ABSTRACT
Objectives: Exposure to 4-vinylcyclohexene diepoxide (VCD) was reported to induce testicular germ cell toxicity in rodents. However, there is paucity of information on the precise biochemical and molecular mechanisms of VCD-induced male reproductive toxicity.
Methodology: This study investigated the influence of VCD on testicular and epidydimal functions following oral exposure of Wistar rats to VCD at 0, 100, 250 and 500 mg/kg for 28 consecutive days.
Results: Administration of VCD significantly decreased the body weight gain and organo-somatic indices of the testes and epididymis. When compared with the control, VCD significantly decreased superoxide dismutase and catalase activities in the testes whereas it significantly decreased superoxide dismutase activity but increased catalase activity in the epididymis. Moreover, while glutathione peroxidase activity and glutathione level remain unaffected, exposure of rats to VCD significantly increased glutathione S-transferase activity as well as hydrogen peroxide and malondialdehyde levels in testes and epididymis of the treated rats. The spermiogram of VCD-treated rats showed significant decrease in epididymal sperm count, sperm progressive motility, testicular sperm number and daily sperm production when compared with the control. Administration of VCD significantly decreased circulatory concentrations of follicle-stimulating hormone, luteinizing hormone and testosterone along with testicular and epididymal degeneration in the treated rats. Immunohistochemical analysis showed significantly increased cyclooxygenase-2, inducible nitric oxide synthase, caspase-9 and caspase-3 protein expressions in the testes of VCD-treated rats.
Conclusion: Exposure to VCD induces testicular and epidydimal dysfunctions via endocrine suppression, disruption of antioxidant enzymes activities, increase in biomarkers of oxidative stress, inflammation and apoptosis in rats.
Introduction
Exposure of humans to environmental contaminants have been related to several reproductive health problems including decreased fertility, impaired spermatogenesis, cryptorchidism and hypospadias, menstrual disorders, pregnancy loss and postnatal developmental disorders [Citation1,Citation2]. The incidence of infertility is on the increase because a considerable amount of the global economy depends on these important but toxic chemicals which are widely used in modern day life [Citation3]. 4-Vinylcyclohexene diepoxide (VCD) is one of the metabolites of 4-vinylcyclohexene which is usually produced during tire curing and manufacture of flame-retardants, plastics and pesticides [Citation4]. Industrially, VCD is used as a diluent in the production of epoxides, epoxy resins and rubber products [Citation5]. VCD is an industrial occupational health hazard chemical because of its widely reported noxious effects on the ovary [Citation6–8] and the few reports on its liver, kidney and testicular toxicities in rodents [Citation9,Citation10]. Previous investigations on male reproductive toxicity of VCD showed that it induced testicular germ cell toxicity in mice. Specifically, VCD treatment (320 mg/kg/d, i.p.) for 30 days caused significant decreases in testis weight and loss of maturing sperm cells due to the destruction of spermatogonia and spermatocytes [Citation11].
The two main functions of mammalian testes namely the spermatogenesis and steroidogenesis are very crucial for reproductive success. The fine balance between reactive oxygen species (ROS) production and scavenging by endogenous antioxidants are of utmost importance for testicular function. Interestingly, elevated levels of reactive oxygen and nitrogen species production with concomitant disruption of the antioxidant system in Drosophila melanogaster exposed to VCH and VCD have been reported by our research group [Citation12,Citation13]. In addition, we recently reported that, exposure to VCD-induced oxidative stress, resulting in hepatic and renal oxidative damage in male and female rats [Citation10]. Excessive production of ROS above normal levels due to exposure to environmental contaminants is well-known to impair the normal testicular function [Citation1]. Apoptosis plays an essential role in spermatogenesis because it regulates the fine balance between the number of germ cells and the somatic Sertoli cells. Apoptosis is a programmed cell death which can be initiated by a variety of signals and pathophysiological conditions, including oxidative stress [Citation14]. Elevated ROS generation and oxidative changes have been associated with apoptosis in spermatogenic cells [Citation15,Citation16] and various pathological states including infertility [Citation17,Citation18].
Although the testicular germ cell toxicity of VCD has been reported, the precise biochemical and molecular mechanisms by which VCD elicits its toxic effects on the testes remains poorly understood. We hypothesized that the exposure to VCD would induce oxidative stress and inflammation in the testes and epididymis resulting in reproductive dysfunction of rats. We report herein some functional alterations in the spermatogenic parameters, hormonal level, antioxidant defense systems, apoptotic biomarkers and histology of the testes and epididymis of rats orally exposed to VCD.
Materials and methods
Chemicals
4-Vinylcyclohexene diepoxide (CAS: 106-86-6: 96%), epinephrine, reduced glutathione, thiobarbituric acid, 5′, 5′-dithiobis(2-nitro-benzoic acid) and 1-chloro-2,4-dinitrobenzene were purchased from Sigma Chemical Co. (St Louis, MO, USA). All other reagents were of analytical grade and purchased from British Drug Houses (Poole, Dorset, UK).
Experimental animals and protocol
Forty sexually matured male Wistar rats (8 weeks old, 146 ± 5 g) were obtained from the Central Animal House Facility of the College of Medicine, University of Ibadan, Nigeria. The rats were housed in plastic cages placed in a well-ventilated animal house and allowed to acclimatize for a week before starting the experiment. The rats were fed with rat pellets and water ad libitum, and subjected to natural photoperiod of 12-hour light: 12-hour dark cycle. The experimental protocols were carried out after approval and in accordance with the guidelines set by the University of Ibadan Ethical Committee. The ethical regulations have been followed according to the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institute of Health.
The rats were randomly assigned into four groups of 10 rats each. Group I animals orally received corn oil alone and served as control. Animals in groups II, III and IV were orally exposed to VCD at 100, 250 and 500 mg/kg body weight respectively for 28 consecutive days. The doses of VCD used in the present study were chosen based on previous studies in which VCD doses ranging from 40 to 1000 mg/kg were administered to rats and mice for different durations [Citation9,Citation19]. Besides, these doses (100, 250 and 500 mg/kg) caused hepatic and renal oxidative damage in male and female rats within 28 days of exposure in our previous study [Citation10].
Animal sacrifice
Twenty-four hours after the last exposure, the final weights of the rats were taken and the blood collected from retro-orbital venous plexus using heparin containing tubes before the rats were sacrificed by cervical dislocation. Plasma samples obtained by centrifugation of the blood at 3000g for 10 minutes were subsequently stored frozen at −20°C prior to the determination of reproductive hormones concentrations using ELISA strip reader (Robonik India Private Limited, Mumbai, India). The testes and epididymis were removed immediately, weighed and subsequently processed for biochemical estimations and histology. The organo-somatic index (OSI) of the testes or epididymis was calculated as follows: OSI = 100 × organ weight (g)/body weight (g).
Estimation of pituitary and testicular hormones
The commercially available enzyme immunoassay kits specific for rats were used to assay for FSH (RPN 2560, Amersham, UK), LH (RPN 2562, Amersham, UK) and testosterone (EIA-5179, DRG Diagnostics GmbH, Marburg, Germany) according to the manufacturer’s protocols. The sensitivity of LH was 0.08 ng at 80%, FSH sensitivity was 0.06 ng at 97% whereas testosterone sensitivity was 0.05 ng/ml with negligible cross-reactivity with other androgen derivatives like androstenedione, methyl testosterone and 5α-dihydrotestosterone. The intra-assay coefficients of variations were 3.3% for LH, 3.5% for FSH and 3.8% for testosterone. All the samples were assayed on the same day to avoid the inter-assay variation.
Sperm progressive motility assay
The assessment of sperm progressive motility of the rats was performed according to the method described by Zemjanis [Citation20]. Briefly, the cauda epididymis was incised with surgical blades and the sperm released onto a sterile clean glass slide. Subsequently, 2.9% (w/v) sodium citrate dehydrate solution which had been pre-warmed to 37°C was added to the sperm, mixed carefully and covered with a 24 × 24 mm coverslip. The sperm motility was assessed by visualizing a minimum of 10 microscopic fields under a phase contrast microscope at 200× magnification. Sperm motility was obtained by counting the number of all progressive sperm, followed by the non-progressive and then the immotile sperm in the same field. The data were expressed as percentage of sperm progressive motility.
Evaluation of epididymal sperm count
The epididymal sperm count of the rats was obtained according to the method described in the WHO manual [Citation21]. Briefly, the sperm was obtained by mincing the caudal epididymis in normal saline and filtering with a nylon mesh. An aliquot of 5 µL of the sperm was subsequently mixed with 95 µl of the diluent (0.35% (w/v) formalin containing 5% (w/v) NaHCO3 and 0.25% (w/v) trypan blue). Ten microliters of the diluted sperm was placed on the hemocytometer, allowed to sediment by standing for 5 minutes in a humid chamber before they were counted using the improved Neubauer chamber (Deep 1/10 m; LABART, Munich, Germany) with a light microscope at 400×.
Sperm viability and morphological abnormalities assay
Sperm viability was determined according to established method [Citation22]. Briefly, a portion of the sperm suspension placed on a glass slide was smeared out with another slide and stained with 1% (w/v) eosin and 5% (w/v) nigrosine in 3% (w/v) sodium citrate dehydrate solution. Sperm morphological abnormalities assay was performed by staining the sperm cells with a reagent containing 0.2 g eosin and 0.6 g fast green dissolved in distilled water and ethanol in a ratio of two to one (2:1). A total of 400 sperm cells from each rat were counted during morphological examination.
Determination of testicular sperm number and daily sperm production
Testicular sperm number and daily sperm production were determined using frozen left testes from rats in each group according to Blazak et al. [Citation23]. Briefly, the testis was decapsulated and homogenized in ice-cold physiological saline containing 0.01% (v/v) Triton X-100. An aliquot of the resulting homogenate was transferred to a glass vial and kept in ice. Sample aliquots were then placed on the Neubauer hemocytometer and counted twice at 100× magnification under a light microscope and the average number of elongated spermatid nuclei with spermatid characteristic of steps 17–19 of spermatogenesis was calculated [Citation24,Citation25]. These values were then used to obtain the total number of spermatids per gram of testes. Moreover, since developing spermatids spend 6.1 days in rats, the values for the number of spermatids per testis were divided by 6.1 to obtain daily sperm production.
Biochemical assays
The testes or epididymis samples were homogenized in phosphate buffer (pH 7.4) and the resulting homogenate was centrifuged at 10,000g for 15 minutes at 4°C. The supernatant obtained was subsequently used for the biochemical estimations. Protein concentration was determined using the method of Lowry et al. [Citation26]. Activity of superoxide dismutase (SOD) was determined according the method of Misra and Fridovich [Citation27]. Catalase (CAT) activity was determined using hydrogen peroxide as substrate according to the method of Clairborne [Citation28]. Glutathione peroxidase (GPx) activity was determined according to the method of Rotruck et al. [Citation29]. Glutathione-S-transferase (GST) activity was determined according to the method of Habig et al. [Citation30]. The level of reduced glutathione (GSH) was determined according to the method described by Jollow et al. [Citation31]. Hydrogen peroxide generation was determined using the method of Wolff [Citation32]. Lipid peroxidation was determined as malondialdehyde (MDA) according to the method described by Farombi et al. [Citation33].
Light microscopic examination
Testes and epididymis samples were fixed with Bouin’s solution and processed for histology according to the method described by Bancroft and Gamble [Citation34]. Briefly, the fixed tissues were dehydrated using increasing concentrations of alcohol, cleared by xylene and embedded in paraffin wax. The tissues were subsequently cut with a microtome to produce 4–5 μm sections which were fixed on the slides and subsequently stained with hematoxylin and eosin. The slides were examined under a light microscope (Olympus CH; Olympus, Tokyo, Japan) and photomicrographs taken using a Sony DSC-W 30 Cyber-shot (Sony, Tokyo, Japan) by pathologists who were blinded to control and treatment groups.
Immunohistochemical evaluations of testicular iNOS, COX-2, caspase-9 and caspase-3 expressions
Bouin-fixed testes were embedded in paraffin, sectioned to a thickness of 5 μm and were mounted on silane-coated slides. Subsequently, the sections were deparaffinized in xylene and rehydrated with graded alcohol and were treated with 10 mM citrate buffer (pH 6.0) in a water bath for 30 minutes at 95°C for antigen retrieval. The endogenous peroxidase activity was quenched by treating the sections with 1.5% (v/v) H2O2 for 10 minutes. Moreover, the tissues were washed in 10 mM PBS-0.05% Tween 20 (pH 7.5) and probed at a dilution of 1/200 with rabbit polyclonal antibodies (ABCAM Scientific Corporation) for iNOS, COX-2, caspase-9 and caspase-3, or without primary antibody (negative control) overnight at 4°C in a humidified chamber. The sections were thereafter washed extensively in PBS-Tween 20, incubated with biotinylated goat anti-rabbit for 30 minutes, and with avidin-biotinylated peroxidase complex for 30 minutes. The bound antibodies were visualized with diaminobenzidine (DAB) in PBS (pH 7.5). Immune complexes were detected using 0.05% of 3, 3-diaminobenzidene (DAB), countered stained with hematoxylin and the slides were visualized under light microscope and images were captured with a Sony Digital Camera. The percentage of tissue stained positive cells was scored by investigators blinded to the study. The protein expressions of COX-2, iNOS, caspase-9 and caspase-3 were quantitatively assessed by the counting of 10 non-continuous sections per eye with a total of 20 eyes per group and the data expressed as the percentage of the total cells counted.
Statistical analyses
The statistical analyses were carried out with Graph Pad Prism (Version 6.0), and the data were expressed as mean ± standard deviation. Statistical significant differences were determined using a one-way analysis of variance (ANOVA) followed by Bonferroni’s test for post hoc comparisons. P < 0.05 was considered statistically significant.
Results
Body weight gain and organo-somatic index of the testes or epididymis
The body weight gain and the organo-somatic index of the testes or epididymis of the rats are presented in . Exposure of rats to VCD at 100, 250 and 500 mg/kg resulted in significant decrease in the body weight gain compared with control. Similarly, VCD-treated rats showed marked decreased in the absolute testes and epididymis weights as well as in the organo-somatic indices of the testes and epididymis compared with control.
Table 1. Body weight gain and organo-somatic indices (OSI) of the testes and epididymis in rats exposed to VCD for 28 consecutive days.
Testicular and epididymal antioxidant status in rats after exposure to VCD
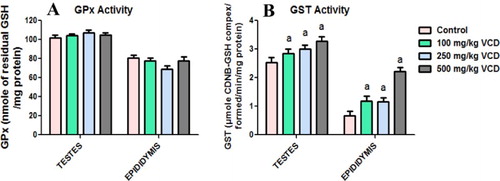
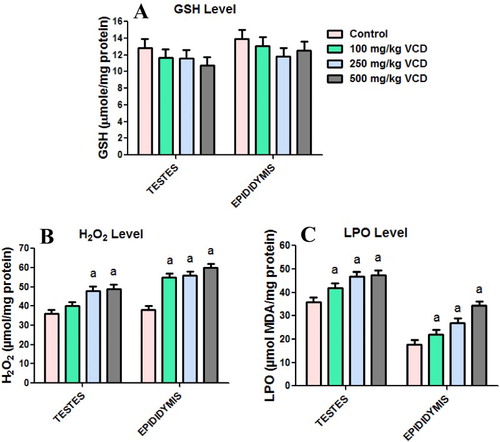
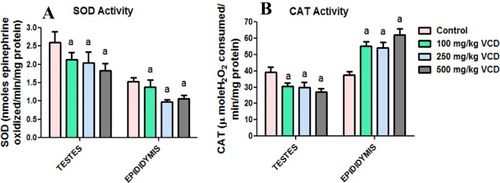
The effects of VCD treatment on antioxidant defense system and oxidative stress indices in testes and epididymis of rats are presented in –. Administration of VCD at all investigated doses significantly (P < 0.05) decreased SOD activity whereas it markedly increased GST activity in both testes and epididymis when compared with the control group. CAT activity decreased significantly in the testis but increased significantly in epididymis of rats exposed to 100, 250 and 500 mg/kg, respectively, when compared with control rats. Moreover, at all the doses of VCD investigated, the GPx activity and GSH level in both testes and epididymis were unaffected when compared with control. Conversely, exposure to VCD caused a significant elevation in the levels of H2O2 and malondialdehyde, an index of lipid peroxidation, in testes and epididymis of treated rats when compared with control.
Figure 1. Superoxide dismutase (SOD) and catalase (CAT) activities in testes and epididymis following 28 consecutive days of VCD treatment in rats. Each bar represents mean ± SD of 10 rats. aP < 0.05 versus Control.
Sperm functional parameters, testicular sperm number and daily sperm production in rats following exposure to VCD
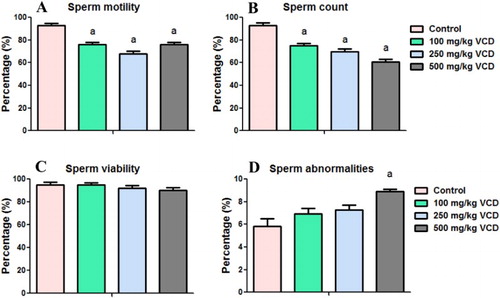
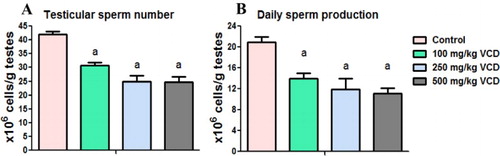
The sperm characteristics, TSN and DSP of rats after 28 consecutive days of exposure to VCD are presented in and . The results showed a significant decrease in sperm count and sperm motility whereas sperm viability was not affected following exposure to VCD when compared with the control. The sperm abnormalities significantly increased in rats treated with VCD at 500 mg/kg whereas the morphology was not affected by VCD at 100 and 250 mg/kg doses when compared with the control.
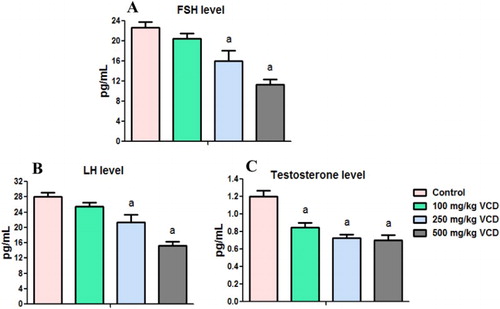
Circulatory levels of LH, FSH and testosterone in rats after exposure to VCD
The levels of circulatory concentrations of reproductive hormones namely LH, FSH and testosterone were measured in plasma of the rats in order to further delineate the mechanisms of VCD-induced testicular toxicity. The effects of VCD treatment on circulatory concentrations of LH, FSH and testosterone in the treated rats are presented in . Administration of VCD adversely affected the hormonal homeostasis in the treated rats. The levels of LH and FSH were not affected in rats treated with VCD at 100 mg/kg dose whereas their levels were significantly decreased in rats treated with 250 and 500 mg/kg VCD when compared with the control. Administration of VCD at all investigated doses significantly decreased the circulatory concentrations of testosterone in the treated rats when compared with the control.
Histopathology of testes and epididymis of VCD-treated rats
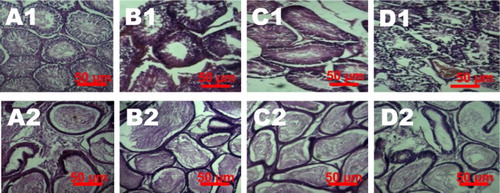
Histopathological alterations observed in the testes and epididymis sections of the rats are presented in . The light microscopic examination revealed normal seminiferous tubules with many sperm and adequate numbers of the testicular epidermal cells. Testes of rats treated with 100 mg/kg VCD showed mild congestion and edema at the interstitium with reduced sperm numbers in the seminiferous tubules. The treatment-related lesions observed in 250 and 500 mg/kg VCD-treated rats includes marked degeneration of the seminiferous tubules. Light microscopy revealed normal epididymal architecture of control rats. However, epididymis of rats treated with 100 and 250 mg/kg VCD showed few viable sperm cells in the lumen whereas reduced epithelia layer integrity with some empty lumen were observed in 500 mg/kg-treated rats.
Figure 7. Representative photomicrographs of testes and epididymis from control and VCD-treated rats. Control testes (A1) and epididymis (A2) showed normal morphology. Treatment-related lesions such as mild congestion, edema and reduced sperm numbers in the testes of 100 mg/kg VCD-treated rats (B1) whereas 250 and 500 mg/kg VCD-treated rats showed marked degeneration of the seminiferous tubules (C1 and D1). Epididymis of 100 and 250 mg/kg VCD-treated rats showed lumen with few viable sperm cells (B2 and C2) whereas 500 mg/kg VCD-treated rats showed reduced epithelia layer integrity with some empty lumen (D2).
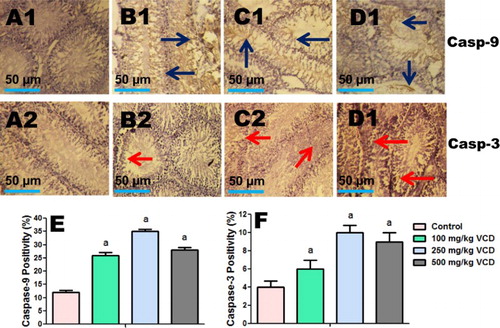
Immunohistochemical expressions of iNOS, COX-2, caspase-9 and caspase-3
The influence of VCD exposure on markers of inflammation (iNOS and COX-2) and apoptosis (caspase-9 and caspase-3) in testes of the treated rats were analyzed using immunohistochemical technique. The immuno reactivity of these proteins is presented in and . The intensity of iNOS, COX-2, caspase-9 and caspase-3 expression was significantly higher on the peritubular regions (spermatogonia and spermatocytes) of the testes of VCD-treated rats when compared to control.
Figure 8. Testes histopathology guide showing the influence of VCD on COX-2 and iNOS expression in control and treated rats. Percentage of positive cells is presented in the graphs (e and f). Each bar represents mean ± SD of 10 rats per group. aValues differ significantly from control (P < 0.05).
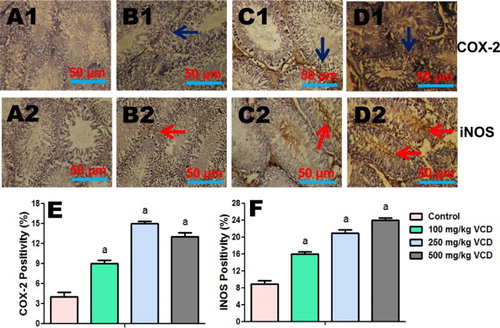
Figure 9. Testes histopathology guide showing the influence of VCD on caspase-9 and caspase-3 expression in control and treated rats. Percentage of positive cells is presented in the graphs (e and f). Each bar represents mean ± SD of 10 rats per group. aValues differ significantly from control (P < 0.05).
Discussion
4-Vinylcyclohexene diepoxide is a widely used industrial solvent that adversely impacts a variety of organs. Despite the usefulness of VCD, its exposure has been associated with oxidative stress resulting in oxidative damage in kidney and liver tissues of rats [Citation10]. However, no study has been reported on the involvement of oxidative stress in male reproductive toxicity due to VCD exposure. The testes and epididymis are very sensitive to oxidative stress due to high concentration of unsaturated lipids. The testes and epididymis are endowed with ROS detoxifying system consisting of the antioxidant enzymes SOD, CAT, GPx and GST and non-enzymatic antioxidant GSH [Citation3]. The decrease in testicular and epididymal SOD activity in VCD-treated rats in the present study indicates enzyme inhibition which may enhance superoxide radical accumulation and a state of oxidative stress in the tissues. Moreover, the reduction in the testicular CAT activity suggests the inability of the testes to eliminate hydrogen peroxide possibly generated due to VCD exposure or inactivation of the enzyme due to increased generation of ROS in the testes. However, increase in epididymal CAT activity in the present investigation may indicate its induction so as to diminish the effects of H2O2, a potent oxidant at high cellular concentration. Although the reason for this differential response of testicular and epididymal SOD activity to VCD exposure is not understood at present, a possible explanation may be related to the organ differences.
Administration of VCD increased GST activity without affecting GSH level and GPx activity in the testes and epididymis of rats in this study. These findings indicate an adaptive response to oxidative stress which is targeted to detoxify peroxide-containing metabolites with concomitant compensatory GSH synthesis via GSH cycle [Citation35,Citation36]. Lipid peroxidation is a process which involves oxidation of polyunsaturated fatty acids by ROS to produce lipid peroxyl radicals and lipid hydroperoxides which are subsequently converted to reactive aldehydes, such as 4-hydroxy-2 nonenal and MDA [Citation37,Citation38]. Thus, the elevated level of MDA in the testes and epididymis of VCD-treated rats clearly demonstrated the induction of oxidative damage in testes and epididymis following exposure to VCD in the present study.
The mechanisms by which environmental contaminants elicit anti-gonadal actions vary. Some environmental contaminants act directly to exhibit inhibitory effect on the testis whereas some act indirectly by altering gonadotrophin homeostasis and consequently impairs spermatogenesis [Citation39]. Excessive production of ROS has been implicated in the suppression of gonadotropins (LH and FSH) release by the pituitary [Citation2]. The normal production of FSH and LH by the pituitary is a key factor required for spermatogenesis by seminiferous tubules [Citation40,Citation41]. LH induces Leydig cell to secrete testosterone which regulates spermatogenesis via androgen receptors in the seminiferous epithelium. FSH on the other hand regulates spermatogenesis by stimulating the production of several Sertoli cell factors. Thus, the reduction in the FSH and LH in VCD-treated rats possibly indicates the adverse effect of VCD exposure on the anterior pituitary gland which could impair the endocrine regulation of spermatogenesis and consequently affects the reproductive function. The significant reduction in the level of circulatory FSH and LH may be related to the marked decrease in spermatogenesis as evidenced by reduction in the TSN and DSP in VCD-treated rats.
The biosynthesis and secretion of testosterone, the male primary steroid hormone, is exclusively executed by the Leydig cells. The decrease in testicular steroidogenesis which, is evidenced by the reduced testosterone level in VCD-treated rats in the present investigation, demonstrates the anti-androgenic nature of VCD. Further, testosterone plays an important role in the regulation of spermatogenesis and weights of testes and epididymis [Citation42,Citation43]. In agreement with previous study [Citation11], VCD treatment significantly decreased the testes weight in this study. The significant decrease in the sperm progressive motility, sperm count, absolute weights and organo-somatic indices of testes and epididymis in VCD-treated rats is attributable to inadequate hormonal levels. One of the interesting findings of the present investigation was the histopathological lesions in the testes and epididymis of the rats treated with VCD. The observed histopathological lesions in testes of VCD-treated rats in this study are in agreement with previous studies [Citation9,Citation11]. The reduced sperm density and the marked degeneration seen in the histology of the testes and epididymis of the VCD-treated rats are indicators of reduced spermatogenesis owing to the VCD-induced oxidative damage of the biological membranes in the testes and epididymis. Moreover, the lack of effects of VCD on sperm viability and abnormality in the present investigation showed that VCD specifically affected sperm motility and count while sparing the normal morphological structure of the sperm in the treated rats. The reduction in epididymal sperm count and motility observed following VCD treatment may result in infertility owing to insufficient sperm quantity and inability of the sperm to reach the site of fertilization and to penetrate zonal pellucida [Citation44].
The increase in the testicular expression of COX-2 and iNOS in the VCD-treated rats is indicative of inflammatory response. Moreover, excessive production of NO by iNOS exerts detrimental effects due to its ability to react with superoxide anion to generate peroxynitrite radical and thus indicates another mechanism of VCD-induced testicular toxicity. Apoptotic cell death plays a pivotal function in the regulation of testicular germ cell population both in physiological conditions and under stress induced by external factors. Mitochondrial damage by extracellular stress is well-known to cause cytochrome c release from mitochondria into the cytoplasm. Cytochrome c subsequently recruits procaspase-9 and activates ‘apoptosome’ and the downstream effectors including caspase-3 which eventually executes apoptotic cell death [Citation45]. Immunohistochemical analysis revealed an increased expression of caspase-9 and caspase-3 in the testes of rats treated with VCD, thus suggesting activation of caspase-9 and the downstream signaling events which triggered the final executioner caspase-3 and subsequently apoptosis [Citation45,Citation46].
In conclusion, the findings from the present study demonstrated for the first time that VCD treatment induces testicular and epidydimal dysfunctions via endocrine suppression, disruption of antioxidant enzymes activities, increase in biomarkers of oxidative stress, inflammation and apoptosis in rats.
Acknowledgment
This research was done without specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Isaac A. Adedara, B.Sc., M.Sc., Ph.D., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Dr Amos O. Abolaji, B.Sc., M.Sc., Ph.D., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Emmanuel O. Ladipo, B.Sc., M.Sc., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Ore J. Fatunmibi, B.Sc., M.Sc., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Ayodeji O. Abajingin, B.Sc., M.Sc., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Professor Ebenezer O. Farombi, B.Sc., M.Sc., Ph.D., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
References
- Saradha B, Mathur PP. Effect of environmental contaminants on male reproduction. Environ Toxicol Pharmacol. 2006;21:34–41. doi: 10.1016/j.etap.2005.06.004
- Kumar V, Kural MR, Pereira BMJ, et al. Spearmint induced hypothalamic oxidative stress and testicular anti-androgenicity in male rats – altered levels of gene expression, enzymes and hormones. Food Chem Toxicol. 2008;46:3563–3570. doi: 10.1016/j.fct.2008.08.027
- Adedara IA, Farombi EO. Chemoprotection of ethylene glycol monoethyl ether-induced reproductive toxicity in male rats by kolaviron, isolated biflavonoid from Garcinia kola seed. Hum Exp Toxicol. 2012;31:506–517. doi: 10.1177/0960327111424301
- Rappaport SM, Fraser DA. Gas chromatographic–mass spectrometric identification of volatiles released from a rubber stock during simulated vulcanization. Anal. Chem. 1976;48:476–481. doi: 10.1021/ac60367a012
- International Agency for Research on Cancer. 4-Vinylcyclohexene. In: IARC, editor. Monographs on the evaluation of carcinogenic risks to humans: some industrial chemicals, 60. Lyon: IARC; 1994. p. 347–359.
- Hu X, Flaws JA, Sipes IG, et al. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259
- Keating AF, Mark CJ, Sen N, et al. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol. 2009;241:127–134. doi: 10.1016/j.taap.2009.08.012
- Roosa KA, Mukai M, Place NJ. 4-Vinylcyclohexene diepoxide reduces fertility in female Siberian hamsters when treated during their reproductively active and quiescent states. Reprod Toxicol. 2015;51:40–46. doi: 10.1016/j.reprotox.2014.12.003
- Chhabra RS, Elwell MR, Peters A. Toxicity of 4-vinyl-1-cyclohexene diepoxide after 13 weeks of dermal or oral exposure in rats and mice. Fundam Appl Toxicol. 1990;14:745–751. doi: 10.1016/0272-0590(90)90299-Y
- Abolaji AO, Toloyai P, Odeleye TD, et al. Hepatic and renal toxicological evaluations of an industrial ovotoxic chemical, 4-vinylcyclohexene diepoxide, in both sexes of Wistar rats. Environ Toxicol Pharmacol. 2016;45:28–40. doi: 10.1016/j.etap.2016.05.010
- Hooser SB, DeMerell DG, Douds DA, et al. Testicular germ cell toxicity caused by vinylcyclohexene diepoxide in mice. Reprod Toxicol. 1995;9:359–67. doi: 10.1016/0890-6238(95)00022-3
- Abolaji AO, Kamdem JP, Lugokenski TH, et al. Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic Biol Med. 2014;71:99–108. doi: 10.1016/j.freeradbiomed.2014.03.014
- Abolaji AO, Kamdem JP, Lugokenski TH, et al. Ovotoxicants 4-vinylcyclohexene 1,2-monoepoxide and 4-vinylcyclohexene diepoxide disrupt redox status and modify different electrophile sensitive target enzymes and genes in Drosophila melanogaster. Redox Biol. 2015;5:328–339. doi: 10.1016/j.redox.2015.06.001
- Adedara IA, Farombi EO. Influence of kolaviron and vitamin E on ethylene glycol monoethyl ether-induced haematotoxicity and renal apoptosis in rats. Cell Biochem Funct. 2014;32:31–38. doi: 10.1002/cbf.2968
- Saradha B, Vaithinathan S, Mathur PP. Lindane induces testicular apoptosis in adult Wistar rats through the involvement of Fas–FasL and mitochondria-dependent pathways. Toxicology. 2009;255:131–139. doi: 10.1016/j.tox.2008.10.016
- Adedara IA, Vaithinathan S, Jubendradass R, et al. Kolaviron prevents carbendazim-induced steroidogenic dysfunction and apoptosis in testes of rats. Environ Toxicol Pharmacol. 2013;35:444–453. doi: 10.1016/j.etap.2013.01.010
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/S0090-4295(96)00313-5
- Wang X, Sharma RK, Sikka SC, et al. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003;80:531–535. doi: 10.1016/S0015-0282(03)00756-8
- Devine PJ, Sipes IG, Hoyer PB. Effect of 4-vinylcyclohexene diepoxide dosing in rats on GSH levels in liver and ovaries. Toxicol Sci. 2001;62:315–320. doi: 10.1093/toxsci/62.2.315
- Zemjanis R. Collection and evaluation of semen. In: Zemjanis R, editor. Diagnostic and therapeutic technique in animal reproduction. 2nd ed. Baltimore (MD): William and Wilkins Company, Waverly Press, Inc; 1970. p. 139–153.
- World Health Organization. Collection and examination of human semen. In: Aitken RJ, Comhaire F, Baker HWG, Cooper TG, Barratt CLR, De Jonge C, Behre HM, Elasson R, editors. WHO Laboratory Manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. UK: Cambridge University Press; 1999. p. 4–33.
- Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Diary Sci. 1970;53:227. doi: 10.3168/jds.S0022-0302(70)86184-7
- Blazak WF, Trienen KA, Juniewicz PE. Application of testicular sperm head counts in the assessment of male reproductive toxicity. In: RE Chapin, J Heindel, editors. Methods in toxicology, Vol. 3A, Male reproductive toxicology. San Diego: Academic Press; 1993. p. 86–94.
- Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod. 1990;43:525–542. doi: 10.1095/biolreprod43.3.525
- Amann RP, Johnson L, Thompson DL, et al. Daily spermatozoal production, epididymal spermatozoal reserves and transit time of spermatozoa through the epididymis of the rhesus monkey. Biol Reprod. 1976;15:586–592. doi: 10.1095/biolreprod15.5.586
- Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Clairborne A. Catalase activity. In: AR Greewald, editor. Handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1995. p. 237–242.
- Rotruck JT, Pope AL, Ganther HE, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139.
- Jollow DJ, Mitchell JR, Zampaglione N, et al. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485
- Wolff SP. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. doi: 10.1016/S0076-6879(94)33021-2
- Farombi EO, Tahnteng JG, Agboola AO, et al. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron – a Garcinia kola seed extract. Food Chem Toxicol. 2000;38:535–541. doi: 10.1016/S0278-6915(00)00039-9
- Bancroft JD, Gamble M. Theory and practice of histology techniques. 6th ed. London: Churchill Livingstone Elsevier; 2008; p. 83–134.
- Kaur P, Kaur G, Bansal MP. Tertiary-butyl hydroperoxide induced oxidative stress and male reproductive activity in mice: role of transcription factor NF-kappaB and testicular antioxidant enzymes. Reprod Toxicol. 2006;22:479–484. doi: 10.1016/j.reprotox.2006.03.017
- Farombi EO, Adedara IA, Akinrinde SA, et al. Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rat. Andrologia. 2012;44:273–284. doi: 10.1111/j.1439-0272.2012.01279.x
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001
- Demir E, Kaya B, Soriano C, et al. Genotoxic analysis of four lipid-peroxidation products in the mouse lymphoma assay. Mutat Res. 2011;726:98–103. doi: 10.1016/j.mrgentox.2011.07.001
- Adedara IA, Awogbindin IO, Adesina AA, et al. Municipal landfill leachate-induced testicular oxidative damage is associated with biometal accumulation and endocrine disruption in rats. Arch Environ Contam Toxicol. 2015;68:74–82. doi: 10.1007/s00244-014-0075-x
- Russell LD, Alger LF, Naquin LG. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987;120:1615–1632. doi: 10.1210/endo-120-4-1615
- Sriraman V, Anbalagan M, Rao AJ. Hormonal regulation of Leydig cell proliferation and differentiation in rodent testis: a dynamic interplay between gonadotrophins and testicular factors. Reprod Biomed. 2005;11:507–518. doi: 10.1016/S1472-6483(10)61147-9
- Yakubu MT, Akanji MA, Oladiji AT, et al. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullockex Hoyl stem in male Wistar rats. J Ethnopharmacol. 2008;118:508–513. doi: 10.1016/j.jep.2008.05.020
- Yoshida S, Hiyoshi K, Ichinose T, et al. Effect of nanoparticles on the male reproductive system of mice. Int J Androl. 2009;32:337–342. doi: 10.1111/j.1365-2605.2007.00865.x
- Farombi EO, Adedara IA, Ebokaiwe AP, et al. Nigerian Bonny light crude oil disrupts antioxidant systems in the testes and sperm of rats. Arch Environ Contam Toxicol. 2010;59:166–174. doi: 10.1007/s00244-009-9443-3
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7
- Gao HB, Tong MH, Hu YQ, et al. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 2003;199:153–163. doi: 10.1016/S0303-7207(02)00290-3