Abstract
The focus of the present study is to determine proteins responsible for the oxidative and toxic stress response in proliferating and stationary phase (G0) cultures. Therefore, the yeast Saccharomyces cerevisiae was treated with oxidative and drug compounds (H2O2, menadione, zeocin, and ibuprofen) in both phases. These substances were chosen to determine the redox status of the yeast. S. cerevisiae appeared to employ different strategies to ensure their antioxidant defence metabolism. Analysis, including sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) coupled with mass spectrometry, was used in the search. The proteins were identified by SDS-PAGE, matrix-assisted laser desorption/ionization time-of-flight/time-of-flight (MALDI-TOF/TOF) mass spectrometry analysis, and Mascot database-fingerprint. The final step was determination of protein profiles of yeast S. cerevisiae in proliferating (M) and stationary phase (G0). Seven bands were determined and the corresponding proteins were proposed: cytochrome c peroxidase, glutathione S-transferase omega-like, NAPDH-dependent diflavin reductase, DNA replication fork-blocking protein, putative aryl alcohol dehydrogenase, AP-1-like transcription factor YAP5, GTP-binding protein. All putative proteins coincide with the literature database. A typical example of such an adaptation mechanism in the defence against oxidative damage is the synthesis of several glutathione and thioredoxin peroxidases in the yeast cell. A deeper investigation of the conserved mechanisms responsible for entry into, survival, and exit from quiescence in higher eukaryotes will help the development of new anticancer therapies, the study in the process of ageing and neurodegenerative diseases.
Introduction
In view of the fact that all living organisms are subject to changing environmental conditions, knowledge of stress and stress response is critical to understand how single and multicellular organisms adapt to endo- and exogenous stress factors.
Yeast Saccharomyces cerevisiae, established as the most broadly researched microorganism concerning stress responses, during starvation, cease growth and enter a non-proliferating state denoted as a stationary phase or quiescence [Citation1,Citation2]. Moreover, cells in stationary phase develop specific differentiation programmes which allow preservation of viability for prolonged time without the presence of nutrients. And quiescent cells possess the capacity to restart growth immediately in the presence of nutrients [Citation3]. The mechanisms by which eukaryotic microorganisms survive prolonged periods of nutrient limitation and resume proliferation remain unclear. Stationary phase cells are also unbudded and contain unreplicated DNA, characteristic of the regulatory step in G1 phase of the mitotic cell cycle [Citation4].
Both the common mechanisms of stress response among eukaryotes and the genetic and molecular advantages of S. cerevisiae allow us to use them as a model system for better understanding the stationary phase in yeast cells as well as to provide novel insights into non-proliferating states in other eukaryotic cells [Citation4]. Of considerable interest is the comprehension of different mechanisms implicated in the stress response of yeast S. cerevisiae, caused by different endogenous and exogenous toxic compounds. The cells of the yeast in stationary phase could mirror cells of multicellular organisms regarding different important metabolic characteristics. It is stated that most of the energy in the G0 phase is derived from mitochondrial respiration, a process leading to considerable cellular damage over time [Citation5]. Taking into account that cells from multicellular eukaryotic organisms spend most of their lifetime in the G0 phase, it is reasonable to use S. cerevisiae quiescent cells not only to study transcriptional regulation, but also to get valuable information for other fields like cancer, development, and ageing research [Citation6].
This study investigated the stress response of yeast S. cerevisiae during exposure to different exogenous agents in proliferating and stationary phase. The specific protein profiles were determined in both states triggered by four different toxic compounds: H2O2, menadione, ibuprofen, and zeocin, using proteomic analysis and mass spectrometry.
Materials and methods
Yeast strain and growth conditions/harvesting. Isolation of G0 cells
The yeast strain used in this study was S. cerevisiae BY4741 with a genotype MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, which is an auxotroph and is described by Harsch et al. as haploid [Citation7]. It was obtained from the collection centre EUROSCARF (European S. cerevisiae Archive for Functional Analysis, Institute of Molecular Biosciences, Johann Wolfgang Goethe-University) Frankfurt, Germany. The yeast cells were batch-cultivated in YPD medium (1% yeast extract, 2% bacto/peptone, 2% glucose) with a rotational Schuettel Apparatus (205 rpm) at 30 °C for 20 h for logarithmically growing yeast and for 168 h at 30 °C for cultures reaching stationary phase of growth, respectively. The lab flasks were inoculated with a culture of S. cerevisiae BY4741 grown on YPD agar slant for 24 h. To harvest the biomass, the broth was centrifuged at 5000 rpm for 10 min with centrifuge Sigma 3-30KS (Sigma Laborzentrifugen GmbH, Germany). The pellet was washed twice with distilled water and centrifuged following the same parameters.
The isolation of cells that have entered G0 phase was carried out through fractioning in percoll density gradient according to the method used by Allen et al. [Citation8]. Briefly, 6 mL of the percoll solution (percoll: 1.5 mol/L NaCl) in ratio 9:1 (v/v) were pipetted in four 8-mL conical tubes. To form a gradient, the tubes were centrifuged at 13,800 rpm (19,240 g) for 15 min at 20 °C. The lower and upper cell fractions were separated based on differences in their density. G0 cells formed the denser fraction (respectively the lower one) due to the thickened cell walls and accumulation of storage carbohydrates. The upper fraction containing non-quiescent cells was not a subject of the present study. Each fraction was washed twice in 40 mL 0.1 mol/L Tris-HCl buffer solution with рН 7.5 and centrifuged for 10 min at 4000 rpm at 20°С (Sigma 3-30KS, Sigma Laborzentrifugen GmbH, Germany). The cells in G0 state were presented as the high-density lower fraction [Citation8].
Cell survival after inhibition with H2O2, menadione, ibuprofen, and zeocin
Survival rates of both, proliferating (Log) and G0 cultures of S. cerevisiae BY4741, were tested after treatment with oxidative and drug agents with a wide range of concentrations. IC50 was determined using a spot analysis. The yeast strain was grown on YPD agar medium which was added, after cooling, H2O2 (5, 10, and 20 mmol/L), menadione (30, 40, 50, 100, 200, 300 µmol/L), ibuprofen (0.5, 0.75, 1.1, 1.5 mg/mL), and zeocin (50, 75, 100 µg/mL) were added. Yeast cultures were grown at 30 °C for 48 h together with the control without the addition of oxidative or drug agents.
Oxidative and toxic studies of cells exposed to IC50 doses
Oxidative and toxic studies were performed by incubating the proliferating and G0 state yeast suspensions (0.3 g biomass resuspended in 1 mL of 0.1 mol/L Tris buffer, pH 7.5) for 1 h at room temperature with IC50 doses of the following agents: hydrogen peroxide (5 mmol/L), menadione (100 µmol/L), ibuprofen (1.1 mg/mL), and zeocin (50 µg/mL), respectively. After incubation, the cells were washed twice with distilled water and subjected to mechanical disintegration. Treated and untreated yeast cells (0.3 g) were resuspended in 0.05 mol/L potassium phosphate buffer pH 7.8 and mixed with polyester beads (0.1 ÷ 0.5 mm) (Sigma-Aldrich, Germany) at a ratio of 1:2:1. The resuspended biomass was disintegrated by a Bullet Blender® Storm homogenizer at 8000 rpm, three times for 5 min. Cell debris was removed by centrifugation at 5000 rpm for 15 min, the resulting supernatant was clarified after centrifugation at 13,000 rpm for 20 min at 4 °C and the homogenate was stored at −20 °C.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE (10%) was carried out using a standard procedure to examine the change in the expression of proteins in cells treated with oxidative and drug agents in comparison to non-treated ones [Citation9–11]. The reagents of analytical grade for SDS-PAGE N,N,N′,N′-tetramethylethylenediamine (TEMED) and ammonium persulphate (APS) were supplied from Sigma®, Steinheim, Germany. Rapid Gel™ 40% liquid acrylamide was used as acrylamide-bisacrylamide reagent diluted 19:1 (USB Corporation, Cleveland, OH, USA) for casting the mixture. All bands were visualized by staining with Coomassie Brilliant Blue G-250 Sigma®, Steinheim, Germany. Unstained SDS-PAGE protein marker mixture from SERVA Electrophoresis GmbH, Heidelberg, Germany was applied as a standard identifier, including the following proteins with respective molecular masses: myosin (200 kDa), β-galactosidase (116.3 kDa), albumin bovine (67 kDa), ovalbumin (45 kDa), carbonic anhydrase (29 kDa), trypsin inhibitor (21 kDa), lysozyme (14 kDa), aprotinin (6,5 kDa). The samples were dissolved in Laemmli sample buffer (SERVA Electrophoresis GmbH) in a ratio of 1:1 and an amount of 25 µL was loaded into the wells of the PAG (polyacrylamide gel), whereas only 10 µL of standard mixture was applied. The 10% SDS-PAGE electrophoresis of intracellular crude extracts from S. cerevisiae BY4741 biomass isolated from proliferating and G0 phases was repeated three times for the purpose of statistical confirmation of the results.
Enzymatic digestion of the protein bands
The protease digestion was run according to the work of Rosenfeld et al. [Citation12] with a slight modification. The bands of interest were cut out and decoloured. The dried gel bands were dissolved with a volume of 8 µL digestion buffer, prepared from 50 mmol/L ammonium bicarbonate, pH 7.8, containing sequencing grade modified trypsin from Roche Diagnostics GmbH. The 0.5-mL Eppendorf tubes were placed on ice and kept for 45 min to allow the gel pieces to be effectively saturated with the protease solution. The proteolytic cleavage of the gel bands ran overnight at 37 °C. Two times extraction of the resulting peptides with 40 µL of 60% ACN/0.1% HCOOH was performed. The extracts were once more dried on a speedvac. The extracted peptides were redissolved in 10 µL of 0.1% TFA.
Protein identification and validation
The extracted peptides were prepared by mixing 1.0 µL of the sampling with 1.0 µL matrix solution (10 mg/mL α-cyano-4-hydroxycinnamic acid [CCA] from Fluka Analytical Sigma®, Steinheim, Germany in 50% ACN containing 0.1% TFA) and the whole quantity was tipped onto a stainless steel target plate. The plate was left to dry at room temperature [Citation13]. Consequently, analyses of the prepared mixture were done on an AutoflexTM III, high performance matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF & TOF/TOF) Systems (Bruker Daltonics, Bremen, Germany) which operate at a wavelength of 355 nm, using a 200 Hz frequency-tripled Nd-YAG laser. The Instrument was set at linear mode, collision energy of 4.2 kV with a total of 3000 shots. Peptide Calibration Standard (Bruker Daltonik GmbH, Bremen, Germany) was used to externally calibrate the mass spectrometer. The calibration spectra contained a mixture of angiotensin I (1296.6848 Da), angiotensin II (1046.5418 Da), glu-fibrinopeptide B (1569.65 Da), ACTH clip 1–17(2093.0862 Da), and ACTH clip 18–39 (2465.1983 Da).
Database searches
Mass spectral data were processed through FlexAnalysis 3.4 (Brucker Daltonics) and subsequently subjected to a search against different protein databases using MASCOT server (Matrixscience, London, UK). Additionally, database NCBI port and SwissProt were used to confirm the Mascot searches. For successful analysis and validation of the proteins, the following criteria were applied: (1) Type of search: Peptide Mass Fingerprint; (2) Enzyme: Trypsin; (3) Taxonomy: Saccharomyces cerevisiae (baker’s yeast); (4) Mass values: Monoisotopic, Peptide Charge State: 1; (5) Max Missed Cleavages: 1; (6) Peptide Mass Tolerance: ±2.5 Da; (7) Report top hits: 5 (WC 1229).
Results and discussion
Cell viability under stress conditions
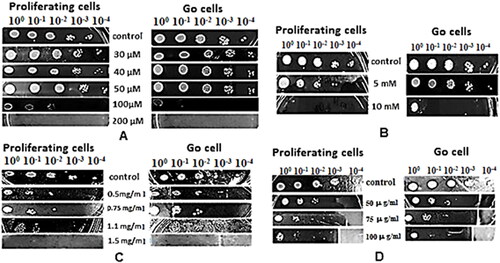
In order to investigate the toxic effect of menadione, hydrogen peroxide, ibuprofen, and zeocin on the viability of proliferating and quiescent S. cerevisiae cells, both cell types were exposed to a range of concentrations of the selected stress agents. The results of the performed spot analyses revealed the degree of sensitivity of the yeast cells to the applied toxic concentrations, and IC50 for each of the tested compounds was defined: menadione (100 µmol/L), H2O2 (5 mmol/L), ibuprofen (1.1 mg/mL), and zeocin (50 µg/mL) (). At IC50, the test compounds inhibited cell growth between 40% and 60% compared to the control cells and were taken as utmost concentrations at which yeast cells still retained the ability to grow.
Figure 1. Sensitivity assay of proliferating and G0 S. cerevisiae BY 4741 cells subjected to: menadione (A); hydrogen peroxide (B); ibuprofen (C); zeocin (D). Control – S. cerevisiae BY 4741 cells cultivated in the absence of toxic agent. The image is representative of one of the three independent experiments.
The toxicity of menadione and H2O2 is related mainly to their ability to produce intracellularly large amounts of reactive oxygen species (ROS). At high concentration, these oxidizing compounds induce strong oxidative stress, which results in disturbance of the cellular redox homeostasis, decrease of cell viability and finally cell death.
The results from the performed spot analyses revealed that both cell types showed relative resistance to exogenous menadione up to 50 µmol/L, with colony growth detected from all dilutions. However, menadione exerted its toxic effect on both proliferating and G0 cells when added to the medium at a concentration of 100 µmol/L. The stronger inhibitory effect on growth (60%) was observed for cells in G0 state, in contrast to proliferating ones (40% growth inhibition). A concentration of 200 µmol/L menadione completely inhibited growth of the two cell types. According to Chuang et al. [Citation14], low concentrations of superoxide-generating menadione (2 µmol/L) induce the formation of ROS which function as signal molecules in the cells. However, at high concentration, menadione triggers strong oxidative stress due to the intense formation of superoxide, radicals, and mitochondrial dysfunction and fragmentation. Having in mind that in G0 state the major source of energy is the mitochondrial respiration [Citation5], it is not surprising that this agent has stronger effect in quiescent yeast cells.
Contrary to the results observed for menadione, the exposure of S. cerevisiae BY4741 to 5 mmol/L exogenous H2O2 led to insignificant inhibition of growth of G0 cells. However, for the exponentially grown yeasts, 40% inhibition was detected at this test concentration. The observed damage rate in proliferating cells was probably due to their higher metabolic activity and the corresponding elevated levels of oxidative damage. The results from the performed analysis with the non-steroidal anti-inflammatory drug ibuprofen revealed that at IC50 (1.1 mg/mL) of this chemical induce stronger toxic effect on proliferating yeast cells, as evidenced by weaker growth (60% growth inhibition). The quiescent S. cerevisiae cells showed visibly greater resistance with 40% inhibition of cell growth. A concentration of 1.5 mg/mL was lethal to both cell types. In the zeocin assay, both types of yeast cells showed relatively equal resistance to its toxic effect at all three tested concentrations. Knowing that zeocin causes cell death by intercalating into and cleaving DNA, it was evident that its toxicity should be comparable among both types of cells.
Comparative analyses of the changes in the expression of proteins in proliferating (LOG) and stationary (G0) phase by SDS-PAGE
The main objective of the present work was to determine the protein profile of the yeast strain undergoing oxidative stress. For that purpose, treated and non-treated homogenates from S. cerevisiae BY4741 were analysed by SDS-PAGE and mass spectrometry. The results from the 10% SDS-PAGE together with the identified MALDI-TOF/MS spectra and the putative proteins and enzymes corresponding to the bands and their function are also presented. The 10% SDS-PAGE in gives a very intriguing visualization of the oxidative and drug stress, and the response to it, respectively. Eight fractions could be identified on lane 9 which was the control in proliferating state. The observed proteins (control in Log state) were in the size range between 25 kDa and 65 kDa. Four strongly stained protein bands at approximately 26, 32, 42, and 65 kDa were detected. Of our interest, Band 1 at 60–65 kDa and Band 6 at 32 kDa, were analysed mass spectrometrically and discussed further below. In contrast to the control in proliferating state (lane 10, control in G0), such intensive bands were not observed, except a protein band at 42 kDa. Obviously, the production of proteins was suppressed in the aforementioned lane, corresponding to the already known fact that at stationary state the cellular metabolism is lowered down [Citation15].
Figure 2. Denaturating 10% SDS-PAGE of proteins isolated from S. cerevisiae in Log and G0 phase: lane 1 Log phase treated with H2O2; lane2 G0 phase treated with H2O2; lane 3 Log phase treated with 100 µmol/L menadione; lane 4 G0 phase treated with 100 µmol/L menadione; lane 5 Log phase treated with 1.1 mg/mL ibuprofen; lane 6 G0 phase treated with 1.1 mg/mL ibuprofen; lane 7 Log phase treated with 50 µg zeocin; lane 8 G0 phase treated with 50 µg zeocin; lane 9 control in Log phase; lane 10 control in G0 phase; St: Standard from Serva.
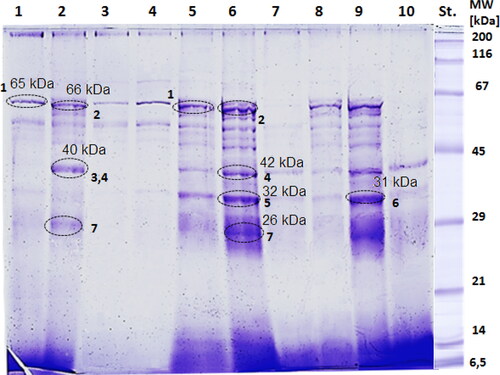
Changes in the expression of proteins were recognized after treatment of the cell suspensions in proliferating and quiescent state with 5 mmol/L hydrogen peroxide observed in lane 1 and lane 2. In lane 2, a considerable increase in the protein synthesis was observed at 40–42 kDa (Band 3 and Band 4) and 26–28 kDa (Band 7). A fraction corresponding to 40–42 kDa was almost absent in lane 1. Identical expression of proteins at 62–65 kDa was recognized in both lanes 1 and 2, Band 1 and Band 2, respectively, although a few additional bands appeared around Band 2 (lane 2). Moreover, a comparison shows that most of the protein bands which were abundantly expressed in the control sample (lane 9) were less intensive in lane 1. On the contrary, in lane 2, three extensively stained bands appeared, compared to lane 10.
In lanes 3 and 4, that there was expression of proteins primarily at ∼62 kDa and ∼50 kDa after treatment of the suspensions of proliferating cells and cells in G0 state with 100 µmol/L menadione. Comparison between both lanes demonstrates that bands were more intensive for cells in quiescent state. Although the preliminary test for cell viability showed a low degree of inhibition both in proliferating and G0 state, it could be assumed that the experimentally determined sublethal concentration of menadione (100 µmol/L) was too high and therefore repetition of the experiments could be done by treating the homogenate with a lower concentration. Moreover, Cyrne et al. [Citation16] conducted such an experiment for viability of the same S. cervisiae strain in stationary phase, performed with 40 µmol/L of menadione. A hypothesis for the behaviour of the cell homogenate is that severe stress from menadione leads to a decrease in intracellular proteolysis. Consequently, severely damaged cellular proteins become resistant to degradation. While mild oxidative stress from menadione increases intracellular proteolysis, consequently, it leads to cellular protein changes.
Considerable changes were noticed by the expression of proteins in lanes 5 (cells at Log phase, treated with ibuprofen) and 6 (cells at G0 phase treated with ibuprofen). One can recognize that the expression of proteins after treatment of quiescent cells with ibuprofen, was elevated. In lane 6, bands at 60–65 kDa (Band 1), 40–42 kDa (Band 4), 30–32 kDa (Band 5), and 26–28 kDa (Band 7) were more abundantly expressed than those bands in lane 5. He et al. [Citation17] in their work mentioned that ibuprofen boosts longevity in yeast, especially in S. cerevisiae. They stated that the pro-longevity function of ibuprofen owes its activity to the noncyclooxygenase activity. Ibuprofen was considered as a drug with decreased risk of causing age-related pathologies that could be taken into account as a stepping stone to finding efficacious and safe therapeutics for humans [Citation17]. What was also observed is that only 3 bands are dominant in lane 2. It was obvious that the fraction at 40–42 kDa was wider than the one found at the same position in lane 6. Therefore, one can assume that two proteins were responsible for responding to hydrogen peroxide stress.
Lanes 7 (metabolically active yeast cells treated with zeocin) and 8 (cells in G0 state treated with zeocin) indicated some changes in the expression of proteins. One can directly spot that proteins expressed in lane 7 were hardly discerned in comparison to bands in lanes 9 and 2. The proteins in lane 8, on the contrary to lane 7, were more visible, where the protein at 60–65 kDa was more intensively expressed. The protein band was observed in Log phase cells treated with zeocin (lane 8) not in the control suspension in G0 state (lane 7). An overall speculation for the zeocin activity proposed by Dialynaki et al. [Citation18] was the discard of the chelated Cu2+ when the drug penetrates the cells. That phenomenon further results in changes in vital cell biological processes. It was also found that zeocin acts especially upon TORC1 conserved signalling pathway. Dialynaki et al. [Citation18] suggested that treatment of yeast cells with zeocin is ensued by a switch of metabolism towards catabolism.
The selected bands marked with black dotted circles on the SDS-PAGE were excised. Consequently, tryptic digestions and mass spectrometric analyses were performed. The aim of the tests was to propose and find expressed enzymes responsible for the adaptation to oxidative stress for the following bands: Band 1 (60–65 kDa), Band 2 (60–63 kDa), Band 3 (40–42 kDa), Band 4 (40–42 kDa), Band 5 (30–32 kDa), Band 6 (30–32 kDa), and Band 7 (26–28 kDa).
Identification of protein profile by mass spectral analysis
The putative proteins and enzymes were experimentally determined through a tryptic digest of the bands after running SDS-PAGE, and subsequently MALDI-TOF-MS data and Mascot analysis. A comprehensive view () gives more information about the proteins and enzymes. Based on the spectra and most intensive peaks, the peptides give а clue as to what enzymes could have a role in the expression of proteins of S. cerevisiae undergoing oxidative and drug stress.
Table 1. Enzymes responding to oxidative stress with significant changes in expression on SDS-PAGE in Log and G0 state after treatment with H2O2, menadione, ibuprofen, and zeocin.
In the paragraphs below, the experimentally determined proteins and enzymes in proliferating and stationary phase cells undergoing oxidative stress are discussed.
Band 1
In Band 1, the analysis determined DNA replication fork-blocking (RFB) protein with a molecular weight around 65 kDa, read from the SDS-PAGE. This is a nucleolar protein and the gene FOB1 shows an essential function for both the decrease and increase of rDNA repeats. FOB1 is required for RFB activity at the RFB site in rDNA and for recombination hot-spot (HOT1) activity [Citation19]. Condensins are necessary for segregation of rDNA repeats in tune with Fob1 [Citation20]. Fob1 has distant, but significant analogy to the retroviral integrase [Citation21]. Another crucial aspect of the protein is its involvement in the regulation of cell ageing, where a null mutant exhibits lengthened replicative lifespan [Citation22]. As far as stress in the cell is concerned, it was observed an increased resistance to methyl methanesulphonate (MMS). An increased general stress and a progressive lengthening of the cell cycle for the last few cell divisions is the essence for ageing of the wild-type cells. Moreover, the aforementioned features are much less apparent in the long-lived FOB1 deletion mutant. The individual cells revealed that there are various forms of cell death being associated with diverse terminal cell morphologies and varying levels of stress and lifespan [Citation22].
Band 2
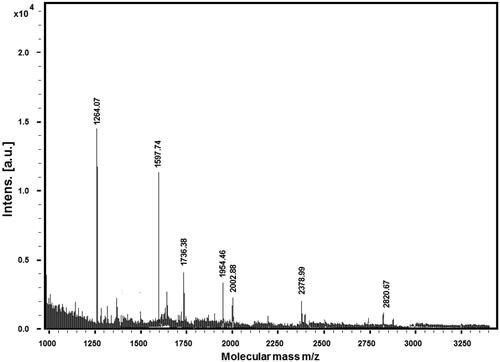
The result from Mascot determined that Band 2 corresponds to the enzyme NAPDH-dependent diflavin reductase with a coding gene TAH18 and an approximate mass of 65 kDa. Fe-S clusters play a role as an inorganic cofactor in proteins taking part in a number of vital cell processes. The essential flavoprotein Tah18 is one of the components of the early step in the cytosolic and nuclear iron–sulphur protein assembly (CIA) machinery. The CIA machinery appears to be functionally conserved from yeast to humans through the human Ndor1–Ciapin1 proteins. The work of Netz et al. [Citation23] pointed out that the complex of Tah18 and Dre2 constitutes an electron transfer chain delivering electrons from NADPH via the FAD (flavin adenine dinucleotide)- and FMN (flavin mononucleotide)-containing Tah18 to the Fe-S cluster of Dre2p. Other studies have shown that the mitochondrion in tah18 mutants owes its protection against H2O2-induced damage to cytochrome c [Citation24]. Tah18 is involved in the positive regulation of hydrogen peroxide-mediated programmed cell death having an apoptotic (pro-death) role under oxidative stress, with a key process to eliminate dead cells. The protein complex, Dre2-Tah18 controls yeast cell death in response to high levels of hydrogen peroxide. In the absence of exogenous oxidative stress, Tah18 and Dre2 physically interact outside the mitochondria. Respectively, in the presence of oxidative stress, Tah18 is targeted to the mitochondria and controls mitochondrial integrity and cell death [Citation24]. Tah18p-dependent nitric oxide synthesis provides high-temperature stress tolerance and could be possibly an applicant for development of antifungal drugs [Citation25]. shows the mass spectrum obtained from Band 2 (lane 2 on the SDS-PAGE) with extensive peptide masses, expressed as [M + H]+ ions.
Band 3
In lanes 2 and 6, two proteins were determined at circa 42 kDa. The first putative protein/enzyme determined from Band 3 is the glutathione S-transferase omega-like GTO3 with a measured mass of ∼42 kDa. The S. cerevisiae genome encodes three proteins that have analogy to human GSTOs (omega-class glutathione S-transferase) hGSTO1-1 and hGSTO2-2. GSTOs possess different features from most of the GSTs and bear a resemblance to Grx (glutaredoxin) proteins which, from the point of detoxifying the cell, are not included in the enzymatic defence of the cell against oxidative stress [Citation26]. The three yeast proteins have been labelled as Gto1, Gto2, and Gto3, and their purified recombinant forms operate as thiol transferase (glutaredoxins) against model disulphide HED (β-hydroxyethyl disulphide), as dehydroascorbate reductases, and as dimethylarsinic acid reductases, while no activity against the standard GST substrate CDNB (1-chloro-2,4-dinitrobenzene) is found [Citation26]. Their glutaredoxin activity is also detectable in yeast cell extracts. The enzyme activity characteristics of the Gto proteins contrast to those of another yeast GST, Gtt1. The latter is active against CDNB and displays glutathione peroxidase activity against organic hydroperoxides such as cumene hydroperoxide, but is not active as a thiol transferase [Citation27–30]. Gtt1 is linked to the endoplasmic reticulum and is involved in thermotolerance. Moreover, according to the work of Choi et al. [Citation31], it is shown that Gtt1 arises after a diauxic shift and its high level of expression lasts during the stationary phase. The GSTOs belong to a family of multipurpose isoenzymes that take part in the cellular defence against several exogenous and endogenous compounds having distinct structural and functional characteristics, which differ from those of other GSTs. Lately, genetic and molecular researches demonstrated evidence that regulating the MAPK signalling pathway GstO1 includes a protective function against H2O2 using the Drosophila system. On the other hand, GstO2 is required to trigger mitochondrial ATP synthase in the Drosophila neurodegenerative disease model [Citation32].
Band 4
The second putative protein is mitochondrial cytochrome c peroxidase with measured mass ∼40 kDa (theoretical mass 40348.3 kDa, ), a haeme oxidoreductase which functions as degrading ROS in mitochondria, thus being involved in the response to oxidative stress [Citation33,Citation34]. This enzyme, determined from Band 4, is localized in the mitochondrial intermembrane space and its main biological function is thought to be reduction of H2O2 generated during aerobic respiration [Citation35]. The determination of that enzyme, having a key function of detoxifying ROS, especially H2O2, is proposed in other papers. van der Klei et al. [Citation36] in their work suggest that the scavenging role of cytochrome c peroxidase is an alternative mechanism of catalase enzyme [Citation36,Citation37]. As mitochondria are one of the most vulnerable organelles to oxidative stress, and cytochrome c peroxidase has no recognizable paralog in the genome, it is assumed that another alternative mechanism should exist which would be responsible for the removal of the hydrogen peroxide generated in this cellular compartment [Citation38]. Our result confirms the idea of Martins et al. [Citation34] that the peroxidase activity from cytochrome c is autonomous and the enzyme also plays a role in sensing hydrogen peroxide and regulation of the catalase activity. Kathiresan et al. [Citation33] assume in their recent studies that CCP1 behaviour reminds more of a mitochondrial H2O2 sensor rather than of a H2O2 detoxifying catalytic protein.
Band 5
Putative aryl alcohol dehydrogenase AAD16 corresponds to Band 5 from the spectral Mascot analysis where the molecular mass is around 32 kDa as determined from the SDS-PAGE. Seven genes encoding proteins are found in S. cerevisiae with a high degree of more than 85% (>85%) of amino acid sequence identity to the aryl alcohol dehydrogenase of the lignin-degrading filamentous fungus, Phanerochaete chrysosporium. It is shown that among the seven-gene set only one is not telomere associated. That means that only one is not related to the other six at the nucleotide sequence grade, but the protein level of that one bears a resemblance to the fungal aryl alcohol dehydrogenase. Moreover, a search in the sequences of AAD genes shows a close match to the DNA-binding site of the Yap1p transcriptional activator upstream of chemicals, such as diamide and diethyl maleic acid ester (DEME) that provoke an oxidative shock by inactivating the glutathione (GSH) reservoir of the cells generated by expression of the AAD genes. On the contrary, the oxidizing agent hydrogen peroxide has no effect on exhibiting these genes [Citation39]. It is evident that the present experimental work shows the evocation of AAD 16 during ibuprofen induced oxidative stress (lane 6) and no expression when S. cerevisiae in G0 is treated with hydrogen peroxide (lane 2).
Experiments of single and multiple aad deletants indicate that only AAD4 (YDL243c) and AAD6 (YFL056/57c) respond to the oxidative stress. Previously, results showed that only AAD4 and AAD6 responded to oxidative stress [Citation39].
Band 6
Band 6 (visible in the untreated proliferating cells) represents an AP-1-like transcription factor with a molecular mass of ≈32 kDa. Specific transcriptional factors emerge when there is a change in the sufficient growth conditions. Iron is an essential element, but on the other hand, could be potentially toxic. Therefore, the iron homeostasis is sustained through transcriptional control. The aforementioned factors include the group of basic leucine zipper (bZIP) iron sensing transcription factors, and the AP-1-like transcription factor YAP5 as well. According to Zampar et al. [Citation40], Yap5p is also involved in the diauxic shift. The diauxic shift in S. cerevisiae happens at the end of the logarithmic and the beginning of the stationary phase of growth as the cell metabolism readjusts from glycolytic to gluconeogenic operation. The diauxic shift is fulfilled by three major events that are organized with growth time. First, the glycolytic flux is diminished and the building of depot compounds is mediated before glucose limitation by down-regulation of phosphofructokinase and pyruvate kinase reactions. Second, when glucose is exhausted, the reversion of carbon flow through glycolysis and beginning of the glyoxylate cycle operation is elicited by an up-regulation of the enzymes. They act as catalysts of malate synthase and cytosolic citrate synthase reactions. The last stage of the adaptation is connected to the quitting of the pentose phosphate pathway with a switch in NADPH regeneration [Citation40–42].
Band 7
In the present study concerning Band 7 (visible in G0 state), a GTP-binding protein (Ypt 53) with molecular mass ≈ 26 kDa is determined in terms of oxidative stress in stationary phase. Representatives of the Rab family of small GTpases are responsible for the regulation of the macromolecular traffic of proteins and lipids in the secretory and endocytic pathway via recruitment of various effectors [Citation43–46]. The functional homology between mammalian and yeast cells is well known. There is high level of identity between rab5 and Vps21p, Ypt52p, and Ypt53p. A considerable increase in Ypt53 over time occurred when cells were grown to post-log phase in glucose-containing medium but was almost undetectable during the log phase. Similar behaviour of the stress-induced GTPase Ypt53 is also observed on lanes 2 and 6 (). Our experimental research partly upholds that hypothesis, taking into account that the enzyme is strongly expressed in G0 state. Yet, it is still not clear how the expression and activity of Rab proteins are adjusted in reply to exogenous, endogenous, or intracellular compounds. Previously, research showed that Ypt53, an isoform of Rab5 in S. cerevisiae, is up-regulated significantly under nutrient stress, which leads to entering the stationary phase. Vps21, a constitutively expressed Rab5 isoform, is pivotal for Golgi-vacuole trafficking and vacuolar hydrolase activity, when stress conditions are absent. Cells exposed to nutrient stress for a long period of time, then overexpressing Ypt53 and constitutive Vps21 function together to maintain the activities, which, in turn, prevent the gain of stress-inducing species and keep mitochondrial respiration [Citation47].
Conclusions
The present work is focused on comparative analysis of the protein expression levels of treated and untreated cells of S. cerevisiae BY4741 in actively proliferating and quiescent state. IC50 doses of four toxic substances (menadione, hydrogen peroxide, ibuprofen, and zeocin) were determined and applied. Through SDS-PAGE and subsequent MALDI-TOF/TOF tests, protein profiles of the cells upon enduring oxidative stress were generated. That allowed the analyses of the key enzymes responsible for overwhelming toxic disbalance in the proliferating and quiescent cells, respectively. That could serve as a basis for further investigations on specific enzyme mechanisms of action and revealing the relationships between them using distinguishing proteomic analyses, 2D SDS-PAGE, and as a result, a specific proteomic map could be defined.
Author contributions
Methodology: V.P., P.D., A.K., and L.V.; Data analysis: L.V., A.D., and V.P.; Writing original manuscript: L.V., A.D., and V.P.; Writing review and editing: L.V., V.P., W.V., P.D., and A.D.; Project administration: V.P. and P.D.
Acknowledgments
We dedicate this article to Prof. Voelter from the University of Tübingen, Germany, who died on 21st January, 2021.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
Notes on contributors
Asya Daskalova
Asya Vladimirova Daskalova, is an assistant at laboratory “Chemistry and Biophysics of proteins and enzymes”, Institute of Organic Chemistry with Centre of Phytochemistry at the Bulgarian Academy of Sciences (BAS). Her research work is focused on mass spectrometric analysis and proteomic analysis of bioactive compounds.
Ventsislava Petrova
Ventsislava Yankova Petrova is an assistant professor at the Department of General and Industrial Microbiology, Faculty of Biology, Sofia University “St. Kliment Ohridski”. Her research activity is concentrated on studying the different aspects of physiology, biochemistry and molecular biology of yeasts; cellular antioxidant defense system; quiescence in yeast; protein targeting and localization; biologically active substances, application of in silico approaches for solving and interpreting biological experimental data.
Lyudmila Velkova
Assoc. Prof. Dr. L. Velkova works in Institute of Organic Chemistry with Centre of Phytochemistry at the Bulgarian Academy of Sciences, laboratory “Chemistry and Biophysics of proteins and enzymes”. Her research interests include isolation, characterization and application of bioactive substances from natural sources, primarily antimicrobial peptides, proteins, and glycoproteins, investigated by mass spectrometry and proteomic analysis. She published more than 30 articles in reputed international journals.
Anna Kujumdzieva
Anna Vangelova Kujumdzieva is an associate professor at the Department of General and Industrial Microbiology, Faculty of Biology, Sofia University “St. Kliment Ohridski”. Her research activity is focused predominantly on studying the physiology and biochemistry of fungi; cultivation of microorganisms, regulation and manipulation of cell antioxidant enzyme system; biosynthesis of secondary metabolites; kinetics of microbial fermentations and microbial biomass processing for obtaining of biologically active substances.
Anna Tomova
Anna Atanasova Tomova is an assistant professor at the Department of General and Industrial Microbiology, Faculty of Biology, Sofia University “St. Kliment Ohridski”. Her research activity is focused predominantly on yeasts as a model system for studying the toxic effect of harmful substances; enzymatic and non-enzymatic antioxidant defense mechanisms; biomarkers of oxidative stress in the presence of stress inducers in the culture media; quiescence state.
Wolfgang Voelter
Wolfgang Voelter was a professor at the Department of Physical Biochemistry in the Tübingen University, Tübingen, Germany. Moreover, he was a head board of the Institute for Scientific Cooperation, Tübingen, Germany (1985–2005). In 2003 he was awarded a Doctor of Science Honoris Causa, Hamdard University, Pakistan. His work was focused on isolation, synthesis and bioactivity of natural products. He is an author of over 1000 research articles.
Pavlina Dolashka
Prof. Dr. P. Dolashka has a wide experience in the isolation, purification, and characterization of biologically active compounds. She has more than 130 publications on these topics, 3 book chapters and 6 patents. Prof. Dolashka is an editor-in-board of 3 journals and representative IUPAC. She is coordinating several international research projects, sponsored by NATO (Brussels), the European Commission, Germany (DFG and BMBF), CNR (Italy), FWO (Belgium), China, and Ukraine. She is also a coordinator from the side of IOCCP–BAS for the National Research Programme “Innovative Low-Toxic Bioactive Systems for Precision Medicine (BioActiveMed)” approved by DCM № 658/14.09.2018 and for the grant project BG05M2OP001-1.002-0019: “Clean Technologies for Sustainable Environment - Water, Waste, Energy for Circular Economy”.
References
- Hartwell L, Culotti J, Pringle J, et al. Genetic control of the cell division cycle in yeast. Science. 1974;183(4120):46–51.
- Pringle JR, Hartwell LH. The Saccharomyces cerevisiae cell cycle. Cold Spring Harb Monogr Arch. 1981;11:97–142.
- Kumar R, Srivastava S. Quantitative proteomic comparison of stationary/G0 phase cells and tetrads in budding yeast. Sci Rep. 2016;6:32031.
- Werner-Washburne M, Braun E, Johnston GC, et al. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57(2):383–401.
- Longo VD, Gralla EB, Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271(21):12275–12280.
- Galdieri L, Mehrotra S, Yu S, et al. Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS. 2010;14(6):629–638.
- Harsch MJ, Lee SA, Goddard MR, et al. Optimized fermentation of grape juice by laboratory strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2009;10(1):72–82.DOI: 10.1111/j.1567-1364.2009.00580.x
- Allen C, Büttner S, Aragon AD, et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J Cell Biol. 2006;174(1):89–100.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685.
- Walker JM. SDS polyacrylamide gel electrophoresis of proteins. In: Walker JM, editors. The protein protocols handbook. Springer protocols handbooks. 3rd ed. Totowa (NJ): Humana Press; 2009. p. 61–67.
- Gallagher SR. SDS-polyacrylamide gel electrophoresis. In: Gallagher SR, Wiley EA, editors. Current protocols essential laboratory techniques. Hoboken (NJ): Wiley; 2008. p. 290–314.
- Rosenfeld J, Capdevielle J, Guillemot JC, et al. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203(1):173–179.
- Beavis RC, Chaudhary T, Chait BT. α‐Cyano‐4‐hydroxycinnamic acid as a matrix for matrixassisted laser desorption mass spectrometry. Org Mass Spectrom. 1992;27(2):156–158.
- Chuang Y, Chen Y, Chandramouli V, et al. Gene expression after treatment with hydrogen peroxide, menadione, or t-butyl hydroperoxide in breast cancer cells. Cancer Res. 2002;62:6246–6254.
- Werner‐Washburne M, Braun EL, Crawford ME, et al. Stationary phase in Saccharomyces cerevisiae. Mol Microbiol. 1996;19(6):1159–1166.
- Cyrne L, Martins L, Fernandes LM, et al. Regulation of antioxidant enzymes gene expression in the yeast Saccharomyces cerevisiae during stationary phase. Free Radic Biol Med. 2003;34(3):385–393.
- He C, Tsuchiyama SK, Nguyen QT, et al. Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLoS Genet. 2014;10(12):e1004860.
- Dialynaki D, Fragiadakis G, Palikaras K, et al. The anti-cancer drug zeocin affects TORC1 pathway, mitochondrial function and autophagy, in S. cerevisiae. 12th Scientific FORTH Retreat, 2019 Oct 14–16; FORTH/ICE-HT, Patras, Greece.
- Kobayashi T, Heck DJ, Nomura M, et al. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev. 1998;12(24):3821–3830.
- Katsuki J, Takashi H. The cis element and factors required for condensin recruitment to chromosomes. Mol Cell. 2009;34:26–35.
- Dlakić M. A model of the replication fork blocking protein Fob1p based on the catalytic core domain of retroviral integrases. Protein Sci. 2002;11(5):1274–1277.
- Xie Z, Zhang Y, Zou K, et al. Molecular phenotyping of aging in single yeast cells using a novel microfluidic device. Aging Cell. 2012;11(4):599–606.
- Netz DJ, Stümpfig M, Doré C, et al. Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat Chem Biol. 2010;6(10):758–765.
- Vernis L, Facca C, Delagoutte E, et al. A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast. PLoS One. 2009;4(2):e4376.
- Nishimura A, Kawahara N, Takagi H. The flavoprotein Tah18-dependent NO synthesis confers high-temperature stress tolerance on yeast cells. Biochem Biophys Res Commun. 2013;430(1):137–143.
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14(16):1511–1527.
- Garcerá A, Barreto L, Piedrafita L, et al. Saccharomyces cerevisiae cells have three omega class glutathione S-transferases acting as 1-Cys thiol transferases. Biochem J. 2006;398(2):187–196.
- Board PG, Coggan M, Chelvanayagam G, et al. Identification, characterization, and crystal structure of the omega class glutathione transferases. J Biol Chem. 2000;275(32):24798–24806.
- Rouimi P, Anglade P, Benzekri A, et al. Purification and characterization of a glutathione S-transferase Omega in pig: evidence for two distinct organ-specific transcripts. Biochem J. 2001;358(Pt 1):257–262.
- Girardini J, Amirante A, Zemzoumi K, et al. Characterization of an omega-class glutathione S-transferase from Schistosoma mansoni with glutaredoxin-like dehydroascorbate reductase and thiol transferase activities. Eur J Biochem. 2002;269(22):5512–5521.
- Choi JH, Lou W, Vancura A. A novel membrane-bound glutathione S-transferase functions in the stationary phase of the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273(45):29915–29922.
- Kim Y, Cha SJ, Choi HJ, et al. Omega class glutathione S-transferase: antioxidant enzyme in pathogenesis of neurodegenerative diseases. Oxid Med Cell Longev. 2017;2017:1–6.
- Kathiresan M, Martins D, English AM. Respiration triggers heme transfer from cytochrome c peroxidase to catalase in yeast mitochondria. Proc Natl Acad Sci USA. 2014;111(49):17468–17473.
- Martins D, Kathiresan M, English AM. Cytochrome c peroxidase is a mitochondrial heme-based H2O2 sensor that modulates antioxidant defense. Free Radic Biol Med. 2013;65:541–551.
- Kwon M, Chong S, Han S, et al. Oxidative stresses elevate the expression of cytochrome c peroxidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 2003;1623(1):1–5.
- van der Klei IJ, Rytka J, Kunau WH, et al. Growth of catalase A and catalase T deficient mutant strains of Saccharomyces cerevisiae on ethanol and oleic acid. Arch Microbiol. 1990;153(5):513–517.
- Petrova VY, Kujumdzieva AV. Robustness of Saccharomyces cerevisiae genome to antioxidative stress. Biotechnol Biotechnol Equip. 2010;24(sup1):474–483.
- Petrova VY, Drescher D, Kujumdzieva AV, et al. Dual targeting of yeast catalase A to peroxisomes and mitochondria. Biochem J. 2004;380(Pt 2):393–400.
- Delneri D, Gardner DC, Oliver SG. Analysis of the seven-member AAD gene set demonstrates that genetic redundancy in yeast may be more apparent than real. Genetics. 1999;153(4):1591–1600.
- Zampar GG, Kümmel A, Ewald J, et al. Temporal system-level organization of the switch from glycolytic to gluconeogenic operation in yeast. Mol Syst Biol. 2013;9:651.
- Martins TS, Costa V, Pereira C. Signaling pathways governing iron homeostasis in budding yeast. Mol Microbiol. 2018;109(4):422–432.
- Rodrigues-Pousada C, Devaux F, Caetano SM, et al. Yeast AP-1 like transcription factors (Yap) and stress response: a current overview. Microb Cell. 2019;6(6):267–285.
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659.
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25(4):414–419.
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009; 10(8):513–525.
- Schwartz SL, Cao C, Pylypenko O, et al. Rab GTPases at a glance. J Cell Sci. 2007;120(Pt 22):3905–3910.
- Nakatsukasa K, Kanada A, Matsuzaki M, et al. The nutrient stress-induced small GTPase Rab5 contributes to the activation of vesicle trafficking and vacuolar activity. J Biol Chem. 2014;289(30):20970–20978.