Abstract
In rainfed lowland rice ecosystem, rice plants are often exposed to alternating recurrences of waterlogging and drought due to erratic rainfall. Such soil moisture fluctuation (SMF) which is completely different from simple or progressive drought could be stressful for plant growth, thereby causing reduction in yield. Root plasticity is one of the key traits that play important roles for plant adaptation under such conditions. This study aimed to evaluate root plasticity expression and its functional roles in dry matter production and yield under SMF using Nipponbare, KDML 105 and three backcross inbred lines (BILs) and to identify QTL(s) associated with root traits in response to SMF at two growth stages using Nipponbare/KDML105 F2 plants. A BIL, G3-3 showed higher shoot dry matter production and yield than Nipponbare due to its greater ability to maintain stomatal conductance concomitant with greater root system development caused by promoted production of nodal and lateral roots under SMF. QTLs were identified for total nodal root length, total lateral root length, total root length, number of nodal roots, and branching index under SMF at vegetative and reproductive stages. The QTLs detected at vegetative and reproductive stages were different. We discuss here that relationship between root system of G3-3 and the detected QTLs. Therefore, G3-3 and the identified QTLs could be useful genetic materials in breeding program for improving the adaptation of rice plants in target rainfed lowland areas.
Keywords:
Classification:
Introduction
In rainfed lowland rice ecosystems, it is believed that the main constraint to production is water deficit mainly due to either erratic rainfall (Ding et al., Citation2011; Gomez et al., Citation2006; Serraj et al., Citation2011; Wade et al., Citation1999) or limited access of water in deep soil layer where water is available because of the presence of hardpan (Bengough et al., Citation2011; Clark et al., Citation2008; Samson et al., Citation2002). Many researchers have pointed out the importance of roots under such conditions, and consequently the main desirable traits proposed include deep, thick (Gomez et al., Citation2010; Henry, Citation2013; Li et al., Citation2011; Liu et al., Citation2008), and strong roots in penetration into hardpan (Cairns et al., Citation2011; Clark et al., Citation2008; Wade et al., Citation2015). In addition, Boling et al. (Citation2004), Wade et al. (Citation1999) and Yadav et al. (Citation2011) indicated that rice plants are often exposed to alternating recurrences of waterlogging and water deficit to various extents in rainfed lowlands, and Suralta et al. (Citation2010) and Niones et al. (Citation2012) showed such soil moisture fluctuation (SMF) had negative effects on dry matter production and yield. On the other hand, in irrigated rice fields, establishment of water-saving technology such as alternative wet and dry (AWD), aerobic culture is one of the most urgent needs (Bouman et al., Citation2005; Kato et al., Citation2009). However, the current technology still often suffer from yield penalty as compared with continuously flooded conditions while it has greatly improved water use efficiency (Patel et al., Citation2010; Peng et al., Citation2006; Tao et al., Citation2006; Zhao et al., Citation2009, Citation2010, Citation2011). The cause of such yield reduction has not yet been specifically identified although a number of studies have been conducted and found out that possible causes for such yield reduction include high soil pH resulting in reduction of nitrogen uptake, decline of soil organic matter which affects soil physical properties such as high bulk density, low porosity and poor structure (Bouman et al., Citation2007; Carrijo et al., Citation2017; Tuong & Bouman, Citation2003). In this aspect, Suralta et al. (Citation2008, Citation2010) suggested that the AWD and aerobic culture may inherently involve SMF, which could be stressful for rice plants and thus cause yield reduction.
These facts strongly suggest that the SMF of various extents can happen under both rainfed lowland, and irrigated lowland with the practice of water-saving technology, and can be stressful for plant growth, which, however, is totally different from the stress due to simple/progressive drought. We have reported that root plasticity that is expressed in response to changing environmental conditions (O’Toole & Bland, Citation1987) is a key trait for crop adaptation and maintenance of productivity (Suralta et al., Citation2016; Wang & Yamauchi, Citation2006). Several studies in rice have demonstrated the contribution of root plasticity of promoted lateral root development in response to varying water-deficit stress intensities to the plant growth and yield under drought conditions (Kano et al., Citation2011; Kameoka et al., Citation2015; Kano-Nakata et al., Citation2011, Citation2013; Tran et al., Citation2014, Citation2015; Menge et al., Citation2016). In contrast, for SMF conditions, Suralta and Yamauchi (Citation2008, Suralta et al. Citation2008, Citation2010) and Niones et al. (Citation2012, Citation2013) found out that root plasticity such as the ability of rapidly forming aerenchyma resulting from cortical parenchyma disintegration when roots were exposed to waterlogging (oxygen deficiency) after being exposed to water deficit, and the ability of promoting lateral root development under water deficit conditions after being exposed to waterlogging is the most desirable root trait for the rice plant adaptation to stressful conditions under SMF. They further discussed that the two abilities may possibly be related with each other in a way that the materials and energy that are released from the cortical disintegration may be utilized for the promoted lateral root development, which enhance water uptake during water deficit periods under SMF.
Khao Dawk Mali 105 (KDML 105), one the most popular varieties in Thailand, has been grown mainly in the Northeastern Thailand since 1959 (Sarkarung et al., Citation2000; Sri-Aun, Citation2005) and shown to be well adapted to the rainfed lowlands conditions (Azhiri-Sigari et al., Citation2000; Fukai & Cooper, Citation1995; Kamoshita et al., Citation2000; Kano-Nakata et al., Citation2013; Wade et al., Citation1999). In addition, a series of our studies have shown KDML 105 to have very sharp root plasticity in response to water stress. For example, KDML 105 increased specific root length or the efficiency for converting dry matter to root length (promoted lateral root production), promoted tiller production and leaf expansion under SMF (Bañoc et al., Citation2000a, Citation2000b). Kameoka et al. (Citation2015) also showed that KDML 105 promoted lateral root production at the shallow soil layer even under the condition in which the water was available in deep soil, which indicates high adaptability of this variety in drought prone areas such as rainfed upland with limited soil depth or rainfed lowland with impenetrable hardpan.
Recently, QTL on root plasticity expressed in response to SMF have been identified in rice such as those in aerenchyma development (Niones et al., Citation2013), lateral root growth (Niones et al., Citation2015; Suralta et al., Citation2015) and yield stability (Sandhu et al., Citation2016). In this aspect, KDML 105 is a potential source of genes for root plasticity expressed under rainfed lowland conditions, which could be introgressed into elite lines. Thus, we generated a potential set of backcross inbred lines (BILs) derived from Nipponbare (japonica, recurrent parent) and KDML 105 (indica, donor parent) through marker-assisted selection. We considered that the BIL(s) that show similar shoot and root growth with the recurrent parent, Nipponbare under non stress conditions but increase shoot and root growth as compared with Nipponbare under SMF are ideal and thus desirable materials for the evaluation of root plasticity (Kano-Nakata et al., Citation2017), and then hypothesized that the improved performance of BILs under SMF would be contributed by one or combinations of the introgressed segments from KDML 105, which could regulate the expression of root plasticity under SMF. In addition, to effectively utilize these genetic information for future breeding, we need to pay careful attention to the fact that the expression of QTL functions often depend on growth stages (Niones et al., Citation2015; Qu et al., Citation2008; Xu et al., Citation2004) and growing conditions like intensity of drought (Zheng et al., Citation2003) in relation to our study.
This study, therefore, aimed (a) to evaluate root plasticity expression of the selected Nipponbare/KDML 105 BILs under SMF condition in field and its functional roles in dry matter production and grain yield, and (b) to identify major QTL(s) associated with several root traits in response to SMF at vegetative and reproductive stages.
Materials and methods
Evaluation of NILs (Experiment 1)
Plant materials and establishment
Three BILs (G3-3, G3-10 and G3-12) (Supplement 1) derived from a cross between Nipponbare and KDML 105 (BC3F3 generation) developed in 2011 at the Laboratory of Project Development, Graduate School of Bioagricultural Sciences, Nagoya University, Japan, and the parents (Nipponbare and KDML 105) were used in this study. Preliminary screenings were done under non-stressed condition for two growth stages suing 93 BILs. The first one was done at seedling stage under hydroponics condition and the second one at vegetative stage under continuously flooded field condition. Three BILs mentioned above were selected since they had similar agronomic traits with the recurrent parent, Nipponbare at seedling and vegetative stages. The selected BILs and Nipponbare were evaluated under field experiments at Togo Field, Field Science Center, Graduate School of Bioagricultural Sciences, Nagoya University, Togo-cho, Aichi, Japan (35°6′42′′N, 137°4′57′′E) in the summer seasons (May to September) of 2012. Further evaluations were repeated for two more years in 2013 and 2014 using Nipponbare, KDML 105 and only one BIL, G3-3. G3-3 was selected because it showed no significant differences in agronomic traits with the recurrent parent, Nipponbare under control (continuous waterlogging) condition but showed greater growth than Nipponbare under CAW-D conditions. We attempted to confirm consistency of such performance of G3-3 relative to Nipponbare under SMF.
Seeds of each genotype were soaked in water containing benomyl fungicide (0.15% w/v) and then incubated in a seed germinator at 28 °C for 72 h prior to sowing. The seeds of each genotype were sown in black plastic trays with soil under well-watered conditions. A waterproof experimental bed (3.6 × 8.4 m) with 30 cm depth of sandy loam soil for controlling soil moisture conditions was established as an experimental field under rain-out shelter. Twenty-one-day-old healthy seedlings of each genotype were transplanted in the field at one seedling per hill. The dates of transplanting were: 10 June 2012; 5 June 2013, and 11 June 2014 at a spacing of 30 cm between rows and 25 cm between hills. A compound chemical fertilizer was applied at a rate of 120 kg N ha−1, 120 kg P ha−1, and 120 kg K ha−1 after transplanting. The transplanted seedlings were allowed to recover from transplanting shock for 14 days before water stress treatment was imposed. Plants were grown until maturity.
In 2012 and 2014, the genotypes except KDML 105 headed at 65 days after transplanting (DAT) and 70 DAT in 2013. KDML 105 did not head due to its sensitivity to photoperiod.
Soil moisture treatments and plant sampling
In 2012 and 2013, the plants were exposed to two soil moisture conditions; continuous waterlogging (CWL; control) and cycles of alternating waterlogging and drought condition (CAW-D) as stress treatment. In CWL, the water level was maintained at 5 cm depth above the soil surface from transplanting until maturity. In CAW-D, the field was first waterlogged for 14 days, then the water was drained until soil water potential dropped down to −30 kPa, which was considered as mild drought stresses in this study, prior to rewatering events to bring back the water level at 5 cm above the soil surface (Niones et al., Citation2012). In 2014, the soil moisture treatments were the same with those of the previous years’ except that in CAW-D, the water was drained until soil water potential dropped to −50 kPa, which was considered severe drought stress. The soil beds were drained at the 8th leaf-stage in 2012 and 2014, and at the 7th leaf-stage in 2013. The cycle was repeated until maturity in 2012 and 2014, and two weeks after heading in 2013 (Supplement 2).
Soil water potential was recorded using a soil tensiometer (Daiki soil and moisture, Daiki Rika Kogyo Co., Japan) and O2 concentration was measured with a soil oxygen meter (E.M.J., Decagon, Utah, USA). Six soil tensiometers and one oxygen meter were installed at 20 cm soil depth in the CAW-D plot only.
Stomatal conductance measurements
The stomatal conductance was measured using a portable photosynthesis analyzer (Li-6400, Li-COR Inc., USA) in 2012 and a leaf porometer (Decagon, Utah, USA) in 2013 and 2014 between 10:00 h and 14:00 h. All measurements were made at the end of each drought cycle just prior to re-watering.
Shoot and root measurements
The shoot and root samples were collected when the set water potential was reached prior to rewatering until 110 DAT in 2012, 85 DAT in 2013 and 105 DAT in 2014. Shoots were cut from the base and oven dried at 70 °C for 72 h before weighing. The root system was extracted as described by Niones et al. (Citation2012) and Kano et al. (Citation2011) using a monolith stainless cylinder (15 cm diameter × 20 cm height) (Kang et al., Citation1994). The collected root samples were washed free of soil with gentle running water. The cleaned root samples were stored in FAA solution (formalin: acetic acid: 70% ethanol = 1:1:18 by volume) for further measurements. The number of nodal roots at the base was manually counted. For the total root length (TRL) measurements, root samples were spread evenly on a transparent tray without overlapping. Digital images were then taken using an image scanner (EPSON Expression 10000XL) at 400 dpi resolution. The TRL was analyzed using WinRhizo software (Regent Instruments Inc., Saint-Foy, Canada) (Kameoka et al., Citation2015; Menge et al., Citation2016). A pixel threshold value was set at 175 for the root length analysis (Suralta et al., Citationin press; Nguyen et al., Citation2018). The root lengths were grouped according to their diameter classes to estimate the lateral root length (LRL). In rice, roots with a diameter of <0.3 mm corresponds to lateral roots (Yamauchi et al., Citation1996).
Yield and yield component measurements
Fifteen plants per genotype per water treatment were sampled at maturity to determine yield and yield components. Yields were determined in 2012 and 2013. Panicles were separated from the shoots, counted and oven dried at 70 °C for 72 h and weighed. The spikelets were collected from the panicles with manual threshing. The spikelets were classified into filled (with developed grains) and unfilled (without developed grains) and counted separately. The 1000 grains were collected from each sample to record the 1000 grain weight. Yield was determined as the weight of filled grains per plant (adjusted to 14% grain moisture content).
Statistical analysis
The field experiments were laid out in a randomized complete block design (RCBD) with three replications. Differences between mean values were compared using the least significant difference (LSD) test at p < 0.05 level to compare genotypes within each water treatment using CropStat version 7.2 (IRRI, Citation2009).
Identification of root trait QTLs under SMF (Experiment 2)
Plant materials and establishment
An F2 mapping population from a cross between Nipponbare and KDML 105 developed at the Laboratory of Project Development, Graduate School of Bioagricultural Sciences, Nagoya University, Japan was used in this study. The F2 plants were evaluated at vegetative stage using soil-filled root boxes and reproductive stage using soil bed field under SMF condition.
SMF treatment at vegetative stage
The seeds of 287 F2 plants and the parents (Nipponbare and KDML 105) were soaked in water mixed with fungicide (benomyl (benlate), 0.15% w/v) and incubated in a seed germinator maintained at 28 °C for 72 h prior to sowing. The pre-germinated seeds were sown in PVC rootboxes (25 × 2 × 40 cm, L × W × H) filled with 2.5 kg of air-dried sandy loam soil following the method as described by Kano-Nakata et al. (Citation2012). The soil in each box was pre-mixed with fertilizer containing 60 mg nitrogen, 80 mg phosphorus and 70 mg potassium. The seedlings were thinned to one seedling per box at 5 days after sowing (DAS). The soil moisture treatments were, continuously waterlogged (CWL) as control and SMF as stress treatment. The parents were grown under CWL and SMF while the F2 plants were grown under SMF only. In CWL, water level was maintained at 2 cm above the soil surface from 5 DAS until the end of the experiment at 38 DAS. In SMF, the plants were first exposed to CWL from 5 to 14 DAS, then watering was withheld until the target soil moisture content (SMC, % w/w) dropped down to 15% and maintained to that level of SMC until 28 DAS. Thereafter, the plants were rewatered back to CWL for 10 days. The boxes were weighed daily using a digital balance to record the wet mass of the soil. As described by Suralta et al. (Citation2015), the SMC (% w/w) in each box was calculated as the proportion of water weight (difference between the wet weights of the soil excluding the box on a given day) to the dry weight of soil. The target SMC during drought period in SMF was maintained at 15% SMC by watering to replace the amount of water lost.
Shoot and root sampling at vegetative stage
Shoot and root samplings were done at 38 DAS. The shoot sampling and measurements were followed as described in Experiment 1. The roots were sampled using a pinboard and transparent perforated plastic sheet (Kono et al., Citation1987; Kano-Nakata et al., Citation2012). The roots embedded between the plastic sheets were washed under running water and then stained with 0.25% Coomassie Brilliant Blue R aqueous solution for 72 h. The stained roots were then rinsed with tap water and placed in a light box for digital imaging using a digital camera (Nikon D3000 DSLR, Nikon Corporation, Japan; 2592 × 3872 resolution). Root measurements were done as described in Experiment 1. Five root traits: TRL, total LRL, total nodal root length (NRL), total nodal root number (NRN), and branching index (BI) were measured. The total NRL was calculated as the difference between the TRL and LRL. The branching index (BI) was computed as the ratio of LRL to NRL.
SMF treatment at reproductive stage
The plant establishment and water treatments were done in the same manner as described in Experiment 1 using 225 F2 plants and the parents; Nipponbare and KDML 105.
Shoot and root sampling at reproductive stage
The plants with flag leaf were selected, and their shoot and roots were sampled at 80 DAT. Shoot and root sampling and measurements were done as described in Experiment 1 while root measurements were done as described in the vegetative stage SMF in this experiment.
Genotyping
The DNA was extracted from lyophilized leaf samples using a modified Dellaporta method. The quality of extracted DNA was checked with electrophoresis on a 0.6% agarose gel in 1 × Tris/Borate/EDTA (TBE; 40 mmol L−1Tris, 20 mmol L−1acetic acid, and 0.5 mmol L−1 EDTA). The QuantiFluor dsDNA System and a Quantus fluorometer instrument (Promega) were used for the quantification of double-stranded DNA (Dellaporta et al., Citation1983).
A GBS library was prepared following the protocol of Poland et al. (Citation2012) with modifications as described by Furuta et al. (Citation2017). The obtained genotypes were filtered using TASSEL5 (Bradbury et al., Citation2007). For filtering individuals, minimum proportion of sites present >0.8, maximum heterozygous proportion of 0.25 was applied. For filtering markers, minimum count >200, and minimum minor allele frequency >0.05 were applied. The markers that looked low in quality were visually removed.
QTL analysis was performed using the R package ‘qtl’ (v1.37.11) (Broman et al., Citation2003). The populations of different plots were analyzed separately. The additive effect was estimated at the maximum LOD score. The critical threshold value of LOD at a genome-wide significant level of p = 0.05 was 2.5. Naming of QTLs with modification followed QTL nomenclature system defined by McCouch et al. (Citation1997). The co-located QTLs were found at the same position between the flanking markers on the same chromosomal region.
Statistical analysis
The F2 plants were grown under SMF only without replication while the parents were replicated three times under both CWL and SMF conditions. Differences between mean genotype within each water treatment were compared using the LSD test at p < 0.05 level, and analyzed using CropStat version 7.2 (IRRI, 2009). Correlation analysis between the root traits was performed using SAS program (SAS version 9.1, SAS Institute, Cary, NC, USA, 2002).
Results
Evaluation of BILs (Experiment 1)
SMF dynamics
The dynamics of SMF and oxygen concentration in the field for 2013 and 2014 are shown in Supplement 2. One complete cycle of SMF comprises waterlogged-to-drought conditions, wherein the water level was raised and maintained at 5 cm depth above the soil surface for 5 days and then drained until the soil water potential dropped down to either −30 kPa in 2012 (data not shown) and 2013 or −50 kPa in 2014 prior to rewatering. In each cycle, the soil water potential dropped from 0 at rewatering to either −30 or −50 kPa at an average duration of 14 (2013) and 22 days (2014), respectively. Soil oxygen concentration increased up to 13 and 16 ppm when soil water potential was either −30 (2013) or −50 kPa (2014), respectively. After rewatering, soil water potential returned to 0 kPa, while the soil oxygen concentration dropped down near 0 ppm at an average of 10 days in 2013 and 11 days in 2014 after rewatering.
Shoot dry matter production
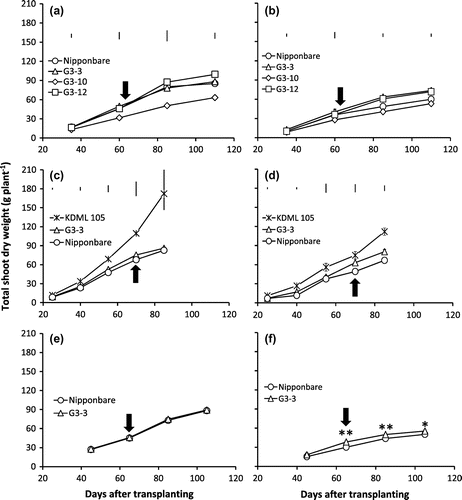
For the three years, G3-3 consistently showed no significant differences in shoot dry weight (SDW) with Nipponbare from early vegetative to maturity under CWL (Figure (a), (c), (e)). On the other hand, G3-12 had significantly higher SDW than Nipponbare under CWL at 85 DAT and G3-10 had significantly lower SDW than Nipponbare under CWL at 110 DAT in 2012 (Figure (a)). Under CAW-D, there were no significant differences in SDW among BILs and Nipponbare from 35 to 60 DAT (almost heading) in 2012 and at 25 DAT in 2013 (Figure (b), (d)). However, in 2012, G3-12 and G3-3 showed significantly greater SDW than Nipponbare by 24 and 30% at 85 DAT; 20 and 22% at 110 DAT (maturity), respectively, and G3-10 had significantly lower SDW than Nipponbare (Figure (b)). Likewise in 2013, G3-3 had significantly higher SDW than Nipponbare by 28% at 70 DAT and 20% at 85 DAT (Figure (d)). In addition, under CAW-D with severe drought stress (−50 kPa), G3-3 showed greater SDW than Nipponbare by 27, 14 and 10% at 65, 85 and 105 DAT (maturity stage), respectively (Figure (f)). Under CAW-D condition, SDW generally was reduced for all genotypes as compared to their CWL counterparts.
Figure 1. Total shoot dry weight of NILs, G3-3, G3-10, G3-12, KDML 105, and Nipponbare genotypes under continuous waterlogging (CWL) (a, c, e) and continuous cycles of alternate waterlogging and drought (CAW-D) of mild stress (b, d) and severe stress (f) in 2012 (a, b), 2013 (c, d) and 2014 (e, f).
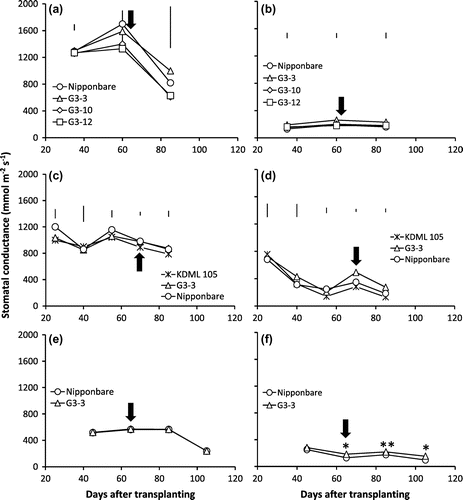
Stomatal conductance
Figure shows the dynamics in stomatal conductance of genotypes under different soil moisture treatments in 2012, 2013, and 2014. Generally, the stomatal conductance was not significantly different among genotypes under CWL regardless of years and growth stages (Figure (a), (c), (e)). In contrast, there were observed differences between only G3-3 and Nipponbare at certain growth stages under CAW-D both with mild and severe drought stresses. For instance, G3-3 showed significantly higher stomatal conductance than Nipponbare at 60 and 85 DAT in 2012 (Figure (b)) and at 70 and 85 DAT in 2013 (Figure (d)) under CAW-D with mild drought stress prior to rewatering. Furthermore, G3-3 had significantly higher stomatal conductance than Nipponbare at 85 DAT and 105 DAT under CAW-D with severe drought stress (Figure (f)).
Figure 2. Stomatal conductance of NILs, G3-3, G3-10, G3-12, KDML 105, and Nipponbare genotypes under continuous waterlogging (CWL) (a, c, e) and continuous cycles of alternate waterlogging and drought (CAW-D) of mild stress (b, d) and severe stress (f) in 2012 (a, b), 2013 (c, d), and 2014 (e, f).
Root system development
Figure shows the TRL of the whole root system under CWL and CAW-D with mild and severe drought stresses at different growth stages in three years. There were no significant differences between the BILs and Nipponbare under CWL in all growth stages for all years except G3-12 that had significantly greater TRL at 85 and 110 DAT in 2012 (Figure (a)). In contrast, under CAW-D, G3-12 and G3-3 had significantly longer TRL than Nipponbare by 30 and 39% at 85 DAT, and 31 and 32% at maturity, respectively, in 2012. The G3-10 had similar TRL with Nipponbare under CAW-D (Figure (b)). In addition, in 2013, G3-3 had significantly greater TRL than Nipponbare at 70 and 85 DAT by 34 and 39%, respectively (Figure (d)). Also in 2014, under severe stress of CAW-D, G3-3 developed significantly greater root system than Nipponbare at 85 and 105 DAT (Figure (f)). Furthermore, under CWL, the genotypes showed an increasing trend in TRL from 60 to 85 DAT in 2012 and 40 to 55 DAT in 2013 (Figure (a), (c)), and from 45 to 65 DAT in 2014 (Figure (e)). On the contrary, under severe stress of CAW-D, G3-3 showed its ability to continue to increase the TRL from heading (65 DAT) to maturity (105 DAT) stages (Figure (f)).
Figure 3. Total root length of NILs, G3-3, G3-10, G3-12, KDML 105, and Nipponbare genotypes under continuous waterlogging (CWL) (a, c, e) and continuous cycles of alternate waterlogging and drought (CAW-D) of mild stress (b, d) and severe stress (f) in 2012 (a, b), 2013 (c, d) and 2014 (e, f).
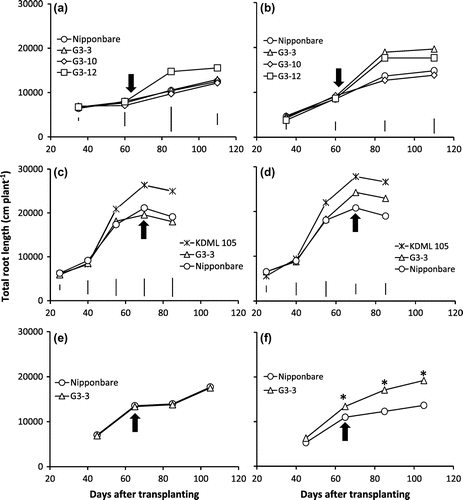
For nodal root production, similar trend was observed as in TRL. Under CWL, there were no significant differences between the BILs and Nipponbare except G3-10, which had significantly lower nodal root production in 2012. On the other hand, under mild and severe stresses of CAW-D, G3-3 significantly produced more nodal roots than Nipponbare especially from heading to maturity growth stages (Supplement 3).
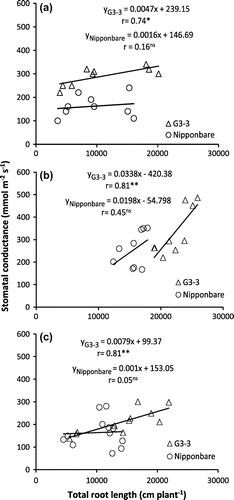
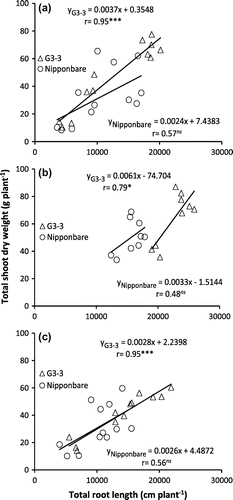
There were positive and significant relationships between TRL and stomatal conductance; TRL and shoot dry weight (Figures and ) as well as stomatal conductance and shoot dry weight (Supplement 5) for G3-3 but no significant correlations were observed in Nipponbare under CAW-D conditions.
Yield and yield components
Tables and show the yield and yield components in 2012 and 2013, respectively. In both years, the genotypes showed no significant differences in yield and yield components under CWL. On the contrary, in 2012 under CAW-D, only G3-3 showed significantly higher yield and yield components than the other genotypes except total number of spikelets (TNS). Similarly in 2013, there were no significant differences among genotypes for TNS under CAW-D. However, G3-3 had significantly higher number of filled spikelet (FS), % FS, 1000 grain weight (GW) and grain yield (GY) under CAW-D (Table ). There was no yield data obtained for KDML105 because this variety did not reach maturity due to its sensitivity to photoperiod. Yield and yield components were not determined in 2014.
Table 1. Yield and yield components of genotypes subjected to continuous waterlogging (CWL) and continuous cycle of alternate waterlogging and drought (CAW-D) in 2012.
Table 2. Yield and yield components of genotypes subjected to continuous waterlogging (CWL) and continuous cycle of alternate waterlogging and drought (CAW-D) in 2013.
Identification of root trait QTLs (experiment 2)
In this experiment, five root developmental traits such as NRL, LRL, TRL, NRN, and BI were phenotyped at vegetative and reproductive growth stages under mild drought stress.,
Phenotypic variation in root traits
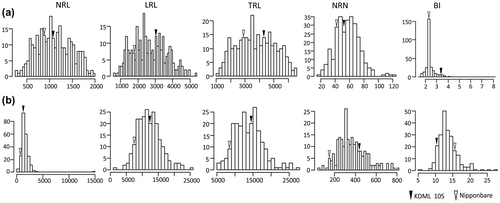
Figure shows normal distributions of variations in root traits among the individuals at vegetative (38 days after sowing, DAS) and reproductive (80 DAT) growth stages. Nipponbare and KDML 105 parents had significant differences in the five root traits measured under SMF conditions. Mean values for F2 mapping population under SMF in terms of root system development were generally higher than Nipponbare but lower or similar to that of KDML 105 in both growth stages (Supplement 8).
Figure 6. Frequency distribution of root traits of F2 mapping population under soil moisture fluctuation (SMF) conditions at (a) vegetative (38 DAS) and (b) reproductive stages (80 DAT). NRL, total nodal root length (cm plant−1); LRL, total lateral root length (cm plant−1); TRL, total root length (cm plant−1); NRN, total nodal root number (No. plant−1) and BI, branching index (cm LRL cm−1 NRL).
Correlations among root traits
The relationships among root traits under SMF at vegetative and reproductive stages are shown in Supplement 9. Significant and positive correlations existed among the root traits except between NRN and BI at the vegetative stage, between TRL and BI, and between LRL and BI at reproductive stage. Significant and negative relationships were observed between NRL and BI at vegetative stage, and between NRN and BI, and NRL and BI at the reproductive stage. At both growth stages, LRL and TRL had very strong positive and significant relationships.
Linkage map and marker segregation
A molecular linkage map was constructed in each growth stage of plants grown under SMF condition using phenotypic and genotypic root data of the F2 mapping population derived from Nipponbare and KDML 105. A total of 1653 polymorphic DNA markers was obtained after filtering and used to segregate the parental lines (Supplement 10).
QTLs associated with root traits at vegetative stage under SMF
A total of 12 QTLs were identified for NRL, LRL, TRL and BI while no QTL was identified NRN under SMF (Table ). Three QTLs for NRL were detected on chromosome (Chr) 5 (qNRL-5), Chr 10 (qNRL-10) and Chr 12 (qNRL-12). The additive effects of QTLs on Chr 5 and Chr 10 were from KDML 105 alleles whereas QTL on Chr 12 was from Nipponbare alleles. The QTLs were detected in the regions flanked by markers S5_2354630 and S5_2967915 on Chr 5, markers S10_22044899 and S10_22565922 on Chr 10, and markers S12_1376460 and S12_2300626 on Chr 12 with phenotypic variations ranging from 4.4 to 5.9%. Also, LRL QTL; qLRL-5 on Chr 5 with phenotypic variation of 4.8% was detected in marker region S5_25208100 and S5_25666504. The QTL for LRL, qLRL-7 on Chr 7 was identified in the marker region S7_17471054 and S7_17802728 with 4.4% phenotypic variation. Again, the QTL, qLRL-10 with phenotypic variation 4.3% was found in marker region S10_21111375 and S10_21269673 on Chr 10. Similarly, three QTLs for TRL were detected on the same chromosomes and marker regions as QTLs for LRL with phenotypic variation ranging from 4.0% to 5.4%. The additive effects were all from KDML 105 alleles. In addition, three QTLs for BI were detected on Chr 1 (qBI-1), 2 (qBI-2) and 5 (qBI-5) with corresponding additive effects of the QTLs, all of which were contributed by Nipponbare alleles. The QTLs were detected in the region flanked by markers S1_35119777 and S1_35880614 on Chr 1, S2_30786098 and S2_31238642 on Chr 2, S5_2354630 and S5_2967915 on Chr 5, which explained 7.0, 4.3, and 5.2% of the phenotypic variation in branching index, respectively (Table ).
Table 3. QTLs associated with the root traits detected using Nipponbare/KDML 105 F2 population at vegetative (38 DAS) and reproductive (80 DAT) stages under soil moisture fluctuation (SMF) condition.
QTLs associated with root traits at reproductive stage under SMF
A total of 15 QTLs were identified for NRN, NRL, LRL, TRL and BI at reproductive stage under SMF (Table ). Three QTLs, qNRL-3 qLRL-3 and qTRL-3 were detected for NRL, LRL and TRL in the same region flanked by markers S3_3152304 and S3_3852048 on Chr 3. These QTLs explained 3.5, 5.7, and 6.0%, respectively, of phenotypic variation with increased effect from KDML 105 alleles. Moreover, two more QTLs for TRL were detected on Chr 1 (qTRL-1) and 10 (qTRL-10) with additive effects contributed by KDML 105. The QTLs were detected in the regions flanked by markers S1_31665708 and S1_32765279 on chromosome 1 and markers S10_22044899 and S10_22565922 on Chr 10, which explained 4.9 and 5.5% of the phenotypic variation, respectively. For NRN, four QTLs contributed by KDML 105 alleles were identified on Chr 1 (qNRN-1), Chr 3 (qNRN-3), Chr 8 (qNRN-8) and Chr 12 (qNRN-12), which explained 6.2 to 10.3% of the phenotypic variation. The QTLs were detected in region flanked by markers S1_35119777 and S1_35880614 on Chr 1, S3_1947308 and S3_2275550 on Chr 3, S8_7779094 and S8_8019735 on Chr 8 and S12_3162247 and S12_3228960 on Chr 12. Furthermore, six QTLs for BI were detected on Chr 2 (qBI-2), Chr 3 (qBI -3), Chr 4 (qBI -4), Chr 6 (qBI -6), Chr 10 (qBI -10) and Chr 12 (qBI-12). The additive effects of QTL on Chr 2 was from KDML 105 alleles whereas those on Chr 3, 4, 6, 10 and 12 were from Nipponbare. The QTLs explained 5.0 to 17.2% phenotypic variation. QTL, qBI -3 that produced the highest phenotypic variation by 17.2% was identified in the region flanked by markers S3_6797449 and S3_7207675 on Chr 3.
Co-locations of identified QTLs
At the vegetative stage under SMF, QTLs, qBI-5 and qNRL-5 for BI and NRL, respectively, were identified in the same region flanked by markers S5_2354630 and S5_2967915 on Chr 5. The QTLs, qLRL-5 and qTRL-5 for LRL and TRL, respectively, were mapped in the same region flanked by markers S5_25208100 and S5_25666504 on Chr 5. Furthermore, QTLs, qLRL-7 and qTRL-7 on Chr 7 overlapped in the same region flanked by markers S7_17471054 and S7_17802728. Likewise, QTLs, qLRL-10 and qTRL-10 on Chr 10 coincided in the region flanked by markers S10_21111375 and S10_21269673. At the reproductive stage under SMF, three QTLs, qNRL-3, qLRL-3 and qTRL-3 overlapped in the same region flanked by markers S3_3152304 and S3_3852048 on Chr 3 (Table , Supplement 10).
However, only two QTLs, qBI-1 and qNRN-1 were mapped for BI and NRN at vegetative and reproductive stages, respectively. These QTLs were detected on Chr 1 region flanked by markers S1_35119777 and S1_35880614. Likewise, two QTLs, qNRL-10 and qTRL-10 for NRL and TRL at vegetative and reproductive stages on Chr 10 overlapped in the same region flanked by markers S10_22044899 and S10_22565922 (Table ).
The QTLs for LRL and TRL on Chr 7, LRL and TRL on Chr 10 and NRL on Chr 10 at vegetative stage were located in the same chromosomal region of BIL, G3-3. Also at the reproductive stage, QTLs for TRL and NRN on Chr 10 and Chr 12, respectively, coincided in the same marker region of BIL, G3-3 under SMF conditions.
Discussion
Root plasticity and its contribution to dry matter production and yield
In this study, the BILs with Nipponbare genetic background carrying introgressed segments from KDML 105 were evaluated under mild or severe drought stresses of CAW-D conditions to examine the expression of root plasticity in response to SMF, and its contribution to dry matter production and grain yield.
Previous studies have reported the reduction of shoot dry matter production (Bañoc et al., Citation2000a, Citation2000b; Suralta & Yamauchi, Citation2008; Suralta et al., Citation2008, Citation2010) and grain yield (Allahyar, Citation2011; Belder et al., Citation2005; Bouman et al., Citation2005; Castillo et al., Citation2006; Niones et al., Citation2012) of rice plants when grown under fluctuating soil moisture conditions, which indicates that such SMF is stressful for rice plants. In this aspect, we have shown evidences on the physiological significance of root plasticity that is expressed in response to heterogeneous soil environments in space and time for the adaptation of rice plants, as well as its genotypic variations (Bañoc et al., Citation2000a; Kano-Nakata et al., Citation2013; Niones et al., Citation2012; Nguyen et al., Citation2018; Siopongco et al., Citation2008; Suralta et al., Citation2010, Citation2016, Citationin press; Tran et al., Citation2014).
In this study, we considered the BIL(s) that showed no significant differences with the recurrent parent under CWL but had greater root system development and shoot dry matter than the recurrent parent, Nipponbare under SMF. As a result, we found that the root system development based on TRL of G3-3 was significantly greater than that of Nipponbare especially from heading to maturity under CAW-D at −30 kPa and even when the drought was intensified to −50 kPa (Figure (b), (d), (f)). This can be attributed to the plasticity in production of nodal roots, resulting from new tillers production, and in the development of lateral roots (Supplements 3 and 4). These observations were similar to those reported by Niones et al. (Citation2012) where the root system development based on TRL of one chromosome segment substitution lines (CSSL47) at 35 days before and after heading were significantly greater than its recurrent parent, Nipponbare under CAW-D of mild stress.
These results imply that the root plasticity that resulted in increased TRL of which lateral roots constitute greater portion (Wang et al., Citation2009; Yamauchi et al., Citation1987), and promoted nodal root development might play a key role in adaptation of rice plants to SMF conditions; i.e. waterlogged conditions followed by drought and rewatering (Bañoc et al., Citation2000a, Citation2000b; Niones et al., Citation2012). This further suggests that the ability of the plant to maintain greater root length (Bañoc et al., Citation2000b) can recompense for the limited water availability in soil under progressive drought stress in SMF conditions by expanding the root surface area and increasing water extraction (Henry et al., Citation2011; Kato et al., Citation2007, Citation2011; Siopongco et al., Citation2005, Citation2006; Suralta et al., Citation2010). Thus, the maintenance or increase in stomatal conductance that reflects root water uptake ability (Kano et al., Citation2011; Matsuo et al., Citation2010) of G3-3 under not only mild stress of CAW-D (in 2012 and 2013) but also severe stress in 2014 (Figure (b), (d), (f)) could be a consequence of greater water supply to the leaves due to the promoted root growth under those conditions. Significant and positive relationships between TRL with stomatal conductance, and that of stomatal conductance with dry matter production and yield under CAW-D indicate that water uptake and dry matter productions were enhanced by increased root length due to the expressed plasticity (Figures and ; Supplement 5).
The BIL, G3-3 had higher grain yield than Nipponbare under CAW-D condition, which was attributed to its significantly higher filled spikelet (FS) and % filled spikelet (FS) since there was no significant difference in TNS between the two genotypes (Tables and ). These results suggest that the promoted root development due to plasticity and the resulting enhanced water uptake contributed to yield through grain filling (Supplements 6 and 7).
Furthermore, the shoot and root development of G3-3 was comparable with that of Nipponbare under CWL conditions, and similar growth performances were also observed in studies using a chromosome segment substitution line (CSSL50) and the recurrent parent, Nipponbare grown under constant drought condition (Kano et al., Citation2011) as well as CSSL47 and Nipponbare under SMF (Niones et al., Citation2012; Suralta et al., Citation2010). This suggests that G3-3 may share similar genetic control with its recurrent parent Nipponbare for root development under constant stress conditions such as waterlogging and drought but not under SMF conditions.
Moreover, we previously reported that waterlogging can be stressful even to rice plants, particularly when this stress is preceded by drought. This is due to the fact that the drought may reduce the plant’s capability of forming aerenchyma under the subsequent waterlogged conditions that cause sudden O2 deficiency (Niones et al., Citation2012; Suralta & Yamauchi, Citation2008). Results of the present study indicate that the reduction in growth especially shoot dry matter production of G3-3 and Nipponbare caused by SMF stress can occur in actual rainfed lowlands and irrigated areas where water saving technology is practiced, and the genotypic differences in the reduction may be attributed to the differences in the ability of root aerenchyma formation, which is an interesting subject for further study.
The BIL, G3-3 has substituted chromosome segments from KDML 105 allele on all chromosomes except chromosomes 3, 8, 9, and 11 regions (Supplement 1). These introgressed segments may be responsible for the root plasticity that was expressed in response to mild and severe drought stresses in CAW-D since in a series of our studies, KDML 105 has consistently shown better root growth adaptation to fluctuating soil moisture conditions due to its high branching ability, especially in the relatively shallow soil layer (Bañoc et al., Citation2000b; Kameoka et al., Citation2015; Kano-Nakata et al., Citation2013). Hence, there is a need to identify QTLs associated with such root traits that are related with plasticity.
Genomic regions related to root traits
Wide phenotypic variation of the target traits of the parental lines is important in QTL analyses (Collard et al., Citation2005), hence the use of parental cultivars, Nipponbare and KDML 105. In this study, using F2 plants (Nipponbare/KDML 105), a total of 27 putative QTLs related to five different root traits; NRL, LRL, TRL, NRN, and BI were identified on almost all of the chromosomes except 9 and 11 (Table , Supplement 10) at vegetative and reproductive growth stages.
At the vegetative stage, two QTLs for LRL and TRL on Chr 7 were found in the same chromosomal region of BIL, G3-3 introgressed for KDML 105. Also, three QTLs for NRL, LRL and TRL on Chr 10 were located in the same chromosomal region of G3-3. In addition, QTLs detected for TRL on Chr 10 and NRN on Chr 12 coincided in the same genomic region of G3-3. These above-mentioned QTLs were found in the region of G3-3 carrying substituted segments of KDML 105. For G3-12, only two QTLs for LRL and TRL on Chr 10 at the vegetative stage were located in the same chromosomal region carrying KDML 105 segments. On the other hand, none of the root trait QTLs identified coincided with the KDML 105 substituted segments of G3-10 which may explain its significantly lower root system development. In view of this, the QTLs identified at the vegetative stage for LRL and TRL on Chr 7and NRL on Chr 10 as well as QTLs detected at reproductive stage for TRL on Chr 10 and NRN on Chr 12 may have contributed to the root plasticity in G3-3 since they were not found in the substituted segments of KDML 105 in G3-10 and G3-12. In addition, the QTL controlling LRL might be the same with that for TRL, and thus the promoted production of lateral roots leading to increase in the whole root system, can contribute to increasing root surface area and soil water extraction. Such increase could contribute to the maintenance of stomatal conductance and hence the dry matter production and yield under SMF condition as observed in G3-3.
Previous studies showed that different root trait QTLs could be detected at different growth stages while only few of them coincided in all stages. For instance, Xu et al. (Citation2004) reported that only one QTL for maximum root length out of seven was co-located at stages of 30 and 40 day after seedling. Likewise, studies by Qu et al. (Citation2008) showed that 12 out of 84 additive QTLs were persistently expressed at two or more growth stages but no QTL was detected at all stages. This corroborates with the present study where only two QTLs each, qBI-1 and qNRN-1 on Chr 1; qNRL-10 and qTRL-10 on chromosome 10 coincided in the same marker region for both growth stages, suggesting that these root traits at different growth stages under SMF may be influenced by QTL x environment interaction (Kamoshita et al., Citation2002; Li et al., Citation2005; Paterson et al., Citation2003; Price et al., Citation2002; Qu et al., Citation2008), which may be related to the low heritability of lateral roots. The aforementioned QTLs for NRL and TRL on Chr 10 which was also located on the same chromosomal region of G3-3 overlapped with QTLs for TRL at 30 cm soil depth in CT9993 and IR62266 genetic mapping population tested under SMF (Suralta et al., Citation2015) (Supplement 11).
Furthermore, QTLs, qLRL-7 and qTRL-7 which were detected in the same chromosomal region of G3-3 coincided with QTLs for number of crown roots per tiller and plant dry weight in panel of rice accessions (Phung et al., Citation2016); maximum root length and basal root thickness in IRAT 109 and Yuefu cross (Qu et al., Citation2008) and grain yield in Vandana and Way Rarem cross (Bernier et al., Citation2007) (Supplement 11). These detected QTLs could play important roles in root system development, thereby increasing water and nutrient uptake, resulting in increased production of dry matter which could be beneficial for stabilizing rice production under various stress conditions. The NRN QTL, qNRN-12 detected on Chr 12 at the reproductive stage which were also found in the same chromosomal region of G3-3 overlapped with QTLs for aerenchyma formation (Niones et al., Citation2013) (Supplement 11), which is useful in oxygen diffusion for rice plants subjected to sudden waterlogged condition after transient drought, thereby improving the adaptation to fluctuating soil moisture stress which occurs in rainfed lowland fields.
Moreover, QTLs detected for NRL, LRL, and TRL on Chr 3 at reproductive stage which were not found in the same chromosomal region of G3-3 carrying substituted segments of KDML 105 coincided with QTLs for biomass yield (Bernier et al., Citation2007) can be important in the development of root system of rice plants grown under SMF condition. Likewise, the BI QTL detected on Chr 3 contributed by Nipponbare alleles with the highest LOD score among the identified QTLs could play functional role in branching ability particularly in shallow soil layer resulting in promoted production of lateral roots and elongation which can enhance nutrient and water uptake under fluctuating soil moisture environment such as rainfed lowland rice ecosystem. This QTL associated with BI to the best of our knowledge could be the first reported on Chr 3 since it did not overlap in the same chromosome region with BI QTLs reported in other studies.
Conclusion
We examined BILs with Nipponbare genetic background carrying chromosome segments of KDML 105 to evaluate the expression of root plasticity and its functional roles, and attempted to identify associated QTLs under SMF conditions. The BIL, G3-3, exhibited improved adaptation to soil moisture fluctuating stress than the recurrent parent, Nipponbare due to its greater root system as a result of promoted production of nodal roots and development of lateral roots, which led to the maintenance of stomatal conductance and consequently dry matter production and grain yield. QTLs were identified for NRL, LRL, TRL, NRN, and BI at vegetative and reproductive stages although the QTLs detected at vegetative and reproductive stages were different. The QTLs for LRL and TRL on Chr 7 and NRL on Chr 10 identified at vegetative stage as well as QTLs detected at reproductive stage for TRL on Chr 10 and NRN on Chr 12 which were located in the same chromosomal region in G3-3 introgressed from KDML 105 could have contributed to the expression of root plasticity in G3-3. The unique characteristics of G3-3 and the associated genomic regions could be useful in breeding programs to help improve the adaptation of rice plants grown in rainfed lowland areas where SMF stresses usually occur. However, further study on genetic analysis and phenotypic evaluation of the detected QTLs is necessary.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Grant-in-Aid for Scientific Research [grant number 15H02644] from the Japan Society for the Promotion of Science; and the Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA) and Science and Technology Research Partnership for Sustainable Development (SATREPS).
Supplemental data
Supplemental data for this article is available online at https://doi.org/10.1080/1343943X.2018.1446759.
PPS2018_007RP-File004.docx
Download MS Word (3.9 MB)Acknowledgments
We are very thankful to Mr. Peter Ofori Amoako and Dr. Daniel Menge for their generous support with conducting the experiments.
References
- Allahyar, F. (2011). Interaction effects of nitrogen and irrigation methods on the growth and yield of rice in Amol area. International Journal of Agriculture and Crop Sceinces, 3(4), 111–113.
- Azhiri-Sigari, T., Yamauchi, A., Kamoshita, A., & Wade, L. J. (2000). Genotypic variation in response of rainfed lowland rice to drought and rewatering. II. Root growth. Plant Production Science, 3, 180–188.10.1626/pps.3.180
- Bañoc, D. M., Yamauchi, A., Kamoshita, A., Wade, L. J., & Pardales Jr., J. R. (2000a). Dry matter production and root system development of rice cultivars under fluctuating soil moisture. Plant Production Science, 3, 197–207.
- Bañoc, D. M., Yamauchi, A., Kamoshita, A., Wade, L. J., & Pardales Jr., J. R. (2000b). Genotypic variations in response of lateral root development to fluctuating soil moisture in rice. Plant Production Science, 3, 335–343.
- Belder, P., Spiertz, J. H. J., Bouman, B. A. M., Lu, G., & Tuong, T. P. (2005). Nitrogen economy and water productivity of lowland rice under water-saving irrigation. Field Crops Research, 93(2–3), 169–185.10.1016/j.fcr.2004.09.022
- Bengough, A. G., McKenzie, B. M., Hallett, P. D., & Valentine, T. A. (2011). Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany, 62, 59–68.
- Bernier, J., Kumar, A., Ramaiah, V., Spaner, D., & Atlin, G. (2007). A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Science, 47(2), 507–518.10.2135/cropsci2006.07.0495
- Boling, A., Toung, T. P., Jatmiko, S. Y., & Burac, M. A. (2004). Yield constraints of rainfed lowland rice in Central Java, Indonesia. Field Crops Research, 90, 351–360.10.1016/j.fcr.2004.04.005
- Bouman, B. A. M., Humphreys, E., Tuong, T. P., & Barker, R. (2007). Rice and water. Advances in Agronomy, 92, 187–237.10.1016/S0065-2113(04)92004-4
- Bouman, B. A. M., Peng, S., Castañeda, A. R., & Visperas, R. M. (2005). Yield and water use of irrigated tropical aerobic rice systems. Agricultural Water Management, 74(2), 87–105.10.1016/j.agwat.2004.11.007
- Bradbury, P. J., Zhang, Z., Kroon, D. E., Casstevens, T. M., Ramdoss, Y., & Buckler, E. S. (2007). TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics, 23(19), 2633–2635.10.1093/bioinformatics/btm308
- Broman, K. W., Wu, H., Sen, S., & Churchill, G. A. (2003). R/qtl: QTL mapping in experimental crosses. Bioinformatics, 19, 889–890.10.1093/bioinformatics/btg112
- Cairns, J. E., Impa, S. M., O’Toole, J. C., Jagadish, S. V. K., & Price, A. H. (2011). Influence of the soil physical environment on rice (Oryza sativa L.) response to drought stress and its implications for drought research. Field Crops Research, 121(3), 303–310.10.1016/j.fcr.2011.01.012
- Carrijo, D. R., Lundy, M. E., & Linquist, B. A. (2017). Rice yields and water use under alternate wetting and drying irrigation: A meta-analysis. Field Crops Research, 203, 173–180.10.1016/j.fcr.2016.12.002
- Castillo, E. G., Tuong, T. P., Singh, U., Inubushi, K., & Padilla, J. (2006). Drought response of dry-seeded rice to water stress timing and N-fertilizer rates and sources. Soil Science and Plant Nutrition, 52, 496–508.10.1111/j.1747-0765.2006.00064.x
- Clark, L. J., Ferraris, S., Price, A. H., & Whalley, W. R. (2008). A gradual rather than abrupt increase in soil strength gives better root penetration of strong layers. Plant and Soil, 307, 235–242.10.1007/s11104-008-9602-8
- Collard, B. C. Y., Jahufer, M. Z. Z., Brouwer, J. B., & Pang, E. C. K. (2005). An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica, 142, 169–196.10.1007/s10681-005-1681-5
- Dellaporta, S. L., Wood, J., & Hicks, J. B. (1983). A plant DNA minipreparation: Version II. Plant Molecular Biology Reporter, 1(4), 19–21.10.1007/BF02712670
- Ding, X., Li, X., & Xiong, L. (2011). Evaluation of near-isogenic lines for drought resistance QTL and fine mapping of a locus affecting flag leaf width, spikelet number, and root volume in rice. Theoretical and Applied Genetics, 123(5), 815–826.10.1007/s00122-011-1629-1
- Fukai, S., & Cooper, M. (1995). Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Research, 40, 67–86.10.1016/0378-4290(94)00096-U
- Furuta, T., Ashikari, M., Jena, K. K., Doi, K., & Reuscher, S. (2017). Adapting genotyping-by-sequencing for rice F2 populations. G3: Genes Genomes Genetics, 7(3), 881–893.10.1534/g3.116.038190
- Gomez, M. S., Kumar, S. S., Jeyaprakash, P., Suresh, R., Biji, K. R., Boopathi, N. M., … Babu, R. C. (2006). Mapping QTLs linked to physio-morphological and plant production traits under drought stress in rice (Oryza sativa L.) in the target environment. American Journal of Biochemistry and Biotechnology, 2(4), 161–169.
- Gomez, S. M., Boopathi, N. M., Kumar, S. S., Ramasubramanian, T., Chengsong, Z., Jeyaprakash, P., … Babu, R. C. (2010). Molecular mapping and location of QTLs for drought-resistance traits in indica rice (Oryza sativa L.) lines adapted to target environments. Acta Physiologiae Plantarum, 32(2), 355–364. 10.1007/s11738-009-0413-1
- Henry, A. (2013). IRRI’s drought stress research in rice with emphasis on roots: Accomplishments over the last 50 years. Plant Root, 7, 5–19.
- Henry, A., Gowda, V. R. P., Torres, R. O., McNally, K. L., & Serraj, R. (2011). Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Research, 120(2), 205–214.10.1016/j.fcr.2010.10.003
- IRRI (2009). CROPSTAT version 7.2. Metro Manila: International Rice Research Institute. Retrieved from http://archive.irri.org/science/software/cropstat.asp
- Kameoka, E., Suralta, R. R., Mitsuya, S., & Yamauchi, A. (2015). Matching the expression of root plasticity with soil moisture availability maximizes production of rice plants grown in an experimental sloping bed having soil moisture gradients. Plant Production Science, 18, 267–276.10.1626/pps.18.267
- Kamoshita, A., Wade, L. J., Ali, M. L., Pathan, M. S., Zhang, J., Sarkarung, S., & Nguyen, H. T. (2002). Mapping QTLs for root morphology of a rice population adapted to rainfed lowland conditions. Theoretical and Applied Genetics, 104(5), 880–893.
- Kamoshita, A., Wade, L. J., & Yamauchi, A. (2000). Genotypic variation in response of rainfed lowland rice to drought and rewatering. III. Water extraction during the drought period. Plant Production Science, 3, 189–196.10.1626/pps.3.189
- Kang, S. Y., Morita, S., & Yamazaki, K. (1994). Root growth and distribution in some japonica-indica hybrid and japonica type rice cultivars under field conditions. Japanese journal of crop science, 63(1), 118–124.10.1626/jcs.63.118
- Kano, M., Inukai, Y., Kitano, H., & Yamauchi, A. (2011). Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant and Soil, 342, 117–128.10.1007/s11104-010-0675-9
- Kano-Nakata, M., Gowda, V. R. P., Henry, A., Serraj, R., Inukai, Y., Fujita, D., … Yamauchi, A. (2013). Functional roles of the plasticity of root system development in biomass production and water uptake under rainfed lowland conditions. Field Crops Research, 144, 288–296.10.1016/j.fcr.2013.01.024
- Kano-Nakata, M., Inukai, Y., Siopongco, J. D. L. C., Mitsuya, S., & Yamauchi, A. (2017). Quantitative evaluation of plastic root responses to contiguous water gradient in rice. Plant Root, 11, 70–78.10.3117/plantroot.11.70
- Kano-Nakata, M., Inukai, Y., Wade, L. J., Siopongco, J. D., & Yamauchi, A. (2011). Root development, water uptake, and shoot dry matter production under water deficit conditions in two CSSLs of rice: Functional roles of root plasticity. Plant Production Science, 14, 307–317.10.1626/pps.14.307
- Kano-Nakata, M., Suralta, R. R., Niones, J. M., & Yamauchi, A. (2012). Root sampling by using a root box–pinboard method. In H. E. Shashidhar, A. Henry, & B. Hardy (Eds.), Methodologies for root drought studies in rice (pp. 3–8). Los Baños: International Rice Research Institute.
- Kato, Y., Henry, A., Fujita, D., Katsura, K., Kobayashi, N., & Serraj, R. (2011). Physiological characterization of introgression lines derived from an indica rice cultivar, IR64, adapted to drought and water-saving irrigation. Field Crops Research, 123(2), 130–138.10.1016/j.fcr.2011.05.009
- Kato, Y., Kamoshita, A., Yamagishi, J., Imoto, H., & Abe, J. (2007). Growth of rice ( Oryza Sativa L.) cultivars under upland conditions with different levels of water supply3. Root system development, soil moisture changeand plant water status. Plant Production Science, 10, 3–13.10.1626/pps.10.3
- Kato, Y., Okami, M., & Katsura, K. (2009). Yield potential and water use efficiency of aerobic rice (Oryza sativa L.) in Japan. Field Crops Research, 113(3), 328–334.10.1016/j.fcr.2009.06.010
- Kono, Y., Tomida, K., Tatsumi, J., Nanoyama, T., Yamauchi, A., & Kitano, J. (1987). Effects of soil moisture conditions on the development of root systems of soybean plants (Glycine max MERR.). Japanese Journal of Crop Science, 56, 597–607.10.1626/jcs.56.597
- Li, J., Wang, D., Xie, Y., Zhang, H., Hu, G., Li, J., … Li, Z. (2011). Development of upland rice introgression lines and identification of QTLs for basal root thickness under different water regimes. Journal of Genetics and Genomics, 38(11), 547–556.10.1016/j.jgg.2011.08.005
- Li, Z., Mu, P., Li, C., Zhang, H., Li, Z., Gao, Y., & Wang, X. (2005). QTL mapping of root traits in a doubled haploid population from a cross between upland and lowland japonica rice in three environments. Theoretical and Applied Genetics, 110(7), 1244–1252.10.1007/s00122-005-1958-z
- Liu, L., Mu, P., Li, X., Qu, Y., Wang, Y., & Li, Z. (2008). Localization of QTL for basal root thickness in japonica rice and effect of marker-assisted selection for a major QTL. Euphytica, 164(3), 729–737.10.1007/s10681-008-9695-4
- Matsuo, N., Ozawa, K., & Mochizuki, T. (2010). Physiological and morphological traits related to water use by three rice (Oryza sativa L.) genotypes grown under aerobic rice systems. Plant and Soil, 335(1–2), 349–361.10.1007/s11104-010-0423-1
- McCouch, S. R., Cho, Y. G., Yano, M., & Blinstrub, M. P. (1997). Report on QTL nomenclature. Rice Genetic Newsletter, 14, 11–13.
- Menge, M. D., Kameoka, E., Kano-Nakata, M., Yamauchi, A., Asanuma, S., Asai, H., … Makihara, D. (2016). Drought-induced root plasticity of two upland NERICA varieties under conditions with contrasting soil depth characteristics. Plant Production Science, 19, 389–400.10.1080/1343943X.2016.1146908
- Nguyen, D. T. N., Suralta, R. R., Kano-Nakata, M., Mitsuya, S., Owusu-Nketia, S., & Yamauchi, A. (2018). Genotypic variations in the plasticity of nodal root penetration through the hardpan during soil moisture fluctutaions among four rice varieties. Plant Production Science. doi:10.1080/1343943X.2018.1439757.
- Niones, J. M., Inukai, Y., Suralta, R. R., & Yamauchi, A. (2015). QTL associated with lateral root plasticity in response to soil moisture fluctuation stress in rice. Plant and Soil, 391(1–2), 63–75.10.1007/s11104-015-2404-x
- Niones, J. M., Suralta, R. R., Inukai, Y., & Yamauchi, A. (2012). Field evaluation on functional roles of root plastic responses on dry matter production and grain yield of rice under cycles of transient soil moisture stresses using chromosome segment substitution lines. Plant and Soil, 359, 107–120.10.1007/s11104-012-1178-7
- Niones, J. M., Suralta, R. R., Inukai, Y., & Yamauchi, A. (2013). Roles of root aerenchyma development and its associated QTL in dry matter production under transient moisture stress in rice. Plant Production Science, 16, 205–216.10.1626/pps.16.205
- O’Toole, J. C., & Bland, W. L. (1987). Genotypic variation in crop plant root systems. Advances in Agronomy, 41, 91–145.10.1016/S0065-2113(08)60803-2
- Patel, D. P., Das, A., Munda, G. C., Ghosh, P. K., Bordoloi, J. S., & Kumar, M. (2010). Evaluation of yield and physiological attributes of high-yielding rice varieties under aerobic and flood-irrigated management practices in mid-hills ecosystem. Agricultural Water Management, 97(9), 1269–1276.10.1016/j.agwat.2010.02.018
- Paterson, A. H., Saranga, Y., Menz, M., Jiang, C. X., & Wright, R. J. (2003). QTL analysis of genotype × environment interactions affecting cotton fiber quality. Theoretical and Applied Genetics, 106, 384–396.10.1007/s00122-002-1025-y
- Peng, S., Bouman, B., Visperas, R. M., Castañeda, A., Nie, L., & Park, H. K. (2006). Comparison between aerobic and flooded rice in the tropics: Agronomic performance in an eight-season experiment. Field Crops Research, 96(2–3), 252–259.10.1016/j.fcr.2005.07.007
- Phung, N. T. P., Mai, C. D., Hoang, G. T., Truong, H. T. M., Lavarenne, J., Gonin, M., … Courtois, B. (2016). Genome-wide association mapping for root traits in a panel of rice accessions from Vietnam. BMC Plant Biology, 16(1), 10.10.1186/s12870-016-0747-y
- Poland, J. A., Brown, P. J., Sorrells, M. E., & Jannink, J. L. (2012). Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS ONE, 7(2), e32253.10.1371/journal.pone.0032253
- Price, A. H., Steele, K. A., Moore, B. J., & Jones, R. G. W. (2002). Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. Field Crops Research, 76(1), 25–43.10.1016/S0378-4290(02)00010-2
- Qu, Y., Mu, P., Zhang, H., Chen, C. Y., Gao, Y., Tian, Y., … Li, Z. (2008). Mapping QTLs of root morphological traits at different growth stages in rice. Genetica, 133(2), 187–200.10.1007/s10709-007-9199-5
- Samson, B. K., Hasan, M., & Wade, L. J. (2002). Penetration of hardpans by rice lines in the rainfed lowlands. Field Crops Research, 76(2–3), 175–188.10.1016/S0378-4290(02)00038-2
- Sandhu, N., Anitha Raman, K., Torres, R. O., Audebert, A., Dardou, A., Kumar, A., & Henry, A. (2016). Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiology, 171(4), 2562–2576.
- Sarkarung, S., Somrith, B., & Chitrakorn, S. (2000). Aromatic rice of Thailand. In R. K. Singh, U. S. Singh, & G. S. Khush (Eds.), Aromatic rices (pp. 180–183). Enfield, NH: Science Publishers.
- Serraj, R., McNally, K. L., Slamet-Loedin, I., Kohli, A., Haefele, S. M., Atlin, G., & Kumar, A. (2011). Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Production Science, 14(1), 1–14.10.1626/pps.14.1
- Siopongco, J. D. L. C., Sekiya, K., Yamauchi, A., Egdane, J., Ismail, A. M., & Wade, L. J. (2008). Stomatal responses in rainfed lowland rice to partial soil drying; Evidence for root signals. Plant Production Science, 11, 28–41.10.1626/pps.11.28
- Siopongco, J. D. L. C., Yamauchi, A., Salekdeh, H., Bennett, J., & Wade, L. J. (2005). Root growth and water extraction responses of double haploid rice lines to drought and rewatering during the vegetative stage. Plant Production Science, 9, 141–151.
- Siopongco, J. D. L. C., Yamauchi, A., Salekdeh, H., Bennett, J., & Wade, L. J. (2006). Growth and water use response of doubled-haploid rice linesto drought and rewatering during the vegetative stage. Plant Production Science, 9, 141–151.10.1626/pps.9.141
- Sri-Aun, V. (2005). Tracing the origin of Khao’Hawm Mali. Bangkok: Group of Rice Economic Research, Rice Research Institute, Department of Agriculture, Ministry of Agriculture and Cooperative.
- Suralta, R. R., Inukai, Y., & Yamauchi, A. (2008). Utilizing chromosome segment substitution lines (CSSLs) for evaluation of root responses to transient moisture stresses in rice. Plant Production Science, 11, 457–465.10.1626/pps.11.457
- Suralta, R. R., Inukai, Y., & Yamauchi, A. (2010). Dry matter production in relation to root plastic development, oxygen transport, and water uptake of rice under transient soil moisture stresses. Plant and Soil, 332(1–2), 87–104.10.1007/s11104-009-0275-8
- Suralta, R. R., Kano-Nakata, M., Niones, J. M., Inukai, Y., Kameoka, E., Tran, T. T., … Yamauchi, A. (2016). Root plasticity for maintenance of productivity under abiotic stressed soil environments in rice: Progress and prospects. Field Crops Research. doi:10.1016/j.fcr.2016.06.023
- Suralta, R. R., Lucob, N. B., Perez, L. M., Niones, J. M., & Nguyen, H. T. (2015). Developmental and quantitative trait loci analyses of root plasticity in response to soil moisture fluctuation in rice. Philippine Journal of Crop Science, 40(2), 12–24.
- Suralta, R. R., Niones, J. M., Kano-Nakata, M., Tran, T. T., Mitsuya, S., & Yamauchi, A. (in press). Plasticity in nodal root elongation through the hardpan was trigerred by rewatering during soil moisture fluctuation stress in rice. Scientific Reports.
- Suralta, R. R., & Yamauchi, A. (2008). Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environmental and Experimental Botany, 64(1), 75–82.10.1016/j.envexpbot.2008.01.004
- Tao, H., Brueck, H., Dittert, K., Kreye, C., Lin, S., & Sattelmacher, B. (2006). Growth and yield formation of rice (Oryza sativa L.) in the water-saving ground cover rice production system (GCRPS). Field Crops Research, 95(1), 1–12.10.1016/j.fcr.2005.01.019
- Tran, T. T., Kano-Nakata, M., Suralta, R. R., Menge, D., Mitsuya, S., Inukai, Y., & Yamauchi, A. (2015). Root plasticity and its functional roles were triggered by water deficit but not by the resulting changes in the forms of soil N in rice. Plant and Soil, 386(1–2), 65–76.10.1007/s11104-014-2240-4
- Tran, T. T., Kano-Nakata, M., Takeda, M., Menge, D., Mitsuya, S., Inukai, Y., & Yamauchi, A. (2014). Nitrogen application enhanced the expression of developmental plasticity of root systems triggered by mild drought stress in rice. Plant and Soil, 378(1–2), 139–152.10.1007/s11104-013-2013-5
- Tuong, T. P., & Bouman, B. A. M. (2003). Rice production in water-scarce environments. Water Productivity in Agriculture: Limits and opportunities for improvement, 1, 13–42.
- Wade, L. J., Fukai, S., Samson, B. K., Ali, A., & Mazid, M. A. (1999). Rainfed lowland rice: Physical environment and cultivar requirements. Field Crops Research, 64(1–2), 3–12.10.1016/S0378-4290(99)00047-7
- Wade, L. J., Bartolome, V., Mauleon, R., Vasant, V. D., Prabakar, S. M., Chelliah, M., … Henry, A. (2015). Environmental response and genomic regions correlated with rice root growth and yield under drought in the oryzaSNP panel across multiple study systems. PLoS ONE, 10(4), e0124127. doi:10.1371/journal.pone.0124127
- Wang, H., Siopongco, J., Wade, L. J., & Yamauchi, A. (2009). Fractal analysis on root systems of rice plants in response to drought stress. Environmental and Experimental Botany, 65(2–3), 338–344.10.1016/j.envexpbot.2008.10.002
- Wang, H., & Yamauchi, A. (2006). Growth and function of roots under abiotic stress soils. In B. Huang (Ed.), Plant-environment Interactions (3rd ed. pp. 271–319). New York, NY: CRC Press, Taylor and Francis Group LLC.10.1201/9781420019346
- Xu, C. G., Li, X. Q., Xue, Y., Huang, Y. W., Gao, J., & Xing, Y. Z. (2004). Comparison of quantitative trait loci controlling seedling characteristics at two seedling stages using rice recombinant inbred lines. Theoretical and Applied Genetics, 109(3), 640–647.
- Yadav, S., Humphreys, E., Kukal, S. S., Gill, G., & Rangarajan, R. (2011). Effect of water management on dry seeded and puddled transplanted rice. Field Crops Research, 120(1), 123–132. 10.1016/j.fcr.2010.09.003
- Yamauchi, A., Kono, Y., & Tatsumi, J. (1987). Quantitative analysis on root system structures of upland rice and maize. Japanese Journal of Crop Science, 56, 608–617.10.1626/jcs.56.608
- Yamauchi, A., Pardales, Jr., J. R., & Kono, Y. (1996). Root system structure and its relation to stress tolerance. In O. Ito, K. Katayama, C. Johansen, J. V. D. K. Kumar Rao, J. J. Adu-Gyamfi, & T. J. Rego (Eds.), Roots and nitrogen in cropping systems of the semi-arid tropics (pp. 211–234). Tsukuba: Japan International Research Center for Agriculture Sciences.
- Zhao, L., Wu, L., Li, Y., Animesh, S., Zhu, D., & Uphoff, N. (2010). Comparisons of yield, water use efficiency, and soil microbial biomass as affected by the system of rice intensification. Communications in Soil Science and Plant Analysis, 41(1), 1–12.10.1080/00103620903360247
- Zhao, L., Wu, L., Li, Y., Lu, X., Zhu, D., & Uphoff, N. (2009). Influence of the system of rice intensification on rice yield and nitrogen and water use efficiency with different N application rates. Experimental Agriculture, 45(03), 275–286.10.1017/S0014479709007583
- Zhao, L., Wu, L., Wu, M., & Li, Y. (2011). Nutrient uptake and water use efficiency as affected by modified rice cultivation methods with reduced irrigation. Paddy and Water Environment, 9(1), 25–32.10.1007/s10333-011-0257-3
- Zheng, B. S., Yang, L., Zhang, W. P., Mao, C. Z., Wu, Y. R., Yi, K. K., … Wu, P. (2003). Mapping QTLs and candidate genes for rice root traits under different water-supply conditions and comparative analysis across three populations. TAG Theoretical and Applied Genetics, 107, 1505–1515.10.1007/s00122-003-1390-1