?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study was focussed on the inhibitory mechanism associated with the ability of an endophytic bacterium - Bacillus altitudinis Q7, obtained from Ginkgo biloba, to inhibit the growth of Alternaria alternata in vitro. A fungus, identified as Alternaria alternata was isolated from the rotten part of an apple. One of the endophytes was identified as Bacillus altitudinis Q7. Effect of Bacillus altitudinis Q7 on Alternaria alternata was characterized by measuring membrane permeability and lipid peroxidation. Antioxidant enzymes in Alternaria alternata were determined before and after exposure to Bacillus altitudinis Q7. Results indicated that cell membranes in Alternaria alternata were damaged, lipid peroxidation was enhanced, and superoxide dismutase, peroxidase, and polyphenol oxidase were significantly inhibited. Content of lipopeptides produced by Bacillus altitudinis Q7 was higher than other metabolites. The FTIR spectra revealed that the prepared antibacterial lipopeptides is a cyclic lipopeptides. The effect of lipopeptides produced by Bacillus altitudinis Q7 at 2 mg/mL against Alternaria alternata in vitro reached 83.2%. The lipopeptide-treated group showed the same trend as Q7 fermentation broth in inhibition mode of action. Moreover, the utilization of proteins and sugars during culturing of Alternaria alternata decreased when adding lipopeptides to the growth medium. Bacillus altitudinis Q7 from Ginkgo biloba significantly inhibited the growth of Alternaria alternata and is a very promising biocontrol strain against Alternaria alternata. The study of endophytic bacteria and its metabolites is being done to identify biological control alternatives. The results provide a theoretical basis for understanding their antagonistic properties.
Introduction
A. alternata is the causal agent of many apple diseases. It can infect both inflorescences and fruits, causing mouldy core after harvest. Apple fruits are generally infected through lenticels, eventually producing visible lesions [Citation1].
Apple rots caused by A. alternata can be especially devastating if the growing season is very rainy which may result in a major economic loss [Citation2]. In countries such as China, physical bagging of fruit combined with chemical control is used to manage disease outbreaks of A. alternata. Physical bagging of fruit, however, if not done properly, can actually enhance infection rates and the use of chemicals can lead to problems with pesticide residues. Therefore, more research is being conducted to identify safe and effective bio-acid, approaches to manage this and other apple diseases.
Plant endophytes can produce the same or similar types of physiologically active ingredients as their hosts [Citation3, Citation4]. Most plant endophytes have also been demonstrated to have unique physiological properties, including antimicrobial, anti-oxidant and tumor-suppressive activity [Citation5, Citation6]. This makes plant endophytes with antimicrobial ability good potential control candidates for controlling a wide range of plant diseases.
G. biloba is a tree with many medicinal properties and contains more than 200 biochemicals, including flavonoids, terpenoid lactones, organic ugars, alkaloids and many other bioactive ingredients [Citation7, Citation8].
The research showed that the antagonistic yeasts used as biological control agents were able to adhere to the mycelia of the pathogenic fungi [Citation9]. The tenacious attachment, along with the secretion of extracellular lytic enzymes of two antagonistic yeasts (Pichia membranefaciens and Cryptococcus albidus), may play a role in the biocontrol activity of yeast antagonists, and the interaction between yeasts and pathogens was hampered by a protein denaturant at low concentrations [Citation10]. Bacillus strains have also been used to control Monilinia fructicola in peach fruit through the activity of volatiles released by the biocontrol strain [Citation11]. Microbial antagonists have also been shown to be able to manage anthracnose in fruits [Citation12]. Two endophytic bacteria, Fy11 and Zy44, obtained from G. biloba were shown to exhibit antagonistic activity against the causal agent (Phytophthora sp.) of pepper blight, and the level of control was better when both bacteria were combined and used together [Citation13]. There have been many reports on the antagonistic properties of endophytic fungi obtained from G. biloba against a variety of plant pathogens, however, few reports have examined the properties of endophytic bacteria obtained from G. biloba. In a previous study, endophytic bacteria were isolated from different healthy tissues of G. biloba growing in different geographical regions and demonstrated to have broad-spectrum antimicrobial activity [Citation14].
Cell membranes are semi-permeable with a high level of selectivity. The permeability of cell membranes, however, can be damaged by some antimicrobial substances, thus altering the normal transport of materials in and out of the cell. Notably, some antimicrobial substances can also be inactivated by binding to pathogen proteins or lead to increased production of reactive oxygen species (ROS) which can damage cell walls, the plasma membrane, inhibit antioxidant enzyme activity and generally, inhibit the growth and the reproduction of a pathogen [Citation15, Citation16]. The vigour of plants cell can increase by inducing physiological protection against oxidative damage. SOD catalyses the dismutation of the superoxide anion to hydrogen and molecular oxygen, the first step in active oxygen-scavenging systems. POD is also important for oxygen scavenging and in protecting cells against many environmental stresses [Citation17].
The objective of the present study was to study the inhibitory mechanism associated with the ability of a strain of an endophytic bacterium, Bacillus altitudinis Q7, obtained from G. biloba to inhibit the growth of A. alternata.
Materials and methods
Organisms and reagents
Ten isolates of endophytic bacteria were isolated and screened from G. biloba (harvested in November) in Dalian City, Liaoning Province, China, 120°58′-123°31′ E, 38°43′-40°10′ N and stored in the Microbiological Culture Collection Center of Dalian Polytechnic University. The pathogenic fungus, A. alternata was isolated from the rotten part of the apple. Kits to assay for superoxide dismutase (SOD), peroxidase (POD) and polyphenol oxidase (PPO) were purchased from the Nanjin Jiancheng Bioengineering Institute, China. All used reagents were analytical grade.
Isolation and purification of Alternaria alternata
The rotten part of the apple was inoculated in a Potato Dextrose Agar medium (PDA, 20% Potato, 2% Dextrose, 2% agar) at 30 °C for 5 days. The colonies showing reddish dark green colour with a whitish border were selected. Single colonies were picked and purified by streaking onto PDA plates at 30 °C for 5 days and examined by optical microscopy (10 × 10). The strain was stored at 4 °C.
Culturing of endophytic bacteria from Ginkgo biloba and Alternaria alternata
Endophytic bacteria derived from G. biloba were maintained on Nutrient Agar culture medium (NA, 10% peptone, 3% beef extract, 5% NaCl, 2% agar) and sub-cultured in Nutrient Broth culture medium (NB, 10% peptone, 3% beef extract, 5% NaCl) at 30 °C for 2 days on a rotary shaker at 120 rpm. A. alternata was pre-cultured on PDA at 30 °C for 5 days and the resulting colonies were used to harvest mycelia. A layer of fermentation broth containing individual isolates of endophytic bacteria was poured over PDA plates and subsequently, a 6 mm diameter portion of a colony of A. alternata was placed in the centre of the PDA plate containing the endophytic bacterial isolate and fermentation broth. The diameter of the colony of A. alternata was measured after cultivation at 30 °C for 3 days and calculated according to the formula [Citation18]:
Identification of the target endophytic bacterium
The target endophytic bacterium from G. biloba exhibiting the greatest inhibitory activity against A. alternata was identified based on morphology, physiology, biochemistry and the sequence of 16S rRNA. The various methods, such as colony morphology, used for microbial identification were as described in Sultana et al. [Citation19]. API 50CH strips (BioMérieux) was used for physiological and biochemical assays. The 16S rRNA sequence analysis was conducted by Huada Gene Technology Co., Ltd (Shanghai, China) of amplicons generated using the following PCR procedure.
PCR procedure: 95 °C for 5 min; 95 °C for 30 s, 55 °C for 30 s and 70 °C for 1 min, for 35 cycles; extension at 72 °C for 10 min. Amplicons were generated using the primers, 27 F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492 R (5′-TACGGYTACCTTGTTACGACTT-3′), following the protocol described by Noha et al. [Citation20]. MEGA 7 software was used to build a phylogenetic tree with the neighbor-joining method.
Preparation of the Q7 fermentation broth
The selected strain Q7, identified as Bacillus altitudinis was activated on NA for 12 h and then in liquid NB culture for 3 days at 28 °C, on a rotary shaker set at 120 rpm [Citation13]. The fermentation broth was then centrifuged for 15 min at 6790 g and the supernatant was filtered through a 0.22 μm filter and stored at 4 °C.
Determination of active metabolites in the Q7 fermentation broth
Total polysaccharides (sugar) in the Q7 fermentation broth was determined using the phenol-sulfuric acid method with glucose as the standard [Citation21]. Protein content in the Q7 fermentation broth was determined by colorimetry using Coomassie Brilliant Blue G-250 (CBB G-250) [Citation22]. The working solution of CBB G-250 was prepared by dissolving 0.100 g of the crystal in 50 mL of 95% ethanol and then mixing with 100 ml 85% phosphoric acid. The mixture was then diluted to 1000 mL with sterile water.
Flavonoid content in the Q7 fermentation broth was determined by aluminium chloride colorimetry [Citation23]. One mL of the fermentation broth was added to 9 mL of a 1% aqueous solution of aluminium trichloride, shaken and incubated for 10 min. Absorbance at 420 nm was then recorded. Rutin was used as the standard.
For detection of lipopeptides, the pH of the fermentation supernatant was adjusted to 2.0 with 6 mol/L hydrochloric acid and left overnight at 4 °C. The fermentation broth was then centrifuged (4 °C) for 15 min at 6790 g and the resulting precipitate was rinsed with sterile water. NaOH (2 mol/L) was then used to adjust the pH to neutral. The precipitate was then dissolved in methanol. Suction filtration, spin-vaporization, and freeze-drying were then used to prepare crude lipopeptides [Citation24].
The concentration of crude lipopeptides was adjusted to 14.4 mg/mL lipopeptides solution with a methanol solution. An X-5 macroporous resin (3 cm × 29 cm) was used to further purify the crude lipopeptides, at a flow rate of 1 mL/min. The column was eluted with absolute ethanol at a flow rate of 2 mL/min. Then the eluent was collected and the excess absolute ethanol was removed by rotary evaporation, the lipopeptides was stored after freeze-drying. Among lipopeptides solutions of different concentrations, the lipopeptides solution of 2 mg/mL showed a significant inhibitory effect, so 2 mg/mL was selected as the lipopeptides concentration in the subsequent experiments.
Infrared spectroscopy analysis of lipopeptides
The infrared (IR) spectrum of lipopeptides was determined using a Fourier transform infrared (FTIR) spectrophotometer (Spectrum One-B, Perkin Elmer, U.S.) for detection of various functional groups. The purified lipopeptides were ground with KBr powder and pressed into pellets for FTIR measurement in the frequency range of 4000–400 cm−1 under vacuum.
Effect of temperature and pH on the inhibitory activity of the Q7 fermentation broth against Alternaria alternata
Effect of temperature on the antimicrobial activity of the Q7 fermentation broth
A flask containing Q7 fermentation broth was placed in a water bath at various temperatures (40, 50, 60, 70, 80, 90 or 100 °C) for 30 min, and was then used to cover PDA medium in a petri dish for half an hour, so that the fermentation broth could penetrate into the medium. The fermentation remaining on the PDA plate was then discarded. A 6 mm diameter colony of A. alternata was then placed in the centre of the plate and colony diameter was measured after 3 days of incubation as described above. Sterile water was used as a control and each three replicates were used for each temperature.
Effect of pH on the antimicrobial activity of the Q7 fermentation broth
The pH of the Q7 fermentation broth was adjusted to 3.0, 5.0, 7.0, 9.0, 11.0 and 13.0 using 6 mol/L HCl and 2 mol/L NaOH. After 2 h, the pH was re-adjusted back to the original value (initial pH of the fermentation broth was about 8.0). The Q7 fermentation broth was then used to cover a PDA plate for 30 min and then the plate was inoculated with a 6 mm colony of A. alternata was inoculated onto the medium after 1/2 h. Plates were incubated for 3 days and colony diameter was then measured. Sterile water was used as a control and three replicates were used for each pH.
Effect of Q7 fermentation broth on the physiology and biochemistry of Alternaria alternata
Effect of Q7 fermentation broth on the colony morphology of Alternaria alternata
A small amount of hyphae with spores was picked from the PDA plate incubated for 3 days by a dissecting needle, and then infiltrated with 50% ethanol. The mycelial residues were washed with distilled water and placed directly onto the slide. The hyphae was carefully scattered using a dissecting needle, gently covered with a cover slip. Microscopic morphology observation was observed under an optical microscope. The hyphae treated without Q7 fermentation broth was used as a control.
Effect of Q7 fermentation broth on the cell membrane permeability of Alternaria alternata
Mycelia (1.5 g) of A. alternata was put into a 250 mL flask, containing 100 mL of liquid PD medium. Two millilitres of fermentation broth was added into the PD medium in the treatment group, and an equal amount of sterile water was added in the control group. Cultures were placed at 30 °C on a rotary shaker (120 rpm) and conductivity of the culture broth was measured at 0, 2, 4, 6, 8, 10, 12, 24 and 48 h using a DDS-307A Conductivity Meter (Changzhou, China).
Effect of Q7 fermentation broth on the content of malondialdehyde in mycelia of Alternaria alternata
Mycelia (1.5 g) of A. alternata was prepared as described in section of cultivation of A. alternata. The culture medium containing an aliquot of the Q7 fermentation broth and mycelia was placed at 30 °C on a rotary shaker (120 rpm) for 3 days. The mycelia was rinsed with Phosphate buffer (pH = 7.4) and surface moisture was removed with filter paper. The mixture was ground with quartz sand. Then the mycelia was added into 5 mL of trichloroacetic acid (5%) and centrifuged for 10 min at 950 g. The 2 mL of thiobarbituric acid (0.67%) was added into 2 mL of the supernatant, and then shaken and cooled in room temperature. The absorbance of supernatant was recorded at A532, A600 and A450. Sterile water was used as a control and three replicates were used for each group.
Effect of Q7 fermentation broth on SOD, POD, and PPO enzyme activity in Alternaria alternata
Mycelia (1.5 g) of A. alternata was incubated in 100 mL of liquid PD medium at 30 °C for 3 days on a rotary shaker set at 120 rpm. Then 2 mL of Q7 fermentation broth was added to the culture and the weight of mycelia was determined at different subsequent time points. The mycelia was rinsed with distilled water and surface moisture was removed with filter paper. The wet mycelium was accurately weighed and mixed with physiological saline at ratio (m/v) of 1:9. The mixture was ground with quartz sand and centrifuged for 10 min at 1700 g. SOD and POD levels were determined using assay kits and the manufacturer’s instructions. Cultures in which sterile water was added instead of Q7 fermentation broth were used as a control. When determining PPO activity, a specific extract provided in the assay kit was added to the tissue homogenate during grinding. PPO activity was also determined with an assay kit following the instructions provided by the manufacturer.
Effect of lipopeptides present in the Q7 fermentation broth on Alternaria alternata
Determination of antibacterial spectrum of lipopeptides
The mycelium growth rate method was used to determine the antimicrobial spectrum of moulds. Two mg/mL lipopeptides solution was cover a PDA plate (sterilized and cooled to about 50 °C), Inhibitory activity of lipopeptides against A. alternata in vitro, was evaluated using the same method as the above section of endophytic bacteria against A. alternata.
Effect of lipopeptides on the physiology and biochemistry of Alternaria alternata
One millilitre of lipopeptides (2 mg/mL) was added into the medium used to culture A. alternata as described in section of cultivation of endophytic bacteria. The mixture was cultured at 30 °C for 72 h on a rotary shaker (120 rpm). Supernatant was subsequently obtained by centrifugation (4 °C) for 15 min at 6790 g. Cultures in which sterile water was added instead of Q7 fermentation broth were used as a control. The physiology and biochemistry were determined as described in section of determination of active metabolites in the Q7 fermentation broth.
Statistical analysis
Each experimental variable utilized three biological replicates. The obtained data were subjected to a one-way analysis of variance (ANOVA) using OriginPro 8 Data Analysis ToolPak (p < 0.05).
Results
Screening and purification of Alternaria alternata
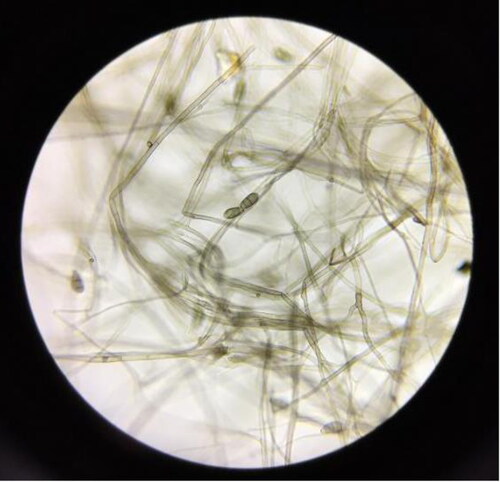
The colonies were off-white in the early stages and dark green after entering the senescence period. The hyphae was slender and the diameters were between 2 and 10 µm. Conidia hyaline were inverted rod-shaped or ovoid with a relatively smooth surface, conidiophores erect with a horizontal septum (). The strain possessed characters typical of the Alternaria alternata. According to Fungal Structure and Morphology [Citation25], the strain was confirmed as Alternaria alternata.
Screening of endophytic bacteria
The antimicrobial activities of 10 endophytic bacteria obtained from G. biloba were presented in . The evaluated endophytic strains of bacteria exhibited a high level of inhibitory activity against A. alternata in vitro, and strain Q7, with the highest level of inhibitory activity, was selected for further study.
Table 1. Inhibition of Alternaria alternata by 10 endophytic bacteria derived from Ginkgo biloba.
Identification of strain Q7
Microscopic examination of Q7 indicated that Gram staining was Gram-positive. The morphology of the cells was rod-shaped. The bacterium formed separate distinct colonies that were smooth in appearance. Based on the physiological and biochemical assessment () strain Q7 was tentatively identified as Bacillus altitudinis, named as Bacillus altitudinis Q7.
Table 2. Physiological and biochemical indexes of Bacillus altitudinis Q7.
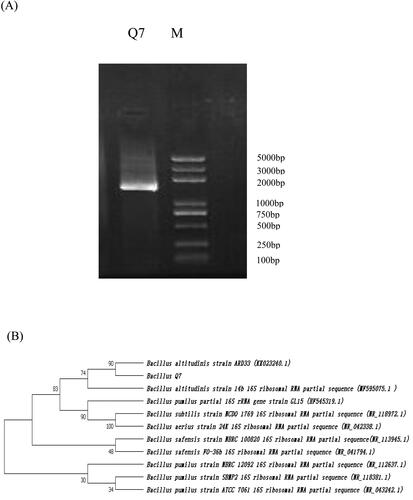
The PCR amplification product of the strain (3 μL) was detected by 1% agarose gel electrophoresis and there was a specific band in the middle of 1000 bp–2000 bp (), the obtained PCR product was sequenced to a sequence of 1438 bp in length. Blast analysis of the 16S rRNA sequence indicated that Q7 was closely related to Bacillus altitudinis ARD33 by 99% similarity. Thus, the collective data, including a phylogenetic tree analysis () confirmed that strain Q7 was an isolated strain of Bacillus altitudinis, which was closely-related to Bacillus altitudinis ARD33.
Figure 2. Identification of strain Bacillus altitudinis Q7 16S rDNA. (A) Agarose gel electrophoresis of PCR-amplified DNA of the strain. (B) Phylogenetic tree based on the sequence similarity of the 16S rRNA; Neighbour-joining tree based on analysis of partial 16S rRNA nucleotide sequences of the selected strain Q7. Numbers at branching points indicate the bootstrap values based on 1000 resampled datasets.
Determination of main antifungal activity metabolites in Bacillus altitudinis Q7 fermentation broth
Several major metabolites were found to be present in the fermentation broth of Bacillus altitudinis Q7. A trace amount of flavonoids (1.13 ± 0.30 mg·L−1) was identified, as well as the presence of total sugars (69.1 ± 1.83 mg·L−1) and proteins (60.7 ± 0.42 mg·L−1). The most abundant metabolites were lipopeptides (1210 ± 5.28 mg·L−1). Since the level of total sugar, proteins and flavonoid in the Bacillus altitudinis Q7 fermentation broth was quite low, further study on inhibitory activity against A. alternata focussed on the lipopeptides.
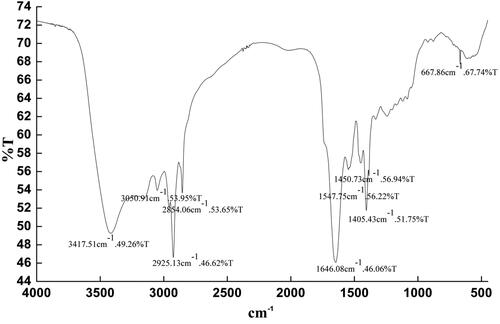
Infrared spectroscopy analysis of lipopeptides
The IR analysis of the lipopeptides was shown in . The peaks assignments of the lipopeptides were as follows: 3417.51 cm−1 was related to the stretch vibration of N-H caused by hydrogen bond, which indicated the existence of N-H group. The stretching vibration band of secondary amide C = O is 1547.75–1646.08 cm−1, which indicated the hydrophilic group of this substance is a peptide chain. The absorption around 2854.06–2925.13 and 1405.43–1450.73 cm−1 was due to the stretching vibration of C-H from aliphatic carbon chain. The signals at 3050.91 cm−1 were related to the stretch vibration of C-H bond. These peaks are consistent with literature reports [Citation26], which indicated that the hydrophobic part of the test substance was a fatty half molecule. The FTIR spectra revealed that the prepared antibacterial lipopeptides were cyclic lipopeptides.
Effect of temperature and pH on the inhibitory activity of the Q7 fermentation broth against Alternaria alternata
Effect of temperature on the inhibitory activity of Bacillus altitudinis Q7 fermentation broth against Alternaria alternata
When the fermentation broth was exposed to temperatures below 70 °C, the inhibitory effect of Bacillus altitudinis Q7 fermentation broth on A. alternata was stable resulting in a 58.8% reduction in colony diameter (). When the fermentation broth was exposed to temperatures greater than 70 °C, however, the inhibitory effect was significantly reduced, with greater reductions in inhibitory activity with increasing temperature. The level of inhibition was only 33.0% when the fermentation broth was exposed to 100 °C ().
Figure 4. Effect of temperature (A) and pH (B) on the inhibitory activity of Bacillus sp Q7 fermentation broth against Alternaria alternata.
Note: Statistical analysis was performed using t test at P < 0.05. Each data point is representative of the mean of three replicates. The different letters (a–d) indicated the temperature and pH have significant differences at different times.
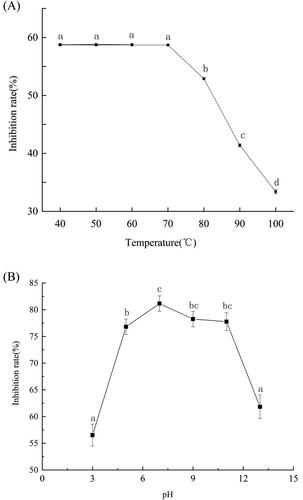
Effect of pH on the inhibitory activity of Bacillus altitudinis Q7 fermentation broth against Alternaria alternata
The effect of the pH of the fermentation broth on the inhibitory activity of Bacillus altitudinis Q7 fermentation broth against A. alternata was shown in . The level of inhibition was over 75% in the pH range 5.0 ∼ 11.0. The level of inhibition was highest (approx. 81.15%) at pH 7.0, but dropped sharply when the pH was lower than 5.0 or higher than 11.0. These results indicated that the inhibitory effect of the Bacillus altitudinis Q7 fermentation broth against A. alternata was active at pH 5.0 ∼ 11.0 but that the inhibitory activity was lost at strong acid or alkaline pH levels.
Inhibitory activity of Bacillus altitudinis Q7 against Alternaria alternata
Effect of Q7 fermentation broth on the colony morphology of Alternaria alternata
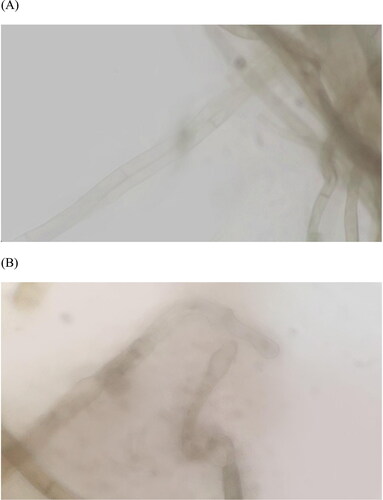
The hyphae treated with Q7 fermentation broth were observed for morphological changes under an optical microscope. Untreated A. alternata was shown in . It can be observed that the appearance of mycelia was clear and complete, and the surface of mycelia was smooth and uniform. shows the treatment group. The comparison between the two group shows that the treated A. alternata has been destroyed, Q7 fermentation broth-treated surfaces was significantly rougher than untreated surfaces, more broken hyphae can be observed and accompanied by extravasation of cellular material.
Effect of Bacillus altitudinis Q7 fermentation broth on Alternaria alternata cell membrane permeability
As illustrated in , the electrical conductivity of the A. alternata culture broth treated with an aliquot of Q7 fermentation broth was much higher than that of the culture broth in the control group, treated with distilled water.
Figure 6. Analysis of the inhibitory effect of the Bacillus altitudinis Q7 fermentation broth on Alternaria alternata. Cell membrane permeability (A); Lipid peroxidation (B); SOD activity (C); POD activity (D); PPO activity (E). The control group (■); the group treated with Q7 (●).
Note: Statistical analysis was performed using t test at P < 0.05. Each data point is representative of the mean of three replicates. The different letters (a–f, A–E) indicated the significant differences at different times.
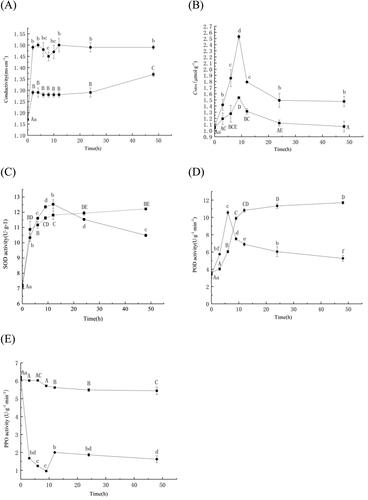
Effect of Bacillus altitudinis Q7 fermentation broth on lipid peroxidation in mycelia of Alternaria alternata
MDA content in the mycelia of A. alternata in the treatment group was significantly higher compared to the control group. As illustrated in , MDA levels content of the cells in the treatment group increased rapidly over the course of 9 h, reaching a peak at 9 h. At that time, MDA levels in the treatment group were 50% higher than in the control group.
Effect of Bacillus altitudinis Q7 fermentation broth on SOD activity in Alternaria alternata
SOD activity in the treatment and control groups were similar during the first 3 h of culture (). After 3 h, SOD activity in the treatment group gradually increased. SOD activity in the treatment group, however, began to decrease with increasing culture time and significantly fell below the control group after 24 h of incubation.
Effect of Bacillus sp. Q7 fermentation broth on POD activity in Alternaria alternata
The effect of Bacillus altitudinis Q7 fermentation broth on POD activity in A. alternata is presented in . The POD activity of the control group increased significantly from 3 to 12 h, while the POD activity enzyme of the treatment group increased rapidly in the first 6 h, and then stabilised after 24 h, The level of the treatment group was significantly lower than the control group, and the enzyme activity is only 44.9% of the control group.
Effect of Bacillus altitudinis Q7 fermentation broth on PPO activity in Alternaria alternata
PPO enzyme activity rapidly decreased in mycelia of A. alternata exposed to an aliquot of the Bacillus sp. Q7 fermentation broth during culture (). PPO activity exhibited a brief, small recovery after 9 h of culture, but the overall level of activity in the treatment group was still much lower than it was in the control group.
Inhibitory activity of Bacillus altitudinis Q7 lipopeptides against Alternaria alternata
Effect of Bacillus altitudinis Q7 lipopeptides on the cell membrane permeability of Alternaria alternata
As illustrated in , the electrical conductivity of the group treated with the lipopeptides produced by Bacillus altitudinis Q7 slowly decreased in 2–10 h and gradually stabilised after 12 h, it exhibited a similar trend consistent with the Q7 fermentation broth.
Figure 7. Analysis of the inhibitory effect of the Bacillus altitudinis Q7 lipopeptide on Alternaria alternata. Cell membrane permeability (A); Lipid peroxidation (B); SOD activity (C); POD activity (D); PPO activity (E). The control group (■); the group treated with lipopeptide (●). Statistical analysis was performed using t test at P < 0.05. Each data point is representative of the mean of three replicates.
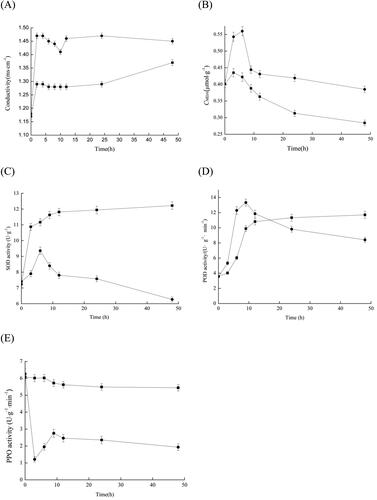
Effect of Bacillus altitudinis Q7 lipopeptides on the content of malondialdehyde in mycelia of Alternaria alternata
The malondialdehyde content in treatment group increased rapidly and reached the peak in the first 2 h, then slowly decreased until 9 h reached a plateau, MDA levels were 35.6% higher in the treatment group than in the control group ().
Effect of Bacillus altitudinis Q7 lipopeptides on SOD, POD and PPO enzyme activity in Alternaria alternata
The change trends in the treatment were consistent with the control group in the first 12 h (), showing an upward trend. Compared with the control group, SOD activity was significantly lower than the control group and decreased with increasing the time . At 48 h, the SOD enzyme activity in the treatment group was 51.3% of the control group.
As shown in , the activity of the POD enzyme of A. alternata in the treatment group increased rapidly and was higher than the control group. After 9 h, POD activity began to decrease until about 16 h. The POD enzyme activity of the treatment group was lower the control.
Taken as a whole, PPO enzyme activity in the mycelia of A. alternata in the treatment group was significantly higher than in the control group. PPO enzyme activity reached the lowest value at 2 h and then decreased slightly and stabilised at 9 h after a brief increase ().
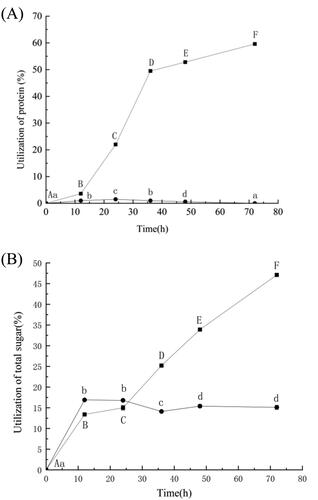
Effect of Bacillus altitudinis Q7 lipopeptides on utilisation of nutrients by Alternaria alternata in culture
The effect of lipopeptides produced by Bacillus altitudinis Q7 at 2 mg/mL against A. alternata in vitro reached 83.2%. The effect of lipopeptides on the utilisation of proteins and sugars by A. alternata was presented in , respectively. The utilisation of proteins and total sugars increased with time in the culture of the control group. The utilisation of proteins and total sugars in the group treated with lipopeptides either remained flat (proteins) or did not increase at the same rate (sugars) as in the control group.
Figure 8. Effect of lipopeptides produced by Bacillus altitudinis Q7 on the utilization of nutrients in the culture medium by Alternaria alternata. Utilization of proteins (A); Utilization of total sugars (B). The control group (■); the group treated with lipopetide (●). Statistical analysis was performed using t test at P < 0.05. Each data point is representative of the mean of three replicates. The different letters (a–d, A–F) indicated the significant differences at different times.
Discussion
Because of their extensive potentiality in enhancing crop protection and food safety, microbial metabolites are used as a substitute for chemical pesticides in organic agriculture [Citation27]. Especially, the genus Bacillus is identified as a producer of a variety of antibiotics, such as antimicrobial peptides, which have antimicrobial activity against many bacteria and fungi, including bacteriocins, lipopeptides and glycopeptides [Citation28]. Saoussen et al. [Citation27] reported Bacillus subtilis V26 strain can produce biosurfactants with anti-fungal and insecticidal activity to inhibit grey mould caused by Botrytis cinerea. Zahoor et al. [Citation29] found that Bacillus licheniformis BL350-2 has the antagonistic activity against the toxin-producing strains of Aspergillus and Penicillium, and can be used as a biological control agent against mycotoxin fungi. In our study, an endophytic strain (Q7) of Bacillus altitudinis exhibited the highest level of inhibitory activity against A. alternata in vitro.
The inhibitory effect of Bacillus altitudinis Q7 fermentation broth against A. alternata changed with temperature. These indicated that the fermentation broth contained substances, which actively inhibited the growth of A. alternata. The Q7 fermentation was found to be thermostable as it still retained activity at 70 °C (). This may be caused by the partial heat resistance of the bacteriostatic substances in the fermentation broth. Acidic-alkali conditions also have an effect on the inhibitory activity of Bacillus altitudinis Q7 fermentation broth (). Collectively, Bacillus altitudinis Q7 fermentation broth had strong inhibitory activity against A. alternata and can maintain this activity under a wide range of temperature and acid-alkali conditions. Since the inhibitory activity was relatively stable over a wide temperature pH range. A similar conclusion was reached by Sharma et al. [Citation28], sublancin from Bacillus subtilis A52 was found to be stable up to 70 °C and lost its activity completely after autoclaving. It was also stable between pH 4.0 and 10.0. Based on our research, it can be inferred that substances such as proteins and enzymes, which were sensitive to temperature and pH, were not the main components responsible for the inhibitory activity of the fermentation broth.
The inhibitory mechanism of fungi may be related to the destruction of cell membrane structure and function. Specifically, antifugal substances can destroy the lipid bilayer, leading to changes in the permeability, fluidity and integrity of cell membranes and ultimately, leading to cell leakage and death [Citation30]. Our study showed the substances in the Q7 fermentation broth damaged the permeability of the cell membrane of A. alternata. The overflow of hyphae cell content and hyphae rupture observed under an optical microscope also confirm this conclusion (). Literatures by Sharma et al. [Citation28] reported that the images of untreated S. aureus cells showed a smooth cell surface and appeared to be healthy and intact, while the cells treated with Bacillus subtilis A52 showed altered morphology and shrinkage. Research by Saoussen et al. [Citation27] reported the similar results that the biosurfactant V26 produced by Bacillus sp. changes the morphology of Botrytis cinerea (rare hyphae deformation and the development of hyphal tip swelling). The conductivity in the treatment group increased rapidly during the first 2 h after treatment indicating that the plasma membrane of cells in the mycelia of A. alternata was damaged and that ions had leaked out of the cell, thus increasing the conductivity of the culture medium [Citation31]. Apparently, the conductivity of the culture medium decreased slightly and then again increased slightly over time, which may be caused by A. alternata trying to repair damaged cells and reabsorb leaked ions. It can be seen that the Q7 fermentation broth inhibited the growth of A. alternata by destroying the cell membrane permeability of the mycelium of A. alternata and achieved the effect of inhibiting fruit diseases.
Malondialdehyde (MDA) can be used as an indicator of lipid peroxidation, which can indicate the stability of a biofilm [Citation32], loss of membrane integrity leading to decompartmentation of enzymes and substratesit [Citation33], it can also be used to measure the stress resistance of fungi [Citation34]. The MDA content of A. alternata in the treatment group was significantly higher than that in the control group, which indicating that the degree of lipid peroxidation in the experimental group always maintained at a high level, which further confirmed the destruction of A. alternata cell membrane in the treatment group [Citation35].
Superoxide dismutase (SOD) and peroxidase (POD) was the enzymatic antioxidants, which can offset the oxidative damage caused by reactive oxygen species (ROS) [Citation32]. SOD activity in the treatment group increased significantly after 3 h, suggesting that A. alternata responded to the stress of exposure to the fermentation broth by increasing the activity of stress-related enzymes, such as SOD [Citation36]. Changes in SOD levels in A. alternata may reflect changes in the level of ROS. However, as cell membranes began to lose their permeability, leakage of proteins and enzymes may have increased in the treated group, leading to a decrease in SOD activity, promoted the levels of cell death caused by ROS (). Results of the POD activity test suggested that the mycelia in the A. alternata culture were under stress due to exposure to the Bacillus altitudinis Q7 fermentation broth (). Due to the influence of the Q7 fermentation broth, the oxidative stress response of the cells increased, resulting in a temporary increase in POD enzyme activity. As the degree of cell membrane damage, permeability and lipid peroxidation increased, substances like enzymes, were leaked into the culture medium. POD activity began to decrease, resulting in a reduced cell resistance and inhibited growth [Citation37]. After 24 h, the POD activity became stable in both group.
Polyphenol oxidases (PPO), first reported in mushrooms by Schoenbein in 1856, are a widespread group of enzymes found in plants, fungi, bacteria and animals [Citation38], PPO is also thought to be a key enzyme related to prevent pathogen invasion. It can produce toxic quinones, and quinones can inhibit the growth of cell [Citation39]. In our research, the PPO activity in the control group consistently stabilized at a high level throughout the entire time and the control group was significantly higher compared to the treatment group (), This shows that the treatment of the Q7 fermentation broth increased the PPO activity of A. alternata, enabled the synthesis of a large amount of toxic substances and inhibited the growth of A. alternata, which has a great significance for the control of many apple diseases caused by A. alternata.
Cyclic lipopeptides was one of the main metabolites of Bacillus altitudinis Q7. It is now widely recognized that lipopeptides play a major role in plant disease suppression. Due to these beneficial traits, lipopeptides can be potentially useful as biocontrol agents against plant diseases [Citation40]. Cyclic lipopeptides have a wide range of biological activities, including antimicrobial, antiviral, anticancer and immunosuppressive activities, and have specific mechanisms that affect membrane permeability, eventually leading to cell disuption [Citation41]. The same conclusion was obtained among studies of Fernanda (2020), their result showed the lipopeptides’capability to disturb the structure and functions of biological membranes, leading to the increased membrane permeability [Citation42]. Ongena and Jacques [Citation43] used a versatile weapons for plant disease biocontrol to describe Bacillus lipopeptides and the recent investigations have shed light on the fact that lipopeptides play a key role in the beneficial interaction of Bacillus species with parasitic plants by stimulating its host defence mechanisms. In our research, further investigations were carried on the inhibitory effect of lipopeptides on A. alternata. After treatment with lipopeptides, the permeability of the cell membrane of A. alternata was destroyed, and the degree of membrane lipid peroxidation increased. The activity of SOD and POD enzymes increased at first and then decreased due to the damage of cell membrane, which resulting in the lose resistance of cells, at the same time, the activity of PPO increased and toxic substances gradually produced, which inhibited the growth of A. alternata and eventually lead to the death. The conclusion was the same as the result after Q7 fermentation broth treatment, which further verified that lipopeptides are the main antibacterial substance in Q7 fermentation broth.
The utilisation of proteins and total sugars in the control group suggested that A. alternata was growing normally without any inhibition (). Lipopeptides were the most abundant metabolite secreted into the culture medium by A. alternata. The content of lipopeptides produced and secreted by Bacillus altitudinis Q7 was higher than other metabolites. The FTIR spectra revealed that the prepared antibacterial lipopeptides was a cyclic lipopeptides () and and this cyclic lipopeptides had a significant inhibitory effect on A. alternata in vitro. We hypothesised that the lipopeptides inhibited the growth of A. alternata, slowing down its metabolism so that the nutrients in the culture medium were not utilized. If the lipopeptides damage cell membranes in A. alternata, the fungus may lose its ability to metabolize nutrients. Notably, the lipopeptides also affected the activity of several metabolic enzymes in A. alternata and this may have also hindered the utilisation of nutrients. Studies have shown that lipopeptides are stable against proteolysis, and in addition, the presence of hydrophobic fatty acid chains enhances their antimicrobial activity [Citation44].
In summary, results indicated that in the Q7 trial, cell membranes in A. alternata were damaged and lipid peroxidation was enhanced, the hyphae ruptured and the material within the hyphae cells overflowed. Meanwhile, SOD, POD and PPO activity in A. alternata were also affected by metabolites present in the fermentation broth of Bacillus altitudinis Q7. Based on the data we obtained from further research, we inferred that lipopeptides was the main antibacterial substance in Q7 fermentation broth and the inhibition of growth of A. alternata by lipopeptides produced by Bacillus altitudinis Q7, was mainly due to the breakdown of cell membranes, a influence in its metabolic enzymes and a reduction in its metabolic capacity.
Present methods used to control apple diseases in the field primarily rely on the use of synthetic fungicides, however, the use of synthetic chemical pesticides can have adverse impacts on human health and the environment. Therefore, considerable research is being done to identify biological control alternatives that are safe and effective. An endophytic strain (Q7) of Bacillus altitudinis obtained from G. biloba significantly inhibited the growth of A. alternata in vitro and is a very promising biocontrol strain against A. alternata. Future studies will continue to investigate the bioactive properties of the metabolites secreted by Bacillus altitudinis Q7 to better understand their ability to inhibit the growth of A. alternata and provide a theoretical basis for the antagonistic properties of endophytic bacteria isolated from G. biloba.
Disclosure statement
No conflict of interest declared.
Data availability statement
The raw data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Additional information
Funding
References
- Gur L, Reuveni M, Cohen Y. Occurrence and etiology of Alternaria leaf blotch and fruit spot of apple caused by Alternaria alternate f. sp.malion cv Pink lady in Israel. Eur J Plant Pathol. 2017;147(3):695–708.
- Amirchakhmaghi N, Yousefzadeh H, Hosseinpour B, et al. First insight into genetic diversity and population structureof the Caucasian wild apple (Malus orientalis Uglitzk.) in the Hyrcanian forest (Iran) and its resistance to apple scab and powdery mildew. Genet Resour Crop Evol. 2018;65(4):1255–1268.
- Daulagala PWHKP, Allan-Atkins EJ. Chitinolytic activities of endophytic bacteria isolated from symptom-free Chinese cabbage leaves. Asian J Microbiol Biotechnol Environ Sci. 2015;17(3):603–609.
- Xu FX, Wang SY, Li YJ, et al. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol Lett. 2018;40(5):797–807.
- Chukalo CE, Chalannavar RK. Endophytic mycoflora and their bioactive compounds from Azadirachta Indica: A comprehensive review. JoF. 2018;4(2):42.
- Das G, Park SJ, Baek KH. Diversity of endophytic bacteria in a fern species Dryopteris uniformis (Makino) Makino and evaluation of their antibacterial potential against five foodborne pathogenic bacteria. Foodborne Pathog Dis. 2017;14(5):260–268.
- Shan SJ, Luo J, Xu DR, et al. Elucidation of micromolecular phenylpropanoid and lignan glycosides as the main antioxidants of Ginkgo seeds. Ind Crops Products. 2018;112:830–838.
- Tang WY, Li GZ, Chen BQ, et al. Evaluating ternary deep eutectic solvents as novel media for extraction of flavonoids from Ginkgo biloba. Sep Sci Technol. 2017;52(1):91–99.
- Samir D, Charles W, Michael W. 2000 Biologically based technology for the control of postharvest diseases of fruits and vegetables. Microb Food Contam. Boca Raton, FL: CRC Press, 187–205. https://doi.org/10.1201/9781420039030.ch13.
- Chan Z, Tian S. Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action. Postharvest Biol Tech. 2005;36(2):215–223.
- Liu C, Yin XH, Wang QG, et al. Antagonistic activities of volatiles produced by two Bacillus strains against Monilinia fructicola in peach fruit. J Sci Food Agric. 2018;98(15):5756–5763.
- Grahovac MS, Balaz JS, Grahovac JA, et al. Screening of antagonistic activity of selected microorganisms against apple rot pathogens. Romanian Biotechnol Lett. 2014;19(1):8959–8965. https://doi.org/10.2147/OTT.S38846.
- Yang R, Fan XJ, Cai XQ, et al. The inhibitory mechanisms by mixtures of two endophytic bacterial strains isolated from Ginkgo biloba against pepper Phytophthora blight. Biol Control. 2015;85:59–67.
- Ye S, Ma Z, Liu Z, et al. Effects of carbohydrate sources on biosorption properties of the novel exopolysaccharides produced by Arthrobacter ps-5. Carbohydr Polym. 2014;112:615–621.
- El-Arnaouty MB, Eid M, Tablawy SYEL. Impact of nano-ZnO/grafted textile on the outer membrane permeability of some pathogenic bacteria. Bull Mater Sci. 2017;40(6):1213–1224.
- Li RP, Zhang HY, Liu WM, et al. Biocontrol of postharvest gray and blue mold decay of apples with Rhodotorula mucilaginosa and possible mechanisms of action. Int J Food Microbiol. 2011;146(2):151–156.
- Guo RT, Wang ZY, Zhou C, et al. Biocontrol potential of trichoderma asperellum mutants t39 and t45 and their growth promotion of poplar seedlings. J Forestry Res. 2018;31(03):333–341. https://doi.org/10.1007/s11676-018-0797-0.
- Sui GQ, Song XQ, Zhang BY, et al. Design, synthesis and biological evaluation of novel neuchromenin analogues as potential antifungal agents. Eur J Med Chem. 2019;173(3):228–239.
- Sultana R, Islam MS, Islam MA, et al. Identification of pathogen causing common bacterial blight (CBB) of bean through the biochemical and molecular pathway and their management system. J Entomol Zool Stud. 2018;6(3):752–757.
- Noha AM, Hossam MH, Mostafa ER, et al. Diketopiperazine derivatives from Enterobacter cloacae isolated from the red sea alga Cystoseira myrica. Egypt Pharmaceut J. 2013;12(2):163–171.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method of sugars and related substances. Anal Chem. 1956;28(3):350–356.
- Li QF, Liu SH, Zhang HY, et al. Microdetermination of proteins by enhanced resonance light scattering spectroscopy of m-acetylchlorophosphonazo. Anal Lett. 2001;34(7):1133–1142.
- Okuno Y, Marumoto S, Miyazawa M. Antimutagenic activity of flavonoids from Sozuku. Nat Prod Res. 2019;33(6):862–869.
- Saeki A, Sugiyama M, Hasebe A, et al. Activation of NLRP3 inflammasome in macrophages by mycoplasmal lipoproteins and lipopeptides. Mol Oral Microbiol. 2018;33(4):300–311.
- Lehmann PF. 2010. Fungal structure and morphology. In: Mahy BW, Meulen VT, Borriello SP, et al., editors. Topley & Wilson’s microbiology and microbial infections. United States: Wiley, 132–145. https://doi.org/10.1002/9780470688618.taw0130
- Duitman EH, Hamoen LW, Rembold M, et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: A multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc Natl Acad Sci U S A. 1999;96(23):13294–13299.
- Saoussen BK, Hanen BA, Laarif ST. Biosurfactant produced by Bacillus subtilis V26: a potential biological control approach for sustainable agriculture development. Organic Agric. 2020;(3):117–124. https://doi.org/10.1007/s13165-020-00316-0.
- Sharma D, Singh SS, Baindara P, et al. Surfactin like broad spectrum antimicrobial lipopeptide co-produced with sublancin from Bacillus subtilis strain A52: dual reservoir of bioactives. Front Microbiol. 2020;11:1167. https://doi.org/10.3389/fmicb.2020.01167.
- Zahoor UH, Thani RA, Alnaimi H, et al. Investigation and application of Bacillus licheniformis volatile compounds for the biological control of toxigenic aspergillus and Penicillium spp. ACS Omega. 2019;4(17):17186–17193.
- Liu YA, Lu J, Sun J, et al. Membrane disruption and dna binding of Fusarium graminearum cell induced by c16-fengycin a produced by Bacillus amyloliquefaciens. Food Control. 2019;102:206–213.
- Douaiher MN, Nowak E, Durand R, et al. Correlative analysis of Mycosphaerella graminicola pathogenicity and cell wall-degrading enzymes produced in vitro: the importance of xylanase and polygalacturonase. Plant Pathol. 2007;56(1):79–86.
- He YZ, Li ZR, Tan FQ, et al. Fatty acid metabolic flux and lipid peroxidation homeostasis maintain the biomembrane stability to improve citrus fruit storage performance. Food Chem. 2019;292(15):314–324.
- Jing G, Hua H, Yang B, et al. Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage. Chem Central J. 2013;7(1):19.
- Dragun Z, Filipović MV, Krasnići N, et al. Malondialdehyde concentrations in the intestine and gills of Vardar chub (Squalius vardarensis Karaman) as indicator of lipid peroxidation. Environ Sci Pollut Res Int. 2017;24(20):16917–16926.
- Dhindsa RS, Pamela PD, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32(1):93–101.
- Li CW. 2015. Antibacterial activity of strain T-33 and its inhibition mechanism against Populus sinensis. Heilongjiang, China: Heilongjiang University Press.
- Li QQ, Xie F, Zhao YM, et al. Inhibitory effect of postharvest yeast mannan treatment on alternaria rot of tomato fruit involving the enhancement of hemicellulose polysaccharides and antioxidant metabolism. Sci Hortic. 2021;277(3):109798.
- Weerawardana MBS, Thiripuranathar G, Paranagama PA. Natural antibrowning agents against polyphenol oxidase activity in Annona muricata and Musa acuminata. J Chem. 2020;2020(1):1–6.
- Guo YJ, Zhou JX, Zhang JR, et al. Chitosan combined with sodium silicate treatment induces resistance against rot caused by Alternaria alternata in postharvest jujube fruit. J Phytopathol. 2019;167(7–8):451–410.
- Chen MC, Wang JP, Zhu YJ, et al. Antibacterial activity against Ralstonia solanacearum of the lipopeptides secreted from the Bacillus amyloliquefaciens strain. J Appl Microbiol. 2019;126(5):1519–1529. https://doi.org/10.1111/jam.14213.
- Zhou H, Cong B, Tian Y, et al. Characterization of novel cyclic lipopeptides produced by Bacillus sp. SY27F. Process Biochem. 2019;83:206–213.
- de Souza Freitas F, Coelho de Assis Lage T, Ayupe BAL, et al. Bacillus subtilis tr47ii as a source of bioactive lipopeptides against gram-negative pathogens causing nosocomial infections. 3 Biotech. 2020;10(11):1–10.
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–125.
- Fatemeh N, Mohammad-Esmaeel K, Lamrart-Szczapa E. Isolation, identification, and characterization of a novel chemolithoautotrophic bacterium with high potential in biodesulfurization of natural or industrial gasses and biogas. Energy Sources Part A: Recov Utilization Environ Effects. 2017;39(10):971–977. https://doi.org/10.1080/15567036.2016.1263255.
- He FT, Zhao LN, Zheng XF, et al. Investigating the effect of methyl jasmonate on the biocontrol activity of Meyerozyma guilliermondii against blue mold decay of apples and the possible mechanisms involved. Physiol Mol Plant Pathol. 2020;109:101454.