Abstract
To explore the zinc stress response in eddo, plants were grown for 15 d in hydroponic solutions containing 1 (control), 200, and 1000 μM zinc, and the hydathode function and changes in the contents of various elements in these plants were investigated. Under 1000 μM zinc, the dry weights of leaf blades and roots are decreased by 17 and 42%, respectively. The zinc contents in leaf blades, petioles, corms, and roots increased with increasing zinc levels in the solution. The zinc content in roots was 6.57 mg g−1 dry weight, which was 2.8–4.3 times higher than in other plant parts under 1000 μM zinc. These results demonstrate that the severe root damage occurring under 1000 μM zinc is due to higher zinc content in the roots. Under zinc stress, the contents of iron and copper in roots increased, but the contents of magnesium and calcium in petioles, corms, and roots, iron in leaf blades and corms, and manganese in petioles and corms decreased. In the guttation fluid, the concentrations of zinc, magnesium, and potassium increased, while the iron concentration decreased under 1000 μM zinc. Thus, elemental changes occurred in the guttation fluid as well as in different plant parts in eddo. In the 200 and 1000 μM zinc treatments, the amount of zinc eliminated via guttation was 2.8 and 8.5 times higher, respectively, than in the control. The results indicate that guttation partly contributes to the excretion of excess zinc under zinc stress conditions.
Classification:
Eddo [Colocasia esculenta (L.) Schott var. antiquorum Hubbard & Rehder] belongs to the taro group in the family Araceae and is cultivated mainly in Asia as a staple food. Guttation is an overnight process capable of exuding a considerable volume of fluid via hydathode pores and is a common phenomenon in eddo leaves (Hossain et al., Citation2016; Islam & Kawasaki, Citation2015). Hydathode pores are located on the adaxial surface of the leaf blade tip in eddo (Hossain et al., Citation2016). Zinc (Zn) is an essential micronutrient for plant growth and development, but is toxic in excess (Broadley et al., Citation2007; Kochian, Citation1993; Sharma et al., Citation2013; Zhao et al., Citation2003). Although Zn toxicity in crops is far less widespread than Zn deficiency, this element is a major industrial pollutant of terrestrial and aquatic environments in most global regions (Barak & Helmke, Citation1993; Broadley et al., Citation2007; Forstner, Citation1995). Zn concentrations in soil are rising as a result of anthropogenic activities such as mining, coal and waste combustion, steel processing (Bi et al., Citation2006; Wuana & Okieimen, Citation2011), and the addition of sewage sludge to agricultural soils (Chaney, Citation1993). Some crops grown in these contaminated soils and acidic soils may suffer from Zn toxicity (Broadley et al., Citation2007; Chaney, Citation1993). In a previous study, Zn promoted the growth of Brassica juncea at a concentration of .05 mM, but caused significant growth reductions at 5 and 10 mM concentrations (Prasad et al., Citation1999). Other studies have shown that the sufficiency range of Zn in taro leaf blades is .02–.04 mg g−1 dry weight (DW), while the critical toxicity range is .40–.50 mg g−1 DW (Miyasaka et al., Citation2002; O’Sullivan et al., Citation1996). Excess Zn was found to accumulate in the edible parts of taro cultivated in lead/Zn mine waste-affected areas (Liu et al., Citation2005). In plants such as sugar beet, rice, and sorghum, Zn toxicity can reduce growth by disrupting physiological processes such as photosynthesis, respiration, transpiration, and nutrient distribution (Ali et al., Citation2000; Lin et al., Citation2005; Mirshekali et al., Citation2012; Sagardoy et al., Citation2010; Subba et al., Citation2014). Despite these findings, little information is available on the effect of Zn stress on eddo plants.
Several authors have reported on some mechanisms to avoid Zn toxicity in plants. For example, Zn can be sequestered in vacuoles in the epidermal cells of Thlaspi caerulescens (Frey et al., Citation2000; Küpper et al., Citation1999) and accumulated in mesophyll cells in Arabidopsis halleri (Küpper et al., Citation2000; Zhao et al., Citation2000). Some plants compartmentalize excess Zn into central vacuoles (Maeshima, Citation2001; Martinoia et al., Citation2007) and chloroplasts (Kim et al., Citation2009), and/or entrap free Zn in the cytoplasm using small cysteine-rich proteins and peptides (Clemens, Citation2001; Cobbett, Citation2000; Haydon & Cobbett, Citation2007). Guttation can facilitate the excretion of boron (B) in banana (Shapira et al., Citation2013), calcium (Ca) in eddo (Islam & Kawasaki, Citation2015), and arsenic in Pteris vittata fronds (Cantamessa et al., Citation2015) when those elements are present at excessive concentrations. Mizuno et al. (Citation2002) suggested that unbalanced elements could be excreted via guttation to adjust ion concentrations in Petasites japonicus var. giganteus and Polygonum cuspidatum growing on ultramafic soil. Zn has been detected at very low concentrations in the guttation fluid of Thlaspi japonicum growing on ultramafic soil (Mizuno et al., Citation2003). However, it is unclear whether guttation can contribute to Zn homeostasis in plants. Therefore, investigating the functional role of guttation in Zn excretion will help us to understand the response of eddo to Zn stress.
Previous studies have reported that high Zn levels affect the uptake and contents of B, magnesium (Mg), phosphorus (P), potassium (K), Ca, manganese (Mn), iron (Fe), and copper (Cu) in sugar beet, mung bean, and Arabidopsis thaliana (Chaney, Citation1983; Kawachi et al., Citation2009; Sagardoy et al., Citation2009; Samreena et al., Citation2017). Zn was shown to affect the uptake and transport of Fe in maize, leading to decreased growth (Chilian et al., Citation2015). In sugar beet, Zn stress was shown to decrease the concentrations of Mg, K, Mn, and Fe in all plant parts but increase P and Ca concentrations in shoots (Sagardoy et al., Citation2009). These results indicate that Zn stress causes elemental content changes in plants. Therefore, to understand the sensitivity and response to Zn stress, changes in the contents of various elements should be analyzed.
In this study, we aimed to elucidate the mechanism of the Zn stress response in eddo by investigating changes in the contents of various elements in different plant parts as well as in guttation fluid. We also explored whether guttation contributes to ion homeostasis in eddo under Zn stress conditions.
Materials and methods
Plant materials and treatments
Seed corms of eddo cultivar Aichiwase were planted in vermiculite in plastic pots in April 2016. The plants were grown under ambient temperature, light, and humidity conditions in a greenhouse, and watered as required, at Hirosaki University (40°59′N, 140°47′E; 53 m above sea level), Hirosaki, Japan. After 2 months, the plants were removed from the pots, and the roots were washed carefully with distilled water to remove the vermiculite. The plants were then transferred to a hydroponic solution (Kawasaki et al., Citation2008) in a growth chamber (MLR-351H; Sanyo, Osaka, Japan) for 7 d with continuous aeration under the following growth conditions: 25/20 °C light period/dark period temperatures, a 16-h light/8-h dark period with light at approximately 150 μmol m−2 s−1, and 60% relative humidity. The light and dark periods were from 6:00 to 22:00 and from 22:00 to 6:00, respectively. Plants with approximately three leaves and a new, small corm were then treated for 15 d in hydroponic solutions containing at 1 μM (control), 200 μM, or 1000 μM ZnSO4·7H2O. These plants were used to evaluate the effects of Zn treatments in eddo during the vegetative growth period because they had all of the vegetative organs (leaf blade, petiole, corm, and root). The growth conditions were the same as those described above, except that the relative humidity was increased to 85% to create suitable conditions for guttation. The hydroponic solutions were changed every evening to prevent anaerobic conditions and to maintain consistency among treatments. After the Zn treatment, leaf blades and roots were photographed to record Zn stress symptoms. The lengths of all leaf blades, all petioles, and the longest root in each plant were measured using a ruler before the plants were sampled for further analyses.
Collection of guttation fluid and xylem sap
Guttation fluid was exuded during the dark period. Therefore, the guttation fluid from the hydathodes of the leaf tips was collected into a small poly packet during the dark period (8 h) in a growth chamber. To collect xylem sap, the leaf blades were cut crossways at approximately 2 mm inward from the hydathode on the first day of treatment, with cuts made daily thereafter approximately 1 mm along the incision. The cuts were made daily to prevent drying and recovery of the incision site, thereby allowing collection of xylem sap throughout the treatment. During the dark period, the xylem sap was transferred from the vascular bundles of the cut surface into a small poly packet.
Measurement of elemental contents of leaf blades, petioles, corms, and roots
After the Zn treatments, the plants were sampled from 15:00 to 17:00. First, the roots were washed three times with distilled water. The plants were then separated into leaf blades, petioles, corms, and roots. These samples were dried at 60 °C for 3 d in an oven and then pulverized. A portion of the powder (.1 g) was digested with 3-mL (70%, v/v) nitric acid in a sealed decomposition vessel in a microwave oven at 300 W for 10 min. The digest solution was filtered through filter paper (.08 mm diameter) and then diluted to a volume of 25 mL with 5% (v/v) nitric acid. The concentrations of Mg, P, K, Ca, Mn, Fe, Cu, and Zn in the digest solutions of the leaf blades, petioles, corms, and roots were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (iCAP 6300 series; Thermo Fisher Scientific, San Jose, CA, U.S.A.). We analyzed 16, 12, and 14 plants from the 1 μM Zn (control), 200, and 1000 μM Zn treatments, respectively, using all the leaf blades, petioles, corms, and roots collected from each plant. The contents of various elements in the whole plant were calculated based on the total DWs of leaf blades, petioles, corms, and roots of each eddo plant.
Quantification of elements in guttation fluid and xylem sap
The volume of guttation fluid per individual plant was measured with a graduated cylinder. Guttation fluid was analyzed from 12 plants for the 200 μM Zn treatment and 14 plants for the control and 1000 μM Zn treatments, while xylem sap was analyzed from six plants per treatment. To measure the concentrations of elements in the guttation fluid and xylem sap, 714 μL 70% nitric acid was added to 10-mL sample solution in a 100-mL beaker. The beaker was placed on a hot plate in a fume hood and gently heated at 85 °C to reduce the sample volume to approximately 3 mL. After cooling in the fume hood for 30 min, the solution was diluted to a volume of 10 mL with 5% (v/v) nitric acid. The concentrations of Mg, P, K, Ca, Mn, Fe, Cu, and Zn in the guttation fluid and xylem sap digest solutions were then measured by ICP-AES as described above.
The Zn content in each plant was calculated by multiplying the volume of guttation fluid and the Zn concentration in the guttation fluid. The ratios of the total amount of Zn in the guttation fluid eliminated via hydathodes during the treatment period to the post-treatment Zn contents in the leaf blades, shoots, and whole plant were calculated for each plant.
Measurement of weight percentage of Zn relative to the major essential elements in leaf tips (hydathodes) and leaf blade centers
Segments of leaf tips and central portions of leaf blades were sampled from 15:00 to 17:00, frozen rapidly in liquid nitrogen, and freeze-dried under 15–25 Pa pressure at −44 °C for 72 h in a vacuum freeze dryer (FDU-1200; EYEL4, Tokyo Rikakikai Co. Ltd., Japan). The freeze-dried samples were cut crossways, mounted on stubs with conductive carbon tape, and then coated with platinum using an auto fine coater (JFC-1600; JEOL, Tokyo, Japan). The weight percentage of Zn in the analyzed area relative to the total weight of major essential elements (B, carbon, nitrogen, oxygen, Mg, P, S, chlorine [Cl], K, Ca, Mn, Fe, Cu, Zn, and molybdenum) was measured. The areas analyzed for these measurements were the transverse surfaces of mesophyll cells in the center of leaf blades, parenchyma cells in the hydathodes, and vascular bundles in the center of leaf blades and leaf tips (hydathodes). The samples were measured under a scanning electron microscope (JSM-7000F; JEOL) with an energy-dispersive X-ray spectrometer (EDS) at an accelerating voltage of 20 kV. Three to four transverse surface areas of vascular bundles, mesophyll cells, and parenchyma cells of a leaf blade were analyzed per plant, with three plants per treatment, to measure the weight percentage of Zn.
Statistical analysis
All experimental data were subjected to analysis of variance followed by Tukey’s test or Tukey–Kramer’s test.
Results
Effects of Zn treatments on eddo plant appearance and growth
Eddo plants subjected to 200 and 1000 μM Zn treatments formed light-colored dots on the surfaces of older leaf blades (Figure (A)). The plants in the 1000 μM treatment showed a marked reduction in root growth (Figure (B)). The lengths of petiole and root were significantly lower in 1000 μM Zn-treated plants than in 1 μM Zn-treated plants (control) (Table ). The DW of leaf blades was significantly lower in 1000 μM Zn-treated plants than in control plants, but did not differ significantly between control and 200 μM Zn-treated plants nor between 200 and 1000 μM-treated plants (Table ). The DW of roots was significantly lower in 1000 μM Zn-treated plants than in control plants and 200 μM Zn-treated plants. The DWs of petioles and corms did not differ significantly among the different Zn treatments (Table ).
Figure 1. Photographs showing the effects of Zn treatments on (A) leaf blades and (B) roots of eddo.
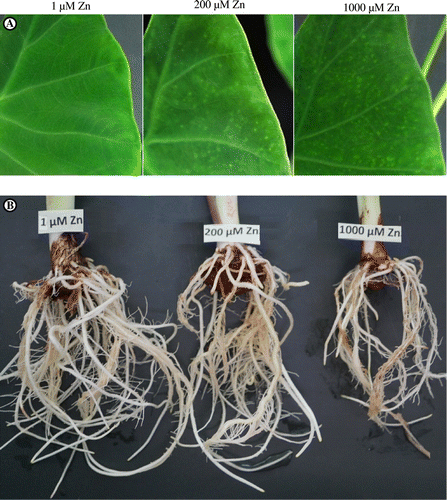
Table 1. Effects of Zn treatments on leaf number, leaf blade length, petiole length, and root length in eddo.
Table 2. Effects of Zn treatments on the dry weights of the leaf blade, petiole, corm, and root in eddo.
Effects of Zn treatments on contents of various elements in leaf blades, petioles, corms, and roots
The Zn contents in leaf blades, petioles, corms, and roots increased significantly with increasing concentrations of Zn in the hydroponic solution (Figure (A)). The Zn contents in leaf blades, petioles, corms, and roots in the 1000 μM Zn treatment were 1.57, 2.37, 1.54, and 6.57 mg g−1 DW, respectively, compared with corresponding values of .052, .074, .12, and .10 mg g−1 DW in the control. Thus, the Zn contents in leaf blades, petioles, corms, and roots in the 1000 μM Zn treatment were 30, 32, 13, and 66 times higher than those in the control, respectively.
Figure 2. Effects of Zn treatments on the contents of (A) Zn, (B) Mg, (C) P, (D) K, (E) Ca, (F) Mn, (G) Fe, and (H) Cu in leaf blades, petioles, corms, and roots of eddo.
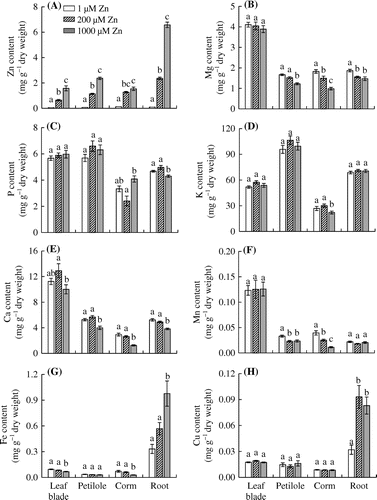
The K content in corms was significantly lower in the 1000 μM Zn treatment than in the control and 200 μM Zn treatment (Figure (D)). The Mg and Ca contents in all plant parts except leaf blades decreased with increasing levels of Zn in the hydroponic solution (Figure (B) and (E)). The P content in roots was significantly lower in 1000 μM Zn-treated plants than in control plants, but the P content in leaf blades and petioles did not differ significantly among the treatments (Figure (C)). The Mn content in petioles was significantly lower in the 200 and 1000 μM Zn treatments than in the control (Figure (F)). The Mn content in corms decreased significantly as the concentration of Zn in the hydroponic solution increased, but the Mn contents in leaf blades and roots did not differ significantly among the treatments. The Fe content was significantly lower in leaf blades and corms but higher in roots in the 1000 μM Zn treatment than in the control and 200 μM Zn treatment (Figure (G)). The Cu content in roots was significantly higher in the 200 and 1000 μM Zn treatments than in the control (Figure (H)).
Effects of Zn treatments on concentrations of elements in guttation fluid and leaf blade xylem sap
The Zn concentrations in the guttation fluid were significantly higher in the 1000 μM Zn treatment (.46 μg mL−1) than in the control and 200 μM Zn treatment (.034 and .090 μg mL−1, respectively), whereas the concentration of Zn in the xylem sap was not affected by the level of Zn in the hydroponic solution (Figure (A)). The Zn concentrations did not differ significantly between the guttation fluid and xylem sap in control plants. However, the Zn concentration in the guttation fluid was significantly higher than that in xylem sap in the 1000 μM Zn treatment.
Figure 3. Effects of Zn treatments on the concentrations of (A) Zn, (B) Mg, (C) P, (D) K, (E) Ca, (F) Mn, (G) Fe, and (H) Cu in the guttation fluid and xylem sap of leaf blades in eddo.
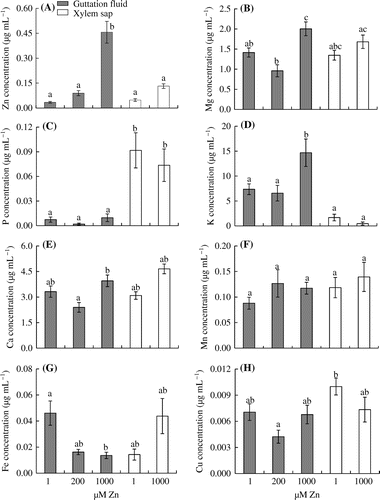
In our experiment, the concentrations of P, Ca, Mn, and Cu in the guttation fluid did not differ significantly among the treatments (Figure (C), (E), (F), and (H)). In contrast, the Fe concentration in the guttation fluid was significantly lower in the 1000 μM Zn treatment than in the control (Figure (G)). The concentrations of Mg and K in the guttation fluid were significantly higher in the 1000 μM treatment than in the control and 200 μM Zn treatment (Figure (B) and (D)). In the xylem sap, none of the elements showed significantly different concentrations between the control and 1000 μM Zn treatment (Figure ). The K concentration in the guttation fluid was significantly higher than that in the xylem sap under higher Zn stress conditions (Figure (D)). Finally, the P concentration in the guttation fluid was always lower than that in the xylem sap (Figure (C)).
Effects of Zn treatments on the weight percentage of Zn relative to the major essential elements in the tips (hydathodes) and leaf blade centers
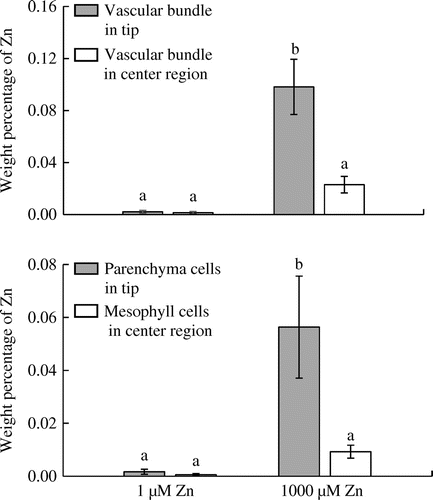
In control plants, there was no difference in the weight percentage of Zn between vascular bundles in the tips and central parts of leaf blades nor between parenchyma cells in tips and mesophyll cells in the central parts of leaf blades (Figure ). The weight percentage of Zn in vascular bundles and parenchyma cells of leaf blade tips was significantly higher in the 1000 μM Zn treatment than in the control. The weight percentage of Zn in vascular bundles of leaf tips was also significantly higher than that in vascular bundles of the central parts of leaf blades in the 1000 μM Zn treatment. The Zn percentage in the parenchyma cells of leaf tips was also significantly higher than that in mesophyll cells in the central regions of leaf blades in the 1000 μM Zn treatment (Figure ).
Figure 4. Effects of Zn treatments on weight percentage of Zn relative to the major essential elements in leaf tips (hydathodes) and leaf blade centers of eddo.
Effects of Zn treatments on volume and Zn content of guttation fluid
As shown in Figure (A), eddo plants treated with 1000 μM Zn exuded less guttation fluid (2.48 mL plant−1 dark period (8 h)−1) than those in the control and 200 μM Zn treatment (4.09 and 3.58 mL plant−1 dark period (8 h)−1, respectively). The Zn content in the guttation fluid increased significantly from .14 μg plant−1 dark period (8 h)−1 in the control to 1.19 μg plant−1 dark period (8 h)−1 in the 1000 μM Zn treatment (Figure (B)).
Figure 5. Effects of Zn treatments on the (A) volume and (B) Zn content of the guttation fluid in eddo.
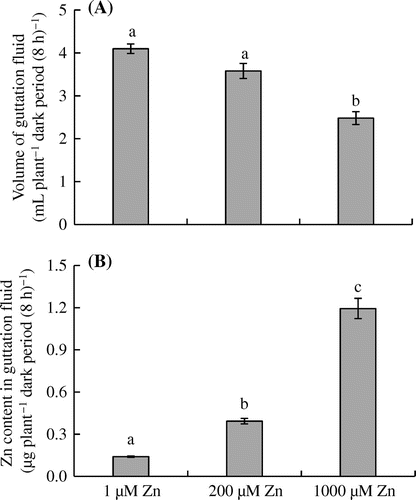
The ratios of Zn content in the guttation fluid to that in the leaf blades in a plant and to that in whole plants were significantly lower in the 200 and 1000 μM Zn treatments than in the control (Table ). The ratio of Zn content in the guttation fluid to that in the shoot (fluid–shoot Zn content ratio) was significantly lower in the 1000 μM Zn treatment than in the control, but was significantly higher in the 1000 μM Zn treatment than in the 200 μM Zn treatment. At the end of the 15-d experiment, the fluid–leaf blade, fluid–shoot, and fluid–whole-plant Zn content ratios were, respectively, 4.13, 1.20, and .98% in the control; 1.20, .30, and .20% in the 200 μM Zn treatment; and 1.57, .55, and .29% in the 1000 μM Zn treatment (Table ).
Table 3. Effects of Zn treatments on the ratios of Zn content in the guttation fluid to that in leaf blades, shoots, and the whole plant of eddo.
Discussion
Zn can be toxic to plants at high concentrations in hydroponic solution (Broadley et al., Citation2007) and soil. For example, Zn concentrations of 100–400 μg g−1 in dried soil caused significant decreases in the root and shoot growth of Artemisia annua plants at different developmental stages (Khudsar et al., Citation2004). O’Sullivan et al. (Citation1996) reported that the complete arrest of plant growth, the development of necrotic lesions along the margins of the oldest leaves, and root death were caused under high levels of Zn (concentrations not shown) in taro. In this study, eddo plants in the 200 and 1000 μM Zn treatments showed light spots on older leaf blades as visible symptoms (Figure (A)). Although the number and length of leaf blades were not affected, petiole length and root length were 24 and 35% lower, respectively, in the 1000 μM Zn treatment than in the control (Table ). The DW of roots did not differ between the control and the 200 μM Zn treatment, but was 42% lower in the 1000 μM Zn treatment than in the control. The DW of leaf blades was 17% lower in the 1000 μM Zn treatment than in the control. Pan et al. (Citation2016) reported that the Zn concentration differed among tissues of Spartina alterniflora, with the highest concentrations in fine roots, followed by leaves, stems, and then rhizomes. Wheeler and Power (Citation1995) reported that the critical toxicity level of Zn was 5.00 mg g−1 in wheat roots. In eddo, the Zn contents in leaf blades, petioles, corms, and roots in the 1000 μM Zn treatment were 30, 32, 13, and 66 times higher than those in the control, respectively. The Zn content in eddo roots in the 1000 μM Zn treatment was 6.57 mg g−1 (Figure (A)). In hydroponic solution containing 1000 μM Zn, the Zn content in roots was 2.8–4.3 times higher than in other plant parts. These results demonstrate that the severe root damage in eddo plants in the 1000 μM Zn treatment was due to higher Zn content in the roots.
Zn stress affects the uptake of nutrient elements by direct competition and/or by altering metabolic processes (Kawachi et al., Citation2009; Sagardoy et al., Citation2009). Shanmugam et al. (Citation2011) reported that excess Zn in A. thaliana reduced Fe accumulation in shoots, leading to a significant Fe deficiency. The critical level of Fe deficiency in leaf blades is .055 to .070 mg g−1 in taro (Ares et al., Citation1996). In eddo leaf blades, the content of Fe was only lower in the 1000 μM Zn treatment than in the control (Figure (G)). The Zn contents were similar in the leaf blade, petiole, and corm in the 1000 μM Zn treatment (Figure (A)), but leaf blades were the only shoot part that showed a reduction in DW (Table ). The growth inhibition of leaf blades was possibly related to the reduced Fe content, because Fe is required for photosynthesis. Feigl et al. (Citation2015) reported that a Zn treatment significantly increased the Cu and Fe contents but decreased the Mn content in the roots of Brassica, compared with the control. Zn and Cu use the same transporters, which can be upregulated by excess Zn, although Cu is preferred (Fraústo da Silva & Williams, Citation2001). In our study, the Fe and Cu contents in roots increased significantly with increasing Zn concentrations in the hydroponic solution (Figure (G) and (H)). The critical toxicity level of Fe is .30−.70 mg g−1 in rice roots (Jugsujinda & Patrick, Citation1993; Stein et al., Citation2014), and the critical toxicity level of Cu is .04 mg g−1 in black gram roots (Kalyanaraman & Sivagurunathan, Citation1993). The Fe and Cu contents in eddo roots were .98 mg g−1 and .08 mg g−1, respectively, in the 1000 μM Zn treatment. Therefore, further research will be required to determine whether the increased levels of Fe and Cu are involved in root growth inhibition under high-Zn conditions. In pea plants, an increase in Zn supply led to decreased concentrations of Mg, P, and Ca in roots and increased concentrations of Ca in stems and leaves (Stoyanova & Doncheva, Citation2002). In this study, the Mg and Ca contents in petioles, corms, and roots of eddo decreased significantly as the Zn concentration in the hydroponic solution increased (Figure (B) and (E)). The P contents in roots, K and Fe contents in corms, and Mn contents in petioles and corms also decreased under Zn stress conditions (Figure (C), (D), (F), and (G)). These results show that the contents of several elements in different parts of eddo were altered under Zn stress conditions.
As Zn levels increased in the hydroponic solution, the volume of guttation fluid decreased but the Zn concentration in the guttation fluid increased (Figure (A) and 5(A)). In the 200 and 1000 μM Zn treatments, the amount of zinc eliminated via guttation during the dark period was 2.8 and 8.5 times higher, respectively, than that in the control (Figure (B)). The ratios of Zn content in the guttation fluid to Zn content in leaf blades, shoots, and whole plants were significantly higher in the control than in the 200 and 1000 μM Zn treatments (Table ), because the Zn contents in all plant organs were much lower in control plants (Figure (A)). However, even though the Zn content in shoots was higher in the 1000 μM Zn treatment than in the 200 μM Zn treatment, the ratio of Zn content in the guttation fluid to that in shoots was significantly higher in the 1000 μM Zn treatment than in the 200 μM Zn treatment. The ratios of Zn content in the guttation fluid to that in leaf blades and whole plants were not significantly different between the 200 and 1000 μM Zn treatments, although the Zn contents in the leaf blade and whole plant were higher in the 1000 μM Zn treatment than in the 200 μM Zn treatment (Table ). These results indicate that guttation partly contributed to the excretion of excess Zn from eddo under Zn stress conditions.
Under the same ultramafic soil conditions, the guttation fluid of P. japonicus var. giganteus contained higher concentrations of Ni, K, Mg, and Ca but a lower concentration of Mn than the guttation fluid of P. cuspidatum (Mizuno et al., Citation2002). In barley, hydathodes stored or re-exported phosphate and K ions to other plant parts, whereas Cl ions, which are toxic in excess concentrations, were excreted in the guttation fluid via the hydathodes (Nagai et al., Citation2013). These reports suggest that the concentrations of some elements in the guttation fluid are adjusted by plants. In this study, the Zn concentration was significantly higher in the guttation fluid than in xylem sap collected near the tip of the leaf blade in the 1000 μM Zn treatment (Figure (A)). In eddo plants subjected to high Zn levels, more Zn accumulated in vascular bundles and parenchyma cells at the tip of the leaf blade than in vascular bundles and mesophyll cells in the central part of the leaf blade (Figure ). These results suggest that Zn accumulation in the tip of the leaf blade induces a higher Zn concentration in the guttation fluid under Zn stress conditions. In addition, the Fe concentration in the guttation fluid decreased under 1000 μM Zn treatment (Figure (G)). In contrast, the K concentration in the guttation fluid increased and was significantly higher than in the xylem sap in the 1000 μM Zn treatment (Figure (D)). The Mg concentration in the guttation fluid also increased in the 1000 μM Zn treatment (Figure (B)). Thus, our study shows that elemental changes occur in the guttation fluid as well as in different plant parts in eddo. Further research will be required to determine whether these changes affect plant growth under Zn stress conditions.
In this study, we analyzed the changes in the contents of various elements in different plant parts and the guttation fluid of eddo under Zn stress conditions. The distribution of Zn from the entry point (the roots) to the outlet (hydathodes) and the function of guttation under Zn stress were also investigated. Our findings are helpful for understanding the mechanism of Zn sensitivity and stress response in eddo.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors thank Kouhei Narita, Faculty of Science and Technology, Hirosaki University, for assistance during the sample digestion process for ICP-AES. We thank Yusei Tsushima, Center for Instruments Analysis, Hirosaki University, for technical assistance with the EDS.
References
- Ali, G., Srivastava, P. S., & Iqbal, M. (2000). Influence of cadmium and zinc on growth and photosynthesis of Bacopa monniera L. cultivated in vitro. Biologia Plantarum, 43, 599–601. doi:10.1023/A:1002852016145
- Ares, A., Huang, S. G., & Miyasaka, S. C. (1996). Taro response to different iron levels in hydroponic solution. Journal of Plant Nutrition, 19, 281–192. doi:10.1080/01904169609365122
- Barak, P., & Helmke, P. A. (1993). The chemistry of zinc. In A. D. Robson (Ed.), Zinc in soils and plants, developments in plants and soil sciences (pp. 1–13). New York, NY: Kluwer Academic Press.
- Bi, X., Feng, X., Yang, Y., Qiu, G., Li, G., Li, F., … Jin, Z. (2006). Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environment International, 32, 883–890. doi:10.1016/j.envint.2006.05.010
- Broadley, M. R., White, P. J., Hammond, J. P., Zelko, I., & Lux, A. (2007). Zinc in plants. New Phytologist, 173, 677–702. doi:10.1111/j.1469-8137.2007.01996.x
- Cantamessa, S., D’Agostino, G., & Berta, G. (2015). Hydathode structure and localization in Pteris vittata fronds and evidence for their involvement in arsenic leaching. Plant Biosystems, 150, 1208–1215. doi:10.1080/11263504.2015.1012135
- Chaney, R. L. (1983). Plant uptake of inorganic waste constituents. In J. F. Parr, P. B. Marsh, & J. M. Kla (Eds.), Land treatment of hazardous wastes (pp. 50–76). Park Ridge, NJ: Noyes Data Corporation.
- Chaney, R. L. (1993). Zinc phytotoxicity. In A. D. Robson (Ed.), Zinc in soils and plants (pp. 135–150). Dordrecht: Kluwer Academic.10.1007/978-94-011-0878-2
- Chilian, A., Bancuta, R. O., Bancuta, I., Setnescu, R., Ion, R.-M., Radulescu, C., … Chelarescu, E. D. (2015). Study of the influence of Zn concentration on the absorption and transport of Fe in maize by AAS and EDXRF analysis techniques. Romanian Reports in Physics, 67, 1138–1151.
- Clemens, S. (2001). Molecular mechanisms of plant metal tolerance and homeostasis. Planta, 212, 475–486. doi:10.1007/s004250000458
- Cobbett, C. S. (2000). Phytochelatins and their roles in heavy metal detoxification. Plant Physiology, 123, 825–832. doi:10.1104/pp.123.3.825
- Feigl, G., Lehotai, N., Molnár, A., Ördög, A., Rodríguez-Ruiz, M., Palma, J. M., … Kolbert, Z. (2015). Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Annals of Botany, 116, 613–625. doi:10.1093/aob/mcu246
- Forstner, U. (1995). Land contamination by metals: Global scope and magnitude of problem. In H. E. Allen, C. P. Huang, G. W. Bailey, & A. R. Bowers (Eds.), Metal speciation and contamination of soil (pp. 1–33). Boca Raton, FL: Lewis.
- Fraústo da Silva, J. J. R., & Williams, R. J. P. (2001). The biological chemistry of the elements (2nd ed.). Oxford: Clarendon.
- Frey, B., Keller, C., Zierold, K., & Schulin, R. (2000). Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant, Cell & Environment, 23, 675–687. doi:10.1046/j.1365-3040.2000.00590.x
- Haydon, M. J., & Cobbett, C. S. (2007). A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in arabidopsis. Plant Physiology, 143, 1705–1719. doi:10.1104/pp.106.092015
- Hossain, M. B., Matsuyama, N., & Kawasaki, M. (2016). Hydathode morphology and role of guttation in excreting sodium at different concentrations of sodium chloride in eddo. Plant Production Science, 19, 528–539. doi:10.1080/1343943X.2016.1210990
- Islam, M. N., & Kawasaki, M. (2015). Evaluation of calcium regulating roles of guttation and calcium oxalate crystals in leaf blades and petioles of hydroponically grown eddo. Plant Production Science, 18, 11–21. doi:10.1626/pps.18.11
- Jugsujinda, J., & Patrick, W. H. J. (1993). Evaluation of toxic conditions associated with oranging symptoms of rice in a flooded Oxisol in Sumatra, Indonesia. Plant and Soil, 152, 237–243.10.1007/BF00029093
- Kalyanaraman, S. B., & Sivagurunathan, P. (1993). Effect of cadmium, copper, and zinc on the growth of blackgram. Journal of Plant Nutrition, 16, 2029–2042. doi:10.1080/01904169309364672
- Kawachi, M., Kobae, Y., Mori, H., Tomioka, R., Lee, Y., & Maeshima, M. (2009). A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant and Cell Physiology, 50, 1156–1170. doi:10.1093/pcp/pcp067
- Kawasaki, M., Takatsuji, A., Taniguchi, M., & Miyake, H. (2008). Localization of Casparian bands and crystal cells in relation to aluminum distribution in the primary root of eddo under aluminum treatment. Plant Production Science, 11, 238–242. doi:10.1626/pps.11.238
- Khudsar, T., Mahmooduzzafar, M., Iqbal, R., & Sairam, K. (2004). Zinc-induced changes in morpho-physiological and biochemical parameters in Artemisia annua. Biologia Plantarum, 48, 255–260.10.1023/B:BIOP.0000033453.24705.f5
- Kim, Y.-Y., Choi, H., Segami, S., Cho, H.-T., Martinoia, E., Maeshima, M., & Lee, Y. (2009). AtHMA1 contributes to detoxification of excess Zn (II) in Arabidopsis. The Plant Journal, 58, 737–53. doi:10.1111/j.1365-313X.2009.03818.x
- Kochian, L. V. (1993). Zinc absorption from hydroponic solution by plant roots. In A. D. Robson (Ed.), Zinc in soils and plants (pp. 45–57). Dordrecht: Kluwer Academic.10.1007/978-94-011-0878-2
- Küpper, H., Lombi, E., Zhao, F. J., & McGrath, S. P. (2000). Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta, 212, 75–84. doi:10.1007/s004250000366
- Küpper, H., Zhao, F. J., & McGrath, S. P. (1999). Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiology, 119, 305–31. doi:10.1104/pp.119.1.305
- Lin, C.-W., Chang, H.-B., & Huang, H.-J. (2005). Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiology and Biochemistry, 43, 963–968. doi:10.1016/j.plaphy.2005.10.001
- Liu, H., Probst, A., & Liao, B. (2005). Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Science of the Total Environment, 339, 153–166. doi:10.1016/j.scitotenv.2004.07.030
- Maeshima, M. (2001). Tonoplast transporters: Organization and function. Annual Review of Plant Physiology and Plant Molecular Biology, 52, 469–497. doi:10.1146/annurev.arplant.52.1.469
- Martinoia, E., Maeshima, M., & Neuhaus, E. (2007). Vacuolar transporters and their essential role in plant metabolism. Journal of Experimental Botany, 58, 83–102. doi:10.1093/jxb/erl183
- Mirshekali, H., Hadi, H., Amirnia, R., & Khodaverdi, H. (2012). Effect of zinc toxicity on plant productivity, chlorophyll and Zn contents of sorghum (Sorghum bicolor) and common lambsquarter (Chenopodium album). International Journal of Agriculture: Research and Review, 2, 247–254.
- Miyasaka, S. C., Hamasaki, R. T., & de la Pena, R. S. (2002). Nutrient deficiencies and excesses in taro. Honolulu: College of Tropical Agriculture and Human Resources, University of Hawai’i.
- Mizuno, N., Nosaka, S., Mizuno, T., Horie, K., & Obata, H. (2003). Distribution of Ni and Zn in the leaves of Thlaspi japonicum growing on ultramafic soil. Soil Science and Plant Nutrition, 49, 93–97. doi:10.1080/00380768.2003.10409984
- Mizuno, N., Takahashi, A., Wagatsuma, T., Mizuno, T., & Obata, H. (2002). Chemical composition of guttation fluid and leaves Petasites japonicus v. giganteus and Polygonum cuspidatum ultramafic soil. Soil Science and Plant Nutrition, 48, 451–453. doi:10.1080/00380768.2002.10409225
- Nagai, M., Ohnishi, M., Uehara, T., Yamagami, M., Miura, E., Kamakura, M., … Kitamura, A. (2013). Ion gradients in xylem exudate and guttation fluid related to tissue ion levels along primary leaves of barley. Plant, Cell & Environment, 36, 1826–1837. doi:10.1111/pce.12090
- O’Sullivan, J. N., Asher, C. J., & Blamey, F. P. C. (1996). Diagnostic criteria for nutrition disorders of root crops in the South Pacific. In E. T. Craswell, C. J. Asher, & J. N. Q’Sullivan (Eds.), Mineral nutrient disorders of root crops in the Pacific (pp. 83–90). Nuku’alofa: ACIAR.
- Pan, X., Chen, G., Shi, C., Chai, M., Liu, J., Cheng, S., & Shi, F. (2016). Effects of Zn stress on growth, Zn accumulation, translocation, and subcellular distribution of spartina alterniflora Loisel. Clean – Soil, Air, Water, 44, 579–585. doi:10.1002/clen.201400288
- Prasad, K. V. S. K., Saradhi, P. P., & Sharmila, P. (1999). Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environmental and Experimental Botany, 42, 1–10. doi:10.1016/S0098-8472(99)00013-1
- Sagardoy, R., Morales, F., López-Millán, A.-F., Abadía, A., & Abadía, J. (2009). Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biology, 11, 339–350. doi:10.1111/j.1438-8677.2008.00153.x
- Sagardoy, R., Vázquez, S., Florez-Sarasa, I. D., Albacete, A., Ribas-Carbó, M., Flexas, J., … Morales, F. (2010). Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytologist, 187, 145–158. doi:10.1111/j.1469-8137.2010.03241.x
- Samreena, T., Humairaa, S., Ullahb, H. U., & Javid, M. (2017). Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mung beans plant (Vigna radiata). Arabian Journal of Chemistry, 10, S1802–S1807. doi:10.1016/j.arabjc.2013.07.005
- Shanmugam, V., Lo, J.-C., Wu, C.-L., Wang, S.-L., Lai, C.-C., Connolly, E. L., … Yeh, K.-C. (2011). Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana – the role in zinc tolerance. New Phytologist, 190, 125–137. doi:10.1111/j.1469-8137.2010.03606.x
- Shapira, O., Israeli, Y., Shani, U., & Schwartz, A. (2013). Salt stress aggravates boron toxicity symptoms in banana leaves by impairing guttation. Plant, Cell & Environment, 36, 275–287. doi:10.1111/j.1365-3040.2012.02572.x
- Sharma, A., Patni, B., Shankhdhar, D., & Shankhdhar, S. C. (2013). Zinc – An indispensable micronutrient. Physiology and Molecular Biology of Plants, 19, 11–20. doi:10.1007/s12298-012-0139-1
- Stein, R. J., Lopes, S. I. G., & Fett, J. P. (2014). Iron toxicity in field-cultivated rice: Contrasting tolerance mechanisms in distinct cultivars. Theoretical and Experimental Plant Physiology, 26, 135–146. doi:10.1007/s40626-014-0013-3
- Stoyanova, Z., & Doncheva, S. (2002). The effect of zinc supply and succinate treatment on plant growth and mineral uptake in pea plant. Brazilian Journal of Plant Physiology, 14, 111–116. doi:10.1590/S1677-04202002000200005
- Subba, P., Mukhopadhyay, M., Mahato, S. K., Bhutia, K. D., Mondal, T. K., & Ghosh, S. K. (2014). Zinc stress induces physiological, ultrastructural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiology and Molecular Biology of Plants, 20, 461–473. doi:10.1007/s12298-014-0254-2
- Wheeler, D. M., & Power, I. L. (1995). Comparison of plant uptake and plant toxicity of various ions in wheat. Plant and Soil, 172, 167–173. doi:10.1007/BF00011318
- Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Network Ecology, 2011, 1–20. doi:10.5402/2011/402647
- Zhao, F. J., Lombi, E., Breedon, T., & McGrath, S. P. (2000). Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant, Cell & Environment, 23, 507–514. doi:10.1046/j.1365-3040.2000.00569.x
- Zhao, F. J., Lombi, E., & McGraht, S. P. (2003). Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil, 249, 37–43. doi:10.1023/A:1022530217289