Abstract
Aquaponics is a specific ecosystem that combines aquaculture, hydroponics and beneficial bacteria in a symbiotic relationship. This integrated approach is emerging as a sustainable method of organic food production. This review analyzes the biological and technological parameters of aquaponic systems based on a survey of over 200 scientific papers from 1974 to 2021. The biological parameters include the characteristics of the species in the aquaponic system, their relationship with nutrition and environmental conditions, the content of nutrients and their impact on productivity. The technological parameters focus on the structural and functional components of the aquaponic system, environmental management techniques and economic feasibility. The advances in aquaponics technology contribute to more sustainable food systems. This calls for further research on the biological and technological characteristics of aquaponic systems, as well as on the environmental, operational and socio-economic aspects and their interrelationship, of interest to both researchers and stakeholders.
Introduction
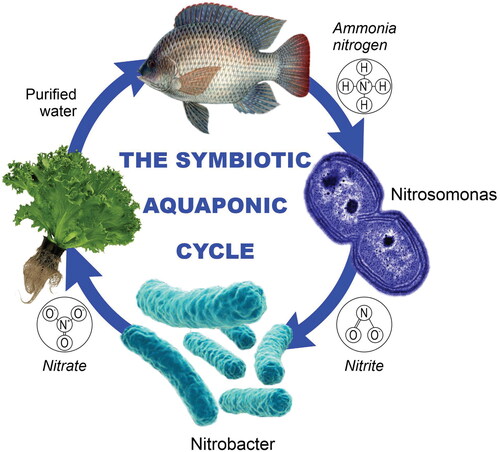
Living conditions at present – climate change, urbanization, pandemics and their economic consequences – pose a need for independent food production within or near urban areas. Urban production in buildings and on rooftops can become more efficient. It can partly contribute to sustainability by reducing the environmental consumption in production and especially the associated food transportation costs and labor shortage [Citation1,Citation2]. Aquaponics – an integrated combination of the recirculation aquaculture system (RAS) and soilless organic farming – is gaining the attention of scientists, entrepreneurs, producers and consumers. It is an important and potentially sustainable method for producing environmentally friendly organic food in close proximity to consumers [Citation3]. By design, aquaponic systems are specific mini-ecosystems, analogous to natural processes [Citation4]. Essentially this technology combines aquaculture, hydroponics and beneficial bacteria in a symbiotic environment (). Within this synergism, the negative environmental impacts of aquaculture and hydroponics become advantages. Aquaponics significantly reduces the requirements for nutrient input and waste disposal, as opposed to the separate operation of the two systems [Citation5]. It is environmentally friendly owing to high levels of water reuse and nutrient recycling [Citation3, Citation6,Citation7]. Aquaponics is a viable alternative to traditional production methods, especially in regions where water scarcity is a problem. The water consumption in aquaponic systems is significantly lower than in traditional soil cultivation [Citation8]. Aquaponic systems produce higher yields in less space, which is a huge advantage over traditional agriculture [Citation9].
The concept of food production through natural processes, known as permaculture, has existed for thousands of years. Scientists have traced forms of organic hydroponics to many ancient peoples [Citation10]. Some notable examples are the so-called ‘stationary islands’ in shallow lakes in Central America (e.g. Chinampas of the Aztecs 1150 − 1350 B.C.E.), the cultures around Inle Lake in Myanmar, the Waru Waru agriculture of the Uru people along the shores of Lake Titicaca in Peru and the introduction of fish into rice fields in Southeast Asia about 1500 years ago [Citation5,Citation10,Citation11]. The first scientist to study soilless agriculture was Francis Bacon, who published Sylva Sylvarum in 1627. In 1842, Justus von Liebing made a list of the elements required for plant growth [Citation12]. Modern aquaponics dates back to the 1970s, when researchers made experiments with aquacultures in a recirculation system and soilless plant systems as a means of removing nitrogen compounds from fish waste [Citation4,Citation5,Citation13,Citation14]. The pioneering researchers were Naegel, Todd, Lewis, Rakocy, MacKay, Van Toever, Watten and Busch, who published their works in 1977 − 1984 [Citation4,Citation14–16]. The New Alchemy Institute of North Carolina State University and the University of the Virgin Islands (UVI), as research organizations, have embraced this technology and refined it [Citation5,Citation17]. Love et al. [Citation4] reported that aquaponics has since gained increasing interest, which is at the heart of its growing importance to society as an innovative response to food security [Citation18]. According to the literature available to us, a significant part of the research on aquaponic systems dates back to after 2010 [Citation15].
Aquaponics has already advanced away from the ‘research project’ phase and is now a viable industry [Citation9]. The excellent scientific basis for its ongoing upgrading is backed by many patents from around the world. The United States and Australia benefited from early research in aquaponics (1980s) and their highly developed entrepreneurial leadership, so many aquaponics facilities began commercial production before their European counterparts [Citation19]. Aquaponics has also attracted interest in Europe as one of the ‘Ten technologies which could change our lives’ [Citation20]. It is essential for science and business to cooperate for aquaponics to fulfill its potential as a viable system for local food production in the European Union. There are currently 52 research centers and 44 companies in Europe, indicating a good balance between research and business interests [Citation5]. Consumer acceptance is crucial in the success of aquaponics. Overall, studies conducted in Canada, Malaysia and Europe have shown a positive attitude of end users toward aquaponics [Citation20].
Commercial interest in aquaponics has grown significantly in recent years. Junge et al. [Citation15] are convinced that this technology has potential and will play a significant role in food production in the future. Here, we aimed to analyze the development of aquaponics according to its biological and technological parameters and their relationship.
For this purpose, we reviewed over 200 research papers and dissertations from 1974 to 2021. We aimed to perform a systematic, strategic and comprehensive review of relevant publications that meet the following requirements: based on research that includes materials related to the current trends and challenges in aquaponics; based on scientifically sound methods; peer-reviewed and published in reputable scientific journals. The bibliographic and reference databases used are: Elsevier, Google Scholar, MDPI, PubMed, ResearchGate, Scopus, Springer and others. The publications were divided into three categories: biological parameters, technological parameters and the relationship between biological and technological parameters of aquaponics.
Biological parameters
Species in aquaponics
Aquaponics is a biointegrated ecosystem of fish, plants, bacteria, in some cases worms and/or other organisms, that grow together symbiotically [Citation4,Citation18].
Aquaculture
Current aquaponic systems use different species of fish () [Citation4,Citation22]. In 2014, a study in 44 countries around the world found that the most common fish species in aquaponics were tilapia (55%) and ornamental fish (koi, goldfish and tropical fish) (48%) [Citation4]. Similarly, Love et al. [Citation21] reported tilapia (69%), ornamental fish (43%), catfish (25%), other aquatic animals (18%), perch (16%), bluegill (15%), trout (10%) and bass (7%). Villarroel et al. [Citation19] reported that tilapia (27%), catfish (10%), ornamental fish (8%), trout (7%), bass (4%) and perch (2%) are the most commonly farmed species in Europe.
Table 1. Most commonly farmed fish in aquaponics.
One of the most common species in aquaponics is tilapia. This is because of its omnivorous nature, rapid reproduction and rapid growth [Citation23]. This species of fish is very resistant and tolerant to a wide range of water parameters, such as wide temperature range (15 − 30 °C) and free ammonia (NH3) concentration (0.2 − 3.0 mg/L) [Citation24]. Farmed trout has shown good results in the aquaponics unit in Nibio, Norway [Citation25]. To choose the right type of aquaculture for a new aquaponic system, it is first important to determine the plants that will be produced. The process optimization depends directly on the symbiosis of the species.
Plants
Many species of plants can grow in aquaponics. The most popular ones are herbs and spices for culinary purposes (basil, coriander, chives, parsley, purslane and mint), lettuce and spinach, chard, pak choi, Chinese cabbage, watercress, calendula and zinnia and medicinal plants, as they have low to medium nutritional requirements. The higher nutritional requirements that vegetables, such as tomatoes, peppers and cucumbers, have make them perform better in well-stocked, well-established aquaponic systems [Citation26]. Danner et al. [Citation27], Love et al. [Citation4,Citation21] and Villarroel et al. [Citation19] outline the most commonly grown plant species in aquaponics ().
Table 2. Most commonly grown crops in aquaponics.
Experimental data generally show a higher yield of crops in aquaponics than in hydroponics or conventional agriculture. Johnson et al. [Citation28] compared the yields of lettuce of different varieties grown in aquaponics and hydroponics. In aquaponic production, the yield was 402.5 g/m2, whereas in hydroponic production it was 468.9 g/m2. However, some varieties, such as butterhead and bibb had higher yields in aquaponics than in hydroponics, and romaine showed similar yields in both systems [Citation29]. Cultivation of edible amaranth (Amaranthus tricolor) by aquaponics is promising. The aquaponics yield may exceed the yield from the field [Citation7]. The choice of suitable plant species and varieties for growth in aquaponic systems is important, so there are prospects for further research into that. Crops are the primary component or focus of production in the aquaponic system. Therefore, their choice must be consistent with the market demand for organic and environmentally friendly food to fall into the upper-class market niche.
Beneficial bacteria
Bacteria play an extremely important role in the optimal development of species in aquaponics [Citation8]. Beneficial bacteria live on the surfaces and begin their development when there is ammonia in the water. Ammonia is the end product of protein catabolism in fish (excretion from gills, urine and feces). The total ammonium nitrogen (TAN) first undergoes conversion to nitrite (NO2) through biological nitrification by ammonia oxidizing bacteria (AOB), such as Nitrosococcus, Nitrosospira and Nitrosomonas. Then, nitrite-oxidizing bacteria (NOB), such as Nitrobacter, Nitrospira, Nitrococcus and Nitrospina, transform nitrite, which is toxic, into nitrate (NO3), which is relatively harmless to fish. When a new aquaponic system starts operating, nitrification begins immediately as a result of AOB and NOB growth, but is slow [Citation7,Citation18,Citation23,Citation30].
The key role of bacterial communities in aquaponics is to transform fish excrement and food debris into macronutrients and micronutrients that plants can assimilate [Citation23]. Representatives of the genera Flavobacterium and Sphingobacterium can take part in the decomposition of organic matter [Citation23,Citation31]. They form a biocontrol formulation for the control of plant pathogens (Phytophtora infestans) [Citation32]. The Saprospiraceae family is commonly present in aquatic environments, such as wastewater treatment plants, and may take part in the breakdown of complex cabiopolymer molecules such as proteins [Citation23]. The two main species of bacteria in the root system of plants are Proteobacteria and Bacteroidetes [Citation23]. Reportedly, members of the Microbacteriaceae family are present in the microbiome of plant roots, together with members of the Comamonadaceae family [Citation21,Citation33]. There are several Comamonadaceae species that produce siderophores for protection against fusarium and rhizoctonia, which is indicative of active biocontrol against fungal pathogens [Citation34]. Lysobacteria are plant-growth–promoting bacteria (PGPB) that enhance plant growth and can help fight plant diseases by producing antibiotics [Citation23,Citation35].
Species/environment relationship and nutrition
Aquaponic systems require balance for optimal species development. Finding the right balance requires basic knowledge and experience in: environmental conditions (temperature and humidity, water temperature; pH; dissolved oxygen [DO]), water quality (ammonia, nitrites, nitrates, alkalinity; heavy metal pollution and microbial contamination), the amount of fish feed, frequency of feeding, the degree of fish waste mineralization and so forth. Environmental parameters such as temperature and humidity, pH and mineral concentrations should be as close as possible to the optimal conditions for species growth [Citation5]. Maintaining an optimal water temperature of 22 − 24 °C, pH in the range of 5.6 − 7.3 and DO of 3 − 10 mg/L for tilapia and crops is a compromise between the needs of fish and plants. The other levels of tolerance are: alkalinity of 50 − 250 mg/L, CO2 of 0 − 30 mg/L, hardness of 50 − 350 mg/L, salinity of 0 − 10 ppt, nitrite concentrations of 0 − 0.8 mg/L [Citation16].
Aquaponics is a closed system, with the only input being fish feed and sunlight [Citation8]. Fish excrement and feed waste undergo microbial degradation to soluble nutrients, which accumulate in concentrations similar to those in commercial hydroponic nutrient solutions [Citation36]. Plants can absorb waste products (pollutants, heavy metals) from soil or water in a process called phytoextraction. The integration of these techniques can contribute to the production of crop yields with less waste and fewer environmental impacts [Citation14,Citation37,Citation38].
The most common fish feed (94%) is in the form of granules. It is possible to supplement the ration with alternative sources: aquatic plants (33%) and live food (worms, etc.) [Citation4]. Neto and Ostrensky [Citation39] define the feed in fish tanks as: consumed (assimilated) feed, feed converted into fish feces, soluble excretion and uneaten feed [Citation5,Citation17,Citation23]. Uneaten feed and solid waste in the form of organic matter must be converted into soluble inorganic forms that plants can assimilate easily. The solubilization rates usually vary, resulting in unequal accumulation of different minerals, which affects their concentration in the water [Citation17]. As reviewed by Gosh and Chowdhury [Citation17], studies have suggested that solid waste could undergo partial solubilization by daily mechanical filtration, followed by external mineralization and reinsertion into the system. Therefore, the solubilization of fish waste needs more research to transform all the nutrients from the input feed into plant biomass.
To strike the right balance between the availability of nutrients and their assimilation by plants, it is important to determine the optimal fish-to-plant ratio and the amount of fish feed. Several studies have defined a rule of thumb for this balance in the system. Leafy crops (e.g. lettuce, spinach, basil) require fish feed of 20–50 g/m2 area, and fruit crops (e.g. tomatoes, eggplants) 50–80 g/m2 [Citation5,Citation26,Citation40,Citation41]. Rakocy et al. [Citation37] obtained an optimal ratio of feed to area (g/day/m2) for the production of tilapia, lettuce, basil and several other plants in an aquaponic system in the range of 60 − 100 g/day/m2. Endut et al. [Citation5] reported an optimal ratio of 15 − 42 g/m2 for African catfish (Clarias gariepinus) and spinach (Ipomoea aquatica). Two independent studies on aquaponic tilapia and green leafy plants, Delaide et al. [Citation42] from Belgium and Love et al., 2015 [Citation43] from the United States, determined that 0.5 kg of feed is needed per 1 kg of crops. In 2015, Kloas et al. [Citation31], from Germany, reported an aquaponic system for integrated cultivation of tilapia and tomato (Solanum lycopersicum) that could produce sustainably 1 kg of tilapia and 5 kg of tomatoes with 1 kg of feed and a small amount of mineral fertilizer.
Tilapia usually has a low feed conversion ratio (FCR: kg feed per kg biomass gained), FCR ∼ 1.0, which is the basis for economical production, since fish feed is the largest cost item [Citation31,Citation37]. Danner et al. [Citation27] determined an FCR of 0.9–1.2 in the production of pak-choi; its yield was approximately four times higher than the weight of the grown tilapia. Silva et al. [Citation30], from Mexico, reported similar results. To optimize a system with plants that have higher nutrient needs, fish usually grow at relatively high densities and need large amounts of feed, leading to high FCR. Fish feed is the main physical input into the aquaponic system, so it can also be a tool to achieve a balance of species symbiosis, leading to maximum productivity and economic feasibility. Aquaponics research mostly aims to demonstrate low FCR, high output and the potential profitability of the system.
Nutrient content and its impact on productivity
Nutrient balance is one of the most important aspects of aquaponic systems. It depends on the sustainable use of nitrogen and phosphorus by the recirculation aquaculture system (RAS) as a source of nutrients for plant production. The suitable ranges of nutrient concentrations for fish farming are nitrates (<20 mg-N/L) and phosphates (<3 mg-P/L) [Citation44]. The nitrate content in aquaponic systems receives extremely great attention. High nitrate concentrations promote the growth of green leafy crops, while low nitrate concentrations influence the development of fruits such as tomatoes [Citation36]. Hussain et al. [Citation45] reported the best growth and significantly higher percentage of nutrient utilization at an optimal density of 1.4 kg/m³ of Koi carp, Cyprinus carpio var. Koi, in combination with spinach (Beta vulgaris var. Bengalensis). A comparative analysis by Maucieri et al. [Citation46], showed that a stocking density of 2.5 kg/m³ gave an optimal production of European carp and leafy vegetables. Paudel [Citation47] reported that a higher ratio of celery (Oenanthe javanica) to Koi carp biomass (Cyprinus carpio) resulted in less N2O, higher nutrient recovery and higher yield. Buzby et al. [Citation48], studied the productivity and concentrations of soluble nutrients in a combined system of trout, three vegetable crops (kohlrabi, lettuce and Swiss chard) and two types of edible flowers (marigold and nasturtium). Their study showed that the kohlrabi yield was significantly higher than that of lettuce or Swiss chard [Citation48]. Salama et al. [Citation49] reported that basil had a higher capacity to remove NH4, NO3, P and K than mint in an integrated aquaponic system with tilapia. According to the findings of Davidson et al. [Citation50] the recommended upper limit of NO3-N for aquaponic systems with rainbow trout is 75 mg/L.
For maximum growth, plants in aquaponic systems require 16 essential nutrients. Three of the macronutrients, carbon (C), oxygen (O) and hydrogen (H), are supplied by water (H2O) and C and O by carbon dioxide (CO2). Other essential elements are present in the water, which is rich in nutrients. Other macronutrients are: nitrogen (N), potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P) and sulfur (S). Seven trace elements – chlorine (Cl), iron (Fe), manganese (Mn), boron (B), zinc (Zn), copper (Cu) and molybdenum (Mo), must be balanced [Citation37]. Most of the essential nutrients for optimal plant growth are present in fish feed, except K and Fe [Citation37,Citation51,Citation52]. Other key elements with low content in the aquaponic system are: P, S, Mn, B and Mo. High levels of one element can affect the bioavailability of others. Nitrate, Ca and Na concentrations increase rapidly and only water exchange can control their accumulation [Citation37]. Ineffective removal of solids increases their accumulation and lowers pH too much, conditions that can lead to deficiencies of Fe, N, P, S, Mg, B, Cu and Mo [Citation23].
Rakocy et al. [Citation37], demonstrated that the external supplementation of nutrients is necessary to ensure adequate plant nutrition. Systems usually require external supplementation of Fe, Ca, K, Mg and B, depending on the crops. In addition, vegetables such as broccoli and lettuce need sulfur and zinc supplementation to maintain high quality (e.g. vitamin B) [Citation53]. Some crops, including Brassica species, such as pak-choi, are dependent on additives [Citation54]. Danner et al. [Citation27] showed that additional P, Mn and B are necessary both for the vegetative growth of arugula and for its flowering stage [Citation17,Citation23]. The supplementation of K, Ca, Mg has a dual purpose: to supplement essential nutrients and adjust pH. Calcium deficiency in lettuce causes burning of the tip, which makes the product unsalable [Citation36]. The Na content in aquaponic systems receives much attention. The Na concentration should not exceed 50 mg/L, because it’s presence in large quantities, in parallel with chloride, results in increased toxicity to plants on the one hand, and on the other, higher levels of Na reduce the absorption of K and Ca. It is common practice to add salt (NaCl) to the fish feed in the final production cycle. Luo et al. [Citation55] demonstrated that adding Se to the aquaponic system could improve the growth, deposition of pigment and health of Koi carp and effectively improve the aquaculture environment without affecting the plants. There is growing evidence that the healthy development of plants depends on a wide range of organic compounds in the root environment that are products of complex biological processes, including microbial decomposition of organic matter, vitamins and other metabolites. Directly assimilated by plants, these compounds stimulate growth, improve yields, increase the content of vitamins and minerals, improve fruit taste and prevent the development of pathogens [Citation36].
Technological parameters
The design of professional aquaponic systems aims at a high degree of independence and self-sustainability. The design of components and their size is such that the system requires minimal intervention to control the conditions within the installation. The effects of the water flow and the nutrient flow in these systems provide an optimal environment for the species and maximum production [Citation2].
Structural and functional elements
The main structural and functional elements of any aquaponic system are: a recirculation aquaculture system (RAS) with fish tanks, a solid waste filter, a bacterial biofilter and plant beds () [Citation36]. Although there are different configurations, typically water is pumped from the lowest component (fish tanks) to the highest one (filter) and flows gravitationally through the plant beds and back into to the fish tanks [Citation7,Citation23]. RAS is a system of tanks in which high-density fish grow under controlled environmental conditions.
The proper functioning of RAS depends on some key factors, such as: mechanical filtration, biofiltration and DO control [Citation4,Citation56]. Mechanical filtration – the removal of solid waste (fish feces and unconsumed feed) – is an essential process to maintain good water quality and good functioning of the system. Waste increases the ammonia content in the water, reduces the concentration of DO and increases the risk of disease [Citation4,Citation25,Citation56]. Solids removed from the system in the form of nutrient-rich sludge can be reused in the beds as a fertilizer [Citation56]. The aim is to obtain a zero-waste system [Citation4,Citation23,Citation25]. There have been continuous improvements in the solids filtration techniques in terms of engineering solutions [Citation37]. Another key feature of the integrated processes in the aquaponics installation is the biofiltration system. As the water recirculates through the aquaponic system, toxic substances will accumulate so they need to be removed. This is done through bio-purification by beneficial bacteria that occur naturally in the environment. The biofilter is a cylindrical tank that contains a porous filter medium, bacteria and water, which require good aeration. The design of the biofilter can be simple or more complex in the case of industrial installations. The size of the biofiltration device depends on several factors such as: temperature; DO concentration in water; biofilter water circulation; water salinity; stocking density and feed rate; surface area of the filter medium; protein content of fish feed [Citation4,Citation56]. The only way to determine the biofiltration efficiency is to monitor continuously the levels of ammonia, nitrites and nitrates in the water.
There are three types of beds that are most common in aquaponics: Media-based grow bed (MGB), Deep-Water Culture system (DWC or RAFT) and Nutrient Film Technique (NFT) [Citation17,Citation37]. There are some modifications of these main designs: Vertical Towers, which are similar to NFT, except that the plants are grown in a vertical tube; Dutch Buckets are containers filled with soilless substrate, and Wicking Beds are functionally like MGB. MGB is a hydroponic trough filled with an inert substrate (e.g. expanded clay, perlite, pumice, gravel) that functions as a root support and microbial substrate. DWC includes a large trough with perforated floating rafts and netted plant pots placed in them. The pots contain a root-supporting substrate (e.g. stone wool, coconut or pumice) [Citation17,Citation57]. NFT is a narrow channel of perforated square tubes where the roots are partially immersed in a thin layer of running water [Citation17,Citation57].
Love et al. [Citation21] reported that among 257 retailers in 23 countries around the world, more than two-thirds of respondents used a combination of two or more techniques, and more than one-third of respondents used a combination of three or more techniques. The choice of methodology, technique and type of beds depends on the high or low assimilation of nutrients by plants, i.e. on the type of crops. When combining several types of crops or different stages of growth, a combination of several methods can optimize the process.
Management of the living environment
Maintaining an optimal habitat for the species is of paramount importance. Some critical parameters that need to remain steady are: temperature, non-ionized ammonia and nitrites, the concentrations of DO and CO2, pH and the hydraulic load rate (HLR), i.e. the water flow rate [Citation5,Citation17,Citation58].
Water temperature is more important than air temperature for plant production [Citation36,Citation59]. Water temperature depends on the ambient temperature, and smaller systems lose heat faster than larger systems [Citation7]. The optimum water temperature for most plants is about 23 °C. Fish have a range of water temperatures that they can tolerate. The local climate is an important factor to consider when deciding which species to grow [Citation7,Citation18,Citation60]. Bernstein, 2011, reported that the development of bacteria, aquaculture species and plants is optimal at higher water temperatures in areas with warmer climates [Citation61]. That is why controlled greenhouse production is common in northern latitudes [Citation7]. Aquaponic systems are most effective where crops can grow continuously, e.g. in tropical, subtropical or temperate areas with environmentally controlled greenhouses [Citation36]. However, an increase in water temperature and pH will lead to an increase in the concentration of toxic NH3. For example, at a water temperature of 20 °C, 0.40% and 1.24% of the total ammonia is non-ionized at pH 7.0 and 7.5, respectively. At 25 °C, these values increase to 0.57% and 1.77%, respectively [Citation7,Citation24]. Maintaining a constant optimal water temperature by heating or cooling is an important part of the environmental management in aquaponic systems.
Recirculation systems must maintain an adequate level of DO, at least 6 mg/L. Installations use diffuse aeration systems for aeration (dissolving oxygen from the atmosphere in the water), which provide low pressure air [Citation37]. Oxygenation systems (transfer of pure oxygen to water) can provide oxygen concentration from 50% to 90% higher than 100 mg/L. Maintaining a high level of dissolved oxygen in the water is extremely important for intensive root respiration and optimal plant growth. If the content of dissolved oxygen is low, the water absorption will decrease and the nutrient uptake will decrease, resulting in loss of root cells and tissues. This leads to reduced plant growth. Low DO levels correspond to high concentrations of CO2, which in turn promotes pathogen growth in plant roots [Citation36].
The ideal solution is an aquaponic system in a tightly sealed greenhouse because CO2 is continuously released from the water. CO2-enriched air leads to significantly increased yields in greenhouses in northern latitudes. Doubling of the CO2 concentration will increase the photosynthesis of plants, thus increasing the nitrogen assimilation so the growth will increase the yields by an average of 30% [Citation36]. On the other hand, elevated CO2 will disrupt fish development, so the RAS design aims to minimize the CO2 levels [Citation23]. The optimum concentration is below 25 mg/L [Citation37].
Goddek et al. [Citation62], found pH 6 − 6.5 as optimal for most crops to improve the nutrient uptake. Boyd and Lichtkoppler [Citation63] described that the optimal development of the genetic potential of fish requires the pH of the medium to be between 7 and 9. Since nitrifying bacteria need a high pH value (>7), the ideal pH range applicable to the entire aquaponic system is in the range of 6.0 − 8.0 and its maintenance is essential [Citation7].
The optimal hydraulic load rate (HLR) – i.e. water flow rate, is an important component in the aquaponic system, as it affects the spatial and temporal characteristics of water and consequently determines the growth and yield of crops [Citation64]. High hydraulic load rates (HFR), 3.3 m³/m2/day, are sufficient to provide better chemical and physical properties of the aquaponics solution for maximum yield and quality of the plants. HLR must maintain a value of at least 2.2 m³/m2/day (6 to 9 h hydraulic retention time) for the production of fast-growing crops (Chinese cabbage (Brassica rapa) and lettuce (Lactuca sativa)) but can be lower for slow-growing crops (basil (Ocimum basilicum) and Swiss chard (Beta vulgaris)) [Citation64]. The water flow affects the efficiency of the system for purification of nutrients and maintenance of water quality parameters. Nuwansi et al. (2014), proposed an optimal water flow rate of 0.8 L/min for carp (Cyprinus carpio var. koi) and goldfish (Carassius auratus) in combination with spinach (Ipomoea aquatica) in an aquaponic system [Citation58]. This report demonstrated that plant growth and nutrient removal increase with decreasing the flow rate.
The use of chemicals for disinfection or treatment of fish diseases can have a devastating effect on the nitrifying bacteria in the biofilter. An alternative approach is the continuous disinfection of recycled water with ozone or ultraviolet (UV) radiation. Ozone is a strong oxidant and has a long history of use in water disinfection. Brazil et al. [Citation65] reported that the effectiveness of ozone treatment depends on the concentration of ozone in the water and the time of contact with microorganisms. However, the entry of residual ozone into aquaculture tanks can have toxic effects. Ozone in the air is also toxic to people in low concentrations. The main advantage of UV treatment is that it is safe and not harmful to cultivated species. Spotte [Citation66] outlined that the effectiveness of UV sterilization depends on the size of the organisms, the dose of UV radiation and the degree of penetration of radiation into the water. The main disadvantage of ozone and UV sterilization is the need for clean water with low concentrations of solids.
Integrated pest management in the aquaponic system should only use non-chemical methods [Citation17]. These include biological control (resistant varieties, predators, pathogens, antagonistic organisms), physical barriers, traps and manipulation of the physical environment. There are more solutions for biological control in sealed greenhouses than in outdoor installations [Citation36].
Economic feasibility of aquaponic systems
The integration of RAS and crops leads to cost savings in both systems. Shared cost savings come from allocating operating costs (e.g. management, water, nutrients and overheads) and capital costs (e.g. system, transport) to the two systems [Citation22].
Aquaponic systems are easier to operate, require less monitoring and are safer than conventional production [Citation36]. Aquaponic systems enjoy growing popularity, on the one hand, because of people’s awareness of food security, water supply and diminishing resources on Earth and, on the other, because of the versatility of aquaponics technology and its use for educational purposes, social development and so forth [Citation23]. The big advantage of aquaponic systems is that the choice of plant and fish species depends entirely on market demand [Citation36]. Culinary herbs and spices grow very quickly in aquaponic systems year round and maintain high market prices. Year-round production is also possible for the cultivation of edible flowers and traditional medicinal plants for extraction modern pharmaceuticals.
The Food and Agriculture Organization of the United Nations (FAO) suggests that tilapia is suitable in poorer regions, as it has a lower feed conversion ratio (FCR) of approximately 1.0 compared to large ruminants, whose FCR is 8.8 [Citation23]. According to a marketing study in Iceland in 2011, there is very good market acceptance for fresh tilapia and it can sell at the same price as cod fillets on the Icelandic market [Citation23]. Javadzadeh et al. [Citation67] optimized the profit of a medium-scale aquaponic system, and concluded that the most profitable combination is tilapia and basil. Economic analysis by Adler et al. [Citation22] on an aquaponic system for integrated production of rainbow trout (Oncorhynchus mykiss), lettuce (Lactuca sativa L.) and sweet basil (Ocimum basilicum L.) demonstrated potential profitability, with a 67% annual return from crop production. Bailey et al. [Citation9] have also reported that a large-scale commercial aquaponic system for the production of tilapia and lettuce can be profitable. Revenues exceed production costs and there is a positive return on investment [Citation9]. Afolabi’s [Citation68] analysis of economic indicators shows that high market value crops can generate profits.
Aquaponics is emerging in the spotlight as a potentially viable approach to sustainable agriculture which meets the understanding of circular economy [Citation69]. An essential factor for the viability of aquaponics is the delicate balance between research, business goals and consumer perceptions [Citation70]. Aquaponic systems are in line with the European Union strategy for a sustainable food system, known as Farm2Fork [Citation71]. Recently researchers have called for attention on the importance of directing efforts toward development of organic aquaponics in a way that meets the certification criteria for organic produce defined in Commission Regulation (EU) 2018/848 [Citation72]. There is an important marketing opportunity for aquaponics because consumers are willing to buy local environmentally friendly, green products free from antibiotics, pesticides and herbicides [Citation73]. Large-scale commercial aquaponic systems can produce high annual yields. Such systems require a particular educational background, so hundreds of secondary and higher schools around the world are already incorporating this in the curricula [Citation36]. Guided by this principle, educational and research centers for aquaponics have opened in many EU member states. For example, one of the latest ones was founded in March 2022, together with a company for production and demonstration of aquaponics products in partnership with the Trakia University (Stara Zagora, Bulgaria). Scientists, investors and manufacturers have joined forces to develop the new version of food systems as an innovation for sustainability and work for the availability of aquaponics technology on the Balkans.
Relationship between biological and technological parameters
The most important principle in the design of aquaponic systems is that, in order to convert the input into the desired output, there needs to be a balance between: nutrient load as a function of fish biomass and feeding rate, metabolism, conversion and subsequent excretion; undigested feed; feces, and the nutritional needs of plants [Citation57]. Therefore, the most common approach to achieving balance in aquaponic systems is the fish-to-plants ratio [Citation4]. However, specific nutritional requirements also depend on the growth stage/life cycle and external factors (e.g. system design) [Citation6,Citation26].
Although aquaponics is relatively new, over the years many experiments have focused on optimizing the model, combined with the most favorable species for cultivation and good results in the production, under different environmental conditions. An approach for optimization of aquaponic systems that emerged in recent years is the Deep-Water Culture system. This technique is more useful than conventional hydroponic systems, as it can produce high yields using suboptimal nutrient solution [Citation3].
A comparative study of two systems (Afolabi, 2020), the Deep-Water Culture (DWC) system and the Integrated AquaVegeculture System (IAVC), for combined production of kale (Brassica oleracea var. acephala) and Nile tilapia (Oreochromis niloticus) showed that the water quality is more efficient for fish growth in DWC compared to IAVC, owing to the presence of mechanical and biological filters in DWC [Citation68]. A study from Romania [Citation12] demonstrated that DWC with floating rafts is easy to install, needs no electricity. It is easy to maintain, and the oxygen supply can come from algae such as Egeria densa. Crops on floating rafts can also be a solution for decorating water surfaces in private or public places [Citation12].
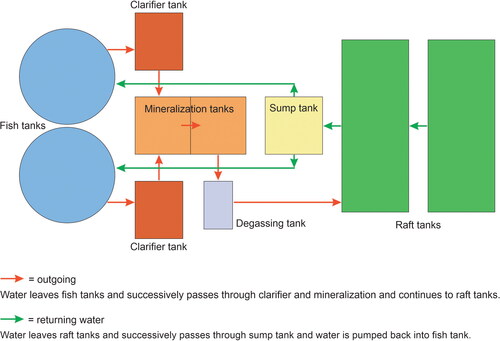
A pilot study in 2017 comparing classical coupled aquaponic systems (CAS) and decoupled aquaponic systems (DAS; see ) obtained a significantly higher yield in DAS (36% higher yield of tomatoes). In contrast to production, there were 31% more leaves, 60% more roots and 50% more stem biomass in CAS. The main reasons behind the higher yield lie in the independent regulation of pH and dynamic adaptation of nutrient concentrations [Citation74]. Another experiment to determine the effects of fish stocking density on water quality, tilapia growth and lettuce cultivation in decoupled recirculation aquaponic systems (DRAPS) showed that higher stocking densities increased the electrical conductivity, total solutes and salinity and decreased the DO. The highest stocking density led to the highest accumulation of nutrients in the form of ammonia-nitrogen (NH3-N), ammonium (NH4), nitrite-nitrogen (NO2-N) and nitrate-nitrogen (NO3-N) and potassium (K), except for phosphorus (P). However, based on the conversion of fish feed to NO3-N and P per kilogram of feed, the lowest stocking density provided the highest concentration of NO3-N and P. Reportedly, DRAPS rely solely on fish waste and produce insufficient concentrations of N, P, K and iron [Citation75].
Figure 3. Schematic illustration of classical (coupled) and decoupled aquaponics. (a): Classic aquaponic system consisting of RAS (blue: fish tanks, brown: clarifier and mineralization tanks and violet: degassing tank) coupled with hydroponics (green: raft tanks). Water is continuously recirculated from RAS to hydroponics and back to RAS. (b): Decoupled aquaponic system consisting of RAS connected to the hydroponic unit (with an additional tank) via a one-way valve. Water is recirculated separately in each system and is delivered to the hydroponics unit if necessary, but not vice versa [Citation74].
![Figure 3. Schematic illustration of classical (coupled) and decoupled aquaponics. (a): Classic aquaponic system consisting of RAS (blue: fish tanks, brown: clarifier and mineralization tanks and violet: degassing tank) coupled with hydroponics (green: raft tanks). Water is continuously recirculated from RAS to hydroponics and back to RAS. (b): Decoupled aquaponic system consisting of RAS connected to the hydroponic unit (with an additional tank) via a one-way valve. Water is recirculated separately in each system and is delivered to the hydroponics unit if necessary, but not vice versa [Citation74].](/cms/asset/e36bd14c-e73d-4564-a980-2ff3aae9057c/tbeq_a_2074892_f0003_c.jpg)
Two new terms, ‘type of coupling’ and ‘degree of coupling’, were introduced in 2021 as qualitative and quantitative parameters to characterize the coupling efficiency of the internal flows in aquaponics subsystems. This new framework provides a uniform indicator for comparing aquaponics facilities and proposes criteria for their optimization [Citation76].
Conclusions
The ongoing research into the biological and technological parameters of aquaponic systems has brought about the understanding that there are many variables that need to be studied. There is a wide range of possible approaches to construct an optimal aquaponic system, which determines its complexity. Future research work may include diverse combinations of aquaculture species and crops and such that have a much higher quality and market value. The development of aquaponics technology, which contributes to more sustainable food systems, needs further research not only on the biological and technological parameters of aquaponic systems, but also on the environmental, operational and socio-economic aspects and their relationship. A key factor for the success of aquaponics is the balance between research and business interests, as well as the positive attitude of end users toward it. It will probably prove to be one of the important modern food production systems needed today and in the future.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study. The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Hendricks P. Life cycle assessment of greenhouse tomato (Solanum lycopersicum L.) production in southwestern Ontario. Guelph, Ontario (Canada): The University of Guelph; 2012.
- Martellozzo F, Landry S, Plouffe D, et al. Urban agriculture: a global analysis of the space constraint to meet urban vegetable demand. Environ Res Lett. 2014;9(6):064025.
- Vermeulen T, Kamstra A. The need for systems design for robust aquaponic systems in the urban environment. Acta Hortic. 2013;1004(1004):71–77.
- Love D, Fry J, Genello L, et al. An international survey of aquaponics practitioners. In PLoS One. 2014;9(7):e102662–10.
- Endut A, Jusoh A, Ali N, et al. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour Technol. 2010;101(5):1511–1517.
- Goddek S, Espinal C, Delaide B, et al. Navigating towards decoupled aquaponic systems: a system dynamics design approach. Water MDPI. 2016;8(7):303–314.
- Medina M. Effect of aquafeed on productivity of red amaranth and on water quality under aquaponic cultivation [thesis]. Miami (FL): Florida International University; 2014.
- Alderman S. The practicality and sustainability of aquaponic agriculture versus traditional agriculture with emphasis on application in the Middle [thesis]. San Marcos, Texas; 2015.
- Bailey D, Rakocy J, Cole W, et al. Economic analysis of a commercial-scale aquaponic system for the production of tilapia and lettuce. Fourth International Symposium on Tilapia in Aquaculture. , St. Croix, U.S. Virgin Islands: Agriculture Experiment Station, University of the Virgin Islands; 1997, Vol. 1. p. 1–12.
- Crossley P. Just beyond the eye: floating gardens in Aztec Mexico. Hist Geogr. 2004;32:112.
- Turcios A, Papenbrock J. Sustainable treatment of aquaculture effluents – what can we learn from the past for the future? Sustainability. 2014;6(2):836–856.
- Molovan I, Bala M. Analysis of aquaponic organic hydroponics from the perspective of setting costs and of maintenance on substratum and floating shelves systems. J Hortic Sci Biotechnol. 2015;19:73–76.
- Bindraban S, van der Velde M, Ye L, et al. Assessing the impact of soil degradation on food production. Curr Opin Environ Sustain. 2012;4(5):478–488.
- Naegel L. Combined production of fish and plants in recirculating water. Aquaculture. 1977;10(1):17–24.
- Junge R, Konig B, Villarroel M, et al. Strategic points in aquaponics. Water. 2017;9(3):182–189.
- Nelson R. Aquaponic food production. Montello (WI): Nelson and Pade; 2008.
- Gosh K, Chowdhury. S. Review of aquaponics system: searching for a technically feasible and economically profitable aquaponics system. J Agric Environ Consumer Sci. 2019;19:5–13.
- Diver S, Rinehart L. Aquaponics – Integration of hydroponics with aquaculture. ATTRA. 2010;28:1–28.
- Villarroel M, Junge R, Komives T, et al. Survey of aquaponics in Europe. Water. 2016;8(10):468.
- Turnsek M, Joly A, Thorarinsdottir R, et al. Challenges of commercial aquaponics in Europe: beyond the hype. Water. 2020;12(1):306.
- Love D, Fry J, Li X, et al. Commercial aquaponics production and profitability: findings from an international survey. Aquaculture. 2015;435:67–74.
- Adler PR, Harper JK, Wade EM, et al. Economic analysis of an aquaponic system for the integrated production of rainbow trout and plants. Int J Recirc Aquac. 2000;1(1):15–34.
- Rakocy J, Shultz R, Bailey D, et al. Aquaponic production of tilapia and basil: comparing a batch and staggered cropping system. Acta Hortic. 2004;648(648):63–69.
- Popma T, Masser. M. Tilapia: life history and biology. Southern Regional Aquaculture Center. SRAC Publication No. 283: United States Department of agriculture. USA; 1999.
- Thorarinsdottir R , editor. Aquaponics guidelines. Reykjavik (Iceland): Haskolaprent, University of Iceland; 2015.
- Licamele J. Biomass production and nutrient dynamics in an aquaponics system [Ph.D. thesis]. Tucson, AZ, (USA): University of Arizona; 2009.
- Danner R, Mankasingh U, Anamthawat-Jonsson K, et al. Designing aquaponic production systems towards integration into greenhouse farming. Water. 2019;11(10):2123.
- Johnson G, Buzby K, Semmens K, et al. Evaluation of lettuce between spring water, hydroponic, and flow-through aquaponic systems. Int J Veg Sci. 2017;23(5):456–470.
- Buzby K, Waterland N, Semmens K, et al. Evaluating aquaponic crops in a freshwater flow-through fish culture system. Aquaculture. 2016;460:15–24.
- Silva L, Escalante E, Valdés-Lozano D, et al. Evaluation of a semi-intensive aquaponics system, with and without bacterial biofilter in a tropical location. Sustainability. 2017;9(4):592.
- Kloas W, Groß R, Baganz D, et al. A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquacult Environ Interact. 2015;7(2):179–192.
- Eck M, Sare A, Massart S, et al. Exploring bacterial communities in aquaponic systems. Water. 2019;11(2):260.
- Bulgarelli D, Garrido-Oter R, Münch P, et al. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe. 2015;17(3):392–403.
- Hynes R, Leung G, Hirkala D, et al. Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil, and chickpea grown in Western Canada. Can J Microbiol. 2008;54(4):248–258.
- Reichenbach. H. The Lysobacter genus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes. New York (NY): Springer; 2006. p. 939–957.
- Losordo T, Masser M, Rakocy J. Recirculating aquaculture tank production systems – a review of component options. Southern Regional Aquaculture Center; SRAC Publication No. 453: United States Department of agriculture. USA; 1999.
- Rakocy J, Masser M, Losordo. T. Recirculating aquaculture tank production systems: aquaponics- integrating fish and plant culture Southern Regional Aquaculture Center. SRAC Publication No. 454: United States Department of agriculture. USA; 2006.
- Trang N, Schierup H, Brix. H. Leaf vegetables for use in integrated hydroponics and aquaculture systems: effects of root flooding on growth, mineral composition and nutrient uptake. Afr J Biotechnol. 2010;9(27):4186–4196.
- Neto R, Ostrensky A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac Res. 2015;46(6):1309–1322.
- Al-Hafedh Y, Alam A, Beltagi M. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J World Aquac Soc. 2008;39(4):510–520.
- Goddek S, Keesman K. The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination. 2018;428:76–85.
- Delaide B, Delhaye G, Dermience M, et al. Plant and fish production performance, nutrient mass balances, energy and water use of the PAFF box, a small-scale aquaponic system. Aquacult Eng. 2017;78:130–139.
- Love D, Uhl M, Genello L. Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquacult Eng. 2015;68:19–27.
- Supajaruwong S, Satanwat P, Pungrasmi W, et al. Design and function of a nitrogen and sediment removal system in a recirculating aquaculture system optimized for aquaponics. Environ Eng Res. 2020;26(2):190494–190490.
- Hussain T, Verma AK, Tiwari VK, et al. Optimizing koi carp, Cyprinus carpio var. Koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J World Aquacult Soc. 2014;45(6):652–661.
- Maucieri C, Nicoletto C, Zanin G, et al. Effect of stocking density of fish on water quality and growth performance of European carp and leafy vegetables in a low-tech aquaponic system. PLoS One. 2019;14(5):e0217561.
- Paudel SR. Nitrogen transformation in engineered aquaponics with water celery (Oenanthe javanica) and koi carp (Cyprinus carpio): effects of plant to fish biomass ratio. Aquaculture. 2020;520:734971.
- Buzby K, West T, Waterland N, et al. Remediation of flow-through trout raceway effluent via aquaponics. North Am J Aquacult. 2017;79(1):53–60.
- Salama S, Kandel A, El-shinawy M, et al. Evaluation of mint and sweet basil herbs production integrated into the aquaponic tilapia production system. AUJASCI. Arab Univ J Agric Sci. 2020;22(2):563–573.
- Davidson J, Good C, Welsh C, et al. Comparing the effects of high vs. low nitrate on the health, performance, and welfare of juvenile rainbow trout Oncorhynchus mykiss within water recirculating aquaculture systems. Aquacult Eng. 2014;59:30–40.
- Saha S, Monroe A, Day M. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann Agric Sci. 2016;61(2):181–186.
- Stathopoulou P, Tsoumalakou E, Levizou E, et al. Iron and potassium fertilization improve rocket growth without affecting tilapia growth and histomorphology characteristics in aquaponics. Appl Sci. 2021;11(12):5681.
- Slosar M, Uher A, Andrejiova A, et al. Selected yield and qualitative parameters of broccoli in dependence on nitrogen, sulfur, and zinc fertilization. Turk J Agric For. 2016;40:465–473.
- Sorin E, Etienne P, Maillard A, et al . Effect of sulphur deprivation on osmotic potential components and nitrogen metabolism in oilseed rape leaves: identification of a new early indicator. J Exp Bot. 2015;66(20):6175–6189.
- Luo X, Rauan A, Xing J, et al. Influence of dietary Se supplementation on aquaponic system: focusing on the growth performance, ornamental features and health status of koi carp (Cyprinus carpio var. Koi), production of lettuce (Lactuca sativa) and water quality. Aquac Res. 2021;52(2):505–517.
- Bandi A, Cristea V, Petrea S, et al. The review of existing and in-progress technologies of the different subsystems required for the structural and functional elements of the model of multi-purpose aquaponic production system. Rom Biotechnol Lett. 2016;21:4.
- Goddek S. Opportunities and challenges of multi-loop aquaponic systems [dissertation]. Wageningen (Netherlands): Wageningen University; 2017.
- Nuwansi K, Verma A, Prakash C, et al. Effect of water flow rate on polyculture of koi carp (Cyprinus carpio var. Koi) and goldfish (Carassius auratus) with water spinach (Ipomoea aquatica) in recirculating aquaponic system. Aquacult Int. 2016;24(1):385–393.
- Rakocy J, Shultz, D. Bailey R. Commercial aquaponics for the caribbean Proceedings of the 51st Gulf and Caribbean Fisheries Institute: Kingshill, U.S. Virgin Islands; 2000. p. 353–364.
- Allen W. Growing the good food revolution. Miami (FL): University of Miami; 2013.
- Bernstein S. Aquaponic gardening: a step-by-step guide to raising vegetables and fish together. Gabriola Island (Canada): New Society Publishers; 2011.
- Goddek S, Delaide B, Mankasingh U, et al. Challenges of sustainable and commercial aquaponics. Sustainability. 2015;7(4):4199–4224.
- Boyd C, Lichtkoppler. F. Water quality management in pond fish culture. Res Dev Seri. 1979;22(43):1–11.
- Yang T, Kim. H. Effects of hydraulic loading rate on spatial and temporal water quality characteristics and crop growth and yield in aquaponic systems. Horticulturae. 2020;6(1):9.
- Brazil B, Summerfelt S, Libey G. Applications of ozone to recirculating aquaculture systems. Successes and failures in commercial recirculating aquaculture Proceeding from the Successes and Failures in Commercial Recirculating Aquaculture Conference. Roanoke, VA. Northeast Regional Agricultural Engineering Service, 152 Riley-Robb Hall, Ithaca, New York; 1996. p. 373–389.
- Spotte S. Fish and invertebrate culture: water management in closed systems. New York (NY): Wiley; 1979. p. 145.
- Javadzadeh P, Park S, Whitworth C. Optimizing the profit of a medium scale home aquaponics system; 2019. DOI: 10.13140/RG.2.2.15448.03849
- Afolabi K. Productivity of Kale (Brassica oleracea var. acephala) and Nile tilapia (Oreochromis niloticus) culture in aquaponic systems [thesis]. Cairo (Egypt): The American University in Cairo; 2020.
- EIP-AGRI Focus Group on Circular Horticulture. Starting Paper: FG27 Circular horticulture. EIP-AGRI, 2017. Available from: https://ec.europa.eu/eip/agriculture/sites/agri-eip/files/eip-agri_fg_circular_horticulture_-starting_paper_2017_en.pdf.
- Suárez-Cáceres GP, Fernández-Cabanás V, Lobillo-Eguíbar J, et al. Consumers’ knowledge, attitudes and willingness to pay for aquaponic products in Spain and Latin America. Int J Gastron Food Sci. 2021;24:100350.
- Directorate-General for Maritime Affairs and Fisheries. Sustainable aquaculture: EU supports promising aquaponics project [Internet]. European Commission, Oceans and Fisheries; [cited 2021 Oct 29]. Available from: https://ec.europa.eu/oceans-and-fisheries/news/sustainable-aquaculture-eu-supports-promising-aquaponics-project-2021-10-29_en
- Fruscella L, Kotzen B, Milliken S. Organic aquaponics in the European Union: towards sustainable farming practices in the framework of the new EU regulation. Rev Aquacult. 2021;13(3):1661–1682.
- Miličić V, Thorarinsdottir R, Santos M, et al. Commercial aquaponics approaching the European market: to consumers’ perceptions of aquaponics products in Europe. Water. 2017;9(2):80–22.
- Monsees H, Kloas W, Wuertz S. Decoupled systems on trial: eliminating bottlenecks to improve aquaponic processes. PLoS One. 2017;12(9):e0183056–18.
- Tawaha A, Wahab P, Jaafar H, et al. Effects of fish stocking density on water quality, growth performance of tilapia and yield of butterhead lettuce grown in decoupled recirculation aquaponic systems. J Ecol Eng. 2021;22(1):8–19.
- Baganz G, Junge R, Portella M, et al. The aquaponic principle – It is all about coupling. Rev Aquacult. 2022;14(1):252–264.