Abstract
In plants, the B-box (BBX) transcription factors (TFs) are a subfamily of zinc-finger TFs that act to regulate diverse plant growth and development processes. The BBX TFs have been the subject of considerable attention, and are well characterized in diverse plant species, including in rice (Oryza sativa) and Arabidopsis thaliana, but less so in the economically important soybean (Glycine max). In this work, we systematically identified and characterized 57 soybean BBX genes (GmBBX1 to GmBBX57). These genes were mapped to all 20 soybean chromosomes and were divided into five clades with high intra-clade intron–exon similarity. The majority of GmBBX gene promoter cis-acting elements were responsive to light, abscisic acid, salicylic acid and methyl jasmonate, as well as a diverse array of other stimuli. Quantitative RT-PCR indicated that several GmBBX genes exhibited tissue-specific and phytohormone- and abiotic stress-responsiveness. The results of this study will be useful in the continued characterization of soybean BBX gene functions and provide new ideas for soybean breeding.
Introduction
Transcription factors (TFs), which bind to specific regions of target gene promoters to activate or inhibit expression, are important regulators of plant growth and development [Citation1, Citation2]. Studies on the structure and function of TFs have shown that they are composed of a nuclear localization signal, oligomerization site, transcription activation domain and DNA binding site [Citation3, Citation4]. One large family of TFs, the zinc-finger TFs, is comprised of several subfamilies which all act to regulate different aspects of development and growth in plants [Citation5]. Among the subfamilies of the zinc-finger family, the B-box (BBX) family is particularly important [Citation6]. In plants, BBX TFs contain one or two N-terminal conserved domains, and sometimes a C-terminal TOC1 (CCT), CONSTANS (CO) or CONSTANS-LIKE (COL) domain [Citation7, Citation8]. BBX TFs are crucial for not only plant development and growth, but also for plant response to external stimuli [Citation8], including stress responses [Citation9] and flower induction [Citation10, Citation11], among others.
The first identified BBX protein, AtBBX1 from Arabidopsis thaliana, regulates the timing of flowering by activating Flowering Locus T (FT) gene expression [Citation12, Citation13]. Two BBX TFs, VvCOL (VviBBX2) and VvCOL1 (VviBBX5), are also involved in regulating the timing of flowering in grapevine (Vitis vinifera) [Citation14]. In rice (Oryza sativa), OsCLO4 negatively regulates flowering during both long and short days [Citation11], OsCO3 negatively regulates flowering during short days [Citation15], and Hd1 (OsBBX18) inhibits flowering during long days while promoting flowering during short days [Citation16]. In chrysanthemum (Chrysanthemum morifolium), CmBBX24 acts to negatively regulate flowering time as well [Citation17]. In soybean, GmCOL5 plays a vital role in the photoperiod response of seedlings [Citation18] and GmCOL9 is involved in regulating flowering time by delaying the floral transition [Citation19]. GmCOL1a and GmCOL1b restore late-flowering Arabidopsis mutants to normal [Citation20].
BBX proteins are specifically related to photomorphogenesis, carotenoid accumulation and anthocyanin synthesis. In Arabidopsis, photomorphogenesis is promoted through the interaction of AtBBX4 with PHYTOCHROME INTERACTING FACTOR 3 (PIF3) [Citation21], and both AtBBX11 and AtBBX21 positively regulate photomorphogenesis [Citation22]. In contrast, AtBBX28 negatively regulates photomorphogenesis by repressing ELONGATED HYPOCOTYL5 (HY5) expression and undergoing CONSTITUTIVELY PHOTOMORPHOGENEC 1 (COP1)-mediated degradation [Citation23]. Furthermore, HY5 regulates BBX30 and BBX31 expression, negatively regulating photomorphogenesis [Citation24]. In tomato (Solanum lycopersicum), SlBBX20 promotes both carotenoid accumulation and chloroplast development [Citation25]. In apple (Malus spp.) MdBBX20 promotes anthocyanin accumulation [Citation26] and MdCOL11 (MdBBX22) modulates the accumulation of anthocyanin pigment [Citation27]. Likewise in pear (Pyrus spp.), PpBBX16 positively regulates anthocyanin accumulation [Citation28].
BBX proteins also act as abiotic stress response regulators in plants. Early studies revealed that Arabidopsis AtBBX2 could enhance yeast (Saccharomyces cerevisiae) salinity tolerance [Citation29]. In Arabidopsis, AtBBX24 overexpression improves the growth of salt-stressed plants [Citation30]. The overexpression of apple MdBBX10 has also been found to enhance tolerance to salinity in Arabidopsis [Citation31]. Furthermore, AtBBX18 acts to negatively regulate heat stress tolerance in Arabidopsis [Citation32]. Grapevine VvZFPL is homologous to Arabidopsis BBX32, and overexpression in transgenic Arabidopsis enhances tolerance to cold stress [Citation33]. Finally, UV-B radiation tolerance in Arabidopsis is enhanced through overexpression of AtBBX31 [Citation34].
Soybean (Glycine max) is currently the world’s leading protein source and second leading source of vegetable oil [Citation35, Citation36]. However, soybean yields are negatively impacted by pests, disease, drought, extreme temperatures and salinity, among other abiotic and biotic stressors. After the successful sequencing of the soybean genome, many soybean gene families have been identified and analyzed, such as the bZIP transcription factors (bZIPs) [Citation37], stress-associated proteins (SAPs) [Citation38] and AT-Hook Motif Nuclear Localized (AHL) genes [Citation39], etc. As outlined above, BBX proteins are crucially important for regulating plant stress response. The BBX TF family has been studied extensively in a diverse array of plants, including A. thaliana [Citation40], tomato (Solanum lycopesicum) [Citation41], rice (O. sativa) [Citation42], peanut (Arachis duranensis) [Citation7], grapevine (V. vinifera) [Citation6], pepper (Capsicum annuum) [Citation43] and apple (Malus domestica) [Citation44]. However, there has been no comprehensive evaluation of this TF family in soybean. To address this pressing need, this study was conducted to characterize the soybean GmBBX genes. We investigated GmBBX gene structures, phylogenetic relationships, chromosomal localizations and subcellular localizations, as well as tissue- and stressor-specific expression patterns. The results of this study will be useful for the continued characterization of soybean BBX gene functions.
Materials and methods
Initial identification of soybean BBX TF family genes and proteins
Genome-wide Glycine max Wm82.a2.v1 data files were acquired from the Phytozome Database (https://phytozome-next.jgi.doe.gov) [Citation45]. A hidden Markov Model (HMM) was utilized to acquire the conserved BBX domain (PF00643) from the Pfam database (Pfam 32.0, http://pfam.xfam.org/). HMMR 3.0 was utilized to perform a BLASTP search of soybean genomic protein databases, using the B-box domain HMM profile. Both NCBI Batch CD-Search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) and SMART (http://smart.embl-heidelberg.de/) were utilized to certify the presence of the BBX domain within obtained GmBBX proteins. The ExPASy proteomics server (https://web.expasy.org/) was utilized for prediction of both the isoelectric points (pI) and molecular weights (kDa) of the obtained GmBBX proteins [Citation46].
Analysis of phylogenetic relationships
To interrogate the phylogenetic relationships between the BBX family genes in both soybean and Arabidopsis, protein sequences were collected from Phytozome v13 (http://www.phytozome.org) [Citation47]. MEGA-X was used to align BBX proteins and construct a neighbor-joining (NJ) phylogenetic tree [Citation48], and bootstrapping was conducted using 1000 replications. Each gene was classified in accordance with its distance homology with Arabidopsis genes.
Analysis of chromosomal location, intron–exon structure and conserved motifs
Phytozome v13 (http://www.phytozome.org) was used to map all GmBBX genes to soybean chromosomes. TBtools was used to create a chromosomal location map, extract soybean genomic full-length and coding sequences, generate intron–exon structure diagrams, and draw a conserved motif map [Citation49]. The MEME analysis tool (https://meme-suite.org/meme/tools/meme) was utilized to identify the conserved motifs of BBX proteins, allowing for a maximum of 10 motifs and otherwise using the default settings [Citation50].
Analysis of GmBBX promoter cis-elements
Genomic sequences (2000 bp) spanning soybean BBX promoter regions were acquired from the soybean genome database. Putative cis-acting elements were predicted with the PlantCARE tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html) [Citation51] and analyzed using TBtools [Citation49].
Analysis of expression patterns
Tissue-specific GmBBX expression data, including for root (SRR037387), root tip (SRR037386), nodule (SRR037385), flower (SRR037382), pod (SRR037383), apical meristem (SRR037381) and leaf (SRR037384), were collected using the soybean database (https://www.soybase.org/). Using these data, TBtools was utilized to create a heatmap of tissue-specific gene expression [Citation49].
Plant materials and experimental treatments
Seeds of soybean variety ‘Williams 82′ were obtained from Liaocheng University (China). Seeds were sown in pots (10*10 *8.5 cm) filled with grass charcoal (a seed per flowerpot) and grown in a climate-controlled greenhouse at 26 °C using a cycle of 10 h dark and 14 h light. At the V1 stage, each seedling was irrigated with 50 mL, specifically, for drought treatment (10% polyethylene glycol-6000 [PEG-6000]) [Citation52], high salinity (100 mmol/L NaCL) [Citation53], 100 μmol/L abscisic acid (ABA), 100 μmol/L salicylic acid (SA) or 100 μmol/L methyl jasmonate (MeJA) [Citation43]. To examine the effects of extreme temperatures, seedlings were kept either at low (4 °C) or high (38 °C) temperatures using climate-controlled growth chambers [Citation54, Citation55]. In all cases, leaves were harvested at 0, 3, 6 and 12 h post-treatment, promptly frozen using liquid nitrogen and then ground with a mortar to extract RNA. Three biological replicates were collected per time point per treatment.
Isolation of RNA and analysis of gene expression using real-time quantitative (RT)-qPCR
An RNAprep pure Plant Total RNA Extraction Kit (Tiangen, Beijing, China) was utilized for RNA extraction from leaf samples. Reverse transcription of mRNA was accomplished using HiScript III RT SuperMix (plus gDNA wiper) for qPCR (Vazyme, Nanjing, China). Gene expression was examined using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). A Bio-Rad CFX96 system was used to carry out quantitative real-time PCR utilizing the following protocol: 95 °C for 30 s, 95 °C for 5 s, 56 °C for 30 s for 39 cycles. The 2−ΔΔCt method was utilized to quantify relative gene expression levels. All qRT-PCR analyses utilized GmACTIN gene and GmTubulin as the internal reference genes. Three technical replicates were carried out per treatment. All primers used in the experiment can be found in Supplemental Table S1.
Results
Systematic identification and characterization of soybean BBX family genes and proteins
In order to determine putative soybean BBX genes, Arabidopsis BBX proteins were searched against the soybean genomic database [Citation40]. Then, the BBX domain (Pfam00643) HMM profile was used to search the soybean genome. After removing redundant sequences and identifying the conserved domain, we identified 57 putative soybean GmBBX genes and named them according to their chromosomal location (GmBBX1-57).
These 57 putative genes were predicted to encode proteins ranging from 125 (GmBBX43 and GmBBX34) to 481 (GmBBX22) amino acids in length. These proteins were predicted to exhibit molecular masses ranging from 14.00827 to 53.91340 kDa and pIs ranging from 4.2 to 9.72. All 57 GmBBX proteins were predicted to have nuclear localization (Supplemental Table S2).
Analysis of evolutionary relationships and conserved domains of soybean BBX proteins
To investigate the evolutionary relationships and functional divergences of the GmBBX gene family, we created a phylogenetic tree illustrating the evolutionary relationships of BBX genes between Arabidopsis and soybean (). The 57 GmBBX proteins were separated into 5 clades: Clade I contained 8 GmBBX proteins (GmBBX7/12/20/38/39/49/51/52) with three protein domains (one CCT domain and two BBX); Clade II contained 13 GmBBX proteins (GmBBX2/3/4/23/24/37/44/46/48/53/54/55/57) with three protein domains (one CCT domain and two BBX); Clade III contained 6 GmBBX proteins (GmBBX8/16/17/22/25/56) with two protein domains (one CCT and one BBX); Clade IV contained 17 GmBBX proteins (GmBBX1/5/6/10/11/21/26/27/28/29/30/31/32/36/41/45/50) with one BBX domain; and Clade V contained 13 GmBBX proteins (GmBBX9/13/14/15/18/19/33/34/35/40/42/43/47) with two BBX domains.
Figure 1. Analysis of phylogenetic relationships of BBX genes between Arabidopsis and soybean. MEGA-X was used to align BBX proteins and construct a neighbor-joining (NJ) phylogenetic tree [Citation48]; bootstrapping was conducted using 1000 replications.
![Figure 1. Analysis of phylogenetic relationships of BBX genes between Arabidopsis and soybean. MEGA-X was used to align BBX proteins and construct a neighbor-joining (NJ) phylogenetic tree [Citation48]; bootstrapping was conducted using 1000 replications.](/cms/asset/df182f2a-4797-4e30-9357-532ad489c7ee/tbeq_a_2155570_f0001_c.jpg)
Investigation of GmBBX gene intron–exon structure and conserved motifs
Representing the same five clades as , a NJ tree was created to evaluate the evolution of the soybean BBX family gene structure, comparing both exon-intron structure and conserved motifs (). We identified ten conserved motifs, with CCT (Motif 2), B-box2 (Morif 3) and B-box1 (Motifs 1 and 8). Intriguingly, Motifs 5 and 10 were found only in Clade V and Motifs 4 and 9 were found only in Clade II, perhaps contributing to the functional divergence of BBX genes. Additionally, both GmBBX9 and GmBBX18 of Clade IV were composed only of Motifs 6 and 8. Gene structure varied considerably between clades, although intra-clade differences were minimal. Overall, the evaluated BBX gene family members contained between one and five exons.
Figure 2. GmBBX gene structures and conserved protein motifs. Analysis of phylogenetic relationships of soybean BBX genes (A). Gene structure of GmBBX (B). Conserved motif analysis of GmBBX genes (C). Tbtools [Citation49] was used for visualization.
![Figure 2. GmBBX gene structures and conserved protein motifs. Analysis of phylogenetic relationships of soybean BBX genes (A). Gene structure of GmBBX (B). Conserved motif analysis of GmBBX genes (C). Tbtools [Citation49] was used for visualization.](/cms/asset/b52cd716-80fc-49a6-92ad-5585ec851e82/tbeq_a_2155570_f0002_c.jpg)
Analysis of phylogenetic relationships and conserved domains of soybean BBX proteins
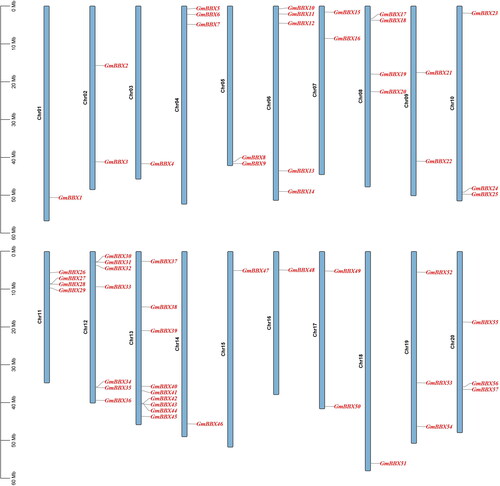
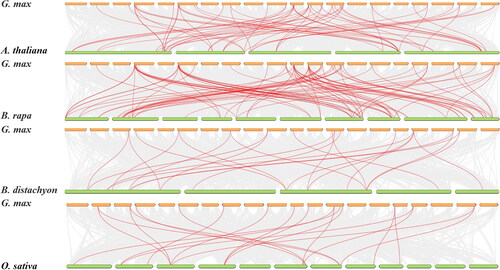
TBtools was used to map the 57 GmBBX genes onto the soybean chromosomes (). Overall, the genes were found to be unevenly distributed across the twenty chromosomes, ranging from one (chromosomes 01, 03, 14, 15, 16 and 18) to nine (chromosome 13) genes per chromosome. Chromosomes 04, 10, 19 and 20 each possessed three GmBBX genes. Two genes were found on chromosomes 02, 05, 07, 09 and 17. To explore the evolutionary relationships of this gene family across plant species, we examined the collinearity relationships of BBXs of two dicotyledonous plants (A. thaliana and Brassica rapa) and two monocotyledonous plants (Brachypodium distachyon and O. sativa; ). Higher homology was found between soybean, B. rapa and A. thaliana than between B. distachyon and O. sativa. Furthermore, dicots contained more homologous BBX genes than monocots.
Cis-element analysis for GmBBX gene promoters
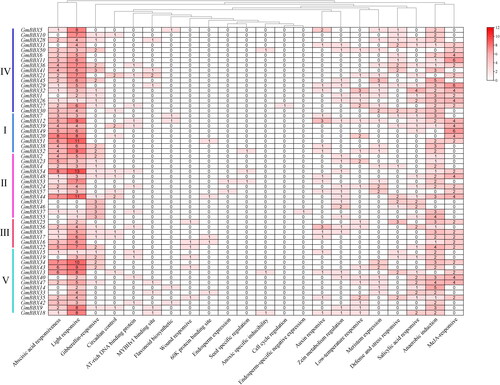
Cis-elements are involved in transcriptional regulation and can respond to a variety of stressors. TFs regulate gene transcription by binding to cis-elements present at the promoter regions of target genes. Sequences 2.0 kb upstream of the GmBBX genes were isolated to explore their potential function (). We found that the GmBBX gene promoters included cis-elements regulating plant defense, circadian rhythm, cell cycling, phytohormones (e.g. ABA, auxin, gibberellin, MeJA and SA), and response to a wide range of stimuli, including wounding, low temperature, anoxia and light, as well as seed-specific regulation, among others. Of these, cis-elements related to light response and SA, ABA and MeJA phytohormone response were the most common in soybean, although the number of each type of element varied greatly between promoters.
Figure 5. Heatmap of putative cis-elements present at GmBBX gene promoter regions. The 22 elements contained in the BBX gene family include zein metabolism regulation, wound responsive, seed-specific regulation, salicylic acid responsive, MYBHv1 binding site, meristem expression, MeJA responsive, flavonoid biosynthetic, endosperm-specific negative expression, endosperm expression, defense and stress responsive, circadian control, cell cycle regulation, auxin responsive, gibberellin responsive, AT-rich DNA binding domain, light responsive, anoxic-specific inducibility, anerobic induction, low temperature responsive, abscisic acid responsive and 60 K protein binding site. The number of cis-acting elements present in the promoter are represented by different values.
Evaluation of tissue-specific GmBBX gene expression
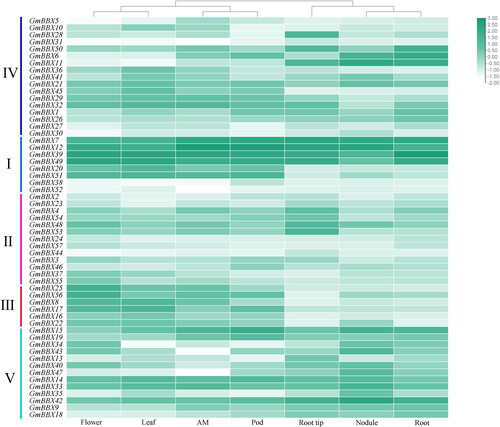
To evaluate tissue-specific GmBBX gene expression patterns, we analyzed expression data for soybean apical meristem (SRR037381), flower (SRR037382), pod (SRR037383), leaf (SRR037384), nodule (SRR037385), root tip (SRR037386) and root (SRR037387) (). Several genes, including GmBBX7, GmBBX12, GmBBX39 and GmBBX49, were highly expressed across tissues, while others, including GmBBX3, GmBBX5, GmBBX10, GmBBX24, GmBBX27, GmBBX30, GmBBX37, GmBBX44, GmBBX46 and GmBBX55, showed low levels of expression. Certain genes exhibited tissue-specific expression patterns, such as GmBBX20 and GmBBX51, which exhibited low expression in roots and high expression in pods, apical meristem, leaves and flowers.
Abiotic stress-responsive expression of GmBBX genes
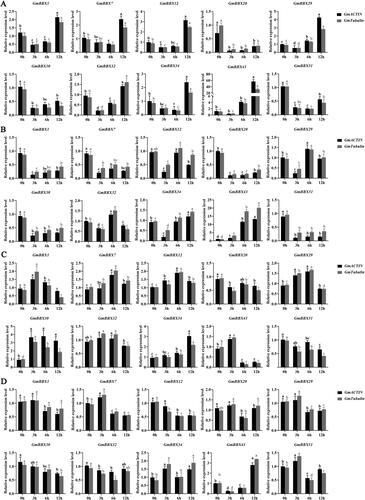
The responsiveness of GmBBX genes to salinity, drought and extreme temperature (both hot and cold) were examined through qRT-PCR (). Specifically, we randomly selected 10 GmBBX genes (GmBBX1, GmBBX7, GmBBX12, GmBBX20, GmBBX29, GmBBX30, GmBBX32, GmBBX34, GmBBX43 and GmBBX51) and examined their expression levels under stress from NaCl, PEG6000, and high and low temperature at several timepoints post treatment (3, 6 and 12 h). Under salt stress, seven genes were upregulated, three of which were rapidly upregulated only after 12 h (GmBBX20, GmBBX30 and GmBBX51; ). Under drought stress, the expression of GmBBX29, GmBBX32, GmBBX34 and GmBBX43 peaked after 6 or 12 h, while seven genes were downregulated (GmBBX1, GmBBX7, GmBBX12, GmBBX20, GmBBX29, GmBBX30 and GmBBX51; ). Under heat stress, eight genes were upregulated (GmBBX1, GmBBX7, GmBBX12, GmBBX29, GmBBX30, GmBBX32, GmBBX34 and GmBBX43) and two genes were downregulated (GmBBX20 and GmBBX51; ). Under cold stress, three genes were downregulated quickly after treatment, at 3–6 h (GmBBX12, GmBBX30 and GmBBX32), while seven were upregulated (GmBBX1, GmBBX7, GmBBX20, GmBBX29, GmBBX34, GmBBX43 and GmBBX51; ).
Figure 7. qRT-PCR gene expression analysis of 10 GmBBX genes in abiotically stressed soybean plants: high salt stress treatment (A); osmotic stress treatment (B); high temperature stress treatment (C); cold temperature stress treatment (D). Different letters indicate significant differences at the p < 0.05 level. All experiments consisted of three biological replicates. GmACTIN and GmTubulin were used as reference genes.
Phytohormone-responsive expression of GmBBX genes
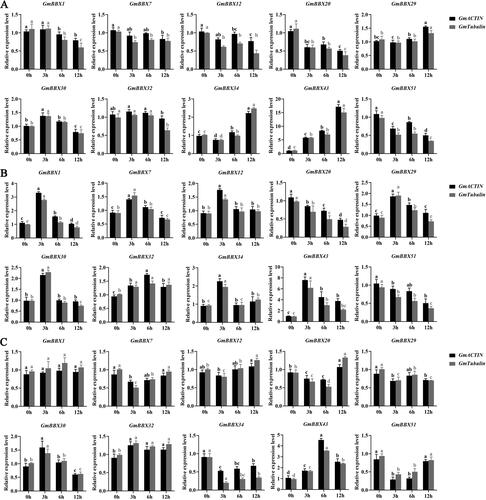
Since the phytohormones ABA, MeJA and SA are crucial not only for plant development and growth, but also for biotic and abiotic stress response, we measured the expression patterns of GmBBX1, GmBBX7, GmBBX12, GmBBX20, GmBBX29, GmBBX30, GmBBX32, GmBBX34, GmBBX43 and GmBBX51 to phytohormone treatment (). Four genes (GmBBX29, GmBBX30, GmBBX34 and GmBBX43) were upregulated at 3 and 12 h after ABA treatment (). Expression of GmBBX20 and GmBBX51 was downregulated after SA treatment (). Interestingly, the expression of eight genes (GmBBX1, GmBBX7, GmBBX12, GmBBX29, GmBBX30, GmBBX32, GmBBX34 and GmBBX43) was upregulated at 3 and 6 h after SA treatment, while the expression of seven of these (GmBBX1, GmBBX7, GmBBX12, GmBBX29, GmBBX30, GmBBX34 and GmBBX43) was decreased rapidly after 6 h (). Four genes were significantly upregulated (GmBBX20, GmBBX30, GmBBX32 and GmBBX43), four genes were significantly downregulated (GmBBX7, GmBBX29, GmBBX34 and GmBBX51), and two genes were unchanged (GmBBX1 and GmBBX12) after MeJA treatment ().
Figure 8. qRT-PCR gene expression analysis of 10 GmBBX genes in phytohormone-treated soybean plants. ABA treatment (A). SA treatment (B). MeJA treatment (C). Different letters indicate significant differences at the p < 0.05 level. All experiments consisted of three biological replicates. The black column indicates the expression level of GmACTIN as an internal reference gene. The grey column indicates the expression level of GmTubulin as an internal reference gene.
Discussion
The BBX TFs of two model plants, Arabidopsis (dicot) and rice (monocot) have received the most study [Citation12, Citation13, Citation45]. Responding to a lack of data concerning the BBX TFs in the economically important soybean, we systematically identified and characterized 57 BBX genes, including their chromosomal position, gene structure, conserved motifs, predicted proteins, subcellular localization, and stress-responsive and tissue-specific patterns of expression.
The majority of the GmBBX promoters were found to contain cis-elements regulating both plant development and responses to diverse stimuli, with the majority of these specifically regulating phytohormones (ABA and MeJa) and light response (). Both ABA and MeJa are critical for regulating the plant response to both biotic and abiotic stress [Citation56]. The high number of these cis-regulatory elements found in the GmBBX promoters indicates that this gene family is critical for soybean plant response to challenging, and changing, environmental conditions. GmBBX7, GmBBX12, GmBBX39 and GmBBX49 are highly expressed in various tissues and may play an essential role in soybean growth and development (Supplemental Table S3).
BBX TFs are key mediators of plant responses to diverse stimuli, including both biotic and abiotic stress and phytohormones [Citation8], as indicated by studies in Arabidopsis [Citation26] and other plants. In soybean, the patterns of GmBBX gene expression were markedly different under different abiotic stress treatments (). However, the expression dynamics of two particular genes, GmBBX34 and GmBBX43, stood out. These two genes exhibited upregulation under drought stress (), salt stress (), and cold stress (). Both GmBBX34 and GmBBX43 are homologues of AtBBX32 (VvZFP), which enhances tolerance to low temperatures in transgenic Arabidopsis [Citation33]. Also of note, both GmBBX1 and GmBBX30, which were upregulated following high temperature stress, clustered with AtBBX18 (), a negative regulator of heat tolerance in Arabidopsis [Citation32]. Patterns of GmBBX gene expression were also markedly different between phytohormone treatments (). For example, GmBBX43 was strongly induced after MeJa, SA and ABA treatments, while GmBBX51 was repressed in response to these treatments. While it is clear that BBX proteins participate in phytohormone signal transduction, the exact BBX gene functions in soybean remain to be determined.
Overall, through bioinformatics analysis and further experimental verification, we believe that GmBBX34 and GmBBX43 can be used as candidate stress-resistance genes for soybean.
Conclusions
In this study, 57 soybean GmBBX genes, and their associated proteins, were systematically identified and characterized. These 57 genes were distributed, albeit unevenly, across all 20 soybean chromosomes. The majority of the predicted BBX proteins were found to have high homology with Arabidopsis BBX proteins, and GmBBXs were grouped into five clades with high intra-clade exon–intron similarity. The majority of the GmBBX gene promoter cis-acting elements were found to be responsive to light, ABA, SA and MeJA, although other elements were found to respond to a variety of stimuli. In addition, several GmBBX genes exhibited tissue-specific, and abiotic stress- and phytohormone-responsive expression patterns. The results of this study will be useful in the continued characterization of soybean BBX gene functions and could be explored further for soybean breeding.
Authors’ contributions
S.G. and P.S. conceived and designed the work; C.S., D.W., L.L., and H.M. performed the bioinformatic analyses and qRT-PCR analysis; S.C., M.D., and J.L. contributed materials and analysis tools; C.S. and S.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download Zip (49.6 KB)Acknowledgments
We thank Yizheng Li in our laboratory for her help in editing the manuscript. We also want to thank the lab members for their help in conducting the experiments related to this work. We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.
Disclosure Statement
The authors declare no conflict of interest.
Data availability statement
The data that support the findings reported in this study are available from the corresponding author (GSJ) on reasonable request.
Additional information
Funding
References
- Riechmann JL, Heard J, Martin G, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290(5499):2105–2110.
- Kiełbowicz-Matuk A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 2012;185–186:78–85.
- Diao WP, Snyder JC, Wang SB, et al. Genome-wide identification and expression analysis of WRKY gene family in Capsicum annuum L. Front Plant Sci. 2016;7:1727.
- Khan SA, Li MZ, Wang SM, et al. Revisiting the role of plant transcription factors in the battle against abiotic stress. IJMS. 2018;19(6):1634.
- Kumagai T, Ito S, Nakamichi N, et al. The common function of a novel subfamily of B-Box zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2008;72(6):1539–1549.
- Zhang X, Zhang L, Ji M, et al. Genome-wide identification and expression analysis of the B-box transcription factor gene family in grapevine (Vitis vinifera L.). BMC Genomics. 2021;22(1):1–16.
- Jin H, Xing M, Cai C, et al. B-box proteins in Arachis duranensis: genome-wide characterization and expression profiles analysis. Agronomy. 2019;10(1):23.
- Gangappa SN, Botto JF. The BBX family of plant transcription factors. Trends Plant Sci. 2014;19(7):460–470.
- Ding L, Wang S, Song ZT, et al. Two B-box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Rep. 2018;25(7):1718–1728.e4.
- González-Schain ND, Díaz-Mendoza M, Żurczak M, et al. Potato CONSTANS is involved in photoperiodic tuberization in a graft-transmissible manner. Plant J. 2012;70(4):678–690.
- Lee YS, Jeong DH, Lee DY, et al. OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J. 2010;63(1):18–30.
- Onouchi H, Igeño MI, Périlleux C, et al. Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell. 2000;12(6):885–900.
- Samach A, Onouchi H, Gold SE, et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288(5471):1613–1616.
- Almada R, Cabrera N, Casaretto JA, et al. VvCO and VvCOL1, two CONSTANS homologous genes, are regulated during flower induction and dormancy in grapevine buds. Plant Cell Rep. 2009;28(8):1193–1203.
- Kim SK, Yun CH, Lee JH, et al. OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta. 2008;228(2):355–365.
- Yano M, Katayose Y, Ashikari M, et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12(12):2473–2484.
- Yang Y, Ma C, Xu Y, et al. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell. 2014;26(5):2038–2054.
- Guo G, Xu K, Zhang X, et al. Extensive analysis of GmFTL and GmCOL expression in Northern soybean cultivars in field conditions. PLoS One. 2015;10(9):e0136601.
- Cheng XF, Wang ZY. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005;43(5):758–768.
- Cao D, Li Y, Lu S, et al. GmCOL1a and GmCOL1b function as flowering repressors in soybean under long-day conditions. Plant Cell Physiol. 2015;56(12):2409–2422.
- Heng Y, Jiang Y, Zhao X, et al. BBX4, a phyB-interacting and modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis. Proc Natl Acad Sci USA. 2019;116(51):26049–26056.
- Zhao X, Heng Y, Wang X, et al. A positive feedback loop of BBX11–BBX21–HY5 promotes photomorphogenic development in Arabidopsis. Plant Commun. 2020;1(5):100045.
- Lin F, Jiang Y, Li J, et al. B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell. 2018;30(9):2006–2019.
- Heng Y, Lin F, Jiang Y, et al. B-Box containing proteins BBX30 and BBX31, acting downstream of HY5, negatively regulate photomorphogenesis in Arabidopsis. Plant Physiol. 2019;180(1):497–508.
- Xiong C, Luo D, Lin A, et al. A tomato B-box protein Sl BBX 20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019;221(1):279–294.
- Fang H, Dong Y, Yue X, et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019;42(7):2090–2104.
- Bai S, Saito T, Honda C, et al. An apple B-box protein, MdCOL11, is involved in UV-B-and temperature-induced anthocyanin biosynthesis. Planta. 2014;240(5):1051–1062.
- Bai S, Tao R, Tang Y, et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol J. 2019;17(10):1985–1997.
- Lippuner V, Cyert MS, Gasser CS. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J Biol Chem. 1996;271(22):12859–12866.
- Nagaoka S, Takano T. Salt tolerance-related protein STO binds to a myb transcription factor homologue and confers salt tolerance in Arabidopsis. J Exp Bot. 2003;54(391):2231–2237.
- Liu X, Li R, Dai Y, et al. A B-box zinc finger protein, MdBBX10, enhanced salt and drought stresses tolerance in Arabidopsis. Plant Mol Biol. 2019;99(4–5):437–447.
- Wang Q, Tu X, Zhang J, et al. Heat stress-induced BBX18 negatively regulates the thermotolerance in Arabidopsis. Mol Biol Rep. 2013;40(3):2679–2688.
- Takuhara Y, Kobayashi M, Suzuki S. Low-temperature-induced transcription factors in grapevine enhance cold tolerance in transgenic Arabidopsis plants. J Plant Physiol. 2011;168(9):967–975.
- Yadav A, Lingwan M, Yadukrishnan P, et al. BBX31 promotes hypocotyl growth, primary root elongation and UV-B tolerance in Arabidopsis. Plant Signal Behav. 2019;14(5):e1588672.
- Philis G, Gracey EO, Gansel LC, et al. Comparing the primary energy and phosphorus consumption of soybean and seaweed-based aquafeed proteins–a material and substance flow analysis. J Clean Prod. 2018;200:1142–1153.
- Zhan J, Twardowska I, Wang S, et al. Prospective sustainable production of safe food for growing population based on the soybean (Glycine max L. Merr.) crops under Cd soil contamination stress. J Clean Prod. 2019;212:22–36.
- Yang Y, Yu TF, Ma J, et al. The soybean bZIP transcription factor gene GmbZIP2 confers drought and salt resistances in transgenic plants. IJMS. 2020;21(2):670.
- Wang M, Chen B, Zhou W, et al. Genome-wide identification and expression analysis of the at-hook motif nuclear localized gene family in soybean. BMC Genomics. 2021;22(1):1–26.
- Zhang XZ, Zheng WJ, Cao XY, et al. Genomic analysis of stress associated proteins in soybean and the role of GmSAP16 in abiotic stress responses in Arabidopsis and soybean. Front Plant Sci. 2019;10:1453.
- Khanna R, Kronmiller B, Maszle DR, et al. The Arabidopsis B-box zinc finger family. Plant Cell. 2009;21(11):3416–3420.
- Chu Z, Wang X, Li Y, et al. Genomic organization, phylogenetic and expression analysis of the B-BOX gene family in tomato. Front Plant Sci. 2016;7:1552.
- Huang J, Zhao X, Weng X, et al. The rice B-box zinc finger gene family: genomic identification, characterization, expression profiling and diurnal analysis. PLoS One. 2012;7(10):e48242.
- Ma J, Dai J, Liu X, et al. Genome-wide and expression analysis of B-box gene family in pepper. BMC Genomics. 2021;22(1):1–18.
- Liu X, Li R, Dai Y, et al. Genome-wide identification and expression analysis of the B-box gene family in the apple (Malus domestica Borkh.) genome. Mol Genet Genomics. 2018;293(2):303–315.
- Sun K, Huang M, Zong W, et al. Hd1, Ghd7, and DTH8 synergistically determine the rice heading date and yield-related agronomic traits. J Genet Genomics. 2022;49(5):437–447.
- Gasteiger E, Gattiker A, Hoogland C, et al. The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–3788.
- Goodstein DM, Shu S, Howson R, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(Database issue):D1178–D1186.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739.
- Chen C, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–1202.
- Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208.
- Lescot M, Déhais P, Thijs G, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327.
- Mohammadi PP, Moieni A, Hiraga S, et al. Organ-specific proteomic analysis of drought-stressed soybean seedlings. J Proteomics. 2012;75(6):1906–1923.
- Jia B, Wang Y, Zhang D, et al. Genome-wide identification, characterization and expression analysis of soybean CHYR gene family. IJMS. 2021;22(22):12192.
- Ding X, Guo J, Zhang Q, et al. Heat-responsive miRNAs participate in the regulation of male fertility stability in soybean CMS-Based F1 under high temperature stress. IJMS. 2021;22(5):2446.
- Dong Z, Wang H, Li X, et al. Enhancement of plant cold tolerance by soybean RCC1 family gene GmTCF1a. BMC Plant Biol. 2021;21(1):1–16.
- Yu X, Zhang W, Zhang Y, et al. The roles of methyl jasmonate to stress in plants. Funct Plant Biol. 2019;46(3):197–212.