ABSTRACT
In this study, red and yellow varieties of djulis (Chenopodium formosanum Koidz) were cultivated in spring and autumn, and the effects of calcium carbonate treatment on their growth traits, antioxidant capacity, and grain maturation were examined. This study showed that calcium carbonate treatment increased the plant height and stem thickness in the red variety of djulis. The treatment also significantly increases the yield components of the red variety of djulis, such as spike length and thousand-grain weight. Treating the red variety of djulis with calcium carbonate in spring significantly increased the antioxidant enzyme activity such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) activities. Interestingly, results indicated that calcium carbonate treatment could promote the early maturation of the mid-late-maturing red variety of djulis and delay the maturation of the early-maturing yellow variety. Hence this experiment concludes that the exogenous calcium treatment in djulis can regulate grain maturation and promote growth, yield, and antioxidant enzyme activity; however, the growing season and the varietal effects play a vital role in determining the overall productivity of the djulis crop.
Graphical abstract
Introduction
Quinoa (Chenopodium quinoa) is a highly nutritive food crop mainly found in South America. On the other hand, djulis (Chenopodium formosanum Koidz.) is a close relative of quinoa and a dicotyledonous plant belonging to the family of Amaranthaceae. The nutritional value and functional components of djulis have gradually gained attention due to its numerous benefits to the human body. The leaves and seeds of djulis are edible where the seeds are rich in vitamins (A, B2, C, E), amino acids, proteins, dietary fiber, and minerals (such as calcium, phosphorus, iron, copper, and zinc) and are the primary source of nutrients. In addition, the shells of djulis contain saponins, betalains, polyphenols, and other antioxidants (Tsai et al., Citation2011), which have antineoplastic, blood pressure-lowering, and anti-inflammatory effects (Hong et al., Citation2016).
Calcium is one of the essential nutrients for plants, and its primary physiological functions include cell division, enzyme activity regulation, and cell wall structure stabilization due to the presence of calcium pectinate in the middle lamella of the cell walls (Quiles et al., Citation2004). The application of exogenous calcium during the growth period of crops can promote crop growth and increase yield, as indicated by several studies. For example, soybean treated with different calcium concentrations significantly increased the dry weight of roots, stems, and leaves, with the yield of seeds increasing with the increase in calcium concentration (Domingues et al., Citation2016). Likewise, exogenous calcium treatment during the growth of tomatoes can increase the volume of roots, fresh weight of roots and leaves, and yield of fruits per plant, and decrease the occurrence of blossom-end rot (Gaion et al., Citation2019; Lopez & Satti, Citation1996). In addition, a significant delay in fruit maturation was also seen as a result of treating papayas with 2.5% CaCl2 for 15 min (Gao et al., Citation2020), indicating that exogenous calcium treatment can also increase crop quality and extend its shelf life (Martin-Diana et al., Citation2007).
Moreover, long-term spraying of exogenous calcium on sugarcane leaves can decrease the number of flowers and delay the flowering period, thereby increasing the sugar content (Endres et al., Citation2016). Tomato leaves sprayed with calcium chloride significantly increased the calcium content in fruits, while the tomato maturation and senescence were delayed, thereby increasing the shelf life of tomatoes (Chéour & Souiden, Citation2015). These studies show that applying calcium can increase crop yield and quality and regulate the harvest time to extend the shelf life of vegetables and fruits.
The calcium ion content in plant cells is associated with the plant’s response to external environmental stimuli (White & Broadley, Citation2003). Djulis seedlings treated with 5 mM calcium carbonate significantly increased the activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) had significantly increased (Chao et al., Citation2021). An increase in the antioxidant activity of plants can aid stress tolerance. Under high temperatures (Tan et al., Citation2011), drought (Xu et al., Citation2013), or salinity stress (Cheng et al., Citation2013), the calcium carbonate treatment of crops can significantly increase the activities of antioxidant enzymes (SOD, CAT, APX, and GR), thereby decreasing the level of the cell damage caused by reactive oxygen species. Hence, these studies indicate that increasing calcium ion content in plants can increase stress resistance in plants.
Furthermore, excessive potassium in the soil has antagonistic effects on calcium uptake by plants. On the other hand, excessive calcium concentration leads to potassium deficiency, affecting crop yield and quality (Madani et al., Citation2013). Therefore, attention must be paid when the exogenous calcium treatment is applied.
Currently, there are very few studies on the effects of exogenous calcium treatment on the growth and physiology of djulis. Therefore, the current research is designed to examine the effects of exogenous calcium treatment on the growth traits and antioxidant activity of djulis seedlings. Two different colored varieties of djulis were used as the study materials, and nano-calcium carbonate treatment was performed.
Materials and methods
Djulis has various spike types, and the color of the spike can be distributed from yellow to red. For this experiment, red and yellow colored djulis were used, which were developed through five years of purification tests; however, the Taiwan Agriculture Research Institute is yet to name them. We named the yellow spike djulis OY line for this study, and the red spike djulis is named the RP line.
Practice Farm of the Department of Plant Industry (DPI), National Pingtung University of Science and Technology (NPUST) (22°38ʹ54.4”N 120°37ʹ02.7”E), was selected as the experimental area. The study was performed in spring (from March to June 2020) and autumn (from September to December 2020). The experimental field was divided into eight plots, with each plot having an area of 10 m × 1 m. Each of these lines was allocated with four plots, with two plots as treatment plots and the other two plots for the control group. In each plot, two rows of djulis were planted with a spacing of 40 cm × 40 cm, making up to 25 plants per row and 50 plants per plot. The Completely Randomized Design (CRD) was used to carry out this study.
Each plot was prepared with 2.5 kg of organic compost containing 65% organic matter, 2.5% total nitrogen, 2.3% total phosphoric anhydride, and 2.0% total potassium oxide. The seeds were sown in the plugs for a month and then transplanted in the field. On the third, sixth, and seventh weeks after transplanting (WAT), 300 g of compound fertilizers (15% N, 15% P2O5, 15% K2O, 3% MgO) were applied in each plot as top-dressing. At WAT 2, 5 mM calcium carbonate (DIAMOND NANO-BIOCHEM CO., LTD. Taichung, Taiwan) was sprayed on the leaves once a week until WAT 9. From WAT 2 to WAT 9, changes in the plant height, branch number, stem thickness, and internode number were recorded. At WAT 6 and 10, about 100–200 mg of the middle part of the fourth or fifth leaf was taken to analyze the antioxidant enzyme activity and the H2O2 content. Four samples per treatment were collected for analysis.
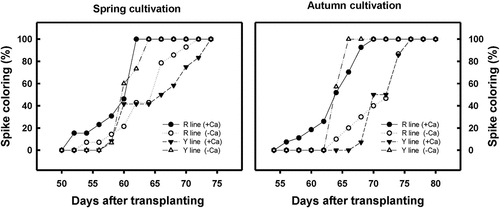
Djulis is ready to be harvested in the second week after two-thirds of the spike inflorescences have undergone the color change (from green to red (for RP line) or yellow (for OY line)). Therefore, the spike color change of red and yellow lines was investigated from the 50th to the 80th day after transplanting (DAT). A combined 15 fixed plants with or without calcium treatment were selected to investigate the changes in spike color, and the results were presented as a percentage.
Analysis of antioxidant enzyme activity
A fresh leaf sample (0.1–0.2 g) was homogenized with sodium phosphate buffer (50 mM; pH 7.4 for SOD and 50 mM; pH 6.8 for CAT, APX, GR) in an ice bath (liquid nitrogen was used for grinding and extraction purposes). The solution was centrifuged (15,000 g for SOD and 12,000 g for CAT, APX, GR) using Velocity 14 R refrigerated Centrifuge (Dymamica Scientific Ltd, UK) at 4°C, and the supernatant was collected. The SOD activity was measured according to Paoletti et al. (Citation1986). The enzyme extract (0.2 mL) was added to the reaction mixture (2.73 mL) containing 100 mM triethanolamine–diethanolamine buffer (pH 7.4), 100 mM/50 mM EDTA/MnCl2 (pH 7.0), and 10 mM β-mercaptoethanol. After the above mixture was mixed evenly, NADH was added to start the reaction. The absorbance was measured at 340 nm for 10 min using Double Beam U-2900 Spectrophotometer (Hitachi High-Tech Corporation, Japan). One unit of SOD was defined as the amount of enzyme that inhibited the rate of NADH oxidation by 50% in the blank sample.
Furthermore, the method of Kato and Shimizu (Citation1987) was used to measure the CAT activity. The reduction in hydrogen peroxide amount was measured at 240 nm, and the extinction coefficient (40 mM−1cm−1) was used to calculate CAT activity. One unit of CAT was defined as the amount of enzyme needed to degrade 1 mole of hydrogen peroxide in 1 min. The APX activity was measured using the method of Nakano and Asada (Citation1981). As the concentration of ascorbate (AsA) decreased, the absorbance at 290 nm also reduced, and the extinction coefficient of AsA (2.8 mM−1cm−1) was used to calculate the APX activity. One unit of APX was defined as the amount of enzyme needed to degrade 1 mole of AsA in 1 min. The GR activity was measured using the method of Foster and Hess (Citation1980). One unit of GR was defined as the amount of enzyme needed to decrease the absorbance at 340 nm at 1 min. The protein content in the enzyme extract was measured using the method of Bradford (Citation1976).
Measurement of hydrogen peroxide content (H2o2)
The method of Jana and Choudhuri (Citation1981) was used to analyze the hydrogen peroxide content. A fresh leaf sample (0.1–0.2 g) was homogenized with 3 mL sodium phosphate buffer (50 mM; pH 6.8, containing hydroxylamine) before centrifuging at 6000 g for 25 min at 4°C, and the supernatant was collected. Further, 2 mL of the collected supernatant and 1 mL of 0.1% titanium chloride (v/v) were dissolved in 20% (v/v) H2SO4. After mixing evenly, centrifugation was performed at 5000 rpm for 15 min at room temperature. Subsequently, the absorbance at 410 nm was measured to calculate hydrogen peroxide content.
Measurement of sodium and potassium ions
After harvesting, djulis plants and seeds were collected and dried at 60°C. Further, 0.5 g of sample was transferred into glass decomposition tubes, and 5 mL of a mixture of two acids [nitric acid: perchloric acid = 4:1 (v/v)] was added. The tubes were then allowed to stand overnight before heating in a calciner. The heating was carried out at 65°C for 15 min before the temperature was increased to 100°C. The tubes were heated until the gas in the tube was almost transparent, and then the tube was again heated to 190°C so that the acidic gases were volatilized entirely. The reaction was completed when the liquid at the bottom was almost transparent. After the decomposition, the tube was cooled, and the liquid in the tube was filtered with filter paper (Whatman 42) and rationed to 50 ml with double-distilled water. Finally, the Model 410 Classic flame spectrometer (Sherwood Scientific Ltd, UK) was used to measure sodium and potassium ions.
Statistical analysis
The collected results were subjected to a two-way analysis of variance (ANOVA) using the International Business Machines SPSS Statistics for Windows, version 26 (International Business Machines Corporation, Armonk, NY, USA), which was used to perform the statistical analysis. The mean separation was performed using Tukey’s honest significance test at p = 0.05, and all the results were expressed as means ± standard error. The charts and graphs were produced using SigmaPlot 10 (Jandel Corporation, Palo Alto, CA, USA).
Results
Agro-meteorological data
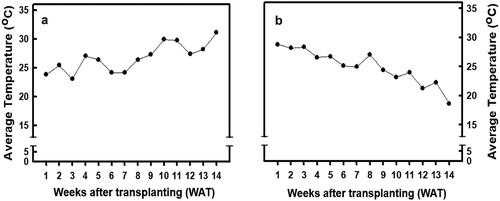
The average weekly temperature data throughout the cultivation period were collected from the meteorological station of the DPI at the NPUST. Fourteen weeks of meteorological data were collected and recorded in each season. Based on the results, a continual increase in the temperature throughout the study period was observed during spring, while a continual decrease in the temperature in autumn was observed ().
Analysis of vegetative growth of djulis
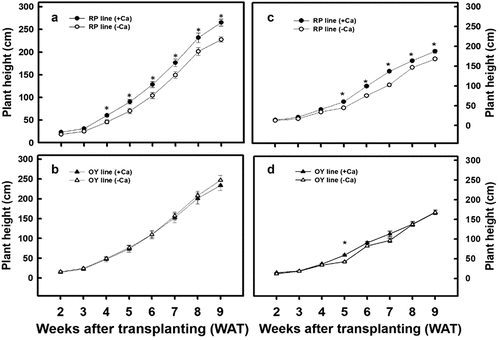
Since spiking occurred at WAT 9, the plant height, number of branches, and stem diameter significantly plateaued, and the number of internodes did not further increase. As a result, agronomic traits were examined until WAT 9. This experiment shows that the RP line treated with calcium carbonate significantly increased the plant height in both spring () and autumn (). However, no significant differences in the plant height of the OY line of djulis were observed irrespective of the treatment applied or the cultivation seasons ().
Figure 2. The effect of calcium carbonate on plant height of different djulis lines. Plant height of the RP line of djulis cultivated in (A) spring and (C) autumn; and the OY line of djulis cultivated in (B) spring and (D) autumn. RP line – red spike djulis; OY line – yellow spike djulis; Bars represent means ± SE; * represent significantly different values between – Ca+2 and + Ca+2 at P < 0.05.
No significant differences in the stem diameter were observed for the OY line of djulis regardless of the treatment used or the cultivated seasons (). However, the stem diameter of only the RP line of djulis cultivated in autumn and treated with calcium carbonate significantly increased the stem diameter (). In addition, no significant difference in the number of internodes was observed for both lines of djulis, irrespective of the treatment used or the cultivated seasons ().
Figure 3. The effect of calcium carbonate on stem diameter of different djulis lines. Stem diameter of the RP line of djulis cultivated in (A) spring and (C) autumn; and the OY line of djulis cultivated in (B) spring and (D) autumn. RP line – red spike djulis; OY line – yellow spike djulis; Bars represent means ± SE; * represent significantly different values between – Ca+2 and + Ca+2 at P < 0.05.
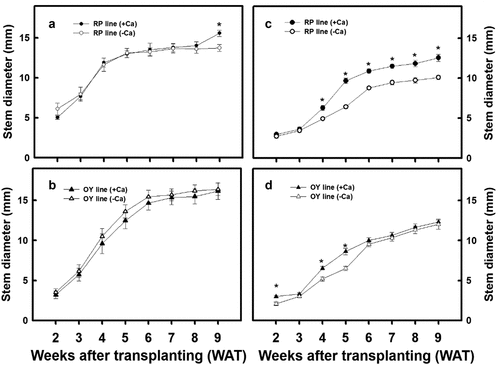
Figure 4. The effect of calcium carbonate on the internode number of different djulis lines. Internode number of the RP line of djulis cultivated in (A) spring and (C) autumn; and the OY line of djulis cultivated in (B) spring and (D) autumn. RP line – red spike djulis; OY line – yellow spike djulis; Bars represent means ± SE; * represent significantly different values between – Ca+2 and + Ca+2 at P < 0.05.
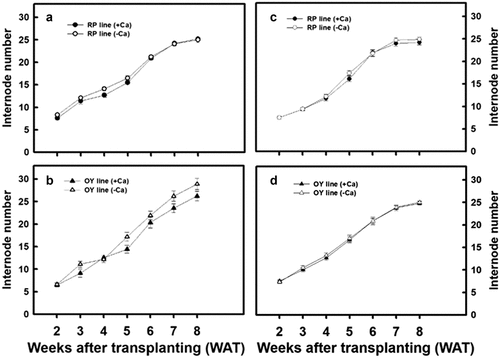
Furthermore, within lines comparison of the agronomic traits of the plants grown in spring and autumn shows that the plant height, stem diameter, and the number of internodes in spring crops were significantly higher than in autumn crops. The increase was more significant in the OY line of djulis (). The above results indicate that calcium carbonate treatment resulted in a varying increase in the agronomic traits in the RP and OY line of djulis, which could be due to the changes in the environmental temperature during the cultivation period.
Hydrogen peroxide (H202) content in djulis
The samples analyzed at WAT 6 indicate that the calcium carbonate treatment in the RP line had no significant effect on the H2O2 content in both spring and autumn cultivated plants (). Similar results were obtained in the autumn crops analyzed at WAT 10, while in spring cultivated plants, the calcium carbonate treatment significantly decreased the H2O2 content. For the OY line, a significant decrease in the H2O2 content was observed after calcium carbonate treatment in spring cultivated plants analyzed at WAT 6 and autumn cultivated plants analyzed at WAT 10. No significant effects of calcium carbonate treatment were observed in plants cultivated in autumn when analyzed at WAT 6 and spring cultivated plants analyzed at WAT 10.
Table 1. Effect of calcium carbonate treatment on hydrogen peroxide (H2O2) activity in RP and OY djulis lines cultivated in spring and autumn.
The two-way ANOVA results indicate that both variety and growing season significantly affected the H2O2 content in plants analyzed at WAT 6. At WAT 10, the crop variety, growing season, and their interaction effects significantly affected the H2O2 content in djulis plants, as shown in .
Analysis of antioxidant enzyme activity in djulis
The results indicate that the calcium carbonate treatment in the RP line of djulis cultivated in spring significantly decreased the SOD activity at WAT 6. No significant effects of calcium carbonate treatment on SOD activity in autumn cultivated plants were observed. On the other hand, the SOD activity significantly increased after calcium carbonate treatment in autumn cultivated plants when analyzed at WAT 10. The results also showed no significant effects of calcium carbonate treatment on SOD activity in spring cultivated plants when analyzed at WAT 10. For the OY line, the calcium carbonate treatment had no significant effect on the SOD activity in djulis plants cultivated in spring or autumn at both WAT 9 and WAT 10. The two-way ANOVA results indicate that the SOD activity in the djulis plant at WAT 6 and 10 were significantly affected by the crop variety, growing season, and their interaction effects, as shown in .
Table 2. Effect of calcium carbonate treatment on antioxidant activity in RP and OY djulis lines cultivated in spring and autumn.
Moreover, the CAT activity of the RP line of djulis cultivated in autumn significantly increased with the calcium carbonate treatment analyzed at WAT 6. No significant effect of calcium carbonate treatment on the CAT activity was observed in plants grown in spring at WAT 6 and both spring and autumn at WAT 10. As for the OY line, no significant effect of calcium carbonate treatment on CAT activity was observed in plants cultivated in spring or autumn at both WAT 9 and WAT 10. The two-way ANOVA results indicate that neither variety, growing season, nor their interaction effects significantly affected the CAT activity of djulis plants, as shown in .
Similar results were obtained for APX activity. The results indicate that the APX activity of the RP line of djulis cultivated in autumn significantly increased with the calcium carbonate treatment analyzed at WAT 6. No significant effect of calcium carbonate treatment on the APX activity was observed in plants grown in spring at WAT 6 and both spring and autumn at WAT 10. As for the OY line, no significant effect of calcium carbonate treatment on APX activity was observed in plants cultivated in spring or autumn at both WAT 9 and WAT 10. However, the two-way ANOVA results indicate that only the growing season significantly affected the APX activity in djulis crops at both WAT 6 and WAT 10, as shown in .
On the other hand, the GR activity in the RP line of djulis cultivated in the spring and autumn seasons was not significantly affected by the calcium carbonate treatment at WAT 6 and WAT 10. As for the OY line, the calcium carbonate treatment did not significantly affect the GR activity in plants cultivated in spring and autumn at WAT 6 and spring at WAT 10. However, the calcium carbonate treatment significantly increased the GR activity in plants cultivated in autumn at WAT 10. The two-way ANOVA results indicate that the GR activity in djulis at WAT 6 was significantly affected by the growing season, while at WAT 10, the variety, growing season, and their interactive effects significantly affected the GR activity, as shown in .
Analysis of potassium and calcium contents in the plants and seeds of djulis
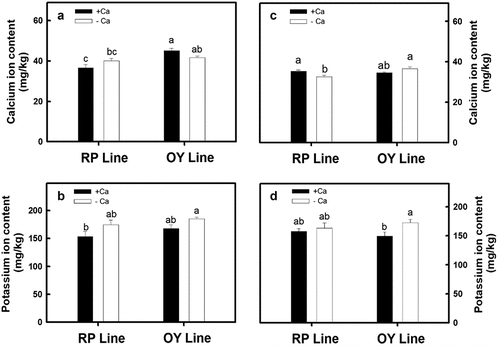
After harvesting djulis seeds, we analyzed the calcium and potassium content changes in the plant tissue and seeds. No significant differences in the calcium ion and potassium ion content in the stem of the RP line in spring and potassium content in autumn of the RP line were observed. However, the calcium content in the stem significantly increased in the RP line treated with calcium carbonate in autumn (). As for the OY line, no significant differences were observed in the calcium ion content in the stem of djulis cultivated in spring and autumn under calcium carbonate treatment (). However, a significant decrease in the potassium ion content in the stem of the OY line in autumn was observed after calcium carbonate treatment (). In contrast, no significant difference in the potassium ion content in the stem of the OY line was observed in spring under calcium carbonate treatment ().
Figure 5. The effect of calcium carbonate on Ca+ and K+ content in the stem of different djulis lines. (A) Ca+ content and (B) K+ content in the stem of the RP and OY djulis lines cultivated in spring. (C) Ca+ content and (D) K+ content in the stem of the RP and OY djulis lines grown in autumn. RP line – red spike djulis; OY line – yellow spike djulis; Bars represent means ± SE; Bars followed by the same letter are not significantly different at P < 0.05.
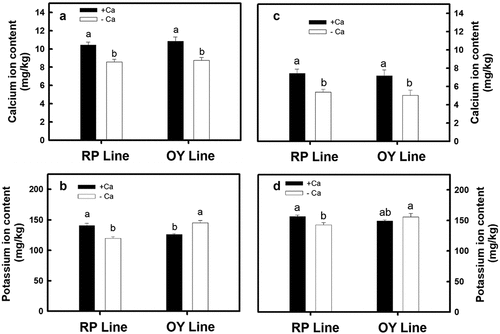
Based on , the calcium contents in seeds from the RP and OY lines planted in spring and autumn significantly increased after calcium carbonate treatment (). The calcium content in seeds of spring plants was 1.2–1.5 times more than in autumn seeds. In addition, the potassium contents in the seeds of the RP line planted in spring and autumn significantly increased after calcium carbonate treatment (). In the OY line, the potassium content in the seeds of the autumn plant did not significantly increase after calcium carbonate treatment (), while the potassium content in the seeds of the spring plant significantly decreased ().
Figure 6. The effect of calcium carbonate on Ca+ and K+ content in the grains of different djulis lines. (A) Ca+ content and (B) K+ content in grains of the RP and OY djulis lines cultivated in spring. (C) Ca+ content and (D) K+ content in the grains of the RP and OY djulis lines grown in autumn. RP line – red spike djulis; OY line – yellow spike djulis; Bars represent means ± SE; Bars followed by the same letter are not significantly different at P < 0.05.
Effects on the spike color change stage of djulis
After djulis have flowered, the color change stage of the spike occurs when two-thirds of inflorescences have undergone the color change. The best time for seed harvesting is the second week after the spike has completely changed color. Therefore, the speed of color change would affect the seed harvest time. Initially, the OY line of djulis is an early maturing line. When planted in autumn without any treatment, the spike color change rate of the OY line was 60% on DAT 60 and 100% on DAT 64. However, after calcium carbonate treatment, the spike color change was only 50% on DAT 67 and 100% on DAT 74 (). It was observed that after treating calcium carbonate on the OY line of djulis planted in autumn, the results obtained were identical to those grown in spring. When the calcium carbonate was treated in the OY line during spring cultivation, the spike color change was 50% on DAT 70; however, the spike color change already reached 100% on DAT 65 in the untreated group (). Therefore, the above results showed that spike color change is delayed when calcium carbonate is treated in the OY line of djulis.
Figure 7. The effect of calcium carbonate treatment on the duration of spike color change in different djulis lines cultivated in (A) spring and (B) autumn. RP line – red spike djulis; OY line – yellow spike djulis.
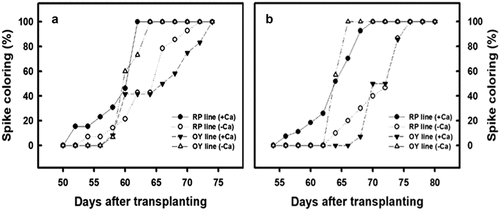
On the other hand, the RP line is initially a mid-late maturing variety. On DAT 64, the rate of the spike color change of the RP line planted in spring was 21%, which increased to 46% when treated with calcium carbonate. On DAT 70, the rate of the spike color change of the RP line was 93% for control and 100% for plants treated with calcium carbonate (). Similarly, on DAT 64, the spike color change of the RP line planted in autumn was 10% for control and 58% for plants treated with calcium carbonate. On DAT 70, the rate of the spike color change of the RP line was 40% for control, whereas it was 100% for those plants treated with calcium carbonate (). The above results showed that calcium carbonate treatment leads to an early occurrence of spike color change in the RP line of djulis.
The overall results show that the calcium carbonate treatment can delay spike color change in the early-maturing OY line of djulis, while it leads to an early occurrence of spike color change in the mid-late-maturing RP line. The effects were highly significant for the results obtained from the autumn cultivated plants.
Effects on the yield-related traits of djulis
The study results show that the calcium carbonate treatment in both RP and OY lines did not significantly affect the spike fresh weight of spring and autumn cultivated crops. The two-way ANOVA results indicate that neither variety, growing season, nor their interaction effects significantly impacted the spike fresh weight of djulis, as shown in .
Table 3. The effect of calcium carbonate on yield components of different djulis lines.
Likewise, for the spike length, the results clearly show that the calcium carbonate treatment did not significantly affect the spike length of both RP and OY lines cultivated in both spring and autumn. The two-way ANOVA results indicate that the variety and growing season significantly impacted the spike length of djulis, while their interactive effects did not, as shown in .
As for the 1000-grain weight, the calcium carbonate treatment did not significantly affect the 1000-grain weight of both RP and OY lines cultivated in spring and the OY line cultivated in autumn. However, the calcium carbonate treatment did increase the 1000-grain weight of the RP line of djulis cultivated in autumn. The two-way ANOVA indicated that both variety and the growing season significantly affected the 1000-grain weight of djulis, as shown in .
Discussions
Calcium is an element that is essential for the growth and development of plants. Its functions include maintaining the cell membrane stability, acting as a cell wall component, participating in signal transduction, and regulating physiological responses under stress conditions to alleviate damage (Thor, Citation2019). Calcium deficiency during plant growth and development will affect the yield and decrease the quality (Olle & Bender, Citation2009). Therefore, exogenous calcium is often applied for extended periods during crop growth to stabilize crop yield and quality. The treatment of djulis seedlings with 5 mM calcium carbonate can increase the root length, plant height, and fresh and dry weights by 55%, 12%, 37%, and 17%, respectively (Chao et al., Citation2021). Kumara et al. (Citation2019) applied calcium carbonate foliarly during the growth phase of rice and found that it increased rice yield and quality and pest resistance in rice. The treatment of strawberries with 0.5% calcium chloride significantly increased the number of leaves per plant, leaf area, and chlorophyll content in the vegetative growth phase, as well as the number of flowers per plant, fruit weight, and fruit weight per plant in the reproductive growth phase (Ahmed et al., Citation2020). Spraying tomatoes with 15 mM calcium chloride could increase the number of branches, plant height, dry weight, chlorophyll, and nitrogen contents (Kazemi, Citation2014). In this study, the treatment of RP and OY lines of djulis with 5 mM calcium carbonate increased the agronomic parameters, such as plant height (), stem diameter (), and the number of internodes (); however, growth-promoting effects varied between the lines. Based on the results, it was found that increasing plant calcium content can promote crop growth regardless of the form of calcium treatment used (calcium carbonate or calcium chloride).
Plants activate defense mechanisms under stress to alleviate the damage caused by reactive oxygen species to plant cells, and exogenous calcium treatment can increase the antioxidant enzyme activity in crops, which helps to increase stress resistance in crops. Increasing plants’ intracellular calcium ion content will activate antioxidant enzymes (White & Broadley, Citation2003). Chao et al. (Citation2021) treated djulis seedlings with 5 mM calcium carbonate and found that the activity of antioxidant enzymes such as SOD, CAT, APX, and GR increased by 42%, 25%, 35.7%, and 56.4%, respectively. Under higher temperature stress, tobacco treatment with 20 mM calcium chloride increased CAT, APX, and GR activities and decreased hydrogen peroxide content, which led to an increase in photosynthesis rate (Tan et al., Citation2011). After tomato seedlings underwent a low night temperature (6°C) treatment for 1 week, the antioxidant enzyme activity increased in calcium chloride-treated seedlings, thereby decreasing cellular damage caused by free radicals, maintaining chloroplast integrity, and increasing photosynthesis under lower temperature stress (Liu et al., Citation2015). These results show that the exogenous calcium treatment of crops can increase antioxidant enzymes to alleviate the damage caused by temperature changes in the external environment. In this study, the growth temperature of spring plants was gradually increased from low temperature (24°C) to high temperature (32°C), whereas the growth temperature of autumn plants was progressively decreased from high temperature (30°C) to low temperature (18°C; ).
The results of this experiment show that the growing season had a higher impact on the antioxidant enzyme activity in djulis plants. In some cases, variety also played a significant role in affecting the antioxidant enzyme activity. For example, the SOD results in show that the spring cultivated plants had the highest SOD activity compared to the autumn cultivated plants at both WAT 6 and WAT 10. The results also show the varietal effects where the RP line shows to be a dominant line with higher SOD activity (in some cases without calcium carbonate treatment) at both WAT 6 and WAT 10, regardless of the growing conditions. However, the OY line is also suitable to be cultivated in spring at WAT 6 and WAT 10 and in autumn at WAT 10 but is not recommended to be grown in autumn at WAT 6 due to the lower SOD activity. The two-way ANOVA also confirmed this result, showing that not only variety or growing season but their interaction effects also significantly impact the SOD activity in djulis crops, as shown in . In the case of catalase activity, the two-way ANOVA ruled out that neither variety, growing season, nor their interaction effect significantly affected the catalase activity in djulis crops, as shown in .
On the other hand, the growing season was the dominant factor impacting the overall APX activity in the djulis crops, as shown in . The results clearly show that both djulis lines (RP and OY), when grown in spring at both WAT 6 and WAT 10, show higher APX activity than when grown in autumn. Opposite results were obtained for GR activity, where only the growing season significantly impacted the GR activity of djulis crops cultivated at WAT 6. At WAT 10, variety, growing season, and their interaction effects significantly impacted the GR activity of djulis crops, as shown in . The results show that djulis grown in autumn at both WAT 6 and WAT 10 and for both lines yield higher GR activity than when grown in spring.
Finally, when focusing on enhancing the overall antioxidant enzyme activity in djulis with calcium carbonate treatment, it is vital to consider the growing season as an important factor determining the success of the treatments. According to the results, the suitable growing season for cultivating djulis is spring to achieve higher antioxidant enzyme activity for both lines and at both WAT 6 and WAT 10. However, the autumn season is more suitable for growing djulis if focusing only on improving the GR activity for both lines. Selecting the RP line would be preferable over the OY line because of the higher antioxidant activity, as shown throughout the growing period.
Calcium is a signaling substance that increases the antioxidant capacity of plants in response to environmental changes, alleviates damage, decreases the incidence of disease, and increases shelf life. After calcium treatment, papayas showed increased rind hardness and reduced incidence of anthracnose. Antioxidant enzyme activity and total phenolic compound levels increased during storage (Madani et al., Citation2013). Calcium treatment in the growth phase of tomatoes increased tomato yield and decreased the incidence of blossom-end rot (Gaion et al., Citation2019; Lopez & Satti, Citation1996). Long-term spraying of exogenous calcium on sugarcane leaves decreased potassium content, delayed flowering by 1 month, and reduced the number of flowers by 50%, thereby increasing the sugar content (Endres et al., Citation2016). Generally, the RP line of djulis is a mid-late-maturing variety due to slow spike color change, whereas the OY line is regarded as an early-maturing variety due to its rapid spike color change (). However, in this study, the spike color change and maturation after calcium carbonate treatment were noted to be early in the RP line, whereas the spike color change and maturation were delayed in the OY line. These effects were most significant in the autumn plants (). This may be attributed to a gradual reduction in the growth temperature. Therefore, the results indicate that calcium carbonate treatment can alter the duration of spike color and maturation period in either the RP or OY line of djulis.
Regardless of the varieties selected, the calcium carbonate treatment in djulis affected the yield under different growing seasons. Both variety and growing season affected the yield of djulis, except for the spike fresh weight, which was neither affected by the variety, growing season, nor their interaction effects. The results show that both varieties can be grown under spring cultivation to achieve a higher spike fresh weight. However, the RP line is highly recommended for autumn cultivation to achieve a higher spike fresh weight, as shown in . As for the spike length, both varietal and growing season effects were shown. Higher spike length was observed in spring cultivated crops than those grown in autumn, demonstrating the seasonal effects. The varietal effects were observed in spring cultivated plants where the RP line had the highest spike length than the OY line. No such effects were shown in the autumn cultivated crops. The thousand-grain weight results show that autumn cultivation of djulis is highly recommended to achieve a higher thousand-grain weight, regardless of the djulis line.
Overall, the yield results indicate that the RP line can be successfully cultivated in autumn to achieve a higher yield; however, it remains dominant when cultivated in spring, as shown in . On the other hand, the OY line can also be cultivated with almost similar yield, particularly to achieve the thousand-grain weight.
Long-term exogenous calcium treatment in crops’ growth phase can stabilize crops’ yield and quality. However, since potassium and calcium have antagonistic effects on crops, potassium uptake may be affected if the calcium concentration is too high, affecting the yield and quality. The yield and quality are affected because plants’ high potassium ion content benefits flowering (Ye et al., Citation2019). In this study, calcium ion content in the seeds of the RP and OY lines planted in both spring and autumn increased after calcium carbonate treatment (). However, there were differences in potassium ion content, which showed an increase in the RP line but a decrease in the OY line (). Since the RP line of djulis is an early-maturing variety, treating with calcium carbonate reduces potassium ion content to delay spike color change and maturation. In contrast, the RP line is mid-late maturing, so treating with calcium carbonate increases potassium ion content, leading to an early occurrence of spike color change and maturation.
Conclusions
The calcium carbonate treatment has shown a minimal effect on djulis during its growth phase but has a more significant impact on the maturation stage of seeds, thereby regulating its production phase. The results also indicate that the growing season significantly impacted plant growth, yield, and antioxidant activity in both lines. Cultivating both lines with calcium carbonate treatment can provide improved growth, yield, and antioxidant enzyme activity, with the highest growth, yield, and antioxidant enzyme activity achieved from spring cultivation of the RP line, as shown by the results obtained from this study. However, further studies are required to understand why potassium ion content increased in djulis when treated with calcium carbonate and how it physiologically interferes with spike color change and maturation in different djulis varieties.
Acknowledgments
The authors thank DIAMOND NANO-BIOCHEM CO., LTD, for providing the calcium carbonate used in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed, A. A. M., Zuhair, A. D., & Wisam, K. K. (2020). Role of Boron and Calcium on growth, flowering and yield of strawberry (Fragaria x ananassa Duch) var. Liberation D’Orleans. Middle East Journal of Agriculture Research, 9, 130–133. https://doi.org/10.36632/mejar/2020.9.1.13
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Chao, -Y.-Y., Wang, W.-J., & Liu, Y.-T. (2021). Effect of calcium on the growth of djulis (Chenopodium formosanum Koidz.) Sprouts. Agronomy, 11(1), 82. https://doi.org/10.3390/agronomy11010082
- Cheng, T.-S., Hung, M.-J., Cheng, Y.-I., & Cheng, L.-J. (2013). Calcium-induced proline accumulation contributes to amelioration of NaCl injury and expression of glutamine synthetase in greater duckweed (Spirodela polyrhiza L.). Aquatic Toxicology, 144, 265–274. https://doi.org/10.1016/j.aquatox.2013.10.015
- Chéour, F., & Souiden, Y. (2015). Calcium delays the postharvest ripening and related membrane-lipid changes of tomato. Journal of Nutrition & Food Sciences, 5(5), 1–6. https://doi.org/10.4172/2155-9600
- Domingues, L. D. S., Ribeiro, N. D., Andriolo, J. L., Possobom, M. T. D. F., & Zemolin, A. E. M. (2016). Growth, grain yield and calcium, potassium and magnesium accumulation in common bean plants as related to calcium nutrition. Acta Scientiarum. Agronomy, 38(2), 207–217. https://doi.org/10.4025/actasciagron.v38i2.27757
- Endres, L., da Cruz, S. J. S., Vilela, R. D., Dos Santos, J. M., de Souza Barbosa, G. V., & Silva, J. A. C. (2016). Foliar applications of calcium reduce and delay sugarcane flowering. BioEnergy Research, 9(98–108), 98–108. https://doi.org/10.1590/1984-70332015v15n3c32
- Foster, J. G., & Hess, J. L. (1980). Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiology, 66(3), 482–487. https://doi.org/10.1104/pp.66.3.482
- Gaion, L. A., Júnior, J. C. M., Barreto, R. F., D’Amico-Damião, V., de Mello Prado, R., & Carvalho, R. F. (2019). Amplification of gibberellins response in tomato modulates calcium metabolism and blossom end rot occurrence. Scientia Horticulturae, 246(27), 498–505. https://doi.org/10.1016/j.scienta.2018.11.032
- Gao, Q., Tan, Q., Song, Z., Chen, W., Li, X., & Zhu, X. (2020). Calcium chloride postharvest treatment delays the ripening and softening of papaya fruit. Journal of Food Processing and Preservation, 44(8), e14604. https://doi.org/10.1111/jfpp.14604
- Hong, Y.-H., Huang, Y.-L., Liu, Y.-C., & Tsai, P.-J. (2016). Djulis (Chenopodium formosanum Koidz.) water extract and its bioactive components ameliorate dermal damage in UVB-irradiated skin models. BioMed Research International, 2, 736–879. https://doi.org/10.1155/2016/7368797
- Jana, S., & Choudhuri, M. A. (1981). Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquatic Botany, 11, 67–77. https://doi.org/10.1016/0304-3770(81)
- Kato, M., & Shimizu, S. (1987). Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Canadian Journal of Botany, 65(4), 729–735. https://doi.org/10.1139/b87-097
- Kazemi, M. (2014). Effect of foliar application of humic acid and calcium chloride on tomato growth. Bulletin of Environment, Pharmacology and Life Sciences, 3(3), 41–46. https://doi.org/10.1007/s00344-014-9462-9
- Kumara, K., Wathugala, D. L., Hafeel, R. F., & Kumarasinghe, H. (2019). Effect of nano calcite foliar fertilizer on the growth and yield of rice (Oryza sativa). Journal of Agricultural Sciences, 14(1), 154–164. https://doi.org/10.4038/tare.v20i1-2.5375
- Liu, Y.-F., Zhang, G.-X., Qi, M.-F., & Li, T.-L. (2015). Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. Journal of Plant Growth Regulation, 34(2), 263–273. https://doi.org/10.1007/s00344-014-9462-9
- Lopez, M. V., & Satti, S. M. E. (1996). Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Science, 114(1), 19–27. https://doi.org/10.1016/0168-9452(95)
- Madani, B., Muda Mohamed, M. T., Awang, Y., Kadir, J., & Patil, V. D. (2013). Effects of calcium treatment applied around the root zone on nutrient concentrations and morphological traits of papaya seedlings (‘Carica papaya’L. cv. Eksotika II). Australian Journal of Crop Science, 7(5), 568–572.
- Martin-Diana, A. B., Rico, D., Frias, J. M., Barat, J. M., Henehan, G. T. M., & Barry-Ryan, C. (2007). Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends in Food Science & Technology, 18(4), 210–218. https://doi.org/10.1016/j.tifs.2006.11.027
- Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant & Cell Physiology, 22(5), 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
- Olle, M., & Bender, I. (2009). Causes and control of calcium deficiency disorders in vegetables: A review. The Journal of Horticultural Science and Biotechnology, 84(6), 577–584. https://doi.org/10.1080/14620316.2009.11512568
- Paoletti, F., Aldinucci, D., Mocali, A., & Caparrini, A. (1986). A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Analytical Biochemistry, 154(2), 536–541. https://doi.org/10.1016/0003-2697(86)
- Quiles, A., Hernando, I., Pérez-Munuera, I., Llorca, E., Larrea, V., & Ángeles Lluch, M. (2004). The effect of calcium and cellular permeabilization on the structure of the parenchyma of osmotic dehydrated ‘Granny Smith’apple. Journal of the Science of Food and Agriculture, 84(13), 1765–1770. https://doi.org/10.1002/jsfa.1884
- Tan, W., Wei Meng, Q., Brestic, M., Olsovska, K., & Yang, X. (2011). Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. Journal of Plant Physiology, 168(17), 2063–2071. https://doi.org/10.1016/j.jplph.2011.06.009
- Thor, K. (2019). Calcium—Nutrient and Messenger. Frontiers in Plant Science, 10, 440. https://doi.org/10.3389/fpls.2019.00440
- Tsai, P.-J., Chen, Y.-S., Sheu, C.-H., & Chen, C.-Y. (2011). Effect of nanogrinding on the pigment and bioactivity of djulis (Chenopodium formosanum Koidz.). Journal of Agricultural and Food Chemistry, 59(5), 1814–1820. https://doi.org/10.1021/jf1041273
- White, P. J., & Broadley, M. R. (2003). Calcium in plants. Annals of Botany, 92(4), 487–511. https://doi.org/10.1093/aob/mcg164
- Xu, C., Li, X., Zhang, L., & Muldoon, M. (2013). The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PloS One, 8(7), e68214. https://doi.org/10.1371/journal.pone.0068214
- Ye, T., Li, Y., Zhang, J., Hou, W., Zhou, W., Lu, J., Xing, Y., & Li, X. (2019). Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Global Ecology and Conservation, 20, e00753. https://doi.org/10.1016/j.gecco.2019.e00753