ABSTRACT
Salinity adaptive responses in halophytes include energy-requiring processes, but the mechanism supplying ATP to meet the increased ATP demand remains unclear. In the present study, we examined the effects of NaCl on ATP synthesis in mitochondria isolated from a halophyte, the common ice plant (Mesembryanthemum crystallinum L.). The ATP synthesis rate was maintained or enhanced with increasing osmotic pressures ranged from 0.2 to 2.0 MPa in the assay mixtures containing NaCl, whereas it was decreased in the mixtures with the equivalent osmotic pressures generated by sorbitol only. Also, the ATP synthesis rate was enhanced with increasing NaCl concentrations ranged from 50 to 350 mM in the assay mixtures with fixed (2.0 MPa) osmotic pressure. These results suggested a mechanism of enhanced ATP synthesis in the mitochondria by the ionic effects of NaCl, which might play an important role in the adaptation of the ice plant under salinity.
Graphical abstract
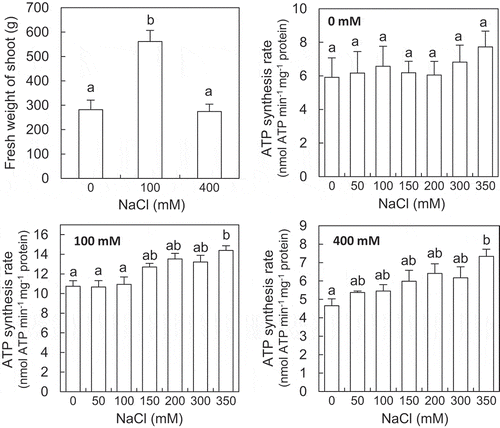
NaCl increased ATP synthesis rate in mitochondria isolated from the ice plants grown with 100 and 400 mM NaCl
Introduction
Salinity, which is caused by the presence of NaCl in soils, is one of the major abiotic stresses limiting plant growth and agricultural production (Flowers & Colmer, Citation2008). Enhancement of salt tolerance in crops and application of salt-tolerant plants as alternative crops are useful solutions to maintain agricultural production (Glenn, Brown & Blumwald, Citation1999; Panta et al., Citation2014; Shabala, Citation2013).
Halophytes are defined as salt-tolerant plants that capable of completing their life cycle under high salinity at which almost all crops die (Flowers & Colmer, Citation2008). Growing under saline conditions, halophytes may operate evolved mechanisms for salt tolerance (Flowers & Colmer, Citation2008; Shabala & Mackay, Citation2011). In the salt-tolerant plant species, Na+ and Cl− are sequestrated into vacuole for preventing toxic effects of the ions on metabolisms in the cytoplasm. The sequestration of Na+ and Cl− is mediated through tonoplast transporters, such as H+/Na+ and H+/Cl− antiporters that are driven by proton gradient generated via ATP hydrolysis of tonoplast H+-pyrophosphatases (V-PPases) and/or H+-ATPases (V-ATPases) (Flowers & Colmer, Citation2008; Flowers, Troke & Yeo, Citation1977). Halophytes also have the ability to maintain active uptake for macronutrient ions and enhance the synthesis of compatible solutes under salt stress conditions for controlling intercellular ion homeostasis and osmotic adjustment (Flowers & Colmer, Citation2008). These processes to increase salt tolerance need much energy to operate; thus, ATP demand in response to salinity of halophytes would considerably increase (Kumari, Das, Parida & Agarwal, Citation2015; Yeo, Citation1983).
In addition to salt tolerance, some halophytes also need salt at some extent for maximum growth (Flowers & Colmer, Citation2008). The enhancement of growth by NaCl that referred to as halophilism is an important trait for the adaptation to salinity, but there have still been few studies on the physiological mechanisms of halophilism (Himabindu et al., Citation2016; Kaburagi, Morikawa, Yamada & Fujiyama, Citation2014; Shabala, Citation2013; Tran et al., Citation2019; Yamada, Kuroda & Fujiyama, Citation2016). In our previous report, NaCl enhanced the growth of suspension-cultured cells of a halophyte, the common ice plant, Mesembryanthemum crystallinum L., and the growth enhancement was attributed to the increase of cell division and cell elongation (Tran et al., Citation2019). ATP is needed in the processes of growth, and thus, ATP synthesis would also be increased for the growth enhancement stimulated by NaCl. Several previous studies have observed variances in mitochondrial respiration rate in some halophyte species under salt stress conditions (Atreya & Bhargava, Citation2008; Jacoby, Taylor & Millar, Citation2011). For example, recent proteomics-based studies have proposed an increase of mitochondrial ATP synthesis in several halophytes under salinity, such as Thellungiella halophila (Wang et al., Citation2013), Kandelia candel (Wang et al., Citation2014), and Puccinellia tenuiflora (Yu et al., Citation2011), through the observation of increased abundances of proteins involved in tricarboxylic acid (TCA) cycle, electron transport chain (ETC) complexes, and ATP synthases. However, there have been no data obtained by direct measurement to demonstrate that NaCl enhances ATP synthesis in halophytes.
The ice plant is known as a facultative halophyte native to South and Eastern Africa and can survive under saline conditions with NaCl concentration equivalent to that of seawater (Adams et al., Citation1998; Bohnert et al., Citation1988). The growth of the ice plant is enhanced in salinized soils contained some amounts of NaCl up to 200 mM (Adams et al., Citation1998; Flowers et al., Citation1977). This halophyte has been cultivated as a vegetable in some countries (Agarie et al., Citation2009) and also used as a useful model halophyte for studies on salt tolerance (Bohnert & Cushman, Citation2000; Vera-Estrella, Barkla, Bohnert & Pantoja, Citation1999). Many of the salt-tolerant responses specific to halophytes have been found in the ice plant (Bohnert & Cushman, Citation2000; Oh et al., Citation2015). To understand the effect of NaCl on ATP synthesis in halophyte for adaptation to salinity, in the present study, we examined the ATP synthesis rate in mitochondria at different levels of NaCl, which were isolated from leaves of the ice plants grown under different NaCl conditions.
Materials and methods
Plant culture and NaCl treatment
Seeds of M. crystallinum L. were surface sterilized and sowed on a germination medium as described by Agarie et al. (Citation2007). Two-week-old seedlings were transferred to pots in 18 cm diameter and grown in a greenhouse at Kagawa University according to the descriptions by Agarie et al. (Citation2009). NaCl treatments were applied to the 6-week-old plants by irrigating the mixed nutrient solutions of Otsuka House No. 1 and No. 2 (Otsuka AgriTechno Co. Ltd., Japan) contained 100 and 400 mM NaCl, while the untreated plants were irrigated with the nutrient solution without NaCl. The fresh and dry weight of shoots were determined at 6 weeks after the onset of the treatments.
Mitochondrial isolation
Mitochondria were isolated from leaves of plants grown without and with 100 and 400 mM NaCl for 8 weeks after the onset of treatment. The mitochondrial isolation was adopted from the method of Hong, Phuong, Thuy, Wheatley and Cushman (Citation2019) with a small modification that the mitochondria were purified by isopycnic density centrifugation with an 18%–23%–40% Percoll step gradient at 25,000 × g for 45 min using an ultracentrifuge (Model CP70MX, Hitachi, Japan). Mitochondrial protein content was determined using a Quick Start Bradford kit (Cat. 500-0205, Bio-Rad) and BSA as a standard. The isolated mitochondria with 80–90% intactness were validated using cytochrome c oxidase assay as described by Keech, Dizengremel and Gardeström (Citation2005).
ATP synthesis of isolated mitochondria in the assay mixtures containing sorbitol and NaCl
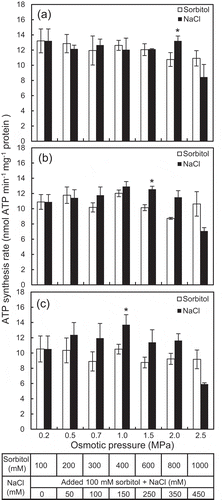
The isolated mitochondria were suspended in the buffer solution as described by Panda, Yamamoto, Kondo and Matsumoto (Citation2008), which contained 100, 200, 300, 400, 600, 800, and 1000 mM sorbitol to generate 0.2, 0.5, 0.7, 1.0, 1.5, 2.0, and 2.5 MPa osmotic pressures in assay mixtures as calculated by the van’t Hoff equation, respectively. Similarly, the isolated mitochondria were also suspended in the buffer solution contained 100 mM sorbitol combined with 0, 50, 100, 150, 250, 350, and 450 mM NaCl, in which the osmotic pressures of assay mixtures were equivalent to that of the sorbitol treatments. On the other hand, the isolated mitochondria were added into the assay mixtures containing 50, 100, 150, 250 and 350 mM NaCl, in which osmotic pressures of these assay mixtures were fixed at 2.0 MPa using different amounts of sorbitol. The assay mixtures were set at 25°C for 10 min. The ATP synthesis was terminated by adding inhibitors as described by Soccio, Laus, Trono and Pastore (Citation2013).
Measurement of ATP content
The ATP content in the assay mixtures was measured on the basis of NADPH fluorescence intensity (ʎex = 360 nm, ʎem = 460 nm) generated via hexokinase (HK)/glucose-6-phosphate dehydrogenase (G6PDH) coupled reactions, according to the procedure described by Soccio et al. (Citation2013). The ATP detection solution was mixed with the assay mixture in a 96-well plate (CellStar, Greiner Bio-one, Germany). Fluorescent intensity measurement was carried out at 25°C with a fluorescence microplate reader (Cyto Fluor 4000, Applied Biosystems). The ATP synthesis rate (nmol ATP min−1 mg−1 protein) was calculated on the basis of the reaction time and mitochondrial protein content (Soccio et al., Citation2013).
Results
Effects of sorbitol and NaCl on ATP synthesis in isolated mitochondria
The growth of the plants grown with NaCl was enhanced approximately twice with 100 mM and maintained with 400 mM NaCl compared to that of the plants grown without NaCl (). The mitochondria were isolated from the leaves of the plants grown without and with NaCl. The ATP synthesis rate was measured on the isolated mitochondria in the assay mixtures with different osmotic pressures ranged from 0.2 to 2.5 MPa, which was generated by sorbitol and NaCl separately. The concentrations of NaCl which was added to the assay mixtures containing NaCl ranged from 0 to 450 mM. The osmotic levels and NaCl concentrations have also been used to study plant mitochondrial responses to salinity in previous reports (Flowers & Hanson, Citation1969; Jacoby, Che‐Othman, Millar & Taylor, Citation2016; Lorimer & Miller, Citation1969). Under the osmotic pressures tested, the ATP synthesis rate tended to be decreased in the assay mixtures containing sorbitol only with increasing osmotic pressure ranged from 1.5 to 2.5 MPa (). However, the ATP synthesis rate was maintained ()) and enhanced (,c)) in the assay mixtures containing NaCl with osmotic pressures ranged from 1.0 to 2.0 MPa compared with that at 0.2 MPa. Also, the ATP synthesis rate in the assay mixtures containing NaCl tended to be increased compared with that containing sorbitol only, and the statistical significances were observed at 2.0, 1.5, and 1.0 MPa of the osmotic pressures in the mitochondria isolated from the plants grown without and with 100 and 400 mM NaCl, respectively (). However, the ATP synthesis rate was decreased in the assay mixtures containing NaCl at 2.5 MPa compared with that in the assay mixtures containing sorbitol only.
Figure 1. Effects of NaCl on the growth of shoots of the ice plant. The fresh weight (a) and dry weight (b) of shoots were observed at 6 weeks after the onset of treatments. Data are mean values ± standard deviations (n = 10). Different letters indicate statistically significant differences among NaCl treatments by Turkey–Kramer test (p < 0.05).
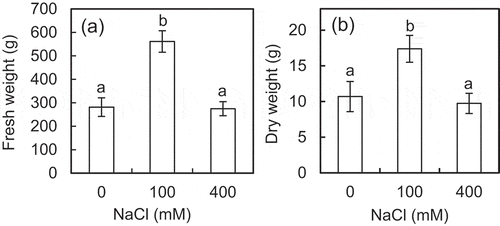
Figure 2. Effects of NaCl and sorbitol on ATP synthesis rate of isolated mitochondria in assay mixtures. The mitochondria were isolated from leaves of the ice plants grown without (a) and with 100 mM (b) and 400 mM NaCl (c). The table below indicates the used amounts of NaCl and sorbitol for generating isosmotic pressures (0.2–2.5 MPa) in the assay mixtures. Data are mean values ± standard errors (n = 3). Asterisks indicate statistically significant differences in the values between the sorbitol and NaCl treatment at the same osmotic pressure by Student’s t-test (p < 0.05).
Effects of NaCl on ATP synthesis in isolated mitochondria
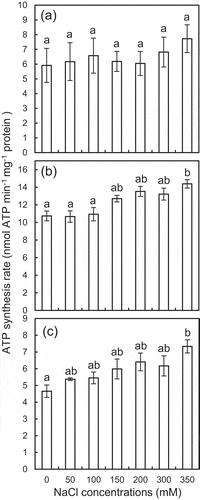
To further examine the effects of NaCl on the ATP synthesis, the ATP synthesis rate was measured on the isolated mitochondria in the assay mixtures containing 50–350 mM NaCl. The osmotic pressures of these assay mixtures were fixed at 2.0 MPa, at which the highest ATP synthesis was observed in the mitochondria isolated from the plants grown without NaCl ()). Under this osmotic pressure, the ATP synthesis rate in the isolated mitochondria tended to increase with the increasing NaCl levels in the assay mixtures (), although there was no statistical significance between the ATP synthesis rates in the mitochondria isolated from the plants grown without NaCl ()). The ATP synthesis rate in mitochondria isolated from the plants grown with 100 and 400 mM NaCl had tendency to gradually increase with the increasing NaCl levels more than 150 and 50 mM, respectively (,c)). The ATP synthesis rate in the plants grown without and with 100 and 400 mM NaCl reached the highest values at NaCl concentration of 350 mM, which was about 31%, 34%, and 58% higher than that in the assay mixtures without NaCl, respectively (). The ATP synthesis rate of the mitochondria isolated from the plants grown with 100 mM NaCl ()) was nearly twice as much as those from the plants grown with 0 and 400 mM NaCl (,c)), irrespective of the NaCl concentrations of the assay mixtures.
Figure 3. Effects of NaCl on ATP synthesis rate of the isolated mitochondria in the assay mixtures. The mitochondria were isolated from leaves of the ice plants grown without (a) and with 100 mM (b) and 400 mM NaCl (c). The osmotic pressure of the assay mixtures was fixed at 2.0 MPa with sorbitol. Data are mean values ± standard errors (n = 3). Different letters indicate statistically significant differences among NaCl treatments by Turkey–Kramer test (p < 0.05).
Discussion
The ice plant has evolved sophisticated mechanisms to adapt to high salinity, such as salt sequestration into the vacuole (Adams, Thomas, Vernon, Bohnert & Jensen, Citation1992; Barkla, Zingarelli, Blumwald & Smith, Citation1995), enhanced synthesis of compatible solutes (Adams et al., Citation1992; Bohnert & Cushman, Citation2000), and salt-inducible switching photosynthesis from C3 to CAM (Niewiadomska, Karpinska, Romanowska, Slesak & Karpinski, Citation2004; Winter & von Willert, Citation1972). These responses require ATP; thus, the ATP synthesis in the mitochondria should be increased to meet the increased energy demand in the ice plants under salinity conditions. As expected, in the present study, we found that the ATP synthesis in the mitochondria isolated from both the plants grown without and with NaCl was enhanced by 50–350 mM NaCl in the assay mixtures ( and ). Although the ATP synthesis enhancement in halophytes has been suggested in some previous reports (Kumari et al., Citation2015; Shabala, Citation2013; Yeo, Citation1983), the present study is the first report of the direct measurement of ATP synthesis that was enhanced by NaCl.
The effects of salinity on the physiological processes in plant cells can be divided mainly into osmotic and ionic effects (Flowers & Colmer, Citation2008). In the present study, the ATP synthesis rate was significantly higher in the assay mixtures containing NaCl than that containing sorbitol only (). These results suggest that the enhancement of the ATP synthesis was caused by the ionic effect, not by the osmotic effect of NaCl. The ATP synthesis rate was also increased with increasing NaCl levels (50–350 mM) under fixed osmotic pressures (2.0 MPa). The maximum ATP synthesis rate in the mitochondria of the plants grown without and with 100 and 400 mM NaCl was obtained with 350 mM NaCl in the assay mixtures, which increased about 31%, 34%, and 58% compared with that in the assay mixtures without NaCl, respectively (). Also, the ATP synthesis rate increased with the highest values at NaCl concentrations of 150 and 250 mM NaCl, but it decreased with 450 mM NaCl compared with that in the assay mixtures without NaCl (). These results suggest that the NaCl concentration ranging from 150 to 350 mM might be optimal for the ATP synthesis enhancement. Jacoby et al. (Citation2016) have observed that the ATP synthesis was enhanced by NaCl in the isolated mitochondria of a glycophyte (wheat) in an assay mixture contained NaCl less than 50 mM, and it decreased with the increase of NaCl concentration. The results of the present study indicate that the ATP synthesis in mitochondria of halophyte could be enhanced by higher salinity level than that in glycophyte, which would contribute to the adaptation to salinity of halophyte (Flowers, Munns & Colmer, Citation2014; Kumari et al., Citation2015).
The response of mitochondria to NaCl differs depending on the growth NaCl conditions. The enhancement of ATP synthesis rate of mitochondria isolated from the plants grown with 0, 100, and 400 mM NaCl, of which the difference in the ATP synthesis between NaCl and sorbitol was statistically significant, was obtained with 350, 250, and 150 mM NaCl, respectively (). These results suggest that an adaptation mechanism which increases the ATP synthesis was induced in response to NaCl in the halophyte. In addition, the ATP synthesis of the mitochondria isolated from the plants grown with 100 mM NaCl ()) was significantly higher than that from the plants grown with 0 and 400 mM NaCl (). The growth was also enhanced with 100 mM NaCl (), suggesting that the ATP synthesis enhancement is an important factor which required for ATP demand of the halophilism in the ice plant (Tran et al., Citation2019). The enhancement of the ATP synthesis would be due to an increase in activity of components related to mitochondrial ATP synthesis, such as ATP synthase, ETC, and TCA cycle (Kumari et al., Citation2015). In some marine bacteria, Na+ motive force instead of H+ is used for ATP synthesis through ATP synthases (Gemperli, Dimroth & Steuber, Citation2003; Mulkidjanian, Dibrov & Galperin, Citation2008), although it has not yet been discovered in higher plants. It would be intriguing to elucidate the components related to the enhancement of ATP synthesis, including the motive force of Na+ in response to salinity.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, P., Nelson, D. E., Yamada, S., Chmara, W., Jensen, R. G., Bohnert, H. J., & Griffiths, H. (1998). Growth and development of Mesembryanthemum crystallinum (Aizoaceae). The New Phytologist, 138(2), 171–190.
- Adams, P., Thomas, J. C., Vernon, D. M., Bohnert, H. J., & Jensen, R. G. (1992). Distinct cellular and organismic responses to salt stress. Plant and Cell Physiology, 33(8), 1215–1223.
- Agarie, S., Kawaguchi, A., Kodera, A., Sunagawa, H., Kojima, H., Nose, A., & Nakahara, T. (2009). Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Production Science, 12(1), 37–46.
- Agarie, S., Shimoda, T., Shimizu, Y., Baumann, K., Sunagawa, H., Kondo, A., … Cushman, J. C. (2007). Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. Journal of Experimental Botany, 58(8), 1957–1967.
- Atreya, A., & Bhargava, S. (2008). Salt-induced respiration in Bruguiera cylindrica—Role in salt transport and protection against oxidative damage. Physiology and Molecular Biology of Plants, 14(3), 217–226.
- Barkla, B. J., Zingarelli, L., Blumwald, E., & Smith, J. A. C. (1995). Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiology, 109(2), 549–556.
- Bohnert, H. J., & Cushman, J. C. (2000). The ice plant cometh: Lessons in abiotic stress tolerance. Journal of Plant Growth Regulation, 19(3), 334–346.
- Bohnert, H. J., Ostrem, J. A., Cushman, J. C., Michalowski, C. B., Rickers, J., Meyer, G., … Schmitt, J. M. (1988). Mesembryanthemum crystallinum, a higher plant model for the study of environmentally induced changes in gene expression. Plant Molecular Biology Reporter, 6(1), 10–28.
- Flowers, T., & Hanson, J. (1969). The effect of reduced water potential on soybean mitochondria. Plant Physiology, 44(7), 939–945.
- Flowers, T., Troke, P., & Yeo, A. (1977). The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology, 28(1), 89–121.
- Flowers, T. J., & Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytologist, 179(4), 945–963.
- Flowers, T. J., Munns, R., & Colmer, T. D. (2014). Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany, 115(3), 419–431.
- Gemperli, A. C., Dimroth, P., & Steuber, J. (2003). Sodium ion cycling mediates energy coupling between complex I and ATP synthase. Proceedings of the National Academy of Sciences, 100(3), 839–844.
- Glenn, E. P., Brown, J. J., & Blumwald, E. (1999). Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences, 18(2), 227–255.
- Himabindu, Y., Chakradhar, T., Reddy, M. C., Kanygin, A., Redding, K. E., & Chandrasekhar, T. (2016). Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environmental and Experimental Botany, 124, 39–63.
- Hong, H. T. K., Phuong, T. T. B., Thuy, N. T. T., Wheatley, M. D., & Cushman, J. C. (2019). Simultaneous chloroplast, mitochondria isolation and mitochondrial protein preparation for two-dimensional electrophoresis analysis of Ice plant leaves under well watered and water-deficit stressed treatments. Protein Expression and Purification, 155, 86–94.
- Jacoby, R., Che‐Othman, M., Millar, A., & Taylor, N. (2016). Analysis of the sodium chloride‐dependent respiratory kinetics of wheat mitochondria reveals differential effects on phosphorylating and non‐phosphorylating electron transport pathways. Plant, Cell & Environment, 39(4), 823–833.
- Jacoby, R. P., Taylor, N. L., & Millar, A. H. (2011). The role of mitochondrial respiration in salinity tolerance. Trends in Plant Science, 16(11), 614–623.
- Kaburagi, E., Morikawa, Y., Yamada, M., & Fujiyama, H. (2014). Sodium enhances nitrate uptake in Swiss chard (Beta vulgaris var. cicla L.). Soil Science and Plant Nutrition, 60(5), 651–658.
- Keech, O., Dizengremel, P., & Gardeström, P. (2005). Preparation of leaf mitochondria from Arabidopsis thaliana. Physiologia Plantarum, 124(4), 403–409.
- Kumari, A., Das, P., Parida, A. K., & Agarwal, P. K. (2015). Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Frontiers in Plant Science, 6, 537.
- Lorimer, G. H., & Miller, R. J. (1969). The osmotic behavior of corn mitochondria. Plant Physiology, 44(6), 839–844.
- Mulkidjanian, A. Y., Dibrov, P., & Galperin, M. Y. (2008). The past and present of sodium energetics: May the sodium-motive force be with you. Biochimica Et Biophysica Acta (bba)-bioenergetics, 1777(7–8), 985–992.
- Niewiadomska, E., Karpinska, B., Romanowska, E., Slesak, I., & Karpinski, S. (2004). A salinity-induced C3-CAM transition increases energy conservation in the halophyte Mesembryanthemum crystallinum L. Plant and Cell Physiology, 45(6), 789–794.
- Oh, D. H., Barkla, B. J., Vera‐Estrella, R., Pantoja, O., Lee, S. Y., Bohnert, H. J., & Dassanayake, M. (2015). Cell type‐specific responses to salinity—The epidermal bladder cell transcriptome of Mesembryanthemum crystallinum. New Phytologist, 207(3), 627–644.
- Panda, S. K., Yamamoto, Y., Kondo, H., & Matsumoto, H. (2008). Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. Comptes Rendus Biologies, 331(8), 597–610.
- Panta, S., Flowers, T., Lane, P., Doyle, R., Haros, G., & Shabala, S. (2014). Halophyte agriculture: Success stories. Environmental and Experimental Botany, 107, 71–83.
- Shabala, S. (2013). Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 112(7), 1209–1221.
- Shabala, S., & Mackay, A. (2011). Ion transport in halophytes. In Cánovas, F. M. (Ed.), Advances in botanical research (Vol. 57, pp. 151–199). Netherlands: Elsevier. doi:10.1016/B9780-12-387692-8.00005-9
- Soccio, M., Laus, M. N., Trono, D., & Pastore, D. (2013). A new simple fluorimetric method to assay cytosolic ATP content: Application to durum wheat seedlings to assess modulation of mitochondrial potassium channel and uncoupling protein activity under hyperosmotic stress. Biologia, 68(3), 421–432.
- Tran, D. Q., Konishi, A., Cushman, J. C., Morokuma, M., Toyota, M., & Agarie, S. (2019). Ion accumulation and expression of ion homeostasis-related genes associated with halophilism, NaCl-promoted growth in a halophyte Mesembryanthemum crystallinum L. Plant Production Science, 1–12. doi:10.1080/1343943X.2019.1647788
- Vera-Estrella, R., Barkla, B. J., Bohnert, H. J., & Pantoja, O. (1999). Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta, 207(3), 426–435.
- Wang, L., Liu, X., Liang, M., Tan, F., Liang, W., Chen, Y., … Chen, W. (2014). Proteomic analysis of salt-responsive proteins in the leaves of mangrove Kandelia candel during short-term stress. PloS One, 9(1), e83141.
- Wang, X., Chang, L., Wang, B., Wang, D., Li, P., Wang, L., … Guo, A. (2013). Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Molecular & Cellular Proteomics, 12(8), 2174–2195.
- Winter, K., & von Willert, D. J. (1972). NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Zeitschrift Für Pflanzenphysiologie, 67(2), 166–170.
- Yamada, M., Kuroda, C., & Fujiyama, H. (2016). Function of sodium and potassium in growth of sodium-loving Amaranthaceae species. Soil Science and Plant Nutrition, 62(1), 20–26.
- Yeo, A. (1983). Salinity resistance: Physiologies and prices. Physiologia Plantarum, 58(2), 214–222.
- Yu, J., Chen, S., Zhao, Q., Wang, T., Yang, C., Diaz, C., … Dai, S. (2011). Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. Journal of Proteome Research, 10(9), 3852–3870.