Abstract
Colorectal cancer is one of the most common cancers and a frequent cause of mortality and morbidity. In this study, we aimed to investigate the Mismatch Repair (MMR) gene products of colorectal adenocarcinomas by immunohistochemistry, to investigate the relationship between deficiency in MMR and clinicopathologic, histomorphologic, and molecular features, and to elucidate the carcinogenesis of colorectal carcinomas in our region. Our study included 224 cases diagnosed with colorectal carcinoma; at Dicle University, Faculty of Medicine, Department of Pathology, between 2008 and 2022. Primary antibodies MLH1, MSH2, MSH6, and PMS2 were used in the immunohistochemical study. In our study, a significant relationship was found between deficiency in MMR and tumour location, tumour size, distant metastasis, stromal ratio, and desmoplasia. As a result, we found that deficiency in MMR is generally associated with good prognostic features, but this may not be the case in all cases and may be influenced by some geographical characteristics.
Introductıon
Colorectal cancer (CRC) is one of the most common cancers and a frequent cause of mortality and morbidity. According to data from the Global Cancer Observatory (GLOBOCAN), over 1.9 million new cases of CRC were diagnosed worldwide in 2020, resulting in over 935,000 deaths [Citation1]. The pathogenesis of CRCs is heterogeneous and involves different mechanisms. CRCs mainly develop through three different pathways: Chromosomal instability (CIN), Microsatellite instability (MSI), and CpG island methylation (CIMP) [Citation2]. In addition, entirely new pathways continue to be recognized. A new subgroup of colorectal tumours with stable genomes without significant aneuploidy or hypermutation has been identified. These APC-initiated tumours have modest levels of DNA hypermethylation (CIMP-low) and show mutations in KRAS, PIK3CA, SOX9, and PCBP1 [Citation3]. When DNA polymerase fails to correctly bind during DNA replication, microsatellite regions are copied incorrectly. The protein products of the DNA Mismatch Repair (MMR) genes (MLH-1, MSH-2, MSH-6, PMS-2), which are tumour suppressor genes, are responsible for correcting these errors. In some cancer patients, the cancer cells are proficient in MMR (pMMR) and the number of repeats in the cancer cells is the same as that in the healthy cells. These cases are also called microsatellite stable (MSS). Some people have mutations in these genes and the cells are deficient in MMR (dMMR); and these cases are called microsatellite instable (MSI). While approximately 15% of CRCs are dMMR, 3% of them are germ-line mutations and cause Lynch Syndrome [Citation4]. It has been reported that when compared to pMMR cases, the treatment algorithms of dMMR CRCs are different and the prognosis is better [Citation5].
Various methods are used for MSI analysis. MMR gene protein analysis is performed by immunohistochemistry (IHC). MLH-1, MSH-2, MSH-6, and PMS-2 are evaluated. It is 95% sensitive, and nuclear staining above 5% is considered as no loss. Stromal cells and lymphocytes can be used as controls [Citation6]. Polymerase Chain Reaction (PCR)-based MSI assay shows instability in microsatellite sequences. MSI is determined by comparing the lengths of nucleotide repeats of normal and tumoural cells. If there is a difference above 30%, it is considered unstable [Citation7]. Sanger sequencing method is used in short tandem repeat (STR)-Fragment analysis. In 1998, international gene loci for MSI were determined. Accordingly, two mononucleotides (BAT25 and BAT26) and three dinucleotides (D5S346, D2S123, D17S250) repeat regions were identified as MSI loci. If two or more of these five loci were unstable, they were considered MSI-High(H), if only one locus was unstable, they were considered MSI-Low(L), and if there was no instability, they were considered MSS. In some cases, more than five loci may be analyzed with alternative loci. In this case, MSI-H was accepted if more than 30% of the analyzed loci were instable, MSI-L if below 30%, and MSS if zero [Citation8]. Next-Generation Sequencing (NGS) can simultaneously detect MSI at a large number of microsatellite loci [Citation9]. It has been observed that solid tumours evaluated with NGS show a high concordance of 97% with PCR and IHC results [Citation7].
In this study, we aimed to to investigate the MMR gene products of CRCs by IHC, to analyze the relationship between dMMR and clinicopathologic, histomorphologic, and molecular features, and to shed more light on the carcinogenesis of CRC in our region.
Subjects and methods
Ethics statement
The study was granted the necessary research permission (1-20.12.23) from the Dicle University Faculty of Medicine Ethics Committee.
Case selection and determination of histopathologic features
In this study, 224 patients diagnosed with CRC based on endoscopic biopsy and resection materials from the archive of the Department of Medical Pathology, Faculty of Medicine, Dicle University, between 2008 and 2022 were retrospectively analyzed. For cases with biopsy materials, those who had been previously studied with the same method and the same branded antibodies were included in the study. Patients without immunohistochemical studies were excluded. Cases with insufficient tissue for immunohistochemical study, with fixation problems, and inaccessible data from the hospital system were excluded. Diagnostic confirmation was performed by two specialized pathologists.
All cases were histopathologically retyped according to the 2019 World Health Organization (WHO) criteria. The diagnoses of mucinous adenocarcinoma and signet ring cell adenocarcinoma were assigned when at least 50% of the tumour consisted of these elements. If tumour cells were isolated or in the form of small aggregates and did not contain signet ring cell morphology, a diagnosis of poorly cohesive carcinoma was given. The grading of tumours was according to WHO 2019. Tumours were classified based on the percentage of gland formation. Those with gland formation above 50% were classified as low-grade and those with gland formation below 50% were classified as poorly high-grade [Citation10]. Tumour localization was classified according to the WHO 2019 guidelines as either right colon (cecum, ascending colon, hepatic flexure, and transverse colon), left colon (from splenic flexure to sigmoid colon), or rectum. Pathologic staging was performed using the American Joint Committee on Cancer TNM staging system as described in WHO 2019 [Citation10, Citation11].
The selected tumour nodules were tumoural foci in pericolonic adipose tissue without residual lymphoid tissue [Citation11]. Tumour budding was evaluated based on the International Tumour Budding Consensus Conference 2016 criteria, with 0–4 buds per 0.785 mm2 classified as low, 5–9 buds as moderate, and 10 or more buds as high [Citation11, Citation12]. Tumour-infiltrating lymphocytes (TIL) were categorized as TIL-low or TIL-high. At the tumour invasion border, the highest number of lymphocytes out of five areas examined with a 40× objective was considered valid. Therefore, the number of lymphocytes below 3 in the tumoural focus in any large magnification area was categorized as low, and the number of lymphocytes above 3 was categorized as high [Citation13]. Dirty necrosis was considered positive when prominent inflammatory cells and cell debris were seen in two or more foci on the 40× objective [Citation14]. Desmoplasia was analyzed in three categories: mature, intermediate, and immature. Immature desmoplasia was defined as the presence of myxoid stroma in at least one large magnification area at the invasion border of the tumour; those with myxoid stroma smaller than the area of the large magnification area and the presence of keloid-like collagen at the invasion border of the tumour were classified as intermediate desmoplasia, and those without keloid-like collagen or myxoid stroma were classified as mature desmoplasia [Citation15]. The tumour-stroma ratio was classified as stroma high or stroma low based on whether the stroma constituted more or less than 50% of the selected area. During the evaluation, we selected foci with a 10× magnification area that were filled with tumour and continued on all edges [Citation16].
The hospital archive records provided information on the patient’s gender, age, location, largest tumour diameter, and presence of polyps.
Immunohistochemical study
Three 5-μm thick sections were taken on adhesive slides (Isotherm, Germany) for each case. The slides were kept in an oven at 65 °C for 45 min and deparaffinized. In the IHC study, anti-MLH1 (Ventana, USA, Mouse monoclonal primary antibody, clone M1, catalogue number: 08033668001, incubation time: 48 min), anti-MSH2 (Ventana, USA, Mouse monoclonal primary antibody, clone G219-1129, catalogue number: 08033684001, incubation time: 32 min), anti-MSH6 (Ventana, USA, Mouse monoclonal primary antibody, clone 44, catalogue number: 05929911001, incubation time: 32 min), and anti-PMS2 (Ventana, USA, Mouse monoclonal primary antibody, clone A16-4, catalogue number: 08033692001, incubation time: 44 min) primary antibodies in ready-to-use form were used. The IHC study was run automatically on the Ventana BenchMark Ultra instrument (Roche Diagnostics, USA). In the IHC study, the absence or less than 5% staining of nuclear expression with one or more of MLH-1, MSH-2, MSH-6, and PMS-2 antibodies was considered MSI. Cases with diffuse nuclear expression with all antibodies were evaluated as MSS.
Statistical analysis
The statistical package program IBM SPSS 21.0 (IBM Corp., Armonk, NY) for Windows was used. Measurable variables are presented as mean values with standard deviation (±SD), while categorical variables are presented as a number and percentage (%). We used the Kolmogorov-Smirnov test to determine whether the data fit a normal distribution. To compare qualitative variables, we used the Pearson Chi-square (χ2) test, Yates Chi-square (χ2) test, and Fisher Chi-square (χ2) test. Pearson/Spearman correlation analysis was conducted to examine the relationship between variables. The Kaplan-Meier test was used to analyze disease-free survival times. Hypotheses were considered two-way and differences were considered significantly different at the p ≤ 0.05 level.
Results
According to WHO 2019, 189 (86.7%) cases were classified as Adenocarcinoma-NOS, 21 (9.6%) as Mucinous Adenocarcinoma, 6 (2.8%) as Signet Ring Cell Carcinoma, and 2 (0.9%) as Poorly Cohesive Carcinoma. No statistically significant correlation was observed between histologic subtypes and dMMR (p = .328). In six cases, histologic subtype and differentiation could not be determined due to the very small size of the tissue. The comparison of age, gender, histologic subtype, and MMR status of the patients is given in . A total of 165 cases (73.7%) were MMR proficient () and 59 (26.3%) were deficient. MLH-1 expression was not detected in 28 (12.5%) cases; PMS-2 expression was not detected in 21 (9.4%) cases; MSH-2 expression was not detected in 15 (6.7%) cases; MSH-6 expression was not detected in 19 (8.5%) cases; both MLH-1 and PMS-2 expression was not detected in 11 (4.9%) cases; and expression of both MSH-2 and MSH-6 was not detected in 13 (5.8%) cases ().
Figure 1. Immunohistochemical PMS2, MLH1, MSH2, and MSH6 study in four different colorectal carcinoma cases. (A) Strong nuclear MLH1 expression, (B) Strong nuclear MSH2 expression, (C) Strong nuclear MSH6 expression, (D) Strong nuclear PMS2 expression in pMMR cases (immunoperoxidase, 100×). Scale bar = 500 µm.
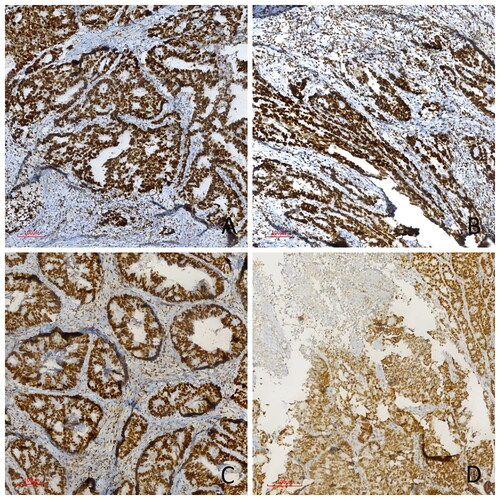
Figure 2. Immunohistochemical PMS2, MLH1, MSH2, and MSH6 study in four different colorectal carcinoma cases. (A) Diffuse loss of MLH1 expression, (B) Diffuse loss of nuclear MSH2 expression, (C) Diffuse loss of nuclear MSH6 expression, (D) Diffuse loss of nuclear PMS2 expression in dMMR cases (immunoperoxidase, 100×) Scale bar = 500 µm.
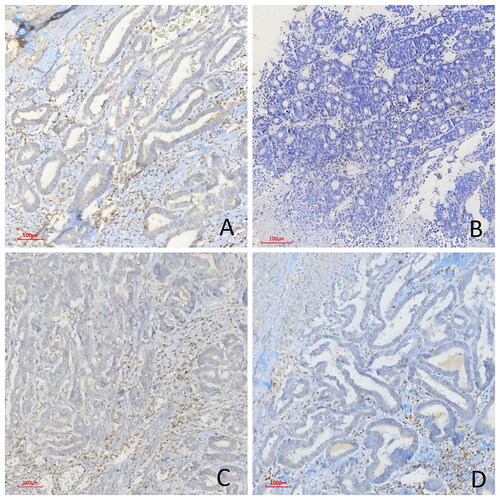
Table 1. The comparison of age, gender, histologic subtype, and MMR status of the patients.
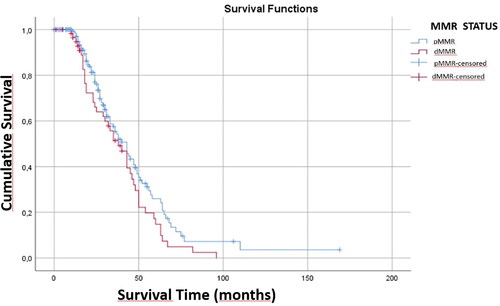
The mean survival was 44.46 ± 2.73 months after diagnosis, 47.70 ± 3.73 months in pMMR patients and 38.71 ± 4.43 months in dMMR patients. The maximum survival after diagnosis was 169 months in pMMR patients and 96 months in dMMR patients. There was no statistically significant relationship between dMMR and survival (p > .05) ().
The comparison of the clinicopathological features of the patients with their MSI status () showed that 60 (34.3%) of our cases were located in the right colon, 58 (33.1%) in the left colon, and 57 (32.6%) in the rectum. Of the dMMR cases, 26 (61.9%) were located in the right colon, 10 (23.8%) in the left colon, and 6 (14.3%) in the rectum. There was a statistically significant correlation between tumour location and dMMR (p = .000). However, statistically, the MMR status was not associated with survival in the tumours located in the right colon (p = .133). Similarly, left colon and rectum localization were not significantly associated with survival (p = .152, p = .911). Four (3.3%) of our cases were below 2 cm, 48 (39%) were between 2 and 5 cm, and 71 (57.7%) were above 5 cm. Among dMMR cases, 1 (2.9%) was below 2 cm, 6 (17.1%) were between 2 and 5 cm, and 28 (80%) were above 5 cm. There was a statistically significant correlation between tumour size and dMMR (p = .006). While accompanying polyps were not observed in 119 (75.8%) of our patients, polyps were observed in 38 (24.2%). There was no statistically significant relation between the presence of polyps and dMMR (p = 1.000). In terms of TNM staging, there was no statistically significant correlation between dMMR and T (p = .391) and N staging, (p = .431), whereas there was a statistically significant correlation between dMMR and M staging (p = .005).
Table 2. The comparison of the clinicopathological features of the patients with their MMR status of the patients.
A comparison of the histomorphologic findings in the cases with MMR is presented in .
Table 3. Comparison of histomorphologic findings of the cases with MMR status of the patients.
A total of 183 cases (83.94%) were low-grade and 35 (16.06%) were high-grade. There was no statistically significant relationship between the degree of differentiation and dMMR (p = .740). However, there was a significant correlation between tumour grade and survival time (p = .002). In high-grade cases with dMMR, the survival time was significantly higher compared to pMMRs (p = .003). In low-grade cases, pMMRs had significantly higher survival time compared to dMMRs (p = .003). Tumour nodules were not observed in 158 (92.9%) of our cases, while 12 (7.1%) had tumour nodules. There was no statistically significant relation between the presence of tumour nodules and dMMR (p = .348). Tumour budding was low in 103 (59.9%) cases, moderate in 50 (29.1%) cases, and high in 19 (11%) cases. There was a statistically significant relationship between the degree of tumour budding and dMMR (p = .033). The grade of TIL was low in 158 (80.6%) and high in 38 (19.4%) of our cases. There was no statistically significant relationship between the degree of TIL and dMMR (p = 1.000). Dirty necrosis was absent in 122 (62.9%) and present in 72 (37.1%) of our patients. There was no statistically significant correlation between the presence of dirty necrosis and dMMR (p = .977). Among our cases, 51 (31.5%) had immature stroma, 28 (17.3%) intermediate stroma and 83 (51.2%) mature stroma. There was a statistically significant relationship between the type of desmoplasia and dMMR (p = .001). When the stromal ratio at the invasive margin was evaluated, 124 (77.5%) of our cases had low stroma and 36 (22.5%) had high stroma. There was a statistically significant correlation between tumour stromal ratio and dMMR (p = .005). While 56 (36.4%) of our cases had pushing borders, 98 (63.6%) were infiltrative borders. There was no statistically significant correlation between growth pattern and dMMR (p = .255).
Despite detailed examination of the tissues and review of all clinical and radiological information of the hospital records, it was not possible to determine the tumour location in 49 patients, tumour size in 101 patients, presence of polyps in 67 patients, pT stage in 78 patients, N stage in 156 patients, M stage in 56 patients, tumour nodules in 54 cases, tumour budding in 52 cases, TIL in 40 cases, desmoplasia in 62 cases, stromal ratio in 64 cases, growth pattern in 70 cases.
Discussion
Although CRC is in the third most common type of cancer, it is the second leading cause of cancer-related deaths [Citation1, Citation17]. CRC is commonly associated with high-income countries and Western lifestyles, leading some to view it as a development indicator [Citation18]. Risk factors for CRC include familial and hereditary factors, as well as lifestyle-related variables. Previous researches have suggested some association between certain lifestyle factors and higher risk of CRC; for example, being overweight or obese, smoking, alcohol abuse, lack of physical activity, and an unhealthy diet [Citation16–18].
When the DNA polymerase fails to bind correctly during DNA replication, microsatellite regions are copied incorrectly. The MMR genes, namely MLH-1, MSH-2, MSH-6, and PMS-2, encode DNA repair proteins that attempt to repair DNA damage through complexing. Loss of function requires a mutation in both alleles [Citation19]. Although PCR is considered to be the gold standard for MMR gene analysis, several studies have been conducted to compare its concordance with the IHC analysis used in routine practice. IHC is a rapid, economical, sensitive (92.3%) and highly specific (100%) method for screening MMR genes in CRC. The predictive value of normal IHC for pMMR patients is 96.7%, and the predictive value of IHC loss for dMMR phenotype is 100% [Citation20]. Our study analyzed the correlation between immunohistochemical MMR gene screening results and clinicopathologic and histomorphologic features in cases of CRC.
In some studies, MSI was found in nearly 15% of CRCs. Approximately 12% of these cases were sporadic, while 3% were due to hereditary Lynch syndrome [Citation4, Citation6]. In our study, we found MMR deficiency in 59 cases (26.3%). Although there are publications in the literature reporting that the rate of MMR gene loss is around 30% [Citation20], the rate of dMMR CRC in our study is higher when compared with studies conducted with large series in the literature. However, it is important to note that our study had a relatively limited number of cases compared to other studies, which may have contributed to this difference.
The National Cancer Institute reports that the average age of diagnosis for CRC is 66 years. Over 90% of new CRC cases occur in people over the age of 50 [Citation21]. People over 65 years of age are estimated to be about three times more likely to develop CRC than those aged 50–64 years and about 30 times more likely than those aged 25–49 years [Citation22]. Wong et al. [Citation23] found that the incidence of CRC continues to increase in young patients and in middle- and high-income countries. In this study, the mean age of the patients was 55 ± 13.63 years, and 63.4% of them were over the age of 50. Although the mean age in our series is lower than that reported in the literature, this difference is not statistically significant. Compared to the literature, the lower mean age in our series supports the observations reported by Wong et al. [Citation23]. We believe this may be due to the red meat-dominant and vegetable-poor diet in our region. Some studies have reported an increased likelihood of MSI in patients younger than 50 years [Citation24]. On the contrary, some studies have shown that there is no increase in MSI in CRC patients younger than 50 years [Citation25, Citation26]. In our study, we did not observe an increase in dMMR in patients under 50 years old.
Studies have shown that dMMR CRCs are more commonly observed in elderly women [Citation27]. In contrast, 62.5% of dMMR cases were male and 37.5% were female in the study by Kang et al. [Citation5]. In our series, 72.9% of dMMR patients were male and 27.1% were female.
MSI is generally associated with early-stage disease [Citation28]. Our study reported that patients with dMMR were generally diagnosed at earlier stages (Stages I and II) and had a lower rate of metastasis. Some studies have reported that dMMR CRCs are observed in more advanced pT stage [Citation5, Citation29]. In our study, 95% of pMMR cases and 89% of dMMR cases were observed at pT 3-4, and no statistically significant relationship was found between pT stage and MSI status. Kang et al. [Citation5] and Xiao et al. [Citation29] showed that dMMR CRCs had significantly less lymph node metastasis. In our study, lymph node metastasis was found in 71% of pMMR cases and 60% of dMMR cases, and although fewer lymph node metastases were observed in dMMR cases, this difference was not considered statistically significant. Metastatic dMMR CRCs constitute approximately 5% of all metastatic CRCs. This may explain the better prognosis of dMMR CRCs [Citation30]. In our series, more metastases were found in pMMR CRCs compared to dMMR CRCs, in agreement with the literature.
Some studies have shown that MSI is associated with the mucinous subtype [Citation25]. Our series showed a lower incidence of the mucinous subtype compared to the literature. Additionally, we did not observe a significant relationship between dMMR and histologic subtype. This difference may be related to geographical characteristics, but larger studies are needed to confirm this regional variation.
There are studies showing that dMMR CRCs are associated with a good prognosis [Citation5, Citation31, Citation32]. Popat et al. [Citation32] reported that dMMR CRCs had a significantly better prognosis than pMMR CRCs. Although not statistically significant Kang et al. [Citation5] found that the prognosis of patients with dMMR CRCs was better than pMMR CRCs. In another study, although fewer distant metastases were observed in dMMR patients, no correlation was found between dMMR and overall, recurrence-free, or cancer-specific survival [Citation26]. Although the metastasis rate was lower in dMMR patients in our study, contrary to the literature the survival of pMMR patients was found to be slightly better than that of dMMR patients.
Most CRCs tend to be seen on the left colon [Citation32]. However, dMMR cases are known to be predominantly located in the right colon [Citation29, Citation34–37]. Right-sided and left-sided colon tumours have different molecular and histological characteristics. In right-sided tumours, mutations in the MMR pathway are common and these tumours usually have a flat histology. On the other hand, mutations related to the CIN pathway such as KRAS, APC, PIK3CA, p53 mutations are observed in left-sided tumours and these tumours show polypoid-like morphology [Citation33, Citation35]. In a study by Deligönül et al. [Citation36], it was found that Turkish patients with proximal colon cancer exhibited a higher frequency of MSI-H compared to those with distal colon cancer. However, the MSI status did not have a significant effect on survival. Similarly, Toyran et al. [Citation37] demonstrated that patients with right colon localization exhibited higher MSI than those with left colon localization. In our study, it was observed that dMMR cases were mostly localized in the right colon, in concordance with the literature. However, in our study, no correlation was shown between MMR status and survival according to tumour localization.
Although there is no direct relationship between tumour diameter and MSI in the literature, it has been reported that mucinous component is more common in dMMR CRCs [Citation8, Citation38]. Maeda et al. [Citation39] showed that a mucinous component greater than 50% was associated with the increase in tumour size. Malik et al. [Citation35] found that 35.3% of dMMR cases and 15.8% of pMMR cases were larger than 7 cm. In our series, we found that 80% of dMMR cases and 48.9% of pMMR cases had a tumour diameter of 5 cm or more. Thus, with this study, we can say that dMMR cases can reach a significantly higher size. Since the diameter of the left colon is smaller, obstructive findings are more common in left colon tumours and are detected earlier [Citation40]. As mentioned in the above studies, tumour diameter may be associated with mucinous adenocarcinoma or right colon localization. In our study, only 7% of dMMR cases were mucinous subtype, but 61.9% were located in the right colon. We thought that the significant difference between the tumour diameters was related to the fact that our cases were located in the right colon and these tumours progressed insidiously without showing signs of obstruction and were diagnosed later.
dMMR CRCs typically tend to be poorly differentiated [Citation29, Citation35]. In our series, the majority of pMMR and dMMR cases were low-grade, 14.9% of pMMR cases and 19.3% of dMMR cases were high-grade. Although our dMMR patients were more likely to be high-grade, this trend is not statistically significant as in the literature. We also found that pMMR was associated with longer survival in cases with high-grade tumours, while dMMR was associated with longer survival in cases with low-grade tumours.
The presence of tumour nodules has been associated with the decrease in disease-free survival and overall survival [Citation41]. Zhang et al. [Citation42] reported the rate of CRCs with tumour nodules to be 25.8% and found MSI in 18% of them. In our study, tumour nodules were observed in 5.9% of pMMR cases and 9.8% of dMMR cases. By these results, we can say that the likelihood of tumour nodules is higher in patients with dMMR, although statistically not significant.
Tumour budding is an important parameter indicating poor prognosis for CRC patients [Citation43]. Topal et al. [Citation44] reported that high tumour budding was directly proportional to APC gene mutation, and dMMR CRCs showed low tumour budding due to intense peritumoural lymphocytic infiltration and the presence of TIL. Lugli et al. [Citation45] reported that budding was less frequent in dMMR CRCs and was an independent prognostic factor. In our series, a lower rate of tumour budding was observed in dMMR cases, in agreement with the literature.
In CRC, TIL have long been known to be a hallmark of dMMR cases. TIL are thought to be a response to neoantigens resulting from the accumulation of MMR mutations and frameshift mutations [Citation45]. Although there are several studies using different algorithms for TIL measurement [Citation13, Citation46–49], none of them could standardize it for routine pathological specimens. In our study, we used the method of Jimenez-Rodriguez et al. [Citation13] for TIL counting, and the results of this method were consistent with studies using IHC and application-based complex counts. In our study, 19.1% of pMMR patients and 20% of dMMR patients were in the TIL-high group, and the small difference was found statistically not significant. We thought that the possible reasons for the difference in the IL counts between our series and the literature may be the difference in the method of lymphocyte counting, subjective counting, the thickness of some H&E sections, and the inability to examine the tumour invasive border in some cases.
The detailed mechanisms of dirty necrosis, which can be seen in CRCs, have not been fully determined [Citation50]. Konishi et al. [Citation14] have shown that dirty necrosis is an indicator of poor prognosis in CRCs metastasizing to the lung. The absence of dirty necrosis was generally associated with dMMR CRCs [Citation24]. Malik et al. [Citation35] detected dirty necrosis in 23.5% of dMMR cases and 59.4% of pMMR cases. Greenson et al. [Citation51] reported that only 2% of cases with dirty necrosis were dMMR. In our study, contrary to the literature, we found the absence of dirty necrosis in 61.8% of dMMR cases and 63.3% of pMMR cases, which demonstrates that there is no relation between dirty necrosis and dMMR.
Desmoplastic reaction (DR) is the proliferation of myofibroblasts in the stroma of invasive cancer [Citation52]. Nearchou et al. [Citation53] showed that DR is the strongest prognostic indicator for disease-free survival in stage II CRC. Fan et al. [Citation15] reported that patients with mature stroma had a significantly better prognosis, but they did not find an association between dMMR and types of desmoplasia. In our study, contrary to these datas, pMMR cases were found to have more immature stroma. The relationship between MSI and type of desmoplasia needs to be clarified with studies including larger case series.
Some studies have shown that tumour stroma ratio is an independent prognostic marker in CRC. A high amount of stroma is an indicator of an unfavourable prognosis [Citation54]. In one study, 71% of patients with high stroma and 82.5% of patients with low stroma were found to be pMMR, and stroma predominance was more common in pMMR patients [Citation15]. In another study, 31.9% of the pMMR group and 19.2% of the dMMR group consisted of patients with high stroma and it was found that low stroma and MSI were related [Citation55]. In our study, the number of high stromal cases was significantly higher in pMMR patients when compared to dMMR patients, in agreement with the literature.
Studies have shown that patients with an infiltrative border have worse survival [Citation56]. It is known that inflammatory response and pushing border are more common in dMMR tumours [Citation57]. In our study, 33 (32.7%) of pMMR cases and 23 (43.4%) of dMMR cases showed pushing border. Although the rate of pushing border was higher in dMMR patients, it did not reach a statistically significant level.
Conclusions
In conclusion, we support the idea that there is a rapid, economical, sensitive, and highly specific method for screening MMR genes in CRC. MMR deficiency should be carefully evaluated in CRC patients, as it influences various factors affecting treatment and prognosis. In many studies and in this study, the association of dMMR with histopathological and prognostic parameters varies. Although it is generally associated with good prognostic features, our study showed that this may not be true for all cases and may be influenced by some geographical characteristics. Consequently, we propose that the MMR status of CRC patients should be investigated by IHC and that a patient-based evaluation should be performed in terms of its relationship with factors affecting prognosis.
Authors’ contribution
All authors contributed significantly to the work and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BT and UA made substantial contributions to the conception and design of the work. BT, ANK, and UA were involved in project management, literature search, data acquisition, interpretation, drafting, and reviewing of the manuscript. BT and UA were involved in visualization. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability Statement
The data that support the findings of this study are available from the corresponding author [UA], upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of ıncidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Pino MS, Chung DC. The chromosomal instability pathway ın colon cancer. Gastroenterology. 2010;138(6):2059–2072. doi: 10.1053/j.gastro.2009.12.065.
- Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158(2):291–302. doi: 10.1053/j.gastro.2019.08.059.
- Carethers J. Heredıtary, sporadıc and metastatıc colorectal cancer are commonly drıven by specıfıc spectrums of defectıve dna mısmatch repaır components. Trans Am Clin Climatol Assoc. 2016;127:81–97.
- Kang S, Na Y, Joung SY, et al. The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors. Medicine (Baltimore). 2018;97(9):e0019. doi: 10.1097/MD.0000000000010019.
- Boland CR, Thibodeau SN, Hamilton SR, et al. A national cancer ınstitute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257.
- Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. doi: 10.1007/s11864-015-0348-2.
- Jung SB, Lee HI, Oh HK, et al. Clinico-pathologic parameters for prediction of microsatellite instability in colorectal cancer. Cancer Res Treat. 2012;44(3):179–186. doi: 10.4143/crt.2012.44.3.179.
- Marques AC, Ferraro-Peyret C, Michaud F, et al. Improved NGS-based detection of microsatellite instability using tumor-only data. Front Oncol. 2022;12:969238. doi: 10.3389/fonc.2022.969238.
- Nagtegaal ID, Arends M, Salto-Tellez M. World Health Organization classification of tumours of the digestive system: tumours of the colon and rectum. 5th ed. Lyon (France): IARC; 2019
- cap.org [Internet]. Northfield (IL): CAP; [cited 2023 Dec]. Available from: https://www.cap.org/.
- Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30(9):1299–1311. doi: 10.1038/modpathol.2017.46.
- Jimenez-Rodriguez RM, Patil S, Keshinro A, et al. Quantitative assessment of tumor-infiltrating lymphocytes in mismatch repair proficient colon cancer. Oncoimmunology. 2020;9(1):1841948. doi: 10.1080/2162402X.2020.1841948.
- Konishi Y, Taki T, Nakai T, et al. Clinicopathological features and prognostic impact of dirty necrosis in metastatic lung cancers from the colon and rectum. Cancer Sci. 2022;114(5):2169–2177. doi: 10.1111/cas.15647.
- Fan S, Cui X, Zheng L, et al. Prognostic value of desmoplastic stromal reaction, tumor budding and tumor-stroma ratio in stage II colorectal cancer. J Gastrointest Oncol. 2022;13(6):2903–2921. doi: 10.21037/jgo-22-758.
- Pelt van GW, Kjær-Frifeldt S, van Krieken JHJM, et al. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch. 2018;473(4):405–412. doi: 10.1007/s00428-018-2408-z.
- Sawicki T, Ruszkowska M, Danielewicz A, et al. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. doi: 10.3390/cancers13092025.
- Hossain M, Karuniawati H, Jairoun AA, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 2022;14(7):1732. doi: 10.3390/cancers14071732.
- Mei WJ, Mi M, Qian J, et al. Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: a narrative review. Front Immunol. 2022;13:1019582. doi: 10.3389/fimmu.2022.1019582.
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry Versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20(4):1043–1048. doi: 10.1200/JCO.2002.20.4.10.
- Thelin C, Sikka S. Screening for colorectal cancer with colonoscopy: epidemiology of colorectal cancer—Incidence, lifetime risk factors statistics and temporal trends. 1 st ed. London (UK): IntechOpen; 2015.
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072.
- Wong MCS, Huang J, Lok V, et al. Differences in ıncidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol. 2021;19(5):955–966.e61. doi: 10.1016/j.cgh.2020.02.026.
- Greenson JK, Huang SC, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33(1):126–133. doi: 10.1097/PAS.0b013e31817ec2b1.
- Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol. 2003;27(11):1393–1406. doi: 10.1097/00000478-200311000-00001.
- Rantanen P, Keränen A, Barot S, et al. The prognostic significance of microsatellite instability in colorectal cancer: a swedish multi-center study. Int J Colorectal Dis. 2023;38(1):197. doi: 10.1007/s00384-023-04480-z.
- Al-Sohaily S, Biankin A, Leong R, et al. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27(9):1423–1431. doi: 10.1111/j.1440-1746.2012.07200.x.
- Battaglin F, Naseem M, Lenz HJ, et al. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol. 2018;16(11):735–745.
- Xiao H, Yoon YS, Hong SM, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140(3):341–347. doi: 10.1309/AJCP8P2DYNKGRBVI.
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29(10):1261–1270. doi: 10.1200/JCO.2010.30.1366.
- Kim CG, Ahn JB, Jung M, et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer. 2016;115(1):25–33. doi: 10.1038/bjc.2016.161.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. doi: 10.1200/JCO.2005.01.086.
- Baran B, Mert Ozupek N, Yerli Tetik N, et al. Difference between left-sided and right-sided colorectal cancer: a focused review of literature. Gastroenterology Res. 2018;11(4):264–273. doi: 10.14740/gr1062w.
- Carneiro F, Arends MJ, Lax SF, et al. World Health Organization classification of tumours of the digestive system: genetic tumour syndromes of the digestive system. 5th ed. Lyon (France): IARC; 2019
- Malik A, Bhatia JK, Sahai K, et al. Evaluating morphological features for predicting microsatellite instability status in colorectal cancer. Med J Armed Forces India. 2022;78(Suppl 1):S96–S104. doi: 10.1016/j.mjafi.2021.03.024.
- Deligonul A, Avci N, Kurtul M, et al. Prognostic significance of microsatellite instability in turkish patients with stage II and III colorectal cancer. Eurasian J Med Invest. 2021;5(1):62–66. doi: 10.14744/ejmi.2021.19999.
- Toyran T, Erdogan K, Kiliç Bağir E, et al. Effect of microsatellite instability on histopathological parameters and prognosis in Colon cancers. Cukurova Med J. 2023;48(1):1–10. doi: 10.17826/cumj.1217604.
- Iacopetta B, Grieu F, Amanuel B. Microsatellite instability in colorectal cancer. Asia Pac J Clin Oncol. 2010;6(4):260–269. doi: 10.1111/j.1743-7563.2010.01335.x.
- Maeda Y, Sadahiro S, Suzuki T, et al. Significance of the mucinous component in the histopathological classification of Colon cancer. Surg Today. 2016;46(3):303–308. doi: 10.1007/s00595-015-1150-2.
- Turner JR. Robbins and cotran pathologic basis of disease: the gastrointestinal tract. 9th ed. Canada: Elsevier Saunders, 2015
- Puppa G, Maisonneuve P, Sonzogni A, et al. Pathological assessment of pericolonic tumor deposits in advanced colonic carcinoma: relevance to prognosis and tumor staging. Mod Pathol. 2007;20(8):843–855. doi: 10.1038/modpathol.3800791.
- Zhang M, Hu W, Hu K, et al. Association of KRAS mutation with tumor deposit status and overall survival of colorectal cancer. Cancer Causes Control. 2020;31(7):683–689. doi: 10.1007/s10552-020-01313-0.
- Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer—ready for diagnostic practice? Hum Pathol. 2016;47(1):4–19. doi: 10.1016/j.humpath.2015.08.007.
- Topal U, Gülcan P, Yüksel S, et al. The relationship between microsatellite instability in colorectal adenocarcinoma and tumor budding and histopathological parameters. Eur Rev Med Pharmacol Sci. 2023;27(20):9793–9800. doi: 10.26355/eurrev_202310_34154.
- Lugli A, Vlajnic T, Giger O, et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol. 2011;42(12):1833–1840. doi: 10.1016/j.humpath.2011.02.010.
- Eriksen AC, Sørensen FB, Lindebjerg J, et al. The prognostic value of tumor-infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl Oncol. 2018;11(4):979–987. doi: 10.1016/j.tranon.2018.03.008.
- Iseki Y, Shibutani M, Maeda K, et al. A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections. PLoS One. 2018;13(4):e0192744. doi: 10.1371/journal.pone.0192744.
- Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–2654. doi: 10.1016/j.ejca.2005.07.017.
- Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147(4):366–372. doi: 10.1001/archsurg.2012.35.
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4.
- Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27(5):563–570. doi: 10.1097/00000478-200305000-00001.
- Angeli F, Koumakis G, Chen MC, et al. Role of stromal fibroblasts in cancer: promoting or impeding? Tumour Biol. 2009;30(3):109–120. doi: 10.1159/000218708.
- Nearchou IP, Kajiwara Y, Mochizuki S, et al. Novel ınternationally verified method reports desmoplastic reaction as the most significant prognostic feature for disease-specific survival in stage II colorectal cancer. Am J Surg Pathol. 2019;43(9):1239–1248. doi: 10.1097/PAS.0000000000001304.
- Mesker W, Liefers GJ, Junggeburt J, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I–II colon cancer patients. Cell Oncol. 2009;31(3):169–178. doi: 10.3233/CLO-2009-0478.
- Huijbers A, Tollenaar RAEM, V Pelt GW, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24(1):179–185. doi: 10.1093/annonc/mds246.
- Zlobec I, Baker K, Minoo P, et al. Tumor border configuration added to TNM staging better stratifies stage II colorectal cancer patients into prognostic subgroups. Cancer. 2009;115(17):4021–4029. doi: 10.1002/cncr.24450.
- Román R, Verdú M, Calvo M, et al. Microsatellite instability of the colorectal carcinoma can be predicted in the conventional pathologic examination. A prospective multicentric study and the statistical analysis of 615 cases consolidate our previously proposed logistic regression model. Virchows Arch. 2010;456(5):533–541. doi: 10.1007/s00428-010-0896-6.