs many temperature changes, requiring an advanced thermal cycling instrument [Citation41]. The amplifier of isothermal nucleic acid is an additional approach that enables magnification at a constant temperature and removes the thermal cycler requirement. Consequently, many methods that are based on this theory have been developed.
Reverse transcription loop-mediated isothermal amplification (RT-LAMP)
RT-LAMP was performed as an alternative approach to SARS-CoV-2 detection for fast and cost-effective research [Citation42]. RT-LAMP uses four specific primers to the targeted gene/area to enhance the response and integrates a reverse transcription stage to detect RNA. To identify the amplification product, this technique measures the turbidity resulting from precipitated magnesium pyrophosphate in solution as an amplification byproduct. To record the process in real-time, the method uses intercalating dyes to calculate the turbidity or fluorescence. Because only heating and optical observation are needed for real-time RT-LAMP diagnostics, its versatility and responsiveness make it an excellent applicant for virus detection [Citation42].
CRISPR-based assays
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) are a group of nucleic acid sequences contained in bacteria. A collection of bacterial enzymes, called CRISPR-associated enzymes, can recognize and cut these sequences identified by Cas9, Cas12 and Cas13. Some enzymes were designed to attack and cut the viral RNA sequence in Cas12 and Cas13 [Citation43]. These CRISPR-based systems do not need complex equipment, and are read utilizing strips of paper to confirm the location of the SARS-CoV-2 virus, avoiding missing of responsiveness or precision. These experiments are low-cost and could be completed in as little as one hour. Such assessments are immensely promising for the diagnostic process at the point of treatment [Citation44–47].
Serological and immunological assays
Although viral RNA detection based on RT-PCR has also been commonly used in the diagnosis of COVID-19, it would not be used to track the progression of the phase of the infection. It cannot be used to identify immunity and past diseases broadly [Citation44,Citation45]. The serological analysis is characterized as blood, serum, or plasma examination. The immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in sputum and saliva can be checked. This assay performs a remarkable role in epidemiology and vaccines' progress, offering an evaluation of both the short-term antibody response, and the abundance and diversity of antibodies. After a few days of infection, IgM becomes observable in serum first, followed by IgG, which appears after a few weeks. So, IgM can be an early-stage disease indicator.
In recent years, not only for the detection of antibodies themselves but also for applying antibodies to the detection of pathogen-based antigens, immunological tests' specificity and responsiveness have been improved. These experiments have tremendous potential for COVID-19 epidemiology. Still, at least three circumstances will affect the test results: (1) a group of subjects with positive molecular and genetic assays for SARS-CoV-2 infections is seronegative due to delays in developing antibodies following infection. (2) Samples could be seropositive but negative for results of a molecular genetic assay representing recent, milder disease. (3) Restriction in precision and specificity of the assessment [Citation48].
Enzyme-linked immunosorbent assay (ELISA)
ELISA is a plate-based approach that evaluates and measures molecules such as proteins, antibodies, peptides and hormones. The test could be qualitative or quantitative, and the time for getting the results usually varies between 1 to 5 h. The plate micro-wells are normally coated with a viral protein as in the case of SARS-CoV-2, as seen in . If antibodies are present in the patients’ specimens, they directly bind to the viral protein coated on the microplate. The complex can be identified with a conjugated secondary antibody. After that, the enzymatic conversion of the substrate generates a measurable colour change. ELISA is fast, has the opportunity to verify different specimens, and can be optimized for enlargement automation but can vary intolerance and is appropriate for point-of-care assessments [Citation32, Citation49–53].
Figure 2. Two different ELISA assays to detect SARS-CoV-2 by indirect assay (A) or direct assay (B) [Citation42].
![Figure 2. Two different ELISA assays to detect SARS-CoV-2 by indirect assay (A) or direct assay (B) [Citation42].](/cms/asset/9a9bac3e-6e69-4066-a4e3-5f5e134841f8/tbeq_a_1865838_f0002_c.jpg)
Different serological assays have been used to identify SARS-CoV-2; including ELISA, immune-fluorescent assay and the immunochromatographic assay [Citation54,Citation55]. The fast and easy approach of SARS-CoV-2 serodiagnosis may aid in diagnosing the disease. Many reports of SARS infection showed that the IgM antibody was detected in serum over 3–6 days [Citation56–58]. The immune-chromatographic approach is considered quick and efficient, commonly utilized in several diseases, including infectious diseases [Citation59]. Its theory is straightforward and facile, and embraced by physicians, making it particularly appropriate for a severe epidemic situation, such as the COVID-19 pandemic caused by SARS-CoV-2, which to spread rapidly across the globe within two months. Accurate detection of the illness and isolation of patients is currently believed to be the best way to prevent and monitor COVID-19 spread. Therefore, a responsive immunofluorescent test approach has been progressed to detect specific IgM and IgG against SARS-CoV-2 in human serum rapidly, within 10 min. The SARS-CoV-2 recombinant nucleocapsid protein has been utilized as a catch antigen. Fluorescent microsphere Lanthanide, Eu(III), has been used to assess the solid phase immune-chromatographic experiment in qualitative/semi-quantitative terms. The results revealed that the quick immune-assay is highly responsive and precise and was valuable for quick serodiagnosis of COVID-19 [Citation60].
Analytical assessment methods for COVID-19 diagnosis
Improvement of the analytical detection methods of SARS-CoV-2
A notable example of this group of analytical methods are the techniques described by Uhlig et al. reported two developed assays for the molecular detection of SARS-CoV-2 [Citation61]. These methods were the ePlex® SARS-CoV-2 and CDC COVID-19 RT-PCR assays. Both of the developed methods were compared to the accredited traditional test (RealStar® SARS-CoV-2 RT-PCR). The clinical specimens used in this study include NP and BAL. The accuracy and the analytical sensitivity of the research study were examined using a negative matrix for SARS-CoV-2 archived frozen specimens (precisely NP and BAL). For the nucleic acid extraction procedure for both RealStar® SARS-CoV-2 and CDC tests, the automated extraction of nucleic acid was conducted applying either the eMAG or NucliSENS easyMag instruments (bioMérieux, Marcy-l'Étoile, France). Briefly, 2 mL of lysis buffer (easyMag/eMAG) was added into 500 µL of the input volume of all the specimens, followed by incubation for 10 min. Then, the bioMérieux nucleic acid extraction protocol was implemented, and 50 µL of the final sample volume was eluted. For the amplification and detection procedures, the 30 µL of total volume sample was prepared by adding 20 µL of MasterMix to 10 µL of extracted sample for the commercial method of RealStar® SARS-CoV-2 RT-PCR. MasterMix has A and B mixed solutions containing primers and probes, PCR buffer, reverse transcriptase, magnesium salt, and DNA polymerase. The Prism 7500 Sequence evaluation system, was applied to perform TaqMan RT-PCR under the following cycling conditions: for 20 min, one cycle at 55 °C followed by 2 min at 95 °C and 45 cycles at 95 °C for 15 s, 55 °C for 45 s and then 72 °C for 15 s. The detectors used FAM, Cy5 and JOE for B-βCoV, SARS-CoV-2 and Internal Control. The volume of the sample in the CDC COVID-19 RT-PCR assay was 20 µL. For control samples, the same procedure was followed by adding a control sample instead of the removed model by using both the Hs_RPP30 Positive Control and IDT 2019-nCoV_N_Positive Control plasmids. IDT primers and probes lot #0000500383 were used with TaqPathTM 1-Step RT-qPCR Master Mix (4x) provided from ThermoFisher to perform the CDC method. The detection was conducted into three reactions, separately per sample for each target (N1, N2 and internal control). The Prism 7500 Sequence detection system (Applied Biosystems, Foster City, CA) was applied to perform TaqMan RT-PCR under the following cycling conditions: for 2 min, one cycle at 25 °C; followed by 15 min with 50 °C, one cycle for 2 min at 95 °C and 45 cycles at 95 °C for 3 s, then 55 °C for 30 s. The detector used for all the targets was FAM. In the ePlex® SARS-CoV-2, the method depends on the automated extraction of nucleic acid, amplification, and detection through a single-use ePlex cartridge [Citation62].
In evaluating and comparing the analytical sensitivity for the methods mentioned above, the authors spiked the eluates with serially diluted SARS-CoV-2 whole viral genomic materials. The results presented that the LOD of the RealStar® SARS-CoV-2 RT-PCR for NP and BAL specimens were at 1200 and 12,000 copies/mL, respectively. While for the CDC COVID-19 RT-PCR test, the LOD for both NP and BAL specimens was 1200 cp/mL. Also, the LOD of the ePlex® SARS-CoV-2 test for NP was 600 cp/mL, whereas there was no LOD for BAL specimens. All three methods were compared in analytical performance, and the results showed 100% agreement for both negative and positive specimens presenting that the developed methods produced accurate results. One of the main important parameters for analytical performance is the reproducibility of the methods and the results' agreement. Replicate testing was performed for all three assays. The authors assessed the test through spiking NP and BAL specimens with SARS-CoV-2 genomic substance and replicated over three separate runs after three-time extractions, separately. The results of spiked NP and BAL specimens for RealStar® SARS-CoV-2 RT-PCR and CDC COVID-19 RT-PCR tests were 1200 cp/mL (n = 20) and 120,000 cp/mL (n = 21) and 1200 cp/mL (n = 26) and 1200 cp/mL (n = 27), respectively. In comparison with the ePlex® SARS-CoV-2 assay, the overall runs showed high precision, and the reproducibility of the results showed 100% agreement. From the results of these experiments, both developed methods and the commercial tests' analytical performance is analogous.
Latest developed analytical detection methods for SARS-CoV-2
Due to RT-PCR tests' limitations in the detection of SARS-CoV-2, especially the long turnaround time, the researchers' efforts have developed serological tests such as immunochromatographic lateral flow assay (LFA) for COVID-19 diagnosis [Citation24–26]. The chromatography techniques generally have been used to separate materials from mixtures on an adsorbent column in a flow system. Immunochromatography is a biophysical method to divide and distribute the components between two phases: the stationary and the mobile phases. This technique is one of the most important strategies that have been used in analytical chemistry and has a wide range of applications. The chromatography technique has been introduced as an alternative method in the SARS-CoV-2 detection (). A basic example of this technique is the assay reported by Demey et al. [Citation63]. The immunochromatographic tests for the IgM and IgG antibodies detection to SARS-CoV-2 and the kinetics of antibody appearance was assessed and evaluated. In their study, the authors used the immunochromatographic tests that were supplied from Asian manufacturers, under the names of Biotime, Autobio, ISIA and Biolidics. The Biotime, Autobio and Biolidics tests were performed using the same analytical strip. Each test's procedure needs 10–20 µL of serum, plasma or whole blood volume and is read 10–15 min after the sample and diluent have been deposited. The results showed that these tests' sensitivity was between 22% and 81% during the post-symptom reporting period. The sensitivity of Biotime, Autobio, ISIA and Biolidics assays reached 100% through 14 to 15 days post-infection. As per the presented results, good analytical performance has been obtained using four immunochromatographic tests for the detection of IgM and IgG antibodies to SARS-CoV-2. The authors suggested these tests were examined as rapid tests of COVID-19 in emergency departments.
Figure 3. The immunochromatographic method as a rapid diagnosis of COVID-19 [Citation63].
![Figure 3. The immunochromatographic method as a rapid diagnosis of COVID-19 [Citation63].](/cms/asset/3508b6f6-077d-4979-986e-4cb6b2ac5482/tbeq_a_1865838_f0003_c.jpg)
Advanced methods based on nanoparticle materials with better accuracy
Although it is difficult to compare the analytical sensitivities and performances of different methods, either the traditional or the developed methods, the other analytical protocols and parameters could explore the analytical performance's best assessment. Metal nanoparticles have been used as a variety of signal reporters to accelerate the detection time and avoid detection efficiency delay [Citation64–68]. A basic example of this method is the assay reported by Huang et al. [Citation69]. An assay for quick detection of IgM antibodies against SARS-CoV-2 via incorporating gold nanoparticle-based lateral-flow (AuNP-LF) as an application of point-of-care testing (POCT) was developed. This method was selected as a representative example of advanced methods based on nanoparticles. In this study, the authors have prepared Au NP as the capture antigen in the immunochromatographic system. The test requires 10–20 µL, and the results are obtained within 15 min. The developed method's analytical performance has been evaluated via SARS-CoV-2 positive, confirmed, COVID-19 patients. The sensitivity was examined and presented exceptional selectivity in the detection of COVID-19 in the presence of coexisting viruses such as dengue virus (DFV) and severe fever with thrombocytopenia syndrome virus (SFTSV). The researchers optimized the reaction condition by adjusting the pH value of the AuNP, and the anti-human IgM amount in µg was to be 8 and 1.5 µg, respectively.
In the developed method [Citation69] briefly, 1 mL of AuNP solution was adjusted to the optimal pH value, and 9 µg of anti-human IgM was added, and the reaction was kept under stirring for 30 min. Then, 250 µL of 5% (w/v) bovine serum albumin (BSA) solution was added to the reaction and kept under stirring for 30 min. After the centrifugation, the sediment products were collected and resuspended in buffer, and then the AuNP−(anti-human IgM) conjugates were attained. The researchers prepared the Au-NP-LF strip by attaching the sample, conjugate, nitrocellulose membrane and absorbent cells to a PVC backing card. The goat anti-mouse IgG and SARS-CoV-2 NP were dispensed on the nitrocellulose membrane at the concentration of 2.0 mg/mL for the Cline and 1.0 mg/mL for the T line were coated, respectively. The nitrocellulose membrane is then blocked using 1% (w/v) BSA for 30 min and dried for 2 h at 37 °C. The developed strip was then assembled into a plastic shell followed by storing under vacuum. As described, in the detection procedure, 100 µL of a mixture of the serum and sample buffer at the ratio of 1:4, is added onto the sample strip. The liquid is transferred towards the absorbent cell under the capillary action that applied thin layer chromatography (TLC). If the sample is positive, IgM in serum will be captured via AuNP−(anti-human IgM) conjugate, and the AuNP−(anti-human IgM)−IgM compound flows to the analytical membrane. The SARS-CoV-2 NP immobilized at the T line held the target IgM against SARS-CoV-2 and formed the AuNP−(anti-human IgM)–(SARS-CoV-2 IgM)–(SARS-CoV-2 NP) compound. Then, the AuNP−(anti-human IgM) will be caught by the goat anti-mouse IgG at line C [Citation69–73].
Only line C will be presented when the sample is negative due to the flowed serum, and the goat anti-mouse IgG will catch only the AuNP−(anti-human IgM) conjugate at line C. The researchers examined the analytical efficiency of the developed approach. The developed method's specificity was tested through six analyzed samples, which were positive SARS-CoV-2 serum, two DFV sera, two SFTSV sera, and one normal human serum. Each piece was placed in a separate strip. The results displayed that only the SARS-CoV-2 strip has a legible red T line confirming that there was no interference with other viruses, and the developed method was selective. The technique showed satisfying stability and reproducibility by repeating the six samples' tests in the first, second and fourth weeks and the same results were collected. The sensitivity presented good consistency by the Kappa test as one of the robust statistics for inter-rater reliability testing, which was found to be 0.872, and the Youden index was 0.933. Both tests presented the sensitivity and accuracy of the method. It seems that the developed immunochromatographic approach via AuNPs incorporated with lateral-flow is a suitable approach for the rapid diagnosis of COVID-19, especially for urgent conditions. Colorimetric bioassays-based nanotechnology is traditional and appealing for its simplification, sensory output, and no need for complex biosensor prototype instruments. Due to its remarkable optical features such as high extinction coefficient, localized surface plasmon resonance, and intrinsic stability, gold nanoparticles (AuNPs) have gained unbelievable significance in the area of colorimetric-based biosensing implementations in recent years. In numerous colorimetric-based biosensing applications, they were used to measure and track a range of chemical and biological objectives like small substances, proteins, metal ions and nucleic acids. The particle alters colour in response to the nanosized particle's reactivity to external circumstances [Citation74–89]. Nevertheless, given these characteristics, this approach still requires preparing ssDNA samples and introducing several key steps, like time-intensive denaturation and annealing following PCR.
Furthermore, because both ssDNA and dsDNA maintain the AuNPs at low salt concentrations, cationic substances are mainly applied to detect the target utilizing probes. Thus, a colorimetric bioassay for identifying DNA/RNA targets was based largely on non-modified AuNP accumulation using disulphide self-assembly DNA. The products created in this methodology showed a significant affinity for the AuNPs surface and effectively protected them from aggregation caused by salt [Citation90].
A notable second example of this group of techniques is the method reported by Parikshit et al. [Citation91]. A colorimetric assay that relies on gold nanoparticles (AuNPs) capped with thiol-modified antisense oligonucleotides (ASOs) specific to the SARS-CoV-2-N-gene that can be utilized to detect COVID-19 positive patients within 10 min from the isolated RNA samples was reported (). In the presence of the target RNA sequence of SARS-CoV-2, the S-modified ASO-capped AuNPs accumulate selectively and display a shift in its surface plasmon resonance. The introduction of RNaseH cleaves the RNA-DNA hybrid strand resulting in a distinctly visible residue from the sample induced by the subsequent AuNP accumulation. In the presence of MERS-CoV viral RNA, the detection limit for SARS-CoV-2 viral load was 0.18 ng/μL, demonstrating that the selectivity of the test was controlled. Thus, the authors propose the method as a selective and sensory ‘naked eye’ identification of the causative virus of COVID-19, SARS-CoV-2, without any advanced techniques required.
Figure 4. Diagrammatic scheme of SARS-CoV-2 RNA selective Naked-Eye assessment mediated by adequately tailored ASO-Capped AuNPs [Citation91].
![Figure 4. Diagrammatic scheme of SARS-CoV-2 RNA selective Naked-Eye assessment mediated by adequately tailored ASO-Capped AuNPs [Citation91].](/cms/asset/3b1ed046-c789-4db6-90cf-867771c2cf0f/tbeq_a_1865838_f0004_c.jpg)
Conclusions
The COVID-19 pandemic has substantially emphasized the crucial role that diagnostics plays in manipulating communicable diseases. The extensive deployment of diagnostics undoubtedly led to the success of a few countries in managing transmission. Emergency clinical and public health requirements are now prompting an ongoing global campaign to enhance the testing capacity for COVID-19. Finally, scientific research should be continuous to fill the gaps in COVID-19 diagnosis capacity and propose potential solutions. COVID-19 diagnosis is still in a period of rapid development. Therefore, more studies must always be performed to find selective, quick, and cost-effective diagnostic tools for COVID-19 that can supply quick and effective test results in much less than an hour and potentially in minutes.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
No data sets were used to support this study.
References
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733.
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523.
- Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544.
- Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936.
- Nowak JA, Kaul KL. The role of community molecular diagnostics laboratories in the H1N1 pandemic. J Mol Diagn. 2009;11(5):369–370.
- Giri B, Pandey S, Shrestha R, et al. Review of analytical performance of COVID-19 detection methods. Anal Bioanalyt Chem. 2020;413(1):1–14.
- Böger B, Fachi MM, Vilhena RO, et al. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2020:1–9.
- Li C, Zhao C, Bao J, et al. Laboratory diagnosis of coronavirus disease-2019 (COVID-19). Clin Chim Acta. 2020;510(2020):35–46.
- Liotti FM, Menchinelli G, Lalle E, et al. Performance of a novel diagnostic assay for rapid SARS-CoV-2 antigen detection in nasopharynx samples. Clin Microbiol Infect. 2020:1–2.
- Pizzol JLD, Hora VPD, Reis AJ, et al. Laboratory diagnosis for Covid-19: a mini-review. Rev Soc Bras Med Trop. 2020;53: 1–6.
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370: 1–13.
- Grassly NC, Pons-Salort M, Parker EP, et al. Comparison of molecular testing strategies for COVID-19 control: a mathematical modelling study. Lancet Infect Dis. 2020;20(12):1381–1389.
- Li N, Wang P, Wang X, et al. Molecular diagnosis of COVID-19: current situation and trend in China. Exp Ther Med. 2020;20(5):1.
- Vandenberg O, Martiny D, Rochas O, et al. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2020;19:1–13.
- Mahapatra S, Chandra P. Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens Bioelectron. 2020;165(2020):112361.
- La Rosa G, Muscillo M. Molecular detection of viruses in water and sewage. In: Viruses in food and water: risks, surveillance and control. Cambridge, UK: Woodhead Publishing Limited, 2013. p. 97–125.
- Sinclair RG, Choi CY, Riley MR, et al. Pathogen surveillance through monitoring of sewer systems. Adv Appl Microbiol. 2008;65:249–269.
- Daughton CG. Monitoring wastewater for assessing community health: sewage chemical-information mining (SCIM). Sci Total Environ. 2018;619–620:748–764.
- Xagoraraki I, O’Brien E. Wastewater-based epidemiology for early detection of viral outbreaks. In: O’Bannon D, editor. Women in water quality. Women Engineering and Science. Cham: Springer; 2020. p. 75–97.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV- 2 RNA in feces of COVID-19 patients. J Med Virol. 2020;92:833–840.
- Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–1707.
- Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697–706.
- Han MS, Seong M-W, Heo EY, et al. Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2020;71(16):2236–2239.
- Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–908.
- Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92(9):1518–1524.
- Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland. Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(11):2000266.
- Siddra I, Imran U. Recombination DNA technology. UK: Cambridge Scholars Publishing; 2019.
- Heid CA, Stevens J, Livak KJ, et al. Real time quantitative PCR. Genome Res. 1996;6(10):986–994.
- Aroca A, Raposo R, Lunello P. A biomarker for the identification of four Phaeoacremonium species using the beta-tubulin gene as the target sequence. Appl Microbiol Biotechnol. 2008;80(6):1131–1140.
- Bilodeau GJ, Pelletier G, Pelletier F, et al. Multiplex real-time polymerase chain reaction (PCR) for detection of Phytophthora ramorum, the causal agent of sudden oak death. Canad J Plant Pathol. 2009;31(2):195–210.
- Cumagun CJ, editor. Plant pathology. UK: IntechOpen; 2012.
- Carter LJ, Garner LV, Smoot JW, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6(5):591–605.
- American College of Physicians. COVID-19 found in sputum and feces samples after pharyngeal specimens no longer positive. Science Daily. 2020 Mar 30. Available from: https://sciencedaily.com/releases/2020/03/200330110348.htm
- Kujawski SA, Wong KK, Collins JP, et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease (COVID-19) in the United States. Nat Med. 2020;26:861–868.
- Rutgers University. New Rutgers saliva test for coronavirus gets FDA approval: emergency use authorization granted for new biomaterial collection approach. Rutgers Today. April 2020. Available from: http://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fdaapproval
- U.S. Food & Drug Administration. Accelerated emergency use authorization (EUA) summary SARS-CoV-2 ASSAY (Rutgers Clinical Genomics Laboratory). FDA, US; 2020. p. 1–8.
- VanGuilde HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44(5):619–626.
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39(1):75–85.
- Jared R, Sugeet J, Jason R, et al. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is comparable in clinical samples preserved in saline or viral transport medium. J Mol Diagn. 2020;22(7):871–875.
- Leland DS. Concepts of clinical diagnostic virology. In: Laboratory diagnosis of viral infections. 2nd ed. New York, NY: Marcel Dekker, Inc.; 1992. p. 3–44.
- Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63–E67.
- Huang WE, Lim B, Hsu C-C, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020;13(4):950–961.
- McGovern Institute, What is CRISPR? Ask the Brain. The McGovern Institute for Brain Research, Massachusetts Institute of Technology. 2019 Jan 1. Available from: http://mcgovern.mit.edu/2019/01/01/crispr-in-a-nutshell/
- Serology-based tests for COVID-19. Johns Hopkins Center for Health Security. 2020. Available from: http://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html
- Pryor J. 3 Questions: How COVID-19 tests work and why they’re in short supply. MIT News: On Campus and around the World. Massachusetts Institute of Technology. 2020 Apr 10. Available from: http://news.mit.edu/2020/how-covid-19-tests-work-why-they-are-in-short-supply-0410
- Broughton JP, Deng X, Yu G, et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38(7):870–874.
- Ali Z, Aman R, Mahas A, et al. iSCAN: an RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020;288:198129.
- La Marca A, Capuzzo M, Paglia T, et al. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41(3):483–499.
- Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9(1):747–756.
- Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835.
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179.
- Serology testing for COVID-19. Johns Hopkins Center for Health Security. 2020. Available from: http://https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-fact-sheets/200228-Serology-testing-COVID
- FDA Fact Sheet: Serological testing for antibodies to SARSCoV- 2 infection. U.S. Food & Drug Administration; 2020. p. 1−2. Available from: http://www.fda.gov/media/137111/download
- Imai K, Tabata S, Ikeda M, et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J Clin Virol. 2020;128:104393
- Chen Z, Zhang Z, Zhai X, et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal Chem. 2020;92(10):7226–7231.
- Wan ZY, Zhang X, Yan XG. IFA in testing specific antibody of SARS coronavirus. South China J Prev Med. 2003;29:36–37.
- Che X, Di B, Zhao G, et al. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003-2004 community outbreak of SARS in Guangzhou, China. Clin Infect Dis. 2006;43:1–5.
- Zhang G, Nie S, Zhang Z, et al. Longitudinal change of SARS-Cov2 antibodies in patients with COVID-19. J Infect Dis. 2020;2:229.
- Barbosa Junior WL, Ramos de Araujo PS, Dias de Andrade L, et al. Rapid tests and the diagnosis of visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome coinfection. Am J Trop Med Hyg. 2015;93(5):967–969.
- Feng M, Chen J, Xun J, et al. Development of a sensitive immunochromatographic method using lanthanide fluorescent microsphere for rapid serodiagnosis of COVID-19. ACS Sens. 2020;5(8):2331–2337.
- Uhteg K, Jarrett J, Richards M, et al. Comparing the analytical performance of three SARS-CoV-2 molecular diagnostic assays. J Clin Virol. 2020;127:104384.
- Schmitz JE, Tang YW. The GenMark ePlex®: another weapon in the syndromic arsenal for infection diagnosis. Fut Microbiol. 2018;13:1697–1708.
- Demey B, Daher N, François C, et al. Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays. J Infect. 2020;81(2):e6–e10.
- Zuo J-Y, Jiao Y-J, Zhu J, et al. Rapid detection of severe fever with thrombocytopenia syndrome virus via colloidal gold immunochromatography assay. ACS Omega. 2018;3(11):15399–15406.
- Ge X, Zhang W, Lin Y, et al. Magnetic Fe3O4@TiO2 nanoparticles-based test strip immunosensing device for rapid detection of phosphorylated butyrylcholinesterase. Biosens Bioelectron. 2013;50:486–491.
- Huang C, Wei Q, Hu Q, et al. Rapid detection of severe fever with thrombocytopenia syndrome virus (SFTSV) total antibodies by up-converting phosphor technology-based lateral-flow assay. Luminescence. 2019;34(2):162–167.
- Lu T, Zhu K-D, Huang C, et al. Rapid detection of Shiga Toxin type II using lateral flow immunochromatography test strips of colorimetry and fluorimetry. Analyst. 2019;145(1):76–82.
- Song C, Liu J, Li J, et al. Dual FITC lateral flow immunoassay for sensitive detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron. 2016;85:734–739.
- Huang C, Wen T, Shi F-J, et al. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega. 2020;5(21):12550–12556.
- Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–582.
- Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111–120.
- Carrio A, Sampedro C, Sanchez-Lopez J, et al. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors (Basel). 2015;15(11):29569–29593.
- Brangel P, Sobarzo A, Parolo C, et al. A serological point-of-care test for the detection of IgG antibodies against Ebola virus in human survivors. ACS Nano. 2018;12(1):63–73.
- Pan D, Schirra CO, Wickline SA, et al. Multicolor computed tomographic molecular imaging with noncrystalline high-metal-density nanobeacons. Contrast Media Mol Imaging. 2014;9(1):13–25.
- Pan D, Pramanik M, Senpan A, et al. Molecular photoacoustic tomography with colloidal nanobeacons. Angew Chem Int Ed Engl. 2009;48(23):4170–4173.
- Pan D, Pramanik M, Senpan A, et al. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials. 2010;31(14):4088–4093.
- Pan D, Pramanik M, Senpan A, et al. A facile synthesis of novel self-assembled gold nanorods designed for near-infrared imaging. J Nanosci Nanotechnol. 2010;10(12):8118–8123.
- Pan D, Pramanik M, Senpan A, et al. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. FASEB J. 2011;25(3):875–882.
- Zeng J, Zhang Y, Zeng T, et al. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today. 2020;32:100855.
- Fraire JC, Perez LA, Coronado EA. Rational design of plasmonic nanostructures for biomolecular detection: interplay between theory and experiments. ACS Nano. 2012;6(4):3441–3452.
- Saha K, Agasti SS, Kim C, et al. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–2779.
- Misra SK, Dighe K, Schwartz-Duval AS, et al. In situ plasmonic generation in functional ionic-gold-nanogel scaffold for rapid quantitative bio-sensing. Biosens Bioelectron. 2018;120:77–84.
- Li Z, Askim JR, Suslick KS. The optoelectronic nose: colorimetric and fluorometric sensor arrays. Chem Rev. 2019;119(1):231–292.
- Curry T, Kopelman R, Shilo M, et al. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol Imaging. 2014;9(1):53–61.
- Peng L, Li BL, Zhou CW, et al. Naked-eye recognition: emerging gold nano-family for visual sensing. Appl Mater Today. 2018;11:166–188.
- Mirkin CA, Letsinger RL, Mucic RC, et al. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382(6592):607–609.
- Elghanian R, Storhoff JJ, Mucic RC, et al. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277(5329):1078–1081.
- Jung YL, Jung C, Parab H, et al. Direct colorimetric diagnosis of pathogen infections by utilizing thiol-labeled PCR primers and unmodified gold nanoparticles. Biosens Bioelectron. 2010;25(8):1941–1946.
- Li H, Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc Natl Acad Sci USA. 2004;101(39):14036–14039.
- Shokri E, Hosseini M, Davari MD, et al. Disulfide-induced self-assembled targets. A novel strategy for the label free colorimetric detection of DNAs/RNAs via unmodified gold nanoparticles. Sci Rep. 2017;7:1.
- Moitra P, Alafeef M, Dighe K, et al. Dipanjan Pan selective naked-eye detection of SARS-CoV‑2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14(6):7617–7627. https://dx.doi.org/10.1021/acsnano.0c03822.
