ABSTRACT
The genus Vigna contains important crops such as cowpea and mungbean. Wild Vigna showing higher salt tolerance than Vigna crops were screened and their tolerance mechanisms are discussed. Primary screening using 7 Vigna crops and 23 wild Vigna under 300 mM NaCl selected V. luteola, V. marina and V. vexillata. A study under different salt concentrations revealed the highest survival ability of V. marina. Diversity of salt tolerance in each species was revealed using a total of 230 accessions. Growth and physiological responses under 150 mM NaCl were then compared using two selected accessions from each species. The pattern of Na+ accumulation in roots, stems and leaves suggested that V. vexillata (V1) and V. luteola (L8, L9) are ‘Na+ excluder’ type, while V. marina (M1, M4) is ‘Na+ includer’ type. V. luteola (L8, L9) showed the highest dry matter production under control condition and well-maintained shoot dry weight under salt stress. Interestingly, V. luteola (L9) accumulated the highest Na+ in roots (3000 μM g−1) and increased root dry weight under salt stress, which might work as Na+ reservoir restricting Na+ transition to the leaves, leading to the increased photosynthetic rate. V. luteola has great potential in areas where moderate salt damage occurs. V. marina (M1, M4) accumulated Na+ at high level in roots, stem, and leaves. Under salt stress, they increased stomatal conductance, transpiration rate, and photosynthetic rate, which suggested the adaptational regulation of aquaporin gene expression. V. marina will be useful as food, pasture and phytoremediation legumes in highly salt-damaged areas.
Introduction
The genus Vigna is an important taxonomic group for agriculture, including several food legumes such as cowpea, mungbean and azuki bean. The genus Vigna consists of about 100 wild species found in tropical and subtropical areas (Takahashi et al., Citation2016). Soil salinity is a serious problem in food production, because high salt concentrations in soil prevent plant growth, delaying maturation and decreasing crop production or causing withering. According to the Food and Agriculture Organization (FAO, Citation2008), more than 800 million hectares of land throughout the world are affected by salt. NaCl is easily soluble in water and is the major composition of seawater, the main cause of salt stress. Some wild Vigna species live in severe natural environments, such as saline land on sandy beaches, acidic land, and flood-prone land beside rivers (Tomooka et al., Citation2014). The wild Vigna species, with adaptability and resistance to severe environmental stress, are an interesting genetic resource for breeding, green manure, and domestication as crops. Research on crop wild relatives has attracted attention recently because some wild species have better tolerance to environmental stress than crops do.
In this study, three experiments were conducted. In ‘Experiment 1ʹ, salt tolerance of 7 Vigna crop species and 23 wild Vigna species were compared. In ‘Experiment 2ʹ, intraspecific diversity in salt tolerance was surveyed using 230 accessions from 3 African wild Vigna species (V. vexillata, V. luteola, V. marina), which were selected as high salt-tolerance species in ‘experiment 1ʹ. In ‘Experiment 3ʹ, selected accessions, respectively, from three wild Vigna species were used to clarify their specific strategy against salt stress by comparing detailed growth characteristics under salt stress. We examined their growth, Na+ accumulation and physiological responses under salt stress in a hydroponic environment to analyze their dry-matter production ability and salt-tolerance mechanism. Studies of wild genetic resources closely related to crops will have possibilities of resistance against salt stress that will not be found from studies of crop varieties, which will be valuable for utilizing such genetic resources to improve the stress tolerance of crops and enhance crop production for foods and feeds in problem soils where show low crop productivity.
Materials and methods
(Experiment 1) comparison of Vigna crops and wild species
Salt tolerance of 7 Vigna crop species (moth bean, azuki bean, black gram, mungbean, creole bean, rice bean, cowpea, 9 accessions in total) and 23 wild Vigna species (31 accessions) were compared (). The experiment was conducted at the Genetic Resources Center, NARO in Tsukuba, Japan (36°03′N, 140°10′E). Two plants per accession without replication (a total of two plants) were grown in plastic pots (7 cm in diameter × 9 cm in height) under natural light condition in a greenhouse from June in 2008. The soil used in the pot was KUREHA-Engei-Baido (KUREHA Inc, Japan). Twenty-one days after sowing, an adequate amount of salt solutions was applied in the pool and then the pots were immersed for 2 weeks. Three concentrations of salt solutions (100, 200 and 300 mM NaCl) were used. Tolerance was evaluated at 2 weeks after treatment started. Average of two plants was visually scored by percent of wilted leaves as follows: R (Resistant) = No leaves wilted, MR (Moderately Resistant) = 1–25% of leaves wilted, MS (Moderately Susceptible) = 26–50% of leaves wilted, S (Susceptible) = 51–100% of leaves wilted ().
Table 1. Salt tolerance of 7 Vigna crop species and 23 wild Vigna species.
(Experiment 2) intraspecific variations in three wild Vigna
(Experiment 2–1) survivability under different NaCl concentrations
Eleven accessions of V. vexillata, five accessions of V. luteola and nine accessions of V. marina were used to clarify survivability of each species under different NaCl concentrations (Supplementary Table 1). Two plants per accession with two replications (a total of four plants) were grown in plastic pots (7 cm in diameter × 9 cm in height) under natural light condition in a greenhouse from June in 2009. The soil used in the pot was KUREHA-Engei-Baido (KUREHA Inc, Japan). Twenty-one days after sowing, an adequate amount of salt solutions was applied in the pool and then the pots were immersed for 4 weeks. Five concentrations of salt solutions (200, 250, 300, 350 and 400 mM NaCl) were used. Tolerance was evaluated at 1-week interval until 4 weeks after treatment started. Average of two plants (with two replications) was visually scored by percent of wilted leaves as follows: R (Resistant) = 0–24% of leaves wilted, MR (Moderately Resistant) = 25–49% of leaves wilted, MS (Moderately Susceptible) = 50–74% of leaves wilted, S (Susceptible) = 75–100% of leaves wilted.
(Experiment 2–2) diversity of salt tolerance within each species
One hundred thirty accessions of V. vexillata, 50 accessions of V. luteola and 50 accessions of V. marina were used to clarify the diversity of salt tolerance within each species (Supplementary Tables 2–1, 2–2, 2–3). Two plants per accession were sown in plastic pots with KUREHA-Engei-Baido supported and kept in greenhouse for 7 days. Each plant was transplanted to a hydroponic culture in greenhouse from August 2009. One culture pool (62.5 cm × 100 cm × 17 cm height) contained 64 plants (8 × 8 plants). The culture pool contained diluted nutrient solution of a 1:1 ratio of Otsuka house No. 1 (1.5 g/L): Otsuka house No. 2 (1 g/L) (Otsuka Chemical Co., Osaka, Japan: N, P, K, Ca and Mg = 18.6, 5.1, 8.6, 8.2 and 3.0 mEq/L, respectively). The nutrient stock solution was diluted to reach EC at 100 mS/m with water. Four days after transplanting, salt stress was initiated. NaCl concentrations were different for each species. Salt stress treatments were applied for 8 weeks with the gradually increasing NaCl concentrations. Based on the survivability to different salt concentrations revealed in Experiment 2–1, different concentrations of salt stresses were applied to each Vigna species. For V. vexillata, salt stress was started at 50mM NaCl for 3 days, followed by 150 mM for 4 days, 250 mM for 3 weeks, and 350 mM for 4 weeks. For V. luteola, salt stress was started at 50mM NaCl for 3 days, followed by 150 mM for 4 days, 300 mM for 3 weeks, and 400 mM for 4 weeks. For V. marina, salt stress was started at 100mM NaCl for 3 days, followed by 200 mM for 4 days, 350 mM for 3 weeks, and 500 mM for 4 weeks. Average of two plants was visually scored by percent of wilted leaves as follows: R (Resistant) = 0–24% of leaves wilted, MR (Moderately Resistant) = 25–49% of leaves wilted, MS (Moderately Susceptible) = 50–74% of leaves wilted, S (Susceptible) = 75–100% of leaves wilted.
(Experiment 3) growth and physiological responses of three Vigna species
Plant materials and NaCl treatment
Six accessions of three Vigna species which showed high level of tolerance or interesting growth response were used, V. vexillata ‘V1ʹ (JP202334) and ‘V5ʹ (JP235869), V. luteola ‘L8ʹ (JP233389) and ‘L9ʹ (JP236246), and V. marina ‘M1ʹ (242,349) and ‘M4ʹ (JP247220).
The experiment was conducted in a greenhouse under natural light and temperature conditions at the Graduate School of Bioresources, Mie University, Tsu, Japan (34º44.666N, 136º31.322E), in August and September of 2013. The daily mean air temperature conditions at the site were 23.3°C to 33.1°C during the experiment. The seeds were washed with a solution of 70% ethanol and 5% sodium hypochlorite and rinsed well with distilled water. The cleaned seeds were scratched on the seed coat for stimulation of germination and then germinated on the surface of tap water in plastic baskets.
About 1 week after germination, the seedlings were transplanted into a hole in a forming polystyrene board and placed on a plastic container (55 × 85 × 21 cm) filled with 60 L of Kimura A culture solution containing (mM) 0.36 (NH4)2SO4, 0.57 K2SO4, 0.73 MgSO4, 1.10 KNO3, 0.36 KH2PO4, 0.36 Ca (NO3), and 0.03 FeO3 (Baba & Takahashi, Citation1958). Four days after transplantation, salt treatment was started with renewed culture solution including 50 mM NaCl; 2 days later, the concentration of NaCl was increased from 50 to 100 mM, and then 2 days later, the concentration of NaCl was increased from 100 to 150 mM to reduce osmotic stress. The plants were then grown there for 15 days until the end of the experiment. For the control group, a culture solution without NaCl was used. The culture solution was adjusted to pH 6.5 by 1N KOH or 1N H2SO4, and the culture solution was changed every 3 days during the treatment. Formerly, we have observed that Vigna plants showed preferable growth without aeration in hydroponics. Thus, we compared the growth of Vigna species with and without aeration in hydroponics and confirmed that they showed preferable growth without aeration (Marubodee, Chakhatrakan & Ehara, Citation2012). Considering our previous results, we did not set aeration system in the hydroponics in this experiment.
Measurement of the photosynthetic rate
The photosynthetic rate of an expanded leaf on the top of the plant was measured at day 13 of the NaCl treatment using a portable photosynthetic meter (SPB-H4, SHIMADZU, Japan). The photosynthetic rate was measured at photosynthetically active radiation of 1300–1500 μmol m−2 s−1, simulating fine seasonal weather, using a light source device (LG-PS2, OLYMPUS, Japan). The SPAD value was measured on the leaves of each accession to measure the photosynthetic rate in the control and the NaCl-treated plant using a chlorophyll meter (SPAD-502, KONICA MINOLTA, Japan) on the last day of NaCl treatment.
Measurement of plant growth and growth analysis
The plants of each accession, both the control and NaCl treated, were sampled twice: at transplantation and at the end of NaCl treatment. Plant samples were rinsed with tap water and then cut into three parts: roots, stem (stem + petioles), and leaves. The total leaf area was determined by scanning the leaves of each plant into image data, and the data were then analyzed with the program software (Scion Image, USA). The dry matter weight was obtained after drying the divided plants at 70°C for 3 days. Growth analysis was conducted in accordance with the protocol of Kevet, Ondok, Necas and Jarvis (Citation1971) to determine the relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), leaf weight ratio (LWR), and specific leaf area (SLA) on the days between transplantation and the end of treatment.
Ion concentrations in the different plant parts
The dried samples, which were ground into a powder, were burned to ashes at 450°C for 8 h in an electric muffle furnace (FO300, Yamato, Japan) and then extracted with 1N HNO3. In this extract, the Na+ and K+ concentration in each plant part was detected using ion chromatography with a conductivity detector (IC-C4, CDD-6A, SHIMADZU, Japan) (Yoshida et al., Citation2016). Oxalic acid (3.3 mM) was used as the mobile phase. The mobile phase was degassed by the degasser (DGU-12A) and pumped with a liquid chromatograph pump (LC-9A) at a speed of 1 μL min-1. This mobile phase was flown to the auto-injector (SIL-6B) and mixed with 10 μL of plant sample solutions after diluted appropriately. The ion concentrations were detected through the analytical column (IC-C4) in the column oven (CTO-10A) at 40°C, and the results were printed by a chromatopac (C-R6A). The standard ion solution was used for calibration and for calculation of the correct ion concentrations of the plant sample solutions.
Statistical analysis
A statistical analysis was performed for the growth parameters by t-test for between the control and the NaCl-treated plants of each accession and for ion concentrations by a Tukey’s honestly significant difference test among different plant parts using the SPSS (IBM SPSS Statistics, USA).
Results
(Experiment 1) comparison of Vigna crops and wild species
All of the six Asian Vigna crop species (moth bean, azuki bean, black gram, mungbean, creole bean and rice bean) were susceptible (S) under 200 mM NaCl stress for 2 weeks (). African Vigna crop species (cowpea) were moderately resistance (MS) under 200 mM stress. Under 300 mM stress, all of the Vigna crop species (seven species) were susceptible (S) (). On the other hand, five wild Vigna species (V. riukiuensis, V. trilobata, V. vexillata, V. luteola, V. marina) showed higher levels of tolerance than Vigna crop species, evaluated as either moderately susceptible (MS), moderately resistance (MR) or resistance (R) ().
(Experiment 2) intraspecific variations of three wild Vigna species
(Experiment 2–1) survivability under different NaCl concentrations
Among five wild Vigna species which showed higher salt tolerance than Vigna crops, three African wild Vigna species (V. vexillata, V. luteola, and V. marina) were used in Experiment 2 and their salt-tolerance levels were clarified (Supplementary Table 1). Most V. vexillata accessions were susceptible (S) under 200 mM NaCl for 4 weeks and could not tolerate higher salt concentrations. In contrast, most V. marina accessions were resistant (R) under 300 mM NaCl for 4 weeks. Even under 400 mM NaCl for 4 weeks, no V. marina accession became wilted (S). V. luteola accessions showed intermediate level of salt tolerance. Some V. luteola accessions could survive under 250 mM NaCl for 4 weeks.
(Experiment 2–2) diversity of salt tolerance within each species
Diversity of salt tolerance within each species was examined under different concentrations of salt stress, i.e. 250 mM (first 4 weeks) to 350 mM NaCl (next 4 weeks) for V. vexillata, 300 mM to 400 mM NaCl for V. luteola, and 350 mM to 500 mM NaCl for V. marina (). Under each salt stress condition, the percentage of susceptible accessions (S) gradually increased. At the end of first 4 weeks salt stress treatment, 74.6% of V. vexillata, 34.0% of V. luteola, and 2.0% of V. marina accessions became wilted (S). At the end of next 4 weeks salt stress treatment, most accessions of V. vexillata and V. luteola (96.9% and 90.0%, respectively) became wilted (S). On the other hand, only 36.0% of V. marina accessions became wilted (S) at the end of stress treatment (350 mM for 4 weeks and 500 mM for the next 4 weeks).
Figure 1. Intra-specific diversity of salt tolerance indicated as change in percent of susceptible accessions within three wild Vigna species.
One hundred thirty accessions of V. vexillata, 50 accessions of V. luteola and 50 accessions of V. marina were used. Salt tolerance was examined under different concentrations of salt stress, i.e. 250 mM (First 4 weeks) to 350 mM NaCl (Next 4 weeks) for V. vexillata (▲), 300 mM to 400 mM NaCl for V. luteola (■), and 350 mM to 500 mM NaCl for V. marina (●).
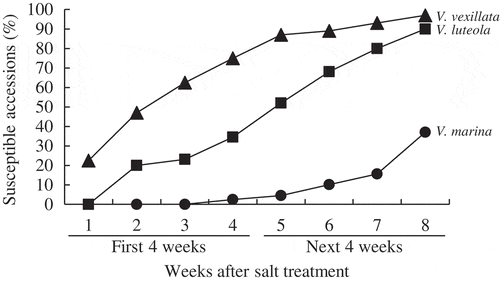
Response of each accession in each species during 8 weeks' salt stress treatment was listed in Supplementary Tables 2–1 (V. vexillata), 2–2 (V. luteola), and 2–3 (V. marina).
(Experiment 3) growth and physiological responses of three Vigna species
Effect of NaCl treatment on dry-matter production
Most of the V. vexillata (V5) plants died under 150 mM NaCl treatment for 15 days and were considered salt-sensitive types (). There was no dead plant in the other five accessions of three Vigna species with 150 mM NaCl treatment until day 15; therefore, these accessions were considered to be salt-resistant (). The total dry weight of the salt-treated plants in V5 was the dry weight of dead plants.
Table 2. Comparison of dry-matter weight between the control and NaCl-treated plants.
Dry-matter production under non-stress condition
Among the five tolerant accessions, the total dry weight of the control plants was highest in L8 (0.79 g), followed by L9 (0.54 g) of V. luteola and V1 (0.46 g) of V. vexillata (). M1 and M4 of V. marina showed comparatively small control plant total dry weights of 0.33 g and 0.35 g, respectively, which meant they had the lowest dry-matter production rate among the three species. In a comparison of dry-matter partitioning of the control plants, V. marina distributed dry matter largely to leaves (56–60%) and less to the stem (26–29%) (). In the case of V. luteola, the dry-matter distribution to leaves was lower (46–48%) and was comparatively high to the stem (41–44%). V. vexillata showed intermediate dry-matter partitioning compared to the other two species.
Table 3. Dry-matter partitioning of leaf, stem and root as percent (%) in the control and NaCl-treated plants.
Ability to maintain dry-matter production under salt stress condition
The total dry weight of the salt-treated plant relative to the control (data were shown as percent of control) was highest in accessions of V. luteola (L8: 66%; L9: 71%), followed by the accessions of V. marina (M1: 61%; M4: 53%) (). The accession of V. vexillata (V1) was highly affected by the NaCl treatment, and its relative total dry weight was 32%. In all accessions, shoot dry weight reduction was severer (29–66%) than root dry weight reduction (59–115%) (). V. luteola could maintain highest shoot dry-matter production (L8: 64%; L9: 66%), followed by V. marina (M1: 57%; M4: 50%). In case of V. vexillata (V1), its shoot growth was depressed to 29%.
The relative root dry-matter production was high in V. luteola (L8: 83%; L9: 115%) and V. marina (M1: 83%; M4: 73%). V. luteola (L9) increased root dry weight and the maximum root length ( and ). V. vexillata (V1) root growth was highly suppressed, and its relative root dry weight was 59%.
Growth analysis to clarify the factors to maintain dry-matter production
The growth analysis was conducted to reveal the factors to maintain dry-matter production of each accession under a saline condition (). The relative growth rate (RGR), expressed as a percentage of the control showed the same tendency as relative dry matter production, i.e. the highest in the accessions of V. luteola (L8: 87%; L9: 88%), followed by those of V. marina (M1: 82%; M4: 77%) and that of the accession of V. vexillata (V1) was the lowest (69%).
Figure 3. Comparison of growth parameters in the control and treated plants. (a) RGR. (b) NAR. (c) LAR. (d) SLA.
White and black bars indicate the control and NaCl-treated plants, respectively. Error bars indicate the standard deviation (n = 10). Numerals in the figure indicate the value of treated plant as percent of control. Asterisks indicate significant difference between the control and the treated plants at the 0.01 (**) probability level.
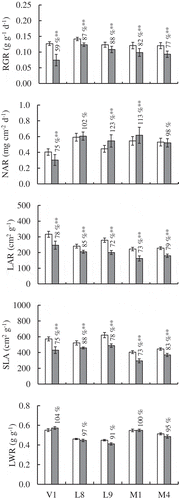
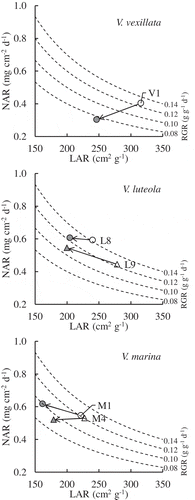
The RGR can be divided into two parameters: the net assimilation rate (NAR: physiological factor) and the leaf area ratio (LAR: morphological factor). In the accessions of V. luteola (L8: 102%; L9: 123%) and V. marina (M1: 113%; M4: 98%), NARs were maintained or even became higher under salt stress condition. In contrast, LARs of V. luteola (L8: 85%, L9: 72%) and V. marina (M1: 73%, M4: 79%) decreased with NaCl treatment ( and ). These data indicated the leaves of V. luteola and V. marina did not suffer physiological damage but leaves morphogenesis was affected by salt stress. In case of V. vexillata (V1), the salt treatment affected both NAR and LAR (75% and 78%, respectively), indicated leaves of this accession were suffered both physiologically and morphologically.
Figure 4. Relationship between LAR and NAR in the resistant accessions.
White and gray symbols indicate the control and NaCl-treated plants, respectively.
The decreases in LARs of all accessions were based on the decrease of the specific leaf area (SLA) under the NaCl treatment (M1: 73% – L8: 88% as a percentage of the control), which meant that the leaves became thicker and the leaf area expansion was depressed. The change in leaf weight ratio (LWR) with the NaCl treatment was negligible in V. marina (M1: 100%; M4: 95%), V. luteola (L8: 97%), and V. vexillata (V1: 104%). In the accession of V. luteola (L9), the LWR decreased slightly to 91% and the root dry weight increased under the NaCl treatment.
Physiological responses in leaves under salt stress
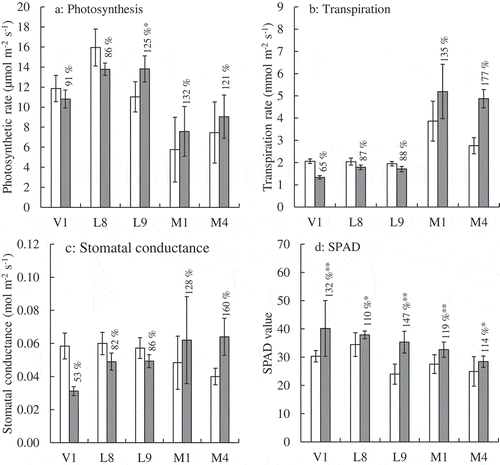
To clarify the physiological responses in leaves under salt stress, the photosynthetic rate, stomatal conductance, transpiration rate, and SPAD (soil plant analysis development) value (index of chlorophyll content) of each leaf were measured ().
Figure 5. Effect of the NaCl treatment for physiological features of leaves. (a) Photosynthetic rate. (b) Transpiration rate. (c) Stomatal conductance. (d) SPAD value. White and gray bars indicate the control and NaCl-treated plants, respectively. Error bars indicate the standard deviation (Ph: n = 3 and SPAD value: n = 10). Numerals in the figure indicate percent of the control. Asterisks indicate significant difference between the control and the treated plants at the 0.01 (**) or 0.05 (*) probability level.
Comparison of leaf physiological characters under non-stress condition
Under non-stress condition (the control plants), the V. marina accessions showed somewhat specific leaf physiological characteristics compared with V. luteola and V. vexillata accessions (, white bars). The V. marina accessions showed lower photosynthetic rate, nearly half that of the other species, and a higher transpiration rate (). There were no distinct differences in the stomatal conductance and chlorophyll content (SPAD values) among accessions of V. marina, V. luteola and V. vexillata ().
The effect of NaCl stress
The photosynthetic rate tended to increase in V. marina (M1: 132%, M4: 121%) and V. luteola (L9: 125%), while it decreased in V. luteola (L8: 86%) and V. vexillata (V1: 91%) (). V. marina (M1, M4) showed an increase in the transpiration rate (M1: 135%, M4: 177%) and stomatal conductance (M1: 128%, M4: 160%) with NaCl treatment (). On the other hand, the transpiration rate and stomatal conductance both decreased in V. luteola (L8: 87% for transpiration and 82% for stomatal conductance, L9: 88% and 86%) and remarkably decreased in V. vexillata (V1: 65% and 53%). The SPAD value increased significantly with NaCl treatment in all of the accessions ().
Mechanism of salt tolerance
Na+ absorption and accumulation
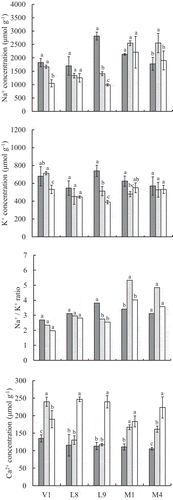
Two patterns of Na+ absorption and accumulation were observed. In V. luteola (L8, L9) and V. vexillata (V1), the concentration was highest in the roots, followed by the stem and the leaves (roots > stem > leaves) (). The Na+ concentration in the roots of V. luteola (L9) was extremely high, up to 2814 μM. By this filtering, the Na+ concentrations in the leaves were lower than 1500 μM in V. luteola (L8, L9) and V. vexillata (V1). In contrast, the Na+ concentration of V. marina (M1, M4) tended to be high in the stem and leaves rather than in the roots (roots < stem = leaves); the value was about 2500 μM and 2000 μM in the stem and leaves, respectively. However, V. marina did not show leaf death and could maintain physiological activities, such as the photosynthetic rate and transpiration rate in Na+ accumulated leaves (). For wilted accession, V. vexillata (V5), the Na+ concentration was higher in the stem and leaves than in the roots (roots < stem = leaves) being over 2000 μM in the leaves.
Figure 6. Na+ and K+ concentration and Na+/K+ ratio in the root, stem and leaf of the treated plants. Na+ concentration (a), K+ concentration (b), Na+/K+ ratio (c) and Ca2+ concentration (d).
Gray, light gray and white bars indicate root, stem and leaf, respectively. Error bars indicate the standard deviation. Different alphabets in the figures indicate significant difference between the root, stem and leaf in each accession at the 0.05 probability level.
K+ absorption and accumulation
The K+ concentration decreased to around half with the NaCl treatment, and its difference in the various plant parts was negligible among un-wilted accessions ().
Na+/K+ ratio
In un-wilted accessions, Na+/K+ ratio values in the roots were lower than those in the top part 4 (). Na+/K+ ratio values in the stem were conspicuously high in V. marina (M1, M4) (). Besides, the Ca2+ concentration was tended to be high in the leaf and low in the roots in the accessions ().
Discussion
Evaluation method of salt tolerance
In this experiment, V. vexillata (V1) showed a comparatively low maintenance ability of dry-matter production, down to a 30% relative total dry weight (), which was lower than that in V. marina (M1, M4) and V. luteola (L8, L9). The physiological activities of the V. vexillata (V1) leaf, such as NAR, decrease to 75% (), and an apparent decrease in stomatal conductance and transpiration rate () was found as compared with M1, M4, L8, and L9. On the contrary, according to Iseki, Takahashi, Muto, Naito and Tomooka (Citation2016), V. vexillata (V1) was classified into the highest salt-tolerance group I. Iseki et al. (Citation2016) reported that the maintenance ability of dry-matter production in V. vexillata (V1) was about 45% relative total dry weight, which was at the same level as V. luteola (L8, L9) after 200 mM NaCl treatment for 2 weeks. Furthermore, the physiological activity of the leaf expressed as the relative quantum yield in V. vexillata (V1) was also maintained at the same level as that in V. luteola (L8, L9).
The cause of the different tendency in salt-tolerance evaluation between our current result and the former report (Iseki et al., Citation2016) was not clear, though we point out a possibility that the different experimental conditions led to the different tendencies. Although Iseki et al. (Citation2016) conducted the evaluation of plants grown in a pot filled with soil and soaked in a salt solution, we conducted the current experiment in a hydroponic system. Yoshida et al. (Citation2016) also reported an example of the different tendency between the results from a soil culture and a hydroponic culture. In their case, Vigna nakashimae was evaluated as highly salt tolerant in a soil culture, but that classification was lowered for the result in a hydroponic culture.
In light of the former report and our current study, we should discuss the result of the salt-tolerance evaluation, keeping in mind the possibility that a highly salt-tolerant accession evaluated in a soil culture may be recognized as sensitive in a hydroponic culture. Yoshida et al. (Citation2016) evaluated salt tolerance of many accessions in both a soil culture and a hydroponic culture and reported that most of the results from the two different culture systems corresponded. Actually, the highly salt-tolerant accessions evaluated in the hydroponic culture showed high salt tolerance in the salt-affected field damaged by the tsunami of the Great East Japan Earthquake (Soma, Fukushima, Japan) (Yoshida et al., Citation2016). Considering these facts, the result of the salt-tolerance evaluation in a hydroponic culture can be employed to conduct a screening for production in a real field.
Potential use of salt-tolerant accessions in saline land
Vigna vexillata
There are plenty of previous reports about the excellent tolerance of wild V. vexillata against diseases such as powdery mildew (James & Lawn, Citation1991), cowpea mottle virus (Ogundiwin, Thottappilly, Aken’Ova, Ekpo & Fatokun, Citation2002; Thottappilly, Ng & Rossel, Citation1994), against pests such as cowpea weevil (Vincenzo et al., Citation2005), pod borer (Jackai, Padulosi & Ng, Citation1996) and environmental stresses such as waterlogging (Miller & Williams, Citation1981), acid soils (United States of America. National Research Council (NRC), Citation1979) and alkaline soils (Lawn & Cottrell, Citation1988; Lawn & Watkinson, Citation2002), hence this species has attracted attention as a genetic resource for cowpea breeding programs. Its genetic diversity revealed by SSR analysis is extra-ordinary high (Dachapak, Somta, Poonchaivilaisak, Yimram & Srinives, Citation2017).
Tubers of wild V. vexillata have been used as protein-rich food in Ethiopia and Sudan (Duke, Citation1981), eastern and northern Indian Himalayas (the United States of America. National Research Council (NRC), Citation1979; Sasikumar & Sardana, Citation1988) and in Australia (Lawn & Cottrell, Citation1988). It has been used also as a forage crop in African countries (Maxted et al., Citation2004; Vanderborght, Citation1989) and in Australia (Miller & Williams, Citation1981). In this study, V. vexillata (V1) was revealed to have higher salt tolerance than all the Vigna crops. It is therefore considered that V. vexillata has high potential as food and/or pasture crop in lands where the major Vigna crops cannot grow because of various environmental problems.
A domesticated strain of V. vexillata called tuber cowpea was recently discovered in Bali, Indonesia, where local farmers still cultivated tuber cowpea mainly to produce high protein edible tubers (Dachapak et al., Citation2018; Karuniawan, Iswandi, Heinzemann & Grüneberg, Citation2006). According to Iseki et al. (Citation2016), the tuber cowpea (Bali) is highly sensitive to salt stress. Consequently, V. vexillata (V1) was considered a promising material to improve the salt-tolerance of Bali cultivars.
Vigna luteola
In this study, the salt-tolerant accessions of V. luteola (L8, L9) showed the highest rate of shoot dry-matter production under the non-stress condition and the best ability of shoot dry weight maintain ability (66% and 71%, respectively) under the salt stress condition (). Based on such results, V. luteola (L8, L9) were considered to be the most suitable and useful for forage production in salt-affected fields. Actually, V. luteola has been used for pasture production in Australia, and cv. Dalrymple was released. This cultivar showed high flood tolerance under short-term (17 days) and long-term (6 months) waterlogging treatment (Miller & Williams, Citation1981; Whiteman, Seitlheko, Siregar, Chudasama & Javier, Citation1984). Therefore, the salt-tolerant accessions of V. luteola (L8, L9) have great potential to be used as genetic materials to improve salt tolerance of V. luteola cv. Dalrymple.
Vigna marina
In this study, V. marina accessions showed the highest survivability under high NaCl concentrations based on soil pot experiment (Supplementary Table 1). In this experiment, even under 400 mM NaCl for 4 weeks, no V. marina accession became wilted (S). In a hydroponic experiment using 50 accessions each of V. marina and V. luteola, the percentage of dead plants in V. luteola was 34% under 300 mM NaCl treatment for 4 weeks and only 2% in V. marina under a higher salt concentration, 350 mM NaCl, for 4 weeks (). Therefore, it is clear that salt-tolerant accessions of V. marina, rather than V. luteola, will be able to maintain dry-matter production in highly salt-damaged areas.
In addition, as shown in this study, the salt-tolerant accessions of V. marina could accumulate a comparatively high level of salt, up to 2500 μM Na+, evenly in leaves, stem, and roots and could maintain the physiological activity of the leaf. Considering these characteristics of V. marina, this species is promising as phytoremediation plant in a saline field. V. marina is a legume distributed in sandy beaches and has evolved symbiosis with root nodule bacteria which show higher salt tolerance than its host plant, V. marina (Elanchezhian, Rajalakshmi & Jayakumar, Citation2009). The use of legume-rhizobial symbiosis for phytoremediation would allow faster plant coverage without expensive artificial nitrogen fertilization of the soil (Checcucci, Bazzicalupo & Mengoni, Citation2017). It is worth to point out that V. marina has been cultivated as a food crop on Foah Mulaku island and Gamu island in the Maldives (Mehra & Ibrahim, Citation1989). Therefore, the utilization of both salt-tolerant edible V. marina and salt-tolerant root nodule bacteria together is recommended for phytoremediation (Tug & Yaprak, Citation2017).
Salt-tolerance mechanisms
There are three types of salt-stress tolerance mechanisms: (1) tolerance to osmotic stress, (2) Na+ exclusion from the leaf blade (excluder type in this study), and (3) tissue tolerance (includer type in this study) (Levitt, Citation1980; Munns & Tester, Citation2008). According to the Na+ accumulation pattern in different plant parts in this study, V. vexillata (V1) and V. luteola (L8, L9) tended to have developed excluder type mechanism, the ability to exclude Na+ from the leaf blade, and V. marina (M1, M4) tended to have developed includer type mechanism with tissue tolerance. In this study, some interesting responses against salt stress were observed in V. luteola (L9) and V. marina (M4, M5) and these will be discussed in detail below.
Response of Vigna luteola (L9)
The response of V. luteola (L9) to salt stress was interesting because it had the highest concentration of Na+ accumulated in the roots (3000 μM g−1, ) and increased its root dry weight and root length under salt stress ( and ). The root dry weight increase under salt stress was observed only for V. luteola (L9). This might lead to the enlargement of root volume being used for Na+ storage and depressing the Na+ transition to leaves. This kind of mechanism is also reported in sorghum (Chaugool, Naito, Kasuga & Ehara, Citation2013): cultivars that showed a larger increase in root dry weight under salt stress had a higher Na+ concentration in the roots but a lower Na+ concentration accumulated in the stem and leaf blades. Moreover, the root-system development under salt stress might contribute not only as Na+ storage but also to the absorption of water and nutrients. Under salt stress, only V. luteola (L9) significantly increased the photosynthetic rate (), suggesting this accession could well maintain water and nutrient absorption.
Soil salinity is always heterogeneous (Munns & Gilliham, Citation2015), sodium distribution in soil is not uniform, and there is usually a mixture of higher and lower sodium concentration spots. The ability to increase root volume and length under salt stress would assist plants to mine non-saline or lower saline areas in soil for water and minerals. In fact, arabidopsis, tomato, and sorghum roots exposed to a band of high-NaCl medium exhibit negative halotropism; that is, their roots grow away from the salt band (Galvan-Ampudia et al., Citation2013). The genetic factors controlling the adaptation abilities of V. luteolla (L9) under salt stress, such as higher Na+ accumulation ability in the roots, the maintenance ability of root physiological activities, root mass increase and root elongation ability, are unknown and are very interesting topics need to be clarified.
Response of Vigna marina (M1, M4)
The V. marina (M1, M4) was considered ‘salt includer’ type, and they might have developed a tissue tolerance mechanism. Although M1 and M4 accumulated Na+ at a higher level in all parts (roots, stem, and leaves) (), their physiological activities, such as the photosynthetic rate, were well maintained under salt stress (). According to Shabala (Citation2013), highly salt-tolerant species, such as halophytes, generally have high Na+ and Cl− concentration in leaves, where the traits of tissue tolerance are evident. Such plants usually compartmentalize most of the leaf Na+ and Cl− in vacuoles to keep the cytosolic and organellar ion concentrations below toxic levels and use organic osmolytes to balance the osmotic pressure.
The most interesting response observed in V. marina (M1, M4) was the increase in stomatal conductance and transpiration rate (). The translocation of Na+ from roots to shoot depends on the vapor pressure gradient in the vessel based on transpiration flux, and the flux will be determined by stomatal conductance (Yoo, Pence, Hasegawa & Mickelbart, Citation2009). Plants usually reduce the stomatal aperture or stomata density to restrict the transpiration rate and prevent water deficiency in the body and restrict Na+ translocation from roots to shoot with transpiration flux to regulate Na+ concentration in the leaf (Hasegawa, Citation2013). However, the reduced transpiration rate will have a negative influence such as the decrease in carbon assimilation rate, nutrient uptake, and latent heat release to maintain leaf temperature (Yoo et al., Citation2009). As far as we know, there are no reports describing the increase in transpiration rate with increased stomatal conductance under salt stress. The responses of V. marina (M1, M4) seemed to be a very unique strategy to salt stress.
The expression regulation of an aquaporin (AQP) gene, which is a membrane protein promoting cell-membrane permeability of water and CO2, might play an important role on the increase in stomatal conductance, transpiration rate, and photosynthetic rate under salt stress (Uehlein, Lovisolo, Siefritz & Kadenhoff, Citation2003). Recently, regulation of PIP gene (member of AQPs) expression under salt stress was reported (Boursiac et al., Citation2005; Postaire, Verdoucq & Maurel, Citation2007; Zhou et al., Citation2014). In soybean, the expression level of PIP (GmPIP1;6) decreased at the early osmotic stress stage and then increased during the salt acclimatization stage (Zhou et al., Citation2014).
Over-expression of AQPs resulted in the improved growth of tomato, soybean, sorghum, and banana under salt stress (Liu et al., Citation2015; Sade et al., Citation2010; Sreedharan, Shekhawat & Ganapathi, Citation2015; Zhou et al., Citation2014). When the aquaporin gene of the tobacco was over-expressed in tomato plants, the stomatal conductance, transpiration rate, and photosynthetic rate became higher than those of the wild type plants under 100 mM NaCl, resulted in two times higher fruit yield compared with the control plant (Sade et al., Citation2010).
An aquaporin (GmPIP1;6) over-expressed soybean plants could maintain higher stomatal conductance, transpiration rate and photosynthetic rate under 100 mM NaCl stress compared with those of the wild type plants (Zhou et al., Citation2014). As a result, PIP over-expressed soybean plants could produce significantly higher shoot fresh weight without showing leaf yellowing (the wild type plants showed severe leaf yellowing), probably because of the lower Na+ concentration in the leaves. Interestingly, PIP over-expressed soybean plants performed better in the non-salt stress field. They showed a significantly higher seed yield, which was attributed to seed size increase (pod and seed number unchanged). These reports suggested that the expression regulation of aquaporin genes in plants could improve their growth both under salt stressed and non-salt stress environments. Since V. marina (especially M1) showed much higher (about twice) transpiration rate compared with V. luteola and V. vexillata even under non-stress condition, V. marina, growing on a sandy beach, seemed to have developed higher expression system of aquaporin genes before encounter the salt stress. They might further increase the expression of aquaporin genes, which led the observed higher stomatal conductance, transpiration rate and photosynthesis rate, resulted in the highest salt tolerance. To clarify the genetic factors underlying high salt tolerance of V. marina, genetic analyses of aquaporin genes as well as other salt tolerance related genes such as SOS (Na+ exclusion) and NHX (Na+ compartmentalization) should be conducted.
Future perspectives
Crop wild relatives have developed much higher levels of stress tolerance compared with domesticated crops. Consequently, they offer us the possibility to find out novel stress tolerance gene(s) which could not be attained by the study targeting only crop gene pool. In the present study, we could find out several salt-tolerant wild accessions showing unique physiological responses under salt stress.
The L9 of V. luteola, which showed an increase in root volume containing a high concentration of Na+, was collected at the clay soil bank of a river around 10km inland from the coast in northern New South Wales, Australia. The M1 of V. marina, that showed an increase in stomatal conductance and transpiration rate under salt stress, adapts to inhabit the coral sandy beach on Iriomote Island in Okinawa, Japan. Studies to clarify the stress tolerance gene(s) of crop wild relatives growing on extremely harsh environment might offer novel clues to develop stress-tolerant crops. The collection, evaluation and use of crop wild relatives should be one of the most important topics for sustainable food production in the coming era of climate change and global warming.
PPS2018_144RP-File015.pdf
Download PDF (270.4 KB)PPS2018_144RP-File014.pdf
Download PDF (274.3 KB)PPS2018_144RP-File013.pdf
Download PDF (309.7 KB)PPS2018_144RP-File012.pdf
Download PDF (312.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
References
- Baba, I., & Takahashi, Y. (1958). Solution culture. In Y. Togari (Ed.), Sakumotsu Shiken Ho (pp. 327–343). Tokyo: Nogyo Gijutsu Kyokai.
- Boursiac, Y., Chen, S., Luu, D. T., Sorieul, M., van den Dries, N., & Maurel, C. (2005). Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology, 139(2), 790–805.
- Chaugool, J., Naito, H., Kasuga, S., & Ehara, H. (2013). Comparison of young seedling growth and sodium distribution among sorghum plants under salt stress. Plant Production Science, 16(3), 261–270.
- Checcucci, A., Bazzicalupo, M., & Mengoni, A. (2017). Exploiting nitrogen-fixing rhizobial symbionts genetic resources for improving phytoremediation of contaminated soils. Enhancing Cleanup of Environmental Pollutants, 1, 275–288. Biological Approaches.
- Dachapak, S., Somta, P., Poonchaivilaisak, S., Yimram, T., & Srinives, P. (2017). Genetic diversity and structure of the zombi pea (Vigna vexillata (L.) A. Rich) gene pool based on SSR marker analysis. Genetica, 145, 189–200. PMID: 28233217.
- Dachapak, S., Tomooka, N., Somta, P., Naito, K., Kaga, A., & Srinives, P. (2018). QTL analysis of domestication syndrome in zombi pea (Vigna vexillata), an underutilized legume crop. PloS One, 13(12), e0200116.
- Duke, J. A. (1981). Handbook of legumes of world economic importance (11th ed., pp. 345 p). New York and London: Plenum Press.
- Elanchezhian, R., Rajalakshmi, S., & Jayakumar, V. (2009). Salt tolerance characteristics of rhizobium species associated with Vigna marina. Indian Journal of Agricultural Sciences, 79(12), 980–985.
- FAO. FAO Land and plant nutrition management service. 2008. http://www.fao.org/ag/agl/agll/spush.
- Galvan-Ampudia, C. S., Julkowska, M. M., Darwish, E., Gandullo, J., Korver, R. A., Brunoud, G., … Testerink, C. (2013). Halotropism is a response of plant roots to avoid a saline environment. Current Biology, 23(20), 2044–2050.
- Hasegawa, P. M. (2013). Sodium (Na+) homeostasis and salt tolerance of plants. Environmental and Experimental Botany, 92, 19–31.
- Iseki, K., Takahashi, Y., Muto, C., Naito, K., & Tomooka, N. (2016). Diversity and evolution of salt tolerance in the genus Vigna. PloS One, 11(10). doi:10.1371/journal.pone.0164711
- Jackai, L. E. N., Padulosi, S., & Ng, Q. (1996). Resistance to the legume pod borer, Maruca vitrata fabricius, and the probable modalities involved in wild Vigna. Crop Protection, 15(8), 753–761.
- James, A. T., & Lawn, R. J. (1991). Inheritance of selected traits in accessions of Vigna vexillata (L.) A. rich of Australian and African origin. Australian Journal of Botany, 39, 415–429.
- Karuniawan, A., Iswandi, P. R. K., Heinzemann, J., & Grüneberg, W. J. (2006). Vigna vexillata (L.) A. Rich. cultivated as a root crop in Bali and Timor. Genetic Resources and Crop Evolution, 53, 213–217.
- Kevet, J., Ondok, J. P., Necas, J., & Jarvis, P. G. (1971). Methods of growth analysis. In Z. Setak, J. Catsky, & P. G. Jarvis (Eds.), Plant photosynthetic production manual of method (pp. 343–391). The Hague: Dr Junk N.V. Publishers.
- Lawn, R. J., & Cottrell, A. (1988). Wild mungbean and its relatives in Australia. Biologist, 35(5), 267–273.
- Lawn, R. J., & Watkinson, A. R. (2002). Habitat, morphological diversity and distribution of the genus Vigna Savi in Australia. Australian Journal of Agricultural Research, 53, 1305–1316.
- Levitt, J. (1980). Response of plants to environmental stress. Volume II. Water, radiation, salt, and other stresses. New York: Academic Press.
- Liu, P., Yin, L., Wang, S., Zhang, M., Deng, X., Zhang, S., & Tanaka, K. (2015). Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environmental and Experimental Botany, 111, 42–51.
- Marubodee, R., Chakhatrakan, S., & Ehara, H. (2012). Comparison of growth of azuki, cowpea and mungbean with aeration and non-aeration under hydroponic technique. Thai Journal of Science and Technology, 1(1), 61–68.
- Maxted, N., Mabuza Dlamini, P., Moss, H., Padulosi, S., Jarvis, A., & Guarino, L. (2004). African Vigna: An ecogeographic study. Rome, Italy: International Plant Genetic Resources Institute (IPGRI).
- Mehra, K. L., & Ibrahim, A. H. (1989). Genetic resources in the Maldives. FAO/IBPGR Plant Genetic Resources Newsletter, 75(76), 42–43.
- Miller, I. L., & Williams, W. T. (1981). Tolerance of some tropical legumes to six months of simulated waterlogging. Tropical Grasslands, 15, 39–43.
- Munns, R., & Gilliham, M. (2015). Salinity tolerance of crops – what is the cost? New Phytologist, 208(3), 668–673.
- Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
- Ogundiwin, E. A., Thottappilly, G., Aken’Ova, M. E., Ekpo, E. J. A., & Fatokun, C. A. (2002). Resistance to cowpea mottle carmovirus in Vigna vexillata. Plant Breed, 121, 517–520.
- Postaire, O., Verdoucq, L., & Maurel, C. (2007). Aquaporins in plants: From molecular structure to integrated functions. In J. C. Kader & M. Delseny (Eds.), Advances in botanical research (Vol. 46, pp. 75–136). Amsterdam: Elsevier
- Sade, N., Gebretsadik, M., Seligmann, R., Schwartz, A., Wallach, R., & Moshelion, M. (2010). The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiology, 152(1), 245–254.
- Sasikumar, B., & Sardana, S. (1988). Vigna vexillata (Fabaceae), a pulse cum tuber crop of northeastern hill region of India. Economic Botany, 42(2), 292.
- Shabala, S. (2013). Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 112(7), 1209–1221.
- Sreedharan, S., Shekhawat, U. K. S., & Ganapathi, T. R. (2015). Constitutive and stress-inducible overexpression of a native aquaporin gene (MusaPIP2;6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Molecular Biology, 88(1–2), 41–52.
- Takahashi, Y., Somta, P., Muto, C., Iseki, K., Naito, K., & Pandiyan, M., Senthil N., & Tomooka N. (2016). Novel genetic resources in the genus Vigna unveiled from gene bank accessions. PloS One, 11(1). doi:10.1371/journal.pone.0147568
- Thottappilly, G., Ng, N. Q., & Rossel, H. W. (1994). Screening germplasm of Vigna vexillata for resistance to cowpea mottle virus. International Journal of Tropical Diseases, 12, 75–80.
- Tomooka, N., Naito, K., Kaga, A., Sakai, H., Isemura, T., Ogiso-Tanaka, E., Iseki, K., & Takahashi, Y. (2014). Evolution, domestication and neo-domestication of the genus Vigna. Plant Genetic Resources Characterization and Utilization, 12, S168–S171.
- Tug, G. N., & Yaprak, A. E. (2017). Halophytes as a potential food source. Anadolu Journal of AARI, 27(2), 78–81.
- Uehlein, N., Lovisolo, C., Siefritz, F., & Kadenhoff, R. (2003). The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature, 425(6959), 734–737.
- United States of America. National Research Council (NRC). (1979). Vigna vexillata in tropical legumes: Resources for the future (pp. 34–36). Washington, DC: National Academy of Science.
- Vanderborght, T. (1989). Some observations on seedlings of Vigna vexillata (L.) A. Rich. (Fabaceae). Bulletin Du Jardin Botanique National De Belgique, 59, 179–187.
- Vincenzo, L., Terzano, R., Cicco, N., Cardinali, A., Di Venere, D., & Linsalata, V. (2005). Seed coat tannins and bruchid resistance in stored cowpea seeds. Journal of the Science of Food and Agriculture, 85, 839–846.
- Whiteman, P. C., Seitlheko, M., Siregar, M. E., Chudasama, A. K., & Javier, R. R. (1984). Short-team flooding tolerance of 17 commercial tropical pasture legumes. Tropical Grasslands, 18(2), 91–96.
- Yoo, C. Y., Pence, H. E., Hasegawa, P. M., & Mickelbart, M. V. (2009). Regulation of transpiration to improve crop water use. Critical Reviews in Plant Sciences, 28(6), 410–431.
- Yoshida, Y., Marubodee, R., Ogiso-Tanaka, E., Iseki, K., Isemura, T., Takahashi, Y., Muto, C., Naito, K., Kaga, A., Okuno, K., Ehara, H., & Tomooka, N. et (2016). Salt tolerance in wild relatives of adzuki bean, Vigna angularis (willd.) Ohwi Ohashi. Genetic Resources and Crop Evolution, 63(4), 627–637.
- Zhou, L., Wang, C., Liu, R. F., Han, Q., Vandeleur, R. K., Du, J., Tyerman, S., Shou, H., Senthil, N., & Tomooka, N. (2014). Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1;6 confers salt tolerance. BMC Plant Biology, 14. doi:10.1186/1471-2229-14-181.
