Abstract
Central nervous system infections continue to be a public health concern owing to the high mortality and the significant neurological sequelae among survivors. The diverse etiology with overlapping clinical and laboratory abnormalities makes the management of such patients challenging. Neuroinflammation plays an essential role in triggering oxidative stress and autophagy dysregulation. Impaired autophagy may lead to abnormal protein aggregation resulting in neurodegeneration. YKL-40 is a secreted glycoprotein, involved in several diseases accompanied by inflammation. The lysosome-associated membrane proteins (LAMPs) 1 and 2 exhibit diverse expression levels in a range of cell processes (including autophagy) and clinical conditions but the complete picture of their biological function is still unknown. This review highlights the role of YKL-40 and LAMPs in central nervous system infections. We suggest that these biomolecules might have a promising value as biomarkers or targets for therapy and could provide additional evidence for inflammatory activity in different neurological diseases.
Introduction
Central nervous system (CNS) infections are potentially life-threatening conditions that can be caused by various pathogens, including bacteria, viruses, fungi and parasites [Citation1,Citation2]. The wide variety of pathogens associated with CNS infections greatly complicates the detection of the specific causative agent and the selection of appropriate antimicrobial therapy. The rapid and precise identification of the etiology is essential for the early targeted therapy thus reducing mortality, improving the clinical course, and reducing the most possible subsequent neurological complications [Citation1,Citation3].
In routine clinical practice, diagnosis of CNS infections is initially made by identifying clinical symptoms, evaluation of cerebrospinal fluid (CSF) and neuroimaging. Clinical data and clinical-laboratory examination of CSF alone do not have sufficiently high specificity and sensitivity to differentiate between etiologies [Citation1–5]. CSF samples are analyzed to search for the protein content, glucose levels, and cellular numbers and types. The microbiological methods based on culture or antigen detection have several limitations related to the identification of diverse potential pathogens [Citation1–5]. Over the past decade, new innovative technologies have been developed including molecular assays able to detect the most common pathogens related to CNS in a single clinical specimen – multiplex PCR assays [Citation6]. Although the utilization of these revolutionary systems in the diagnosis of CNS infections is indeed associated with an increased positive yield, a large proportion of patients remain etiologically unverified. In the developed countries, as high as 68% of meningitis cases and up to 50% of encephalitis cases are etiologically unconfirmed [Citation5,Citation7,Citation8]. In clinical practice, it is essential to have an early differentiation of bacterial from viral neuroinfections, owing to the different prognosis, clinical course, and complications.
To overcome the limitations of conventional approaches to the identification of CNS infections, other strategies emerged aiming to utilize specific biomarkers in the management of acute neuroinfections. The discovery and validation of biomarkers would help clinicians with the choice of therapy, as well as the differentiation of patients at increased risk of developing complications and death. Recently, there has been a growing interest in research focused on biomarkers in various diseases including CNS infection.
The aim of this review is to summarize the available literature on the diagnostic and prognostic potential of YKL-40 and LAMPs proteins during CNS infections.
YKL-40
YKL-40, also known as chitinase 3-like 1 (CHI3L1), is a glycoprotein that has been associated with inflammation, tissue remodeling, and cancer [Citation9]. YKL-40 is an extensively investigated glycoprotein, which has been suggested to have prognostic value in patients with several inflammatory and tumor diseases [Citation10,Citation11]. It is an extracellular matrix glycoprotein with still largely unknown biological functions. IL-13Rα2 is considered the main receptor for YKL-40, which determines its dominant role in inflammatory processes [Citation12]. The conserved amino acid sequence of the glycoprotein suggests involvement and significant function (structural or regulatory) in a signaling pathway activating the MAP-kinase and PI-3K signaling cascade controlling mitogenesis. YKL-40 is expressed and secreted by activated macrophages and neutrophils, chondrocytes, and synovial cells [Citation13,Citation14]. In several neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, etc., YKL-40 is discussed as a potential biomarker, serving to differentiate the individual stages in the development of these diseases, as well as to monitor their progression [Citation9,Citation15]. YKL-40 is proposed to be a non-specific pro-inflammatory protein. Its concentration correlates with established factors such as C-reactive protein and ESR. The results of studies on patients with rheumatoid and psoriatic arthritis, osteoarthritis, and systemic sclerosis prove not only a definite relationship of YKL-40 with proinflammatory cytokines (IL-1β, IL-6, TNF-α) but also with clinical and ultra sonographic parameters [Citation16,Citation17]. Our studies established the local expression and secretion of YKL-40 in CSF from patients with severe brain injury, as well as a correlation with cytokines, which determined the involvement of the glycoprotein in the inflammatory process occurring after the traumatic injury [Citation18].
In the context of meningitis, a disease characterized by a significant inflammatory reaction within the subarachnoidal space and/or the brain parenchyma, YKL-40 has gained interest in several aspects including diagnosis, disease progression, and prognosis. However, in the international scientific literature, the data on the expression and secretion of YKL-40 in neuroinfections are scarce, and in Bulgaria, they are missing. In the CNS, YKL-40 is mostly expressed by microglia, especially when responding to acute and chronic inflammation [Citation19–21]. One study reported that patients with purulent meningitis/encephalitis had significantly higher CSF YKL-40 levels than patients with lymphocytic meningitis or patients without meningitis [Citation19]. Another research group found that changes in CSF levels of the glycoprotein correlated with improvement in meningo-radicular syndrome, suggesting that YKL-40 may be involved in the pathogenesis of the anti-N-methyl-D-aspartate receptor (NMDA-R) encephalitis [Citation21]. Recently, it was reported that CSF YKL-40 levels in autoimmune encephalitis (anti-LGI1) correlated with the severity and prognosis [Citation22]. Interestingly, in tick-borne encephalitis, it has been demonstrated that CSF YKL-40 has the potential to differentiate between meningitis and meningoencephalitis with a sensitivity of 62.5% but provides better specificity of 87.5% [Citation23]. It was shown that YKL-40 can serve as a prognostic biomarker. Kronborg et al. [Citation24] demonstrated that YKL-40 can be elevated in S. pneumoniae bacteremia and the high levels correlate strongly to the poor prognosis.
LAMPs
Neuronal autophagy is crucial for neuronal development and signaling, as well as for their growth and function. It has been shown that the main factors that regulate autophagy also control neuroinflammation aiming to improve the immune system’s ability to respond to changing microenvironment. Many authors suggested that autophagy was an adaptive, pro-survival process during CNS viral infection rather than a response triggering cell death [Citation25]. It was proven that autophagy inhibited the replication of several viruses in neurons [Citation26]. Autophagy can support viral development even it is a cellular reaction involved in the antiviral response [Citation27].
Lysosomes play a major role in autophagy. LAMP-1 and LAMP-2 are conservative proteins of the lysosomal membrane, but little is known about their role. Involvement in processes such as autophagy, cell death, and neoangiogenesis has been proposed [Citation28]. Basic information about YKL-40 and LAMP-1, LAMP-2 regarding gene and protein structure and biological functions is presented in .
Table 1. Basic characteristics of YKL-40, LAMP-1 and LAMP-2.
Although autophagy is intensively investigated in CNS infection, studies on LAMP-1 and LAMP-2 proteins are insufficient in the literature. Our previous experience shows that they change their cellular localization in tumor cell lines and membrane expression promotes binding to extracellular matrix molecules and thus facilitates tumor progression [Citation42]. It was proven that LAMP-2 is involved in the viral cycle of HSV-1. Researchers used a LAMP-2 deficient neuroblastoma cell line in which a lower production of infectious was detected. It was indicated that LAMP2 deficiency significantly attenuates the neurodegenerative causes, increase of phosphorylated tau and inhibition of Aβ secretion induced by HSV-1 in chronic conditions [Citation43].
Autophagy-inflammation interplay during infection
Autophagy is a highly conserved cellular degradation process performed in the autophagosome. It plays a major role during cellular development and differentiation, and tumor suppression. Inflammation is triggered by recognition of the pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) from pattern recognition receptors (PRRs) of immune cells. The increased rate of inflammation resulted in cytokine secretion and higher YKL-40 levels. On the other hand, autophagy inhibits the function of pathogen PAMPs [Citation44].
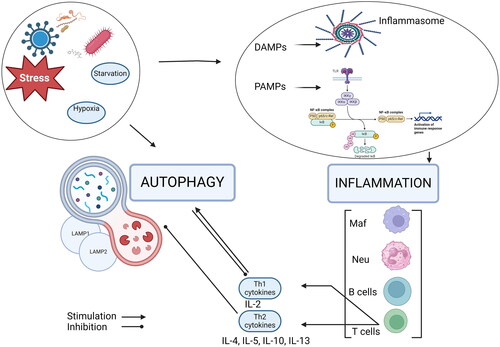
Both autophagy and inflammation could be induced by pathogens, and their interaction persists during the host response. Many participants in the inflammation as receptors (TLRs, NOD-like receptors), proinflammatory cytokines activate autophagy. The autophagic process is triggered also by starvation, cellular damage, and the production of dysfunctional proteins [Citation45]. Removal of pathogens by autophagy eliminates the causative agent and suppresses inflammation, whereas dysfunction in autophagy leads to increased inflammation. Furthermore, it was proven that autophagy modulated inflammation through suppressing the inflammasome or down-regulating proinflammatory cytokines [Citation46]. We attempted to summarize the crosstalk between autophagy and inflammation in .
Inflammation and autophagy are triggered by pathogens (bacteria, viruses), external or internal factors (starvation, stress, hypoxia). Autophagy negatively regulates inflammation by degrading its stimuli, including bacteria, viruses, DAMPs, PAMPs, and inflammasome components. On the other hand, activation of DAMPs, NF-κβ induces immune response leading to Th1- and Th2-related cytokines secretion. The Th2 immune response inhibits LAMPs proteins and autophagy, while Th1-related cytokines trigger autophagy [Citation61].
Autophagy and inflammation are the two sides of the same coin, and their controlled cooperation is required for cellular homeostasis. Dysbalanced autophagy often leads to inflammatory responses, resulting in severe host damage [Citation44]. It is still unclear under which circumstances enhanced autophagy plays a role in cell death or represents a mechanism with protective effects [Citation40].
Late endosomes and lysosomes are rich in LAMPs. Notably, S. pneumoniae has been confirmed to target LAMP-enriched compartments so it can traffic to late phagosomes. On the other hand, Mycobacterium tuberculosis has developed strategies to interfere with phagosome maturation by preventing LAMP integration. Similarly, Neisseria disrupts macrophage-killing mechanisms by slowing LAMP’s integration into phagosomes. LAMP-1 is susceptible to degradation of IgA proteases, produced by Neisseria, S. pneumoniae, and H. influenzae, promoting their survival. These findings demonstrate the complex interactions between pathogens and host cellular defense mechanisms involving phagosome functions [Citation47–50].
It was recently identified that another lysosomal-associated protein (LIMP-2) is a receptor for enterovirus 71 (EV71), a virus known to have the potential to infect the CNS [Citation51–53]. In addition, it was shown that the intracellular development of L. monocytogenes, can involve specific vacuoles, both expressing LAMP-1 [Citation54–56]. These findings suggest the role of this group of proteins in the pathogenesis of CNS infections making them valuable candidates for biomarker research. summarizes studies on YKL-40 and LAMPs in CNS infections.
Table 2. Summary of YKL-40, LAMPs studies in CNS infections.
There are several limitations of using YKL-40 and LAMPs as diagnostic and prognostic biomarkers in patients with CNS infections that should be addressed. There is a lack of specificity since these biomolecules are overexpressed also in a wide range of conditions, including cancer, non-infectious inflammatory diseases, and cardiovascular diseases. The broad expression could limit their use in patients with CNS infection as stand-alone biomarkers. Furthermore, the levels of YKL-40 and LAMPs may show variability among populations and be influenced by age, gender, and other demographic differences. In the context of measuring YKL-40 and LAMPs levels, it is worth mentioning that different methods may yield fluctuating results due to variations in sensitivity, specificity, and calibration. This variability can make it challenging to compare results across studies or clinical settings. In addition, discrepancies could also be attributed to a lack of standardized protocols for such assays, including the time of sample collection, specimen handling, as well as storage conditions.
These limitations could complicate the interpretation of results in patients with CNS infections making it difficult to determine cut-off values for diagnostic and prognostic purposes. However, integrating these proteins in combination with other biomarkers could possibly contribute to a more accurate diagnosis, better disease stratification, and improved monitoring and response to treatment in patients with CNS infections. To address such limitations additional studies in various conditions and populations are needed to integrate more clinical data with biomarker levels. Identifying complementary biomarkers that correlate strongly with specific diseases could be particularly valuable. Moreover, the advances in artificial intelligence and machine learning models could facilitate future application of YKL-40 and LAMPs in patients with neuroinfections.
Conclusions
In order to improve the management of patients with CNS infections, there is a growing interest in studying various biomolecules. YKL-40 and LAMPs have also emerged as potential biomarkers for a number of diseases, also including infections of the CNS. YKL-40 is linked with inflammation and tissue remodeling, while LAMPs are key in autophagy, lysosomal stability, and immune regulation, making them valuable for diagnosing and managing patients with CNS infections. Research on YKL-40 and LAMPs in meningitis is still evolving, with studies aiming to better define their role and application in clinical practice. Current studies potential use in diagnostics and clinical assessment. Despite initial findings, transitioning from research to clinical practice requires further studies to validate and integrate these biomarkers in the care of patients with acute CNS infections. This could lead to improved patient outcomes, personalized treatment approaches, and the development of novel therapeutic targets, thus making significant advances in the field of neuroinfectious diseases.
Author contributions
All the authors contributed to the data research, writing, editing, and reviewing of the manuscript. All authors have read and agreed to the final version of the manuscript.
Acknowledgment
The authors thank Valentina Mihaylova for the help in the design of the figure.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available from the corresponding author upon request.
Additional information
Funding
References
- Giovane RA, Lavender PD. Central nervous system infections. Prim Care Clin off Pract. 2018;45(3):1–9.
- Archibald LK, Quisling RG, Archibald LK, et al. Central nervous system infections. Textb Neurointensive Care. 2013;427–517. https://link.springer.com/chapter/10.1007/978-1-4471-5226-2_22
- Enfermedades Infecciosas y Microbiología Clínica. 2012; [cited 2024 Mar 10]; Available from: www.elsevier.es/eimc.
- Parikh V, Tucci V, Galwankar S. Infections of the nervous system. Int J Crit Illn Inj Sci. 2012;2(2):82–97. https://pubmed.ncbi.nlm.nih.gov/22837896/
- Vila J, Bosch J, Muñoz-Almagro C. Molecular diagnosis of the Central nervous system (CNS) infections. Enferm Infecc Microbiol Clin. 2020;3:1. doi: 10.1016/j.eimc.2020.03.001.
- Ramanan P, Bryson AL, Binnicker MJ, et al. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2017;31(1):77. https://pubmed.ncbi.nlm.nih.gov/29142077/
- Vora NM, Holman RC, Mehal JM, et al. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82(5):443–451. https://pubmed.ncbi.nlm.nih.gov/24384647/ doi: 10.1212/WNL.0000000000000086.
- Sulaiman T, Salazar L, Hasbun R. Acute versus subacute community-acquired meningitis: analysis of 611 patients. Medicine. 2017;96(36):54. https://pubmed.ncbi.nlm.nih.gov/28885354/
- Lautner R, Mattsson N, Schöll M, et al. Biomarkers for microglial activation in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:939426. https://pubmed.ncbi.nlm.nih.gov/22114747/ doi: 10.4061/2011/939426.
- Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4:122. doi: 10.3389/fphys.2013.00122.
- Schultz NA, Johansen JS. YKL-40 – a protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers. 2010;2(3):1453–1491. https://www.mdpi.com/2072-6694/2/3/1453/htm doi: 10.3390/cancers2031453.
- He CH, Lee CG, Dela Cruz CS, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013;4(4):830–841. https://pubmed.ncbi.nlm.nih.gov/23972995/.
- Väänänen T, Koskinen A, Paukkeri E-L, et al. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediators Inflamm. 2014;2014:215140. https://pubmed.ncbi.nlm.nih.gov/25132728/ doi: 10.1155/2014/215140.
- Johansen JS, Jensen BV, Roslind A, et al. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–202. https://pubmed.ncbi.nlm.nih.gov/16492905/ doi: 10.1158/1055-9965.EPI-05-0011.
- Wennström M, Surova Y, Hall S, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PLoS One. 2015;10(8):e0135458. doi: 10.1371/journal.pone.0135458.
- Kazakova MH, Batalov AZ, Mateva NG, et al. YKL-40 and cytokines – a new diagnostic constellation in rheumatoid arthritis? Folia Med. 2017;59(1):37–42. https://pubmed.ncbi.nlm.nih.gov/28384116/ doi: 10.1515/folmed-2017-0013.
- Karalilova R, Kazakova M, Sapundzhieva T, et al. Serum YKL-40 and IL-6 levels correlate with ultrasound findings of articular and periarticular involvement in patients with systemic sclerosis. Rheumatol Int. 2019;39(11):1841–1848. https://pubmed.ncbi.nlm.nih.gov/31375891/ doi: 10.1007/s00296-019-04402-9.
- Kazakova MH, Pavlov GA, Dichev VD, et al. Relationship between YKL-40, neuron-specific enolase, tumor necrosis factor-a, interleukin-6, and clinical assessment scores in traumatic brain injury. Arch Trauma Res. 2021;10(1):23. https://www.archtrauma.com/article.asp?issn=2251-953X;year=2021;volume=10;issue=1;spage=23;epage=29;aulast=Kazakova doi: 10.4103/atr.atr_43_20.
- Østergaard C, Johansen JS, Benfield T, et al. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Diagn Lab Immunol. 2002;9(3):598–604. https://pubmed.ncbi.nlm.nih.gov/11986266/ doi: 10.1128/cdli.9.3.598-604.2002.
- Craig-Schapiro R, Perrin RJ, Roe CM, et al. YKL-40: a novel prognostic fluid biomarker for preclinical alzheimer’s disease. Biol Psychiatry. 2010;68(10):903–912. https://pubmed.ncbi.nlm.nih.gov/21035623/
- Chen J, Ding Y, Zheng D, et al. Elevation of YKL-40 in the CSF of anti-NMDAR encephalitis patients is associated with poor prognosis. Front Neurol. 2018;9(OCT):727. www.frontiersin.org doi: 10.3389/fneur.2018.00727.
- Li J, Li H, Wang Y, et al. CHI3L1 in the CSF is a potential biomarker for anti-leucine-rich glioma inactivated 1 encephalitis. Front Immunol. 2022;13:1071219. doi: 10.3389/fimmu.2022.1071219.
- Czupryna P, Kulczyńka-Przybik A, Mroczko B, et al. Assessment of the YKL-40 concentration in patients with tick-borne encephalitis. Ticks Tick Borne Dis. 2022;13(2):101895.
- Kronborg G, Ostergaard C, Weis N, et al. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated with the outcome of the disease. Scand J Infect Dis. 2002;34(5):323–326. https://pubmed.ncbi.nlm.nih.gov/12069012/
- Orvedahl A, Levine B. Autophagy and viral neurovirulence. Cell Microbiol. 2008;10(9):1747–1756.
- Moy RH, Gold B, Molleston JM, et al. Antiviral autophagy restrictsRift valley fever virus infection and is conserved from flies to mammals. Immunity. 2014;40(1):51–65. https://pubmed.ncbi.nlm.nih.gov/24374193/ doi: 10.1016/j.immuni.2013.10.020.
- C Bílý T, Palus M, Eyer L, et al. Electron tomography analysis of tick-borne encephalitis virus infection in human neurons. Sci Rep. 2015;5(1):10745. doi: 10.1038/srep10745.
- Cheng XT, Xie YX, Zhou B, et al. Revisiting LAMP1 as a marker for degradative autophagy-lysosomal organelles in the nervous system. Autophagy. 2018;14(8):1472–1474. https://www.tandfonline.com/doi/abs/ doi: 10.1080/15548627.2018.1482147.
- Geng B, Pan J, Zhao T, et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through β-catenin/erk/akt signaling in gastric cancer. J Exp Clin Cancer Res. 2018;37(1):208. https://pubmed.ncbi.nlm.nih.gov/30165890/ doi: 10.1186/s13046-018-0876-2.
- Rehli M, Niller HH, Ammon C, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278(45):44058–44067. https://pubmed.ncbi.nlm.nih.gov/12933821/
- Connor JR, Dodds RA, Emery JG, et al. Human cartilage glycoprotein 39 (HC gp-39) mRNA expression in adult and fetal chondrocytes, osteoblasts and osteocytes by in-situ hybridization. Osteoarthr Cartil. 2000;8(2):87–95. https://pubmed.ncbi.nlm.nih.gov/10772238/
- Ringsholt M, Høgdall EVS, Johansen JS, et al. YKL-40 protein expression in normal adult human tissues–an immunohistochemical study. J Mol Histol. 2007;38(1):33–43. https://pubmed.ncbi.nlm.nih.gov/17242979/ doi: 10.1007/s10735-006-9075-0.
- Zhao T, Su Z, Li Y, et al. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;51(1):1–20. https://www.nature.com/articles/s41392-020-00303-7
- Kawada M, Seno H, Kanda K, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31(26):3111–3123. https://pubmed.ncbi.nlm.nih.gov/22056877/ doi: 10.1038/onc.2011.498.
- Peters PJ, Borst J, Oorschot V, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173(5):1099–1109. https://pubmed.ncbi.nlm.nih.gov/2022921/ doi: 10.1084/jem.173.5.1099.
- Krzewski K, Gil-Krzewska A, Nguyen V, et al. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood. 2013;121(23):4672–4683. https://pubmed.ncbi.nlm.nih.gov/23632890/ doi: 10.1182/blood-2012-08-453738.
- Cohnen A, Chiang SC, Stojanovic A, et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122(8):1411–1418. https://pubmed.ncbi.nlm.nih.gov/23847195/ doi: 10.1182/blood-2012-07-441832.
- Künzli BM, Berberat PO, Zhu ZW, et al. Influences of the lysosomal associated membrane proteins (lamp-1, lamp-2) and mac-2 binding protein (mac-2-BP) on the prognosis of pancreatic carcinoma. Cancer. 2002;94(1):228–239. https://pubmed.ncbi.nlm.nih.gov/11815981/ doi: 10.1002/cncr.10162.
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31(21):7817–7830. https://pubmed.ncbi.nlm.nih.gov/21613495/
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. https://pubmed.ncbi.nlm.nih.gov/21884931/ doi: 10.1016/j.cell.2011.07.030.
- Bar-Yosef T, Damri O, Agam G. Dual role of autophagy in diseases of the Central nervous system. Front Cell Neurosci. 2019;13:196. doi: 10.3389/fncel.2019.00196.
- Sarafian V, Jadot M, Foidart J-M, et al. EXPRESSION oF lamp-1 and lamp-2 and their interactions with galectin-3 in human tumor cells. J Cancer. 1998;75:105–111. https://onlinelibrary.wiley.com/terms-and-conditions
- Kristen H, Sastre I, Aljama S, et al. LAMP2 deficiency attenuates the neurodegeneration markers induced by HSV-1 infection. Neurochem Int. 2021;146:105032. doi: 10.1016/j.neuint.2021.105032.
- Pang Y, Wu L, Tang C, et al. Autophagy-Inflammation interplay during infection: balancing pathogen clearance and host inflammation. Front Pharmacol. 2022;13:832750. www.frontiersin.org doi: 10.3389/fphar.2022.832750.
- Jin M, Liu X, Klionsky DJ. SnapShot: selective autophagy. Cell. 2013;152(1-2):368–368.e2. Available fromhttps://pubmed.ncbi.nlm.nih.gov/23332767/
- Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. https://pubmed.ncbi.nlm.nih.gov/33163006/
- Clemens DL, Horwitz MA. Characterization of the Mycobacterium mberctdosis phagosome and evidence that phagosomal maturation is inhibited. Available from: http://rupress.org/jem/article-pdf/181/1/257/1676619/257.pdf.
- Gordon SB, Irving GRB, Lawson RA, et al. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68(4):2286–2293. doi: 10.1128/IAI.68.4.2286-2293.2000.
- Read RC, Zimmerli S, Broaddus C, et al. The (alpha2–>8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64(8):3210–3217. https://pubmed.ncbi.nlm.nih.gov/8757855/ doi: 10.1128/iai.64.8.3210-3217.1996.
- Jonsson S, Musher DM, Chapman A, et al. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J Infect Dis. 1985;152(1):4–13. https://pubmed.ncbi.nlm.nih.gov/3874252/ doi: 10.1093/infdis/152.1.4.
- Liou B, Haffey WD, Greis KD, et al. The LIMP-2/SCARB2 binding motif on acid β-Glucosidase. J Biol Chem. 2014;289(43):30063–30074. http://www.jbc.org/article/S0021925820373385/fulltext doi: 10.1074/jbc.M114.593616.
- Guo H, Zhang J, Zhang X, et al. SCARB2/LIMP-2 regulates IFN production of plasmacytoid dendritic cells by mediating endosomal translocation of TLR9 and nuclear translocation of IRF7. J Immunol. 2015;194(10):4737–4749. https://pubmed.ncbi.nlm.nih.gov/25862818/ doi: 10.4049/jimmunol.1402312.
- Yamayoshi S, Yamashita Y, Li J, et al. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med. 2009;15(7):798–801. https://pubmed.ncbi.nlm.nih.gov/19543282/ doi: 10.1038/nm.1992.
- Pizarro-Cerdá J, Jonquières R, Gouin E, et al. Distinct protein patterns associated with Listeria monocytogenes InlA- or InlB-phagosomes. Cell Microbiol. 2002;4(2):101–115. https://onlinelibrary.wiley.com/doi/full/ doi: 10.1046/j.1462-5822.2002.00169.x.
- Birmingham CL, Canadien V, Kaniuk NA, et al. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451(7176):350–354. https://pubmed.ncbi.nlm.nih.gov/18202661/ doi: 10.1038/nature06479.
- Bleves S, Alonzo F, Bierne H, et al. To be cytosolic or vacuolar: the double life of Listeria monocytogenes background: the prototype of a cytosolic and disseminating bacterium. Front Cell Infect Microbiol. 2018;1:136. www.frontiersin.org
- Villar-Piqué A, Schmitz M, Hermann P, et al. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J Neuroinflammation. 2019;16(1):145. https://jneuroinflammation.biomedcentral.com/articles/ doi: 10.1186/s12974-019-1531-3.
- Pinteac R, Montalban X, Comabella M. Chitinases and chitinase-like proteins as biomarkers in neurologic disorders. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e921. doi: 10.1212/NXI.0000000000000921.
- Hermansson L, Yilmaz A, Axelsson M, et al. Cerebrospinal fluid levels of glial marker YKL-40 strongly associated with axonal injury in HIV infection. J Neuroinflammation. 2019;16(1):16. https://pubmed.ncbi.nlm.nih.gov/30678707/ doi: 10.1186/s12974-019-1404-9.
- Laforge M, Limou S, Harper F, et al. DRAM triggers lysosomal membrane permeabilization and cell death in CD4+ T cells infected with HIV. PLoS Pathog. 2013;9(5):e1003328. https://pubmed.ncbi.nlm.nih.gov/23658518/ doi: 10.1371/journal.ppat.1003328.
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nat. 2011;469(7330):323–335. https://www.nature.com/articles/nature09782 doi: 10.1038/nature09782.