Abstract
The abnormal invasive placenta is a unique pathological condition, and it occurs only in humans. The definitions, classifications, and ultrasound markers for pathological placental attachment are being developed to unify and standardize. Placenta accreta spectrum (PAS) is a clinical situation rather than an isolated condition in which the placenta does not separate spontaneously from the uterine wall after the delivery of the fetus, and all added forceful methods for its separation lead to massive life-threatening hemorrhage. Morbidity and mortality in such situations are increasing; therefore, the involvement of a multidisciplinary team is important. The main risk factors are previous caesarean section and placenta previa. The PAS rate increases in proportion to the increase in the frequency of Sectio Caesarea (SC). The final diagnosis is made by ultrasound Grayscale color Doppler and 3D in the third trimester after 28–30 weeks of gestation. The most common and correct treatment is peripartum hysterectomy. This mini-review provides an overview of PAC, interpreted as a life-threatening disorder (the new terminology for placenta accreta). We present ultrasound findings collected over an 11-year prospective period of examination of over 58 cases of placenta accreta, of which 53 ended with peripartal hysterectomy and 5 partial accreta, which ended with preservation of the uterus, histologically approved. The markers of PAS are presented based on the criteria of FIGO and ACOG in correlation with intraoperative findings and pathohistological examination.
Introduction
Placenta accreta is an abnormal trophoblastic invasion of part or all of the placenta into the myometrium of the uterine wall. Morbidity adherent placenta (MAP) is a term used by pathohistologists at the beginning related to the degrees of invasion in depth. Morbid ingrowth of the placenta includes placenta increta, placenta percreta, and placenta accreta. Some previously used terminologies are now considered incorrect. The new terminology (after 2016) is Placenta Accreta Spectrum (PAS), which is associated with severe clinical and pathohistological features and includes both MAP and abnormal invasive placenta in depth. PAS has been confirmed by many associations, e.g. FIGO (Federation of Gynecology and Obstetrics), ACOG (American College of Obstetricians and Gynecologists), and FMFS (The Fetal Medicine Foundation). As reviewed [Citation1–3], when pathologic findings are reported, the term PAS should be applied only to cases with specific macroscopic and histologic features of abnormal placentation and/or those that meet the FIGO clinical classification criteria. Not all cases with clinically kept placental tissue are part of PAS.
Retention of all or part of the placenta is most often associated with uterine atony, chronic desquamation, chorion leave accreta histologically, as well as other structural abnormalities of the uterus or abnormalities in the shape of the placenta, such as retained lobes (often with associated fibroblastic reaction), presentation of the placenta or abnormalities in the adhesion of the membranes, which are often interpreted as a pathological placental attachment and changes the frequency of placenta accreta in general [Citation1–3].
Research during the 19th and 20th centuries gave more information about the pathological conditions of the placenta. Because of the first histological classification made in 1966 by Lukes et al. [Citation4] based on the depth of villous penetration into the myometrium, placenta accreta has been divided into 3 categories (and this terminology is used nowadays):
Placenta adherent or creta: the villi attach directly to the myometrium without a decidual interface;
Placenta increta: the villi invade the myometrium;
Placenta percreta: the villi penetrate deeply and cover the entire uterine wall, including the serosa. In this category, the villi can grow and invade adjacent organs, tissues, and pelvic vessels beyond the uterine serosa. Also, evidence proved by Lukes and other pathologists has shown that the penetration of villi in the myometrium is rarely the same, and both adherent and invasive villi may co-exist in the same pathological specimen.
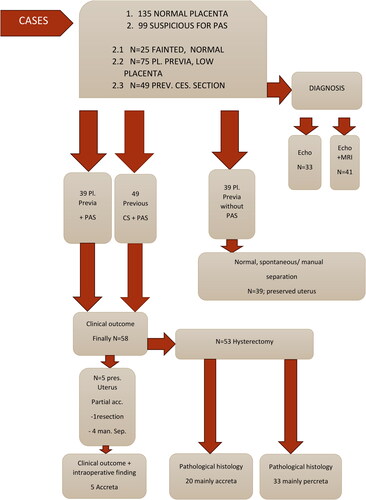
This paper presents a review of the literature on PAS and specific ultrasound markers for that condition according to the association of abnormally invasive placenta (AIP) since 2016. These markers are standardized from the association and correlated with intraoperative and pathohistological findings in our practice. The presented results are part of more than 11 years of investigations in the University Hospital ‘Maichin Dom’ (Sofia, Bulgaria). The ‘gold standard’ in treatment is perinatal hysterectomy. The cases and the clinical outcome are organized in the study design ().
Epidemiology
The diagnosis of PAS is a challenge because of the population. Most of the studies are from the last three or four decades and present no evidence of a correlation between prenatal sonographic markers, clinical symptoms, and pathological findings at birth [Citation5, Citation6]. Massive obstetric hemorrhage is a potentially life-threatening maternal factor; retained placental parts cause such hemorrhage. Manual removal of retained placental tissue also carries a high maternal risk. The two conditions, placenta adherens and kept placenta, are similar in symptomatology; therefore, pathohistology is established as the main definitive diagnosis [Citation7, Citation8].
Many studies confirm the theory that earlier Sectio Caesarea (SC) causes PAS. Delivery by SC is the main risk factor for PAS. The number of PAS cases has increased in the last 30 years, according to FIGO and AIP Association, affecting 1 in 2510 births at the end of the twentieth century and 1 in 272 births in 2016 because of the increasing delivery by SC [Citation9–14].
Other risk factors for PAS are previous uterine surgery for bicornuate uterus, adenomyosis, submucosal fibroids, and myotonic dystrophy. In these cases, they suggest trophoblastic invasion deep into the myometrium in primipara because of specific decidual autoregulation of implantation. Before the twentieth century, there was not enough evidence in the literature for PAS after uterine reconstructive surgery. According to FIGO guidelines, myomectomy brings low risk for PAS. On the other hand, IVF (in vitro fertilization) procedures have become a new and more common observed risk factor, which is independent [Citation15–17].
Another risk factor, which is considered of lesser importance and has been less studied, is age. An IVF (in vitro fertilization) procedure is also a risk factor, which is individual risk [Citation18, Citation19].
According to the international experience documented in many studies, the second main risk factor for PAS is placenta previa. The risk of placenta previa increases as the percentage of SC increases. One SC confers a 50% risk of placenta previa; after two SCs, the risk of placenta previa increases twofold compared to findings after vaginal delivery [Citation20].
However, the generally accepted conclusion is that SC is the primary and most important risk factor.
Pathophysiology
The pathophysiology of PAS has been extensively reviewed before [Citation21, Citation22]. Briefly, there are different hypotheses for PAS. The one that dominates is an iatrogenic endometrial-myometrial defect, especially in the area of the uterine cicatrix, where decidualization can be impaired and cause abnormal trophoblastic invasion. This is demonstrated because of missing decidua and ectopic pregnancy; in places where it is absent, there is an opportunity for the trophoblast to grow aggressively, like abdominal pregnancy, with extension to the abdominal cavity or the fallopian tubes. Deep penetration in the myometrium is associated with the degree of decidua-myometrial damage. The placenta is attached abnormally also after cases of instrumental uterus revision and/or manual extraction of the placenta, uterine curettage after abortion, and endometritis in previous pregnancies.
The uterine cicatrix itself is associated with a lack of endometrial epithelialization and vascular remodeling around the cicatrix itself, resulting in abnormal placental invasion. This mechanism is also assumed in ART—hormonal effects during implantation and placentation after IVF lead to proliferation and trophoblastic invasion, i.e. PAS. The process may be affected by aberrant placentation because of increased estrogen levels and/or, alternatively, low serum estradiol levels, along with the presence of a thin endometrium.
The pathophysiology of the finding in abnormally invasive placenta (AIP) can be explained by the transformation of the spiral arteries. According to Jauniaux et al. [Citation21], the remodeling is characterized by progressive loss of myocytes from the media and internal elastic lamina, replaced by fibrinoid material. Because of that, these vessels lose their responsiveness to circulating vasoactive compounds and become a low-resistance vascular network through dilatation [Citation21]. Not all pregnancies implanted in the cicatrix end with PAS. In the case of surgical intervention in the uterine cavity, the integrity of the endometrium and smooth muscle is broken, for example, in CS, which directly leads to pathological placental attachment. Under the influence of the angiogenic growth factor, uteroplacental physiological oxygenation is lost, which leads to trophoblastic proliferation. The dilated radial arcuate arteries are the basis of the phenomenon of neovascularization that we observe intraoperatively [Citation23–25].
Ultrasound findings in PAS
Ultrasound is the first line for screening and diagnosis of PAS. The sensitivity and specificity of ultrasound diagnosis are 90% when it is performed by experts in ultrasound and 50% in all other cases. Various medical terms have been used to describe a change in the placenta. The basis of the sonographic and clinical study, namely the EWG-AIP-created standardized ultrasound markers for the placenta accreta spectrum (PAS), is briefly described below. For review, see Ref. [Citation21], and for case reports, see Ref. [Citation26, Citation27].
Based on pathophysiology
Marker 1—loss of clear zone—loss or interruption of the hypoechoic retroplacental zone in the myometrium below the placental bed and interruption of normal decidual blood flow. This marker is present when the placental villi enter through the decidua basalis into the myometrium, and the zone is lost in the Grayscale image. Anatomically, at the end of the 1st trimester, when the placenta is fully formed, two lines are observed: thick decidua, containing a variety of glands and terminal branching of the spiral arteries; and superficial myometrium, containing basal arteries. During the pregnancy, the decidual line becomes thinner and breaks in places; therefore, the myometrium thins and becomes more heterogeneous due to progressive dilatation of the uteroplacental circulation [Citation21]. This generates echo lucent signals under the placenta. Loss of clear space is reported in 70% of PAS cases [Citation7, Citation8].
Marker 2—Myometrial thinning below 1 mm is a marker for PAS, presented in 50% of cases according to studies (for example, see Ref. [Citation26]). The change is observed mostly in the area of the uterine cicatrix, under the bladder, i.e. in an area of the lower uterine segment, as a result of abnormal villous invasion. This marker is observed in the third trimester around 32–34 GA in the lower uterine segment. The myometrium can thin so that it looks like dehiscence, described as the ‘uterine window’ near term [Citation26–30].
Marker 3—Placental lacunae—different in number, size, irregular sonolucent, intraplacental spaces, and bizzard, giving the placenta the appearance of being ‘eaten by a moth’ or ‘Swiss cheese.’ The most described symptom of PAS (in 80% of cases) is the placental ponds. The lacuna grading classification described by Finberg is used. ‘Lacunae are often located in a given part of the placenta and are irregular in size.’ They develop secondarily in one or more cotyledons, including intervillous septa. Doppler characteristics show high-frequency blood flow, with high speed (high-peak systolic velocity flow, PSVF), and maternal blood flow results from radial and arcuate arteries [Citation31–36].
Marker 4—Uterine serosa bladder wall interface—described on the gray scale as a loss or irregularity of the zone between the bladder wall and the hyperechoic uterine serosa line [Citation21]. Interruption of this interface is a direct result of villous invasion in the musculature of the back wall of the bladder. The echogenicity changes are the most common sonographic findings that result from massive neovascularization between the anterior wall of the uterus and the back wall of the bladder. The multitude of vessels reaching the surface of the area creates a light line-type effect and changes the regularity of the zone, invading it. The examiner should pay attention to the image of this echolucent zone, which is strongly dependent on the insonation angle [Citation37, Citation38].
Marker 5—Placental bulge—described as a ballooning of the uterine wall containing placental tissue to underlying tissues, most commonly the bladder. It is a prominent sonographic marker, but most often, on MRI, it is a marker of deep invasion through the myometrium as a result of loss of the underlying uterine musculature. This phenomenon is well seen during laparotomy in the area of the lower uterine segment and is described as the Snowman sign [Citation39].
Marker 6—Exophytic mass—described as a villous invasion of tissue through the myometrium and the serosa to adjacent extrauterine organs, usually the bladder [Citation21]. According to studies, this one focal exophyte mass is a marker for invasive placenta accreta, and it indicates the depth of invasion and destruction of the bladder wall. The focal exophytic mass as an independent marker is described more rarely compared to the placental bulge. It is described as present in 33%. The main symptom is a hematuria [Citation40–42].
Marker 7—Subplacental hypervascularity—the result of excessive dilatation of vessels and uteroplacental circulation away from the spiral arteries, involving vessels with a larger caliber: the radial and arcuate arteries. It combines with the phenomenon of neovascularization, observed specifically between the anterior and posterior uterine wall bubble wall. Uteroplacental circulation is observed in 81% of cases of placenta previa and 75% of PAS cases and is a finding located in the placental bed, the so-called subplacental zone. On Doppler, the image shows an extensive network of arterio-venous shunts that normally exist in the myometrium of the placental bed [Citation22, Citation42].
Marker 8—Feeding vessel with velocity blood flow and turbulence in the lacunes—this is a vessel with a high speed of blood flow originating from the deep arterial vessels of the myometrium, i.e. radial and arcuate arteries and feeding lacunae (according to Jauniaux et al. [Citation21]). An ultrasound study found that such finding can be observed in a normal placenta in the placental bed, but these are vessels with a smaller lumen compared to the size of the feeding vessel in invasive placenta. The presence of feeding vessels explains the abnormal hemodynamics of lacunar changes in invasive placenta [Citation21].
Marker 9—Bridging vessels—diagnosed with color Doppler signals originating from the myometrium and passing outside the uterine serosa in the wall of the bladder with multidirectional blood flow; ‘bridging’ is an echographic artifact because these vessels do not cover and do not connect the space between the myometrium and the bladder, and they are actually transitional vessels, deformed vessels as a result of neovascularization under the peritoneum, which are ‘cut through’ in the 2D image; in some cases described as ‘varicose veins of the bladder’ [Citation40].
In total, color Doppler vascular changes are reported in 66% of PAS cases prenatally and are due to dilatation of large vessels located deep in the myometrium, which are of normal size in a normal pregnancy. The above is based on summarized studies of FIGO and ACOG studies by leading experts E Jauniaux and S Collins and relevant recommendations from a pathophysiological point of view [Citation21, Citation22].
Ultrasound Grayscale markers according to FIGO and AIP (2016):
2D gray scale:
Loss of clear zone/space
Abnormal placentae lacunae
Bladder wall interruption
Myometrial thinning
Color Doppler US markers:
Uterovesical hypervascularity—color
Subplacentae hypervascularity
Bridging vessels
Placental lacunae feeder vessels
3D US Rower Doppler
Intra-placental hypervascularity—numerous intra-placental vessels exhibiting tortuous courses and varying calibers
Placental ‘bulge’—as in 2D
Focal exophytic mass—as in 2D
Bridging vessels—as in 2D
The Expert Group of the Association for AIP added new PAS tags to existing ones (from the year 2023) [Citation43]:
Intracervical lakes
Obliteration of the retroplacental clear space
Real sign
Increased parametrial hypervascularity
Missing decidual signal on the Doppler image
Unsharpened placental edges
High elastography score
A recent study (Colins et al. [Citation44]) on the role of three-dimensional ultrasound in combination with energy Doppler for the diagnosis of abnormally invasive placenta mainly studied the uteroplacental interface compared to a normal placenta and histopathologically proven placenta accreta after hysterectomy. The study introduces a new 3D Power Doppler marker—‘numerous coherent vessels’ over a large area of the examined placenta, giving grounds for better predictability of the diagnosis and the degree of the affected part of the placenta. Here, the authors report that the diagnosis relies on the typical ultrasound markers for a spectrum of placenta accreta, and the accuracy of the diagnosis is directly dependent on the sonographer’s experience; the limitation arises, as has been repeatedly reported, from the small number of cases [Citation45].
The following steps are suggested by the PAS diagnosis expert panel after laparotomy [Citation46, Citation47]:
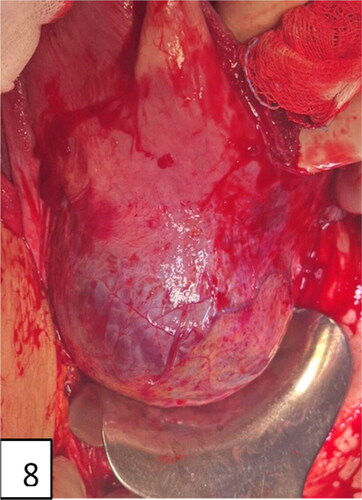
Inspection of the lateral and front wall of the uterus—for an abnormal appearance of the uterus—livid/purple coloration on the surface in the placental site evidence of ‘bulge’/snowman sign/visible invasion of placental tissue with or without passage of the serosa; these are evidence of PAS; also, neovascularization with yellowish and prominent small-caliber livid vessels in the area of the lower uterine segment, the uterus with a pale ‘jelly or jellyfish’ appearance (this name is suggested by the author—Tsankova, M).
If there is no visible evidence from the above, a uterine incision is made carefully and without aggressively separating the placenta (cord-traction). If the cord traction is visible, the uterine wall moves together with the placenta and apparently no evidence of separation—dimple sign, as well as available contraction for separation, the diagnosis is PAS.
If steps 1 and 2 are missing, separation should be done digitally between the uterus and placenta.
These recommendations are especially valid in surprising cases of unexpected PAS when the diagnosis can only be suggested by the intraoperative finding, the type of uterus before the uterine incision, and the subsequent events related to the placenta.
We represent ultrasound images and clinical correlations of the placenta accreta spectrum based on our own prospective clinical experience (study design shown in ). The ultrasound devices used were Samsung A380 Hera and Philips Affinity. The diagnoses were made by one expert or two at most over a period of 11 years (from 2012 to the beginning of 2023) to minimize the error. The different studies emphasize different markers; some of them find the lacunae with high predictability for PAS between 70 and 100%. The European Working Group on AIP Gray scale, Color Doppler 3D, suggests: ultrasound sensitivity, 90.7%; specificity, 96.9%. The highest sensitivity and specificity are for uterine serosa bladder wall disorders—99.7%; abnormal vascularization—sensitivity 90.7%, specificity 87%. The combination of the two main risk factors suggests a risk of 97% of PAS. We investigated 58 cases of PAS, clinically and pathologically confirmed, of which 53 had an outcome with peripartum hysterectomy (91.3%) and 5 partial accreta with preservation of the uterus. The ultrasound detection in our study was 90%. MRI detection shows recognizability in 50–72% of the cases. Ultrasound specificity is 97%, positive predictive value is 96%, and negative predictive value is 91%. We found the following ultrasound markers in the area of the lower uterine segment, the area of the uterine cicatrix, with the highest specificity and sensitivity: uterine serosa bladder wall disorders, bridging vessels, subplacental hypervascularity 90.7%, loss of clear zone 88%. The intraoperative findings suggested the following markers as sensitive: placental bulge and neovascularization (83.3%). Based on the results of our observation, we represent our interpretation of the criteria for PAS in correlation with the intraoperative and pathohistological findings. Normal and abnormal sonographic images of the placenta on a gray scale are presented in . Placenta accreta markers on color Doppler and three-dimensional ultrasound images are shown in .
Figure 2. Normal appearance of the anterior placenta: homogeneous, hypoechoic, and subplacental zone (‘clear zone’ with subchorionic, continuously normal blood flow).
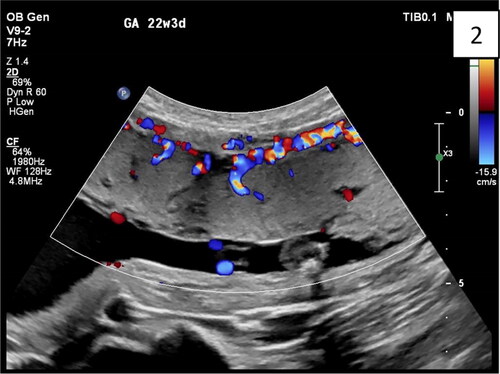
Figure 3. Sagittal transabdominal plan of abdominal invasive anterior placenta with interruption together with a uterus: serosa bladder face with irregular shape with different size, grade 3 lacunae, according to Finberg classification. The abnormal lacunae are mostly at the lower uterine segment under the hyperechoic line between the uterus and bladder. There are four typical markers: loss of ‘clear zone,’ abnormal placental lacunae, bladder wall interruption, and myometrial thinning.
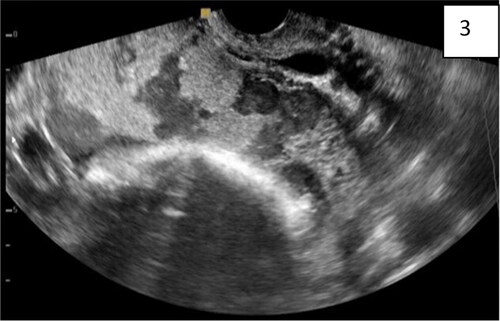
Figure 4. (Same findings as ): transvaginal sonography, abdominal lacunae grade III; specific wall of ‘clear space’ around the cervix (red arrow); subplacental hypervascularity, placenta previa totalis: anterior and posterior.
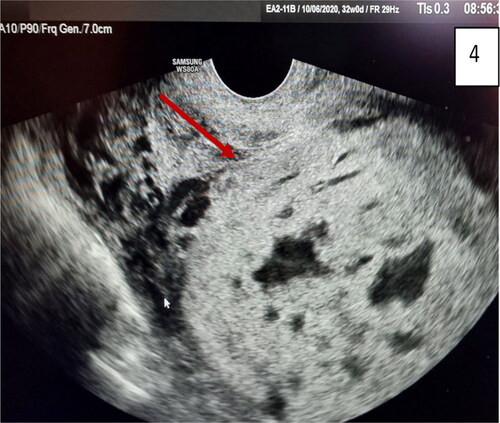
Figure 5. Gray-scale and 3D ultrasound with Power Doppler: multiple images of placenta accreta with bridging vessels on Color Doppler; loss of regularity and interruption of uterine serosa bladder face, demonstrated on 3D. (red arrow).
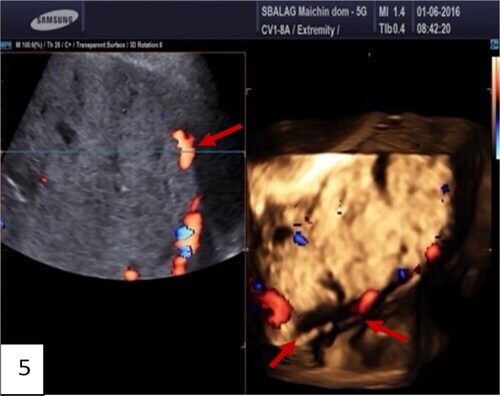
Figure 6. Ultrasound and pathohistological correlation of total placenta accreta in the lower segment. Lacunae, bridging vessels, and myometrial thinning on ultrasound with loss of anatomical space around the cervix, demonstrated on pathohistological specimen—hysterectomy of the uterus with avoiding of placental space (inset). The placenta is left in situ without separation.
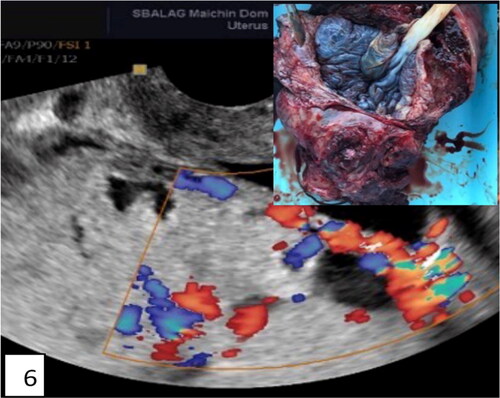
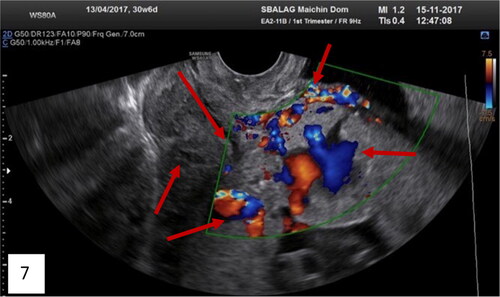
Figure 7. Transvaginal ultrasound of total placenta previa (anterior and posterior) with many ultrasound markers, including the new marker intracervical lakes on Gray-scale; on Color Doppler of the lower uterine segment: bridging vessels, loss of clear space, lacunae with turbulent blood flow inside, feeder vessels and analyzing phenomenon, intraplacental hypervascularity, and intravascular lakes (red arrows).
Antenatal ultrasound diagnosis of PAS is observed in correlation with intraoperative findings before peripartal hysterectomy. We represent intraoperative findings of PAS according to AIP association in .
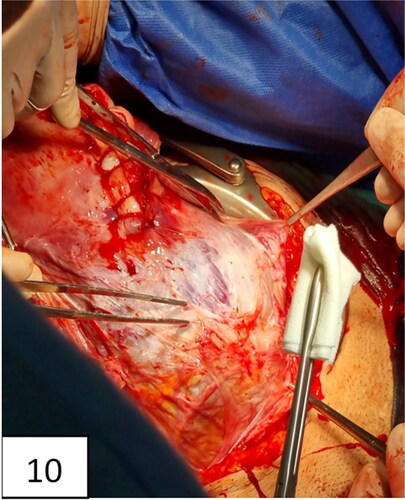
Figure 10. Uterine incision, distraction, extension, and bulging of the lower uterine segment and neovascularization (intraoperative signs of placenta accreta: snowmen sign, distraction, lividity, fading, and ‘jellyfish’—name suggested by M. Tsankova).
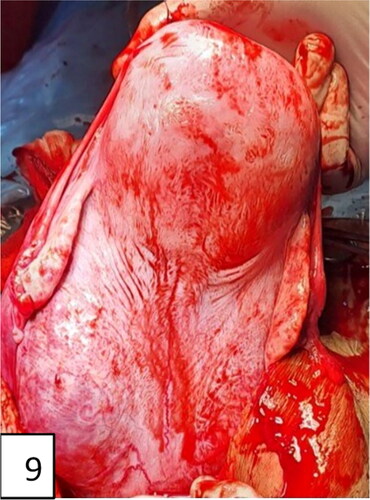
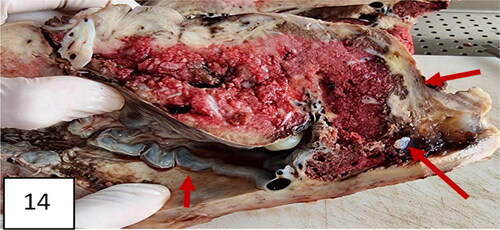
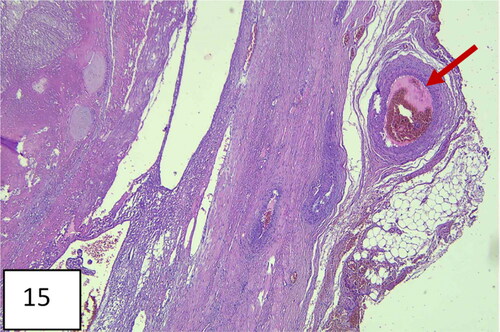
The hysterectomized uterus in and corresponding with pathohistological specimens are shown in and , as well as a histological image ().
Figure 11. Hysterectomy with signs and markers of , bulging in the lower uterine segment, including the cervix, specific for placenta accreta/percreta.
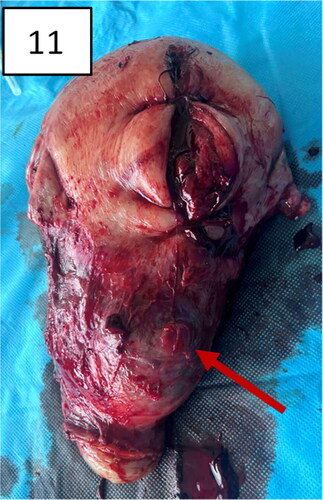
Figure 12. The uterus after hysterectomy with the placenta in situ (pathohistological specimen): thinning of the lower uterine segment and placental tissue, invading the myometrium.
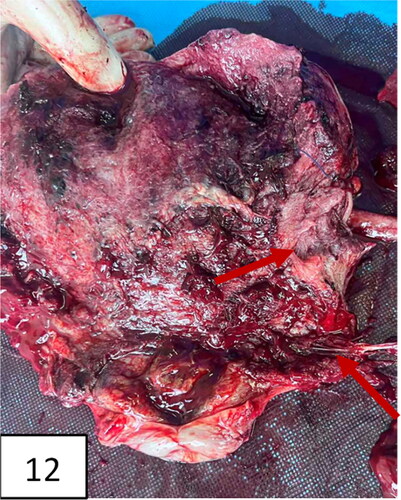
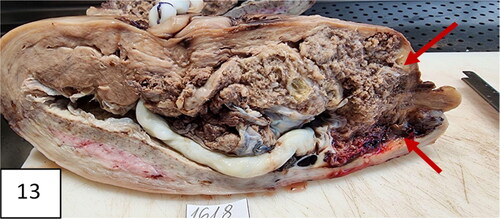
Figure 13. A uterus with invasion of placenta to the uterus cicatrix. Thinning of the cicatrix with loss of myometrium in the zone (arrows). Placenta previa, more anterior and less posterior; vasa previa.
Management and delivery [Citation46, Citation47]
Effective teamwork among surgeons, anesthesiologists, nurses, and blood banks is widely recognized as essential to good outcomes [Citation46]. This is achieved through practice, experience, as well as honest debriefing of each case with efforts made to constantly improve. Each case should undergo review with a feedback regarding team interactions and performance [Citation46].
The optimal management of placenta accreta, according to Silver et al. [Citation46], remains uncertain with regard to the timing of delivery and optimal surgical approach. Indicated preterm delivery at 34–35 weeks [Citation46] has been proposed as a means to decrease the risk of having to perform emergency surgery, given the increasing risk of spontaneous bleeding >34 weeks’ gestation. However, many cases progress to 36 weeks’ gestation without complications, and the issue remains controversial. Most of the cases finished with planned cesarean hysterectomy prior to the onset of labor rather than emergency delivery, which is often necessitated by contractions or when clinically significant bleeding occurs.
Criteria for consideration of delivery in PAS [Citation46]:
Suspicion for placenta accreta on sonogram
Placenta previa with abnormal ultrasound appearance
Placenta previa with ≥3 prior cesarean deliveries
History of classical cesarean delivery and anterior placentation
History of endometrial ablation or pelvic irradiation
Inability to adequately evaluate or exclude findings suspicious for placenta accreta in women with risk factors for placenta accreta
Any other reason for suspicion of placenta accreta
The delivery is determined by the short bleeding episodes and the patient`s health (hemodynamic status). The approach is surgical. The delivery is considered in two different scenarios [Citation48]:
The myometrium underlying the lower uterine segment is extremely thin and destroyed with massive hypervascularization.
Vascular component of placental invasion in the area of uterus and bladder.
The type of invasion usually results from vascular neovascularization, as a result of intense production of stretch factors, which leads to vascular anastomoses between the bladder, uterus, and vagina, additionally involving the superior and lateral vaginal walls and the underlying inferior bladder wall. In the presence of placenta accreta, the increased production of angiogenic factors leads to an increase in the size of the vessels. In this case, the scenario is massive and unexpected blood loss, especially at the end of the 3rd trimester.
In lower uterine segment involvement, bulging and neovascularization of the lower uterine segment at laparotomy are recommended transfundal incision, clamping of the umbilical cord close to the placenta without an attempt to remove it, leaving the placenta in situ, closure of uterine incision with two subsequent options: hysterectomy or wait-and-see behavior until reabsorption of the placenta. Conservative behavior is associated with acute hemorrhage in many cases and risk of second-stage hysterectomy. Hysteroscopic removal of placental tissue has also been reported in the literature. A Bakri balloon is also used for uterine tamponade to compress the bleeding areas in the conservative approach. However, the gold standard behavior remains hysterectomy.
The frequency of PAS in our study, from 2012 to 2022, was from 0.5 per 1000 deliveries to 2.9 per 1000 deliveries. The delivery by Caesarean Section for the same period rose from 46 to 53%.
Conclusions
PAS is a multifactorial life-threatening condition for the mother and the fetus. Diagnosis is important but very difficult to make and represents a real challenge in ob./gyn. practice. According to our study, the frequency of PAS, which is the first of its kind in Bulgaria, increased proportionally with the increase of delivery by Caesarean Section. Exact antenatal diagnosis and a multidisciplinary approach are conditions for a good perinatal outcome. Ultrasound is the ‘gold standard’ and the first line of diagnosis; MRI supports the diagnosis in cases of need to determine lateralization and the depth of invasion, especially in the posterior location. The most predictive ultrasound markers are in the region of the lower uterine segment and the uterine cicatrix. Placenta ‘bulge’ and neovascularization are pathognomonic for the intraoperative diagnosis. Pathologists postulate that different degrees of trophoblastic invasion can be found in the same specimen, and this explains the presence of several ultrasound markers in one placental area. The suggested echograph diagnostic markers in correlation with intraoperative and pathohistological findings support and confirm the opinion of the AIP Association regarding the clinical approach and standardized criteria for PAS.
Author contribution statements
M.Ts., M.K., and N.G. contributed to the design and implementation of the study, to the analysis of the results, and to the writing of the manuscript. All authors have read and approved the final version of the paper.
Data availability
All anonymized data that support the findings from this study are available from the corresponding author [N.G.] upon reasonable request.
Disclosure statement of interest
The authors report no conflict of interest.
Ethics statement
The appropriate ethics review and informed consent protocols have been followed.
Disclosure statement
No potential conflict of interest was reported by the author(s)
Additional information
Funding
References
- Jauniaux E, Bunce C, Grønbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221:1–12. doi: 10.1016/j.ajog.2019.01.233.
- Collins S, Chantraine F, Morgan T, et al. Abnormally adherent and invasive placenta: a spectrum disorder in need of a name. Ultrasound Obstet Gynecol. 2018;51:165–166. doi: 10.1002/uog.18982.
- Hecht J, Baergen R, Ernst L, et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod Pathol. 2020;33:2382–2396. doi: 10.1038/s41379-020-0569-1.
- Luke R, Sharpe J, Greene R. Placenta accreta: the adherent or invasive placenta. Am J Obstet Gynecol. 1966;95:660–668. doi: 10.1016/s0002-9378(16)34741-x.
- Jauniaux E, Chantraine F, Silver R, et al. FIGO placenta accreta diagnosis and management expert consensus panel FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynaecol Obstet. 2018;140:265–273. doi: 10.1002/ijgo.12407.
- Zahn C, Ramsey P, Toy E, et al. Placenta accrete spectrum disorder clinical and Designation Guideline ACOG webinar December 2, 2022.
- Sentilhes L, Merlot B, Madar H, et al. Postpartum haemorrhage: prevention and treatment. Expert Rev Hematol. 2016;9:1043–1061. doi: 10.1080/17474086.2016.1245135.
- Kamara M, Henderson J, Doherty D, et al. The risk of placenta accreta following primary electve caesarean delivery: a case-control study. BJOG. 2013;120 :879–886. doi: 10.1111/1471-0528.12148.
- Wu S, Kocherginsky M, Hibbard J. Abnormal placentaton: twenty year analysis. Am J Obstet Gynecol. 2005;192:1458–1461. doi: 10.1016/j.ajog.2004.12.074.
- Higgins M, Monteith C, Foley M, et al. Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur J Obstet Gynecol Reprod Biol. 2013;171:54–56. doi: 10.1016/j.ejogrb.2013.08.030.
- Cheng K, Lee M. Rising incidence of morbidly adherent placenta and its association with previous caesarean secton: a 15-year analysis in a tertiary hospital in Hong Kong. Hong Kong Med J. 2015;21:511–517. doi: 10.12809/hkmj154599.
- Klar M, Michels K. Cesarean secton and placental disorders in subsequent pregnancies—a meta-analysis. J Perinat Med. 2014;42:571–583. doi: 10.1515/jpm-2013-0199.
- Colmorn L, Petersen K, Jakobsson M, et al. The nordic obstetric surveillance study: a study of complete uterine rupture, abnormally invasive placenta, peripartum hysterectomy, and severe blood loss at delivery. Acta Obstet Gynecol Scand. 2015;94:734–744. doi: 10.1111/aogs.12639.
- Silver R, Landon M, Rouse D, et al. Maternal morbidity associated with multiple repeat cesarean delivery. Obstet Gynecol. 2006;107:1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84.
- Jauniaux E, Burton G. Pathophysiology of placenta accreta spectrum disorders: a review of current findings. Clin Obstet Gynecol. 2018;61:743–754. doi: 10.1097/GRF.0000000000000392.
- Piñas Carrillo A, Chandraharan E. Placenta accreta spectrum; risk factors, diagnosis and management to the triple P procedure. Womens Health (Lond). 2019;15:1745506519878081. doi: 10.1177/1745506519878081.
- Eshkoli T, Weintraub AY, Sergienko R, et al. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am J Obstet Gynecol. 2013;208:219.e1-7–219.e7. doi: 10.1016/j.ajog.2012.12.037.
- Fitzpatrick K, Sellers S, Spark P, et al. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS One. 2012;7:e52893. doi: 10.1371/journal.pone.0052893.
- Kaser D, Melamed A, Bormann C, et al. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril. 2015;103:1176–1184.e2. doi: 10.1016/j.fertnstert.2015.01.021.
- Marshall N, Fu R, Guise J. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1-8–262.e8. doi: 10.1016/j.ajog.2011.06.035.
- Jauniaux E, Collins S, Burton G. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87. doi: 10.1016/j.ajog.2017.05.067.
- Jauniaux E, Collins S, Jurkovic D, et al. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016;215:712–721. doi: 10.1016/j.ajog.2016.07.044.
- Benirschke K, Burton G, Baergen R. Pathology of the human placenta, 6th edition. Berlin: Springer-Verlag, 2012.
- Morlando M, Collins S. Placenta accreta spectrum disorders: challenges, risks, and management strategies. Int J Womens Health. 2020;12:1033–1045. doi: 10.2147/IJWH.S224191.
- Jauniaux E, Jurkovic D, Hussein AM, et al. New insights into the etiopathology of placenta accreta spectrum. Am J Obstet Gynecol. 2022;227:384–391. doi: 10.1016/j.ajog.2022.02.038.
- Findeklee S, Costa S. Placenta accreta and total placenta previa in the 19th week of pregnancy. Geburtshilfe Frauenheilkd. 2015;75:839–843. doi: 10.1055/s-0035-1557763.
- Chou M, Ho E. Prenatal diagnosis of placenta previa accreta with power amplitude ultrasonic angiography. Am J Obstet Gynecol. 1997;177:1523–1525. doi: 10.1016/s0002-9378(97)70102-9.
- Hamada S, Hasegawa J, Nakamura M, et al. Ultrasonographic findings of placenta lacunae and a lack of a clear zone in cases with placenta previa and normal placenta. Prenat Diagn. 2011;31:1062–1065. doi: 10.1002/pd.2833.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559. doi: 10.1002/jcu.20524.
- Rac M, Dashe J, Wells C, et al. Ultrasound predictors of placental invasion: the placenta accreta index. Am J Obstet Gynecol. 2015;212:343.e1-7–343.e7. doi: 10.1016/j.ajog.2014.10.022.
- Comstock C, Love J, Bronsteen R, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140. doi: 10.1016/j.ajog.2003.11.024.
- Nunes C, Carvalho R, Araújo C, et al. Diagnosis of placenta accreta by ultrasonography: a ‘gold standard’. Acta Obstet Ginecol Port. 2014;8:136–140.
- Japaraj R, Mimin T, Mukudan K. Antenatal diagnosis of placenta previa accreta in patients with previous cesarean scar. J Obstet Gynaecol Res. 2007;33:431–437. doi: 10.1111/j.1447-0756.2007.00549.x.
- Jauniaux E, Campbell S. Ultrasonographic assessment of placental abnormalities. Am J Obstet Gynecol. 1990;163(5 Pt 1):1650–1658. doi: 10.1016/0002-9378(90)90645-n.
- Cramer S, Heller D. Placenta accreta and placenta increta: an approach to pathogenesis based on the trophoblastic differentiation pathway. Pediatr Dev Pathol. 2016;19:320–333. doi: 10.2350/15-05-1641-OA.1.
- Jauniaux E. Partial moles: from postnatal to prenatal diagnosis. Placenta. 1999;20:379–388. doi: 10.1053/plac.1999.0390.
- Schiffer V, van Haren A, De Cubber L, et al. Ultrasound evaluation of the placenta in healthy and placental syndrome pregnancies: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2021;262:45–56. doi: 10.1016/j.ejogrb.2021.04.042.
- Shih J, Jaraquemada J, Su Y, et al. Role of three-dimensional power doppler in the antenatal diagnosis of placenta accreta: comparison with gray-scale and color doppler techniques. Ultrasound Obstet Gynecol. 2009;33:193–203. doi: 10.1002/uog.6284.
- Matsuo K, Conturie C, Lee R. Snowman sign: a possible predictor of catastrophic abnormal placentation. Eur J Obstet Gynecol Reprod Biol. 2014;181:341–342. doi: 10.1016/j.ejogrb.2014.07.035.
- Chantraine F, Blacher S, Berndt S, et al. Abnormal vascular architecture at the placental-maternal interface in placenta increta. Am J Obstet Gynecol. 2012;207:188.e1-9–188.e9. doi: 10.1016/j.ajog.2012.06.083.
- Schaaps J, Tsatsaris V, Goffin F, et al. Shunting the intervillous space: new concepts in human uteroplacental vascularization. Am J Obstet Gynecol. 2005;192(1):323–332. doi: 10.1016/j.ajog.2004.06.066.
- Krapp M, Baschat A, Hankeln M, et al. Gray scale and color doppler sonography in the third stage of labor for early detection of failed placental separation. Ultrasound Obstet Gynecol. 2000;15:138–142. doi: 10.1046/j.1469-0705.2000.00063.x.
- Jauniaux E, D'Antonio F, Bhide A, et al. Modified Delphi study of ultrasound signs associated with placenta accreta spectrum. Ultrasound Obstet Gynecol. 2023;61:518–525. doi: 10.1002/uog.26155.
- Collins S, Stevenson G, Al-Khan A, et al. Three-Dimensional power doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653. doi: 10.1097/AOG.0000000000000962.
- Rossetti D, Vitale S, Bogani G, et al. Usefulness of vessel-sealing devices for peripartum hysterectomy: a retrospective cohort study. Updates Surg. 2015;67:301–304. doi: 10.1007/s13304-015-0289-0.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accrete. Am J Obstet Gynecol. 2015;212:561–568. doi: 10.1016/j.ajog.2014.11.018.
- Warshak C, Ramos G, Eskander R, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115:65–69. doi: 10.1097/AOG.0b013e3181c4f12a.
- D'Antonio F, Palacios-Jaraquemada J, Lim P, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301. doi: 10.1002/uog.14950.