Abstract
In the recent years, the application of new antitumor drugs has focused on the replacement of conventional chemotherapeutics with compounds derived from natural products. Cannabidiol (CBD) is one of the 113 cannabinoids derived from the plant Cannabis sativa and is characterized with complex and not entirely understood biological function. Unlike the other most abundant cannabinoid in Cannabis sativa – tetrahydrocannabinol, cannabidiol has low affinity to the endocannabinoid receptors and the manifestation of its activity does not appear to rely on the endocannabinoid system. Cannabidiol is used in the treatment of many diseases including some types of cancer. The aim of our study was to evaluate the cytotoxic activity of cannabidiol and its effect on the process of programmed cell death. This process is directly involved in the antitumor effect of many drugs. Two lung cancer cell lines, A549 expressing р53 and H1299-p53 negative, were used. Apoptosis was monitored by Anexin V assay and the activation of Caspase 3/7. We found that CBD treatment led to a dose-dependant apoptosis increase in p53 positive A549 cells. The level of apoptosis in p53 negative H1299 cells was much lower and did not seem to be dose dependent. However, the total cell viability was similar for the two cell lines, which was due to the increased necrosis observed in H1299 cells.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide [Citation1,Citation2]. Non-small cell lung cancer (NSCLC) represents 85% of all lung cancers and is characterized by genetic abnormalities, which lead to alterations in signalling pathways that are targets for drug therapies – as a result leading to drug resistance [Citation3]. Therefore, the development of novel therapeutic alternatives for NSCLC patients is an area of intense investigation.
Cannabinoids have been used for many centuries to counter pain. The endocannabinoid system (ECS) is an evolutionarily conserved signalling pathway, which has diverse regulatory functions [Citation4]. In the last two decades, a growing body of evidences had shown the role of ECS in a diverse scope of diseases, such as psychological disorders, motion disorders like Parkinson’s and Huntington’s disease, neuropathy, disseminated sclerosis, spinal cord trauma, atherosclerotic disease, heart attack, hemorrhagic stroke, high blood pressure, eye disease, abnormal weight and bone diseases. For each of these diseases the potential positive effect of cannabidiol (CBD), a good candidate for ECS modulator and/or regulator, has been demonstrated [Citation4–9]. Several studies have demonstrated that cannabinoids also have antineoplastic effect and are usually accompanied with no negative side effects such as the ones produced by the conventional chemotherapy treatment [Citation10,Citation11].
Cannabidiol (CBD) exerts selective cytotoxicity and halts the proliferation of many types of cancer. These effects mainly rely on processes such as apoptosis and autophagy and they are observed in many types of cancer [Citation12–16]. In in vitro experiments CBD showed the most powerful antineoplastic activity compared to other phytocannabinoids [Citation11]. In in vitro experiments CBD has been established as a potent anti-metastatic agent in treatment of cervical cancer cells [Citation17], alveolar and lung cancer cells [Citation18,Citation19] and breast carcinoma cells [Citation20]. Furthermore, CBD has demonstrated antineoplastic effects on solid tumors in vivo [Citation12,Citation18,Citation19].
The aim of our study was to evaluate the cytotoxic activity of cannabidiol and its effect on the process of programmed cell death – a process directly involved in the antitumor effect of many drugs. Two lung cancer cell lines, A549 expressing р53 and H1299-p53 negative, were used as a model of NSCLC. Apoptosis was monitored by Anexin V assay and the activation of Cas3/7. Caspase-3 (Cas-3) and caspase-7 (Cas-7) can directly carry out apoptosis, which makes them great targets for apoptosis monitoring [Citation21].
Materials and methods
Cell cultures
A549 (p53 positive, ATCC, LGC STANDARDS) and H1299 (p53 negative, ATCC, LGC STANDARDS) lung cancer cells plated in F-12K Medium (Thermo Fisher Scientific) and RPMI-1640 Medium (Thermo Fisher Scientific) respectively supplemented with 10% (v/v) fetal calf serum (FCS, Thermo Fisher Scientific) and 1% penicillin/streptomycin solution (Thermo Fisher Scientific) were maintained at 37 °C in 5% CO2 incubator.
Cannabidiol ethanol solution
CBD ethanol solution (10 mg/mL) was bought from Sigma-Aldrich.
Etoposide
(Sigma-Aldrich) was used as a positive control in the treatment of cells, as it is a chemotherapeutic with known apoptotic action.
Cell viability assay
The cytotoxic activity of CBD was tested on a panel of two human lung cancer cell lines using the MTT assay as described previously [Citation22]. Briefly, 2000 cells were seeded into each well in 100 µL in octuplicates in 96-well flat-bottom plates and incubated for 24 h (37 °C, 5% CO2) to allow attachment of the cells. After 24 h the media were replaced with 100 µL of fresh media containing the solution of the tested compound in serial dilutions with concentration from 16 to 512 µmol/L. Cannabidiol was initially dissolved in 70% ethanol and then diluted to the final concentration with media. After 72 h of incubation, the medium was changed with media containing MTT (Invitrogen) at a final concentration of 0.5 mg/mL (a soluble tetrazolium salt). The microplates were further incubated for 2 h at 37 °C and the formed formazan crystals were dissolved through addition of 100 µL dimethyl sulfoxide (DMSO) into each well. The absorbance was measured on an ELISA plate reader Varioscan Lux (Thermo Fisher Scintific) at a test wavelength of 570 nm and a reference wavelength of 630 nm to obtain sample signal. The statistical software GraphPad Prism v.7 was used for data analysis.
Immunofluorescence microscopy
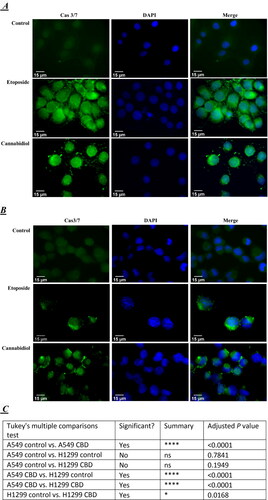
Cells of lung cancer cell lines H1299 and A549 were seeded in numbers of 25 000 per well in a 24-well plate (Corning Costar Flat Bottom Cell Culture Plate) on glass coverslips previously sterilized with ethanol and with ultraviolet (UV) light for 20 min each side. After 24 h, the cells were treated with 10 µmol/L cannabidiol solution and with etoposide at concentrations of 200 µmol/L and 120 µmol/L, respectively, for H1299 and A549 cell lines. CellEvent Caspase-3/7 Green Detection Reagent (Invitrogen, Thermo Fisher Scientific) was used according to the manufacturer’s protocol for detection of activated caspase 3/7 during apoptosis. The coverslips were treated as described previously [Citation23], after 40 min the cells were fixed with 3.7% paraformaldehyde in 1xPBS for 10 min at room temperature (RT) and afterwards permeabilized with 0.1% Triton X-100 in 1xPBS at RT for 2 × 5 min. The glass coverslips were mounted in ProLong Diamond Antifade Mountant (Thermo Fisher Scintific) containing 10 μg/mL DAPI on microscope slides. The signals were obtained with filter for the Alexa Fluor™ 488 dye on Zeiss AxioVert 200 M microscope using a 63 × objective lens, equipped with a CCD camera AxioCam MRm. The images were processed with Image J.
Flow cytometry for apoptosis detection
For apoptosis detection a FITC Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher Scientific) was used [Citation24]. Approximately 3 × 105 cells/mL treated with cannabidiol concentrations corresponding to IC25, IC50 and IC75 values were stained in green with FITC Annexin V followed by staining the DNA in red with propidium iodide according to the manufacturer’s protocol. The measurement was performed on a flow cytometer FACSCalibur BD. The percentage of the cells at each stage of apoptosis was determined by FlowJo v.9 software.
Statistical analysis
For statistical analysis, GraphPad Prism v.8 software was used. Statistical differences in flow cytometry and microscopic analyses were calculated by multiple comparisons function of one-way analysis of variance (ANOVA). Differences were considered statistically significant at P-values ≤ 0.05.
Results
Cell viability under cannabidiol exposure
Our foremost goals were focused on setting up a framework within which to determine the cytotoxicity of CBD in our cell cultures. To that end, we performed an MTT assay, which allowed us to establish the half-maximal inhibitory concentration (IC50) for CBD against the two cell lines after 72 h’ treatment. CBD showed cytotoxic effect for both cell lines. We performed this assay with the assumption that the IC50 values will give us basic information about the cytotoxicity of this compound, which is crucial in the development of more complex and informative experimental settings. The data we got from this experiment showed that the cells more resistant to the CBD treatment in in vitro conditions are H1299, which originated from a more aggressive form of lung cancer. These cells have a homozygous partial deletion of the p53 protein, and lack expression of p53 protein. Thus, the lack of expression of the tumor suppressor gene probably necessitates the administration of higher doses of CBD to achieve cell death. Our results support the idea that CBD has the properties for more intricate research and the potential of becoming a powerful tool in the battle against lung cancer in particular, but also against many other types of cancer.
Assessment of cell apoptosis after cannabidiol treatment by flow cytometry
After establishing the 50% lethal dose of cannabidiol for both lung cancer lines, we check the level of apoptosis in both lines as the cause of cell mortality. Both lung cancer cell lines were treated with three different concentrations of cannabidiol, IC25, IC50 and IC75 (calculated from the cell viability curves, ) for 24 h. To measure the apoptotic cell number, we used a method which relies on the staining with Annexin V conjugated with FITC and a following DNA staining with propidium iodide. We performed this assay in order to verify the data that cannabidiol triggers apoptosis in lung cancer cells ().
Figure 1. Half-maximal inhibitory dose IC50 values of cannabidiol treated cells.
Note: The two lung cancer cell lines A549 and H1299 were treated with different concentrations of CBD in a range from 16 to 512 µmol/L for 72 h exposure and were analyzed by MTT test. The data are normalized to the corresponding non-treated control cells. The mean values of three independent experiments performed in quadruplicates are presented with standard deviation (n = 3) ±SD.
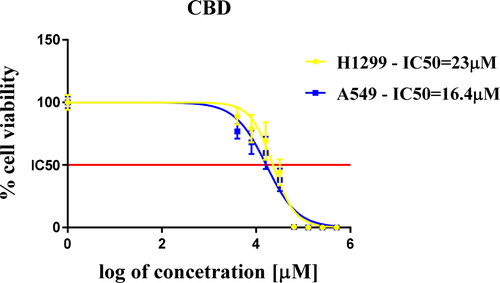
Figure 2. Detection of apoptosis with FITC Annexin V/Dead Cell Apoptosis Kit - cells from the two cancer cell lines were treated with three different concentrations of CBD (IC25, IC50, IC75). Apoptotic cells were identified by the increase in the fluorescence intensity of FITC-labeled Annexin V and necrotic cells were detected by increase in the fluorescence intensity of PI. Representative scatter plots showing the distribution of Annexin V and PI staining for control cells and CBD treated cells. Cells are classified as “viable” (bottom left), “early apoptotic” (bottom right), “late apoptotic” (top right) or “necrotic” (top left).
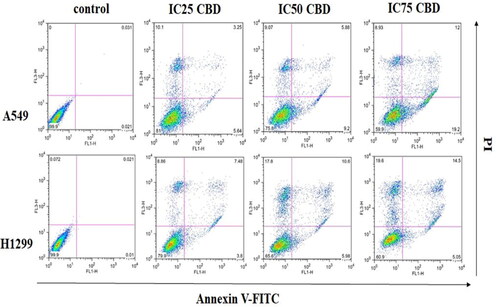
We observed that the percentage of apoptotic cells in the A549 cancer line was higher at all three applied CBD concentrations compared to H1299 (). Moreover, in A549 there was a significant increase in the number of cells in early and late apoptosis between treatments. In contrast, the percentage of necrotic cells in the H1299 cell line was significantly higher, especially at a higher concentration of the applied dose of CBD but the apoptotic cells were less. We determined that the number of cells in apoptosis increased in a dose-dependent manner, which suggests that apoptosis is the main cause of the cytotoxic effect that CBD has on A549 cancer cells. The difference in the response of both cancer cell lines, however, may be due to the fact that A549 is a cancer line with active p53 – one of the main player in apoptosis. This gives us reason to assume that cell apoptosis after treatment with CBD is regulated in a p53 dependent manner.
Table 1. Percentage distribution of cells in early/late apoptosis and necrosis in A549 and H1299 cancer cell line.
Immunofluorescent analysis of caspase 3/7 expression and localization
For immunofluorescent analysis we used CellEvent Caspase-3/7 reagent. Caspase 3 and caspase 7 share similar substrate specificity by recognizing the tetra-peptide motif Asp-x-x-Asp (D-x-x-D) [Citation25]. Caspase-3/7 Green Detection Reagent [Citation26] (Thermo Fisher Scientific) is intrinsically non-fluorescent, as the DEVD peptide inhibits the ability of the dye to bind to DNA. However, after activation of caspase-3/7 in apoptotic cells, the DEVD peptide is cleaved, enabling the dye to bind to DNA and produce a bright, fluorogenic response. We cultured cells on glass coverslips and treated them with IC25 concentration of CBD for 24 h. The concentration was chosen lower than the IC50, so that we can initiate a process of apoptosis without causing massive cell death. The localization of Cas3/7 was detected with a CellEvent® Caspase-3/7 kit and visualized under an epifluorescent microscope Zeiss AxioVert 200 M equipped with a CCD camera AxioCam MRm. For a positive control, we treated the cells with etoposide at previously established IC25 concentrations: 60 µmol/L and 100 µmol/L for A549 and H1299 respectively ().
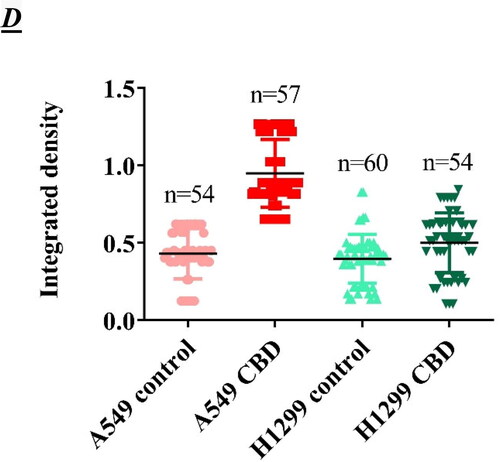
Figure 3. Subcellular localization of cleaved, active caspase-3 in A549 (A) and H1299 (B) lung carcinoma cells. A549 and H1299 cells were cultured on glass chamber slides overnight at 37 °C. They were then analyzed using CellEvent® Caspase-3/7, a green detection cell permeable reagent for the intracellular distribution of cleaved caspase-3, under a fluorescence microscope. Green indicates cleaved caspase-3 and blue indicates nuclei. Quantification of the signal intensity of the active caspase-3/7 in control and CBD treated cells and corresponding ANOVA table (C and D). N is the number of cells that were scored for each tested group. Probability values were considered significant at the level of *p < 0.05, **p < 0.01, ***p < 0.005, **** p < 0.001; ns, non-significant.
The Cas3/7 level in untreated A549 () and H1299 () cells was low close to background. The nuclear signal assigned to Cas3/7 activation was much more intense upon treatment with both etoposide and CBD compared to that in the control cells. We found Cas3/7 activation after treatment with etoposide or CBD in both cell lines, but apparently a stronger signal was observed in the A549-p53 positive cell line, compared to the H1299 cell line (). This result correlates with our results from flow cytometry, in which we also observed a stronger Anexin V signal in the p53 positive cancer cell line A549. It is interesting to note that the nuclei of cells treated with both CBD and etoposide appeared morphologically enlarged (swollen) compared to control untreated cells ().
Discussion
Every year around 1.8 million people are diagnosed with lung cancer. The high mortality rate which comes with this disease is often associated with the cancer cells becoming resistant to the commonly prescribed chemotherapy agents or the low selective cytotoxic capacity of these drugs. That is why, the search for new more selectively acting bio-derived medicines is on the rise.
In recent years, the medical and health-related applications of or CBD, has garnered tremendous attention. The most recent findings strongly support the further development of CBD as a promising anti-cancer drug. The mechanism of action of CBD and its potential applications in cancer therapy are the major focus of many authors [Citation10].
In this study, we determined the toxicity of CBD on two lung cancer cells A549 and H1299. We found that the p53 positive cancer line A549 showed a lower IC50 value, probably due to the fact that H1299 cells have a homozygous partial deletion of the p53 protein, and lack expression of p53 protein. In our previous studies, it was demonstrated that H1299 cells are less sensitive to the treatment with classical chemotherapeutic drugs such as cis-platin and Tamoxifen [Citation22,Citation27] as well; thus, the lack of expression of p53 is probably responsible for the observed higher drug resisters. It was interesting to examine the extent to which this cellular toxicity (mortality) is caused by the process of apoptosis. We performed an experiment which showed that indeed in A549 the percentage of apoptotic cells in early and late apoptosis was higher than in H1299. In contrast to the p53 positive cancer line, in H1299 the percentage of cells that were in the necrotic stage was higher. This required to assay the expression and localization of the Cas3/7 apoptotic markers in both cancer cell lines. Immunofluorescence analysis showed that in A549 (p53+) the expression of caspase 3/7 was much higher compared to H1299 cell line. The results also showed that the effect of CBD on lung cancer cell lines was dose dependent (). In addition, the response of cancer cells depends on the presence of the tumor suppressor protein p53. However, not much has been reported on the induction of apoptosis via activation of p53 by Cannabis sativa-extracts.
The tumor suppressor protein p53 is one of the many substrates of caspase-3 [Citation28,Citation29]. p53 can direct a cell toward apoptosis by trans-activating a large number of pro-apoptotic genes and also this protein may induce programmed cell death by trans-activating the genes for some caspases themselves [Citation29]. A good examples are the executioner caspase-6 and −7 which have been identified as transcriptional targets of p53 in cisplatin injury [Citation30]. This is a probable explanation of the stronger signal observed in p53 positive cancer cell line A549 treated with CBD. An interesting perspective for future research is the in-depth study on the antitumor effect the CBD exerts on H1299 cells, which seem to be more resistant to the treatment. The level of apoptosis was twice to four times less depending on the dose in H1299 cells (); however the total cell viability was similar for the two cell lines. This is due to the increased necrosis observed in H1299 cells.
Conclusions
Having in mind the sheer volume of unknowns that come with these first data, we see many potential perspectives for future investigation of this phytocannabinoid. CBD could turn out a good tool in the fight with cancer. Despite the preliminary state of this study and the non-quantitive data it presents, it supports the idea that cannabidiol has the properties for more intricate research and the potential of becoming a powerful tool in the battle against lung cancer in particular, but also against many of the other types of cancer.
Data availability statement
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- The American Cancer Society. Key statistics for lung cancer; 2021. Official statistics: USA. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Terlizzi M, Colarusso C, Pinto A, et al. Drug resistance in non-small cell lung Cancer (NSCLC): Impact of genetic and non-genetic alterations on therapeutic regimen and responsiveness. Pharmacol Ther. 2019;202:140–148.
- Schurman LD, Lu D, Kendall DA, et al. Molecular mechanism and cannabinoid pharmacology. Handb Exp Pharmacol. 2020;258:323–353.
- Pacher P, Kogan NM, Mechoulam R. Beyond THC and endocannabinoids. Annu Rev Pharmacol Toxicol. 2020;60:637–659.
- Cooray R, Gupta V, Suphioglu C. Current aspects of the endocannabinoid system and targeted THC and CBD phytocannabinoids as potential therapeutics for Parkinson’s and Alzheimer’s diseases: a review. Mol Neurobiol. 2020;57:4878–4890.
- Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants (Basel). 2019;9. doi:10.3390%2Fantiox9010021.
- Straiker A, Dvorakova M, Zimmowitch A, et al. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol. 2018;94:743–748.
- Taylor L, Gidal B, Blakey G, et al. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–1067.
- Seltzer ES, Watters AK, MacKenzie D, Jr, et al. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers (Basel). 2020;12:3203.
- Javid FA, Phillips RM, Afshinjavid S, et al. Cannabinoid pharmacology in cancer research: a new hope for cancer patients?Eur J Pharmacol. 2016;775:1–14.
- Ligresti A, Moriello AS, Starowicz K, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387.
- Massi P, Vaccani A, Bianchessi S, et al. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63:2057–2066.
- McKallip RJ, Jia W, Schlomer J, et al. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908.
- Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172.
- De Petrocellis L, Ligresti A, Schiano Moriello A, et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol. 2013;168:79–102.
- Ramer R, Merkord J, Rohde H, et al. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–966.
- Ramer R, Rohde A, Merkord J, et al. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res. 2010;27:2162–2174.
- Ramer R, Bublitz K, Freimuth N, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. Faseb J. 2012;26:1535–1548.
- McAllister SD, Christian RT, Horowitz MP, et al. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–2927.
- Shim MK, Yoon, HY, Lee S, et al. Caspase-3/-7-specific metabolic precursor for bioorthogonal tracking of tumor apoptosis. Sci Rep. 2017;7:16635.
- Kamenova-Nacheva M, Schröder M, Pasheva E, et al. Synthesis of ferrocenylmethylidene and arylidene substituted camphane based compounds as potential anticancer agents. New J Chem. 2017;41:9103–9112.
- Ugrinova I, Monier K, Ivaldi C, et al. Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. Bmc Mol Biol. 2007;8:1–16.
- Schroder M, Yusein-Myashkova S, Petrova M, et al. The effect of a ferrocene containing camphor sulfonamide DK-164 on breast cancer cell lines. Anticancer Agents Med Chem. 2019;19:1874–1886.
- Agniswamy J, Fang B, Weber IT. Plasticity of S2-S4 specificity pockets of executioner caspase-7 revealed by structural and kinetic analysis. Febs J. 2007;274:4752–4765.
- Thermophisher Scientific. CellEvent™ caspase-3/7 green detection reagent product overview. Available from: https://www.thermofisher.com/order/catalog/product/C10423#/C10423.
- Yusein-Myashkova S, Stoykov I, Gospodinov A, et al. The repair capacity of lung cancer cell lines A549 and H1299 depends on HMGB1 expression level and the p53 status. J Biochem. 2016;160:37–47.
- Sayan BS, Sayan AE, Knight RA, et al. p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem. 2006;281:13566–13573.
- Heyne K, Roemer K. Caspase-3 joins the p53 interactome. Cancer Biol Ther. 2011;11:762–764.
- Yang C, Kaushal V, Haun RS, et al. Transcriptional activation of caspase-6 and -7 genes by cisplatin-induced p53 and its functional significance in cisplatin nephrotoxicity. Cell Death Differ. 2008;15:530–544.