ABSTRACT
Objective: This study aimed to determine the capability of newly designed 3-methoxy derivatives of salicylaldehyde benzoylhydrazone to influence the oxidative stress processes and to test their in vitro cytotoxicity.
Methods: We have used chemiluminescent and spectrophotometric model systems containing different types of reactive oxygen species (OH●, OCl─ and O2─●). The hydrazones effect on the viability of Hep-G2, HEK-293 and SH-SY5Y cell lines was determined via MTT assay.
Results: The comparative analysis of the C50 values of the chemiluminescent investigation demonstrated moderate activity against the hydroxyl radicals (C50 > 50 μmol/L) and remarkable reactivity in the systems containing a superoxide radical and a hypochlorous anion (C50 < 3.7 μmol/L). Further experiments in the spectrophotometric system of UV-induced OH● generation and consequent 2′-deoxyribose oxidative damage excluded the possibility of quenching effect and proved the direct interaction of the studied compounds with that generated in the system reactive oxygen species (ROS). The encapsulation of the studied derivatives into chitosan-alginate particles led to the protection and stabilization of their antioxidant activity as revealed by a one-month study using the ABTS ●+ method. The cytotoxic study revealed less pronounced effects against the non-malignant cell line (HEK-293) compared to Hep-G2 and SH-SY-5Y cells.
Discussion: The incorporation of a hydroxyl group in the hydrazide part of a parent molecule which relates to better antioxidant effect in most of the studied systems is associated with higher IC50 values in all cytotoxicity experiments and relates to the cytoprotective effect against N-methyl-D-aspartate-induced excitotoxicity in SH-SY5Y human neuronal cells.
Introduction
Free radicals are chemical particles playing dual role in all biological systems. Their beneficial effects occur at low or moderate concentration and involve a physiological role in cellular response against infectious agents and signal transduction pathways [Citation1]. The harmful effect of reactive oxygen and nitrogen species is due to the participation of one or more unpaired electrons in their molecular structure making them highly unstable and defining their direct attack against cellular components. The resulting oxidative damage is a common cellular event implicated in a broad spectrum of pathological circumstances, including all infectious, inflammatory, neurodegenerative and cardiovascular diseases, cancer and diabetes [Citation2,Citation3].
Hydrazones are considered as important class of organic compounds possessing diverse biological activities like analgesic [Citation4], anti-inflammatory [Citation5], anticancer [Citation6], antimicrobial [Citation6,Citation7], antibacterial [Citation8], vasodilative [Citation9] and antioxidant activity [Citation10]. Some investigations have proved that the structural characteristics of aroylhydrazones turn them into promising candidates for the development of novel synthetic antioxidants retarding the cellular damage induced by free radicals [Citation11,Citation12]. Aroylhydrazones derived from salicylaldehyde are biologically active molecules and one of the most interesting compounds is salicylaldehyde benzoylhydrazone (SBH) which has been proved to inhibit the process of DNA synthesis and cell growth of malignant cells in vitro and in vivo [Citation6,Citation13].
The active pharmacophore (-CONH-N=CH-) of aroylhydrazones is mainly responsible for the significant biological activities although the attached neighbouring groups may also contribute. Different functional substitutes in the molecule of the compounds could enable structure optimization that could further improve the pharmacological effect, as well as beneficially modify the solubility and bioavailability.
Recently, our group synthesized a series of structurally similar hydrazones by the Schiff-base condensation between 3-methoxysalicylaldehyde and different benzhydrazides [Citation13]. Knowing the powerful antioxidant properties of the phenolic compounds and the remarkable activity of isoniazid itself, we chose to explore three hydrazones derived by benzhydrazide, 4-hydroxybenzhydrazide and isonicotinoyl hydrazide and consequently to compare how the replacement of an H-atom on 4th position in hydrazide affects the antioxidant properties and cytotoxic effect. At the same time, however, the derivatives are easily oxidized by atmospheric oxygen and their solutions could be unstable. In this view, encapsulation of these derivatives in polymeric particles could lead to their protection during storage.
The aim of the present investigation was to determine the radical scavenging properties of 3-methoxy derivatives of SBH against different types of reactive oxygen species (ROS) and to perform a comparative evaluation of their cytotoxic effects on Hep-G2, HEK-293 and SH-SY-5Y cell lines. The capability of these derivatives to influence oxidative stress processes was determined using five model systems of diverse mechanism of free radical generation. Additionally, the opportunity to preserve their antioxidant activity by encapsulation into polysaccharide particles was evaluated. Polysaccharides are attractive carriers or surface-modifying agents for the preparation of micro- and nanoparticles (NPs) [Citation14,Citation15]. Chitosan and sodium alginate were selected as carriers for the NPs taking into account their non-toxic, biodegradable and biocompatible properties [Citation16,Citation17]. The cytotoxicity of the studied derivatives was determined by the MTT-dye reduction assay following a 72 h continuous exposure.
Experimental
Chemicals
All chemicals and solvents used in the performed experiments were of analytical grade. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2′-deoxyribose, 2′2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), luminol, potassium superoxide and chitosan were obtained from Sigma-Aldrich USA. 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), ethylenediaminetetraacetic acid (EDTA), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoluim bromide) and FeCl3.6H2O were purchased from Genaxxon GmBH, (Ulm, Germany). Sodium alginate (very low viscosity) was supplied by Alfa Aesar GmBH & CoKG (Karlsruhe, Germany). SH-SY-5Y cell line was purchased from ATCC (American Type Culture Collection). Hep-G2 and HEK-293 were obtained from DSMZ, Braunschweig, Germany.
Methods
Antioxidant methods
The detection of the chemiluminescent response was performed with an LKB 1251 luminometer (BioOrbit, Turku, Finland) with automatic injector set at 37 °C. The apparatus was connected with AT-type computer via serial interface and MultiUse program ver. 1.08 (BioOrbit, Turku, Finland) used for the collection of the obtained experimental data. In all the assays, the CL response was measured using the ‘flash assay’ option of the MultiUse program. The chemiluminescent response was calculated by determination of the area under the obtained chemiluminescent curve. Negative controls have been included with each run of samples. The chemiluminometer software requires the negative control results for the estimation of the chemiluminescent response. The studied hydrazone derivatives do not generate chemiluminescent signal if the free radical sources have been omitted in the sample – the results obtained were identical to the ones of the negative controls. The CL ratio in the presence/absence of the investigated compounds in percentage was named chemiluminescent scavenging index (CL-SI) and indicated the scavenging properties of the derivative [Citation18].
Assay I-luminol-dependent chemiluminescence in a system of KO2 produced O2–●
One milliliter samples of phosphate saline buffer (PBS) – 50 mM K2HPO4/KH2PO4, pH 7.4, containing 0.1 mM luminol and the tested 3-methoxy derivatives were prepared. In the control samples, the studied compounds were omitted. Owing to the fast release of superoxide, the CL response was measured instantly after the addition of 20 µl KO2 solution dissolved in DMSO. The chemiluminescence was registered for 1 min. every 50 milliseconds after the addition of KO2.
Assay II-luminol-dependent CL in a system of iron-dependent hydroxyl radical formation
The assay was carried out using 1 ml samples of PBS, containing 0.1 mM luminol, 0.1 mM Fe3+ (FeCl3.6H2O), 0.1 mM EDTA, 0.1 mM ascorbate, 1 mM H2O2 and any of the tested derivatives. The chemiluminescence was registered for 1 min. every 50 milliseconds after the addition of H2O2.
Assay III-luminol-dependent CL in a system of NaOCl-generated hypochlorite
One milliliter samples of PBS, pH 7.4, containing 0.1 mM luminol, 0.06 mM NaOCl and either of the hydrazones at the tested concentrations (or buffer for control) were prepared. The chemiluminescence was registered for 1 min. every 50 milliseconds after the addition of NaOCl.
UV-induced deoxyribose damage
The deoxyribose assay was performed as described by Halliwell et al. [Citation19] with some small modifications. The studied derivatives and 0.6 mmol/L 2-deoxyribose were added in phosphate buffer (50 mM K2HPO4/KH2PO4, pH = 7.4). In the control sample, the tested hydrazones were omitted. After 30 min of UV irradiation (UV 220–400), 0.6 ml of 1% TCA and 0.6 ml of 0.6% of thiobarbituric acid were added to 1 ml of the irradiated sample solution. The mixture was vortexed, heated in a 100 °C water bath for 20 min and cooled in cold water. The chromophore absorbance was measured at 532 nm. Balanced irradiance was provided during the UV illumination of all the samples yielding to absorbance of the control sample between 0.3 and 0.4. This confirms the linear dependence between the absorbance and the concentration of the damaged molecules. The results were presented as damage of 2-deoxyribose (% from control) and calculated according to the equation:
Antioxidant stability evaluation
The stability of antioxidant properties of the studied hydrazones was evaluated using the ABTS method. Stock solutions of the tested hydrazones and hydrazones encapsulated into polysaccharide NPs were prepared and stored at 4°C. The NPs were prepared by electrostatic gelation of sodium alginate and chitosan. In particular, sodium alginate solution (3 mg/ml) was incubated with the respective hydrazone derivative for 30 min at a ratio 1:10 with respect to polymer concentration. After that, the solution of calcium chloride (3.35 mg/ml) was slowly added to their mixture under stirring (700 rpm). Finally, the solution of chitosan (0.75 mg/ml) was added drop by drop in order to accomplish gelation between both polymers.
The interaction of non-encapsulated and encapsulated hydrazones with the stable ABTS●+ radical was investigated according to Re et al. [Citation20]. The interactions were evaluated immediately after the preparation of stocks on the first, tenth, twentieth and thirtieth day of storage. The reduction of the absorbance of 2 ml of the ABTS ●+ by 100 µl of the tested hydrazones/NP solutions was measured at 734 nm exactly after 1 hour incubation in the dark. Fresh ABTS ●+ solution was made for each experiment.
Calculation of C50 values
The concentration of the studied derivative causing 50% reduction of the chemiluminescent response was termed C50. The methodology of estimation requires fitting of the experimental data to the ‘sigmoid model’ [Citation21]. The determination of the concentration providing 50% AOA activity (C50) was performed using the same formula.
Cell lines and culture conditions
In the present investigation, we performed a comparative evaluation of the cytotoxic activity of the newly designed 3-methoxy derivatives on three human cell lines, using the MTT-dye reduction method for cell viability [Citation22]. For these experiments, we have used human hepatocellular carcinoma cell line – HEP-G2, human embryotic kidney cell line HEK-293 and human neuroblastoma cells – SH-SY-5Y.
All cell lines were grown in a controlled environment medium under standard conditions– cell culture flasks at 37°C in an ‘BB 16-Function line’ Heraeus incubator with humidified atmosphere and 5% carbon dioxide. RPMI 1640 liquid medium was additionally supplemented with 10% foetal bovine serum and 2 mM L-glutamine was used.
Cytotoxicity assessment (MTT-dye reduction assay)
The cytotoxic effect of the studied hydrazone derivatives was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoluim bromide) dye reduction assay. The method is based on the reduction of the yellow tetrazolium salt MTT to violet formazan via the mitochondrial succinate dehydrogenases activity of viable cells. Exponentially growing cells were seeded into a 96-well micropaletes (100 µl/well) at a final density of 1 × 105 cells per milliliter, incubated for 24 hours at 37°C and subsequently treated with various concentrations of the tested derivatives for 72 hours. After the incubation period with the hydrazones, 10 µl MTT solution (10 mg/ml in PBS) was added to each well. After a third incubation for 4 hours at 37°C during which the preformed MTT-formzan crystals were dissolved using 100 µl/well 5% HCOOH 2-propanol, the absorbance of the samples was measured using the microprocessor controlled microplate reader (Labexim LMR-1) at 580 nm.
The MTT-bioassay data were finally expressed as the percentage of the untreated control sample and subsequently fitted to a sigmoidal concentration–response curve. IC50 values were calculated using the non-linear response analysis (GraphPad prizm Software).
Apoptosis assay
The hallmark for apoptotic DNA fragmentation after the exposure of HEP-G2 cells was determined using a commercially available ‘Cell-death detection’ ELISA kit (Roche Applied Science). The method is based on a quantitative sandwich ELISA to detect internucleosomal degradation of genomic DNA, a hallmark feature of apoptosis. The procedure was carried out according to the manufacturer’s instructions. In brief, exponentially HEP-G2 growing cells were treated with equieffective concentrations of the tested compounds (1/2 IC50 or IC50) and thereafter cytosolic fractions of 1 × 104 cells per group (treated or untreated) served as an antigen source in a sandwich ELISA, employing primary anti-histone antibody-coated microplate and a secondary peroxidase-conjugated anti-DNA antibody. The photometric immunoassay for histone-associated DNA fragments was carried out at 405 nm, using ELISA reader (Labexim LMR-1). The results are expressed as the oligonucleosome enrichment factor (representing a ratio between the absorption in the treated vs. the untreated control samples) and normalized as percentage of control.
Cytoprotective effect against NMDA-induced excitotoxicity in SH-SY5Y human neuronal cells
Exponentially growing SH-SY5Y cells were plated in 96-well microplates and after a 24 h adaptation period they were exposed to NMDA (at 250 µmol/L) alone or in combination with 10 or 20 µmol/L of 3mShBH. After 72 h continuous exposure, the cell survival fractions were determined by the MTT-dye reduction assay.
Results and discussion
Antioxidant activity
The three derivatives of SBH studied in the present work – 3-methoxysalicylaldehyde benzoylhydrazone (3mSBH), 3-methoxysalicylaldehyde-4-hydroxybenzoylhydrazone (3mShBH) and 3-methoxysalicylaldehyde isonicotinoylhydrazone (3mSIH) – were previously synthesized and structurally characterized by elemental, thermo-gravimetric analyses, IR, 1H and 13C-spectroscopy [Citation13]. The compounds were obtained by the Schiff-base condensation method ().
Scheme 1. Synthesis of the studied 3-methoxy derivatives – the type of the substituents and its structure are presented in the table.
In order to characterize more accurately the nature of the antioxidant properties of the 3-methoxy structural analogues and their potential beneficial in vivo effect, we studied their scavenging properties using biological relevant chemiluminescent and spectrophotometric model systems. During the years, luminol-dependent chemiluminescence has been proved as a valuable and rapid detection method for the determination of antioxidant properties possessing specific advantages including low limits of detection and wide linear dynamic range [Citation23–25]. In our work, we have chosen three chemiluminescent assays with different ROS in order to evaluate the diverse aspects of the antioxidant activity of the compounds under study. As shown in , all the studied compounds could be considered as effective scavengers.
Figure 1. 3-methoxy SBH derivatives induced reduction of luminol-dependent chemiluminescent response in model system with different ROS: HO• – system of iron-dependent (0.1 mM Fe3+ (FeCl3.6H2O), 0.1 mM EDTA, 0.1 mM ascorbate, 1 mM H2O2) hydroxyl radical formation; O2–● – system of KO2 (1 mM solution dissolved in DMSO) produced superoxide formation; OCl– – system of NaOCl [0.06 mM] -generated hypochlorite. The assays were carried out using 1 ml samples of 50 mM K2HPO4/KH2PO4, pH 7.4, containing 0.1 mM luminol. Results are presented as percentage from the control sample which does not contain hydrazones as the mean ± S.D. of one experiment performed in triplicate. Only concentrations where the effect is statistically distinguishable from the control are presented.
![Figure 1. 3-methoxy SBH derivatives induced reduction of luminol-dependent chemiluminescent response in model system with different ROS: HO• – system of iron-dependent (0.1 mM Fe3+ (FeCl3.6H2O), 0.1 mM EDTA, 0.1 mM ascorbate, 1 mM H2O2) hydroxyl radical formation; O2–● – system of KO2 (1 mM solution dissolved in DMSO) produced superoxide formation; OCl– – system of NaOCl [0.06 mM] -generated hypochlorite. The assays were carried out using 1 ml samples of 50 mM K2HPO4/KH2PO4, pH 7.4, containing 0.1 mM luminol. Results are presented as percentage from the control sample which does not contain hydrazones as the mean ± S.D. of one experiment performed in triplicate. Only concentrations where the effect is statistically distinguishable from the control are presented.](/cms/asset/ddef60ac-d9bb-45d0-a7e2-ccd7e258ffe1/yrer_a_1276256_f0001_b.gif)
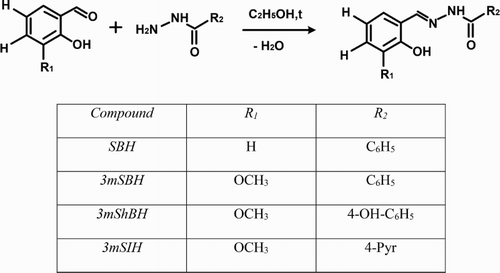
In order to compare the influence of the studied 3-methoxy derivatives on the chemiluminescent scavenging (CL-SI% – see experimental part) index and to determine the impact of the performed structural modifications, we have calculated the C50 values on the basis of the data presented at ().
Figure 2. Comparison between the C50 values of the studied derivatives obtained in chemiluminescent model system with different ROS (hydroxyl radicals – HO•; superoxide – O2–● and hypochlorite – OCl–). Concentrations (C50) of hydrazones leading to 50% reduction of the chemiluminescent response were determined using the results presented in . Estimation of C50 for each condition was carried out by a non-linear regression analysis and the calculations were used to best fit the experimental data to the ‘sigmoid model’.
ROS are constantly being produced in the human body through various mechanisms. Highest generation and respectively highest local concentration are observed in activated phagocytic cells [Citation26–30]. The respiratory burst resulting from the activation of a dormant in resting cells enzyme catalyzes the one electron reduction of oxygen to O2─● in the presence of NADPH. This superoxide radical is the basic responsible stage initiating ROS generations, suggesting its role as a therapeutic target in the prevention of the subsequent formation of H2O2 and OCl─. Our experiments, investigating the radical scavenging properties of the studied derivatives against the superoxide radical in the chemiluminescent model system of KO2 generated O2●─, revealed strong inhibitory effect, remaining statistically distinguishable even at the lowest concentration of 0.1 μmol/L (). Statistical difference between the data for chemiluminescent inhibition of the 3-methoxy derivatives at concentration higher than 30 μmol/L was not observed. The C-50 values revealed that the presence of hydroxyl group or hetero atom in the SBH derivatives led to the improvement of the investigated properties. This effect was more pronounced in the case of the hydroxyl-bearing compound (3mShBH) (). The studied methoxy SBH compounds can be considered as potential agents tackling superoxide-induced via interaction with NO● peroxynitrite radical formation, possessing an exceedingly powerful harmful effect.
The final stage of the oxidative burst is associated with the production of hypochlorite by myeloperoxidase. At the highest studied concentration, the three compounds decreased equally the value of CL-SI to less than 1.5%. In the whole concentration range, 3mShBH demonstrated the strongest scavenging properties. This fact suggested that the 3-methoxy derivatives of SBH exhibited excellent scavenging effect against OCl─ which makes them promising agents capable of reducing the effect of free radical-induced cellular damage resulting from phagocyte activation at the sites of chronic inflammation. As shown in , the subsequent modification of 3mSBH, in particular associated with hydroxyl group incorporation (3mShBH), enhanced the observed effect.
Free radical damages of biological molecules in living systems may be caused by the highly reactive hydroxyl radical. One potential mechanism of hydroxyl radical generation in all biological systems is from H2O2 via the Fenton reaction which we have used in our chemiluminescent investigation of the methoxy derivatives. The observed CL-SI index in the HO generation system is lower compared to the one obtained using the other chemiluminescent assays. At the lowest tested concentration of 3 μmol/L, only 3mSIH demonstrated any scavenging effect (CL-SI = 87.7%). At the highest investigated concentration, the data indicated different scavenging activity for the three compounds. The most potent was 3mSIH (CL-SI = 15.87%), to be exact – two times stronger than 3mShBH and three times more powerful than 3mSBH.
To obtain more information about the antioxidant properties of the compounds, we have tested their potential in iron-free spectrophotometric system with UV irradiation-induced OH● generation. The formation of ROS was measured using 2-deoxyribose oxidative degradation product malondialdehyde by its condensation with TBA ().
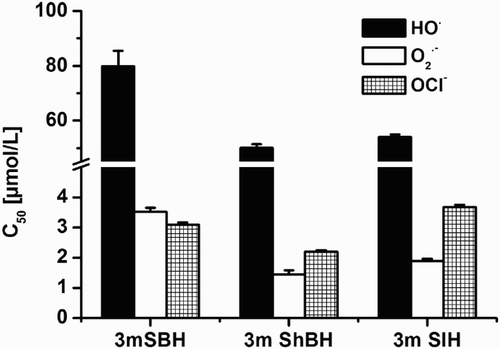
Figure 3. Effect of SBH and its 3-methoxy derivatives on 2-deoxyribose degradation induced by UV-irradiation. After 30 min of UV irradiation (UV 220–400) to 1 ml of the sample containing the studied derivatives and 0.6 mmol/L 2-deoxyribose were added 0.6 ml of 1% TCA and 0.6 ml of 0.6% of thiobarbituric acid. The mixture was vortexed, heated in a 100 °C water bath for 20 min and the quantitative determination of the thiobarbituric acid reactive substances was measured at 532 nm. The degree of the oxidative 2-deoxyribose damage was presented as percentage of the control.
All derivatives have demonstrated remarkable activity in protecting the 2′-deoxyribose molecules in the model system of UV irradiation (). At concentration 100 µmol/L, all of them decreased the deoxyribose damage by more than 45%. We compared the antioxidant efficiency of the methoxy derivatives with that of the initial compound SBH and classical OH● scavengers, e.g. DMSO (43.13 ± 0.61)% and Trolox (34.42 ± 0.38)%, at the same concentration and we concluded that their concentration range of effectiveness was found to be equivalent in aqueous solutions.
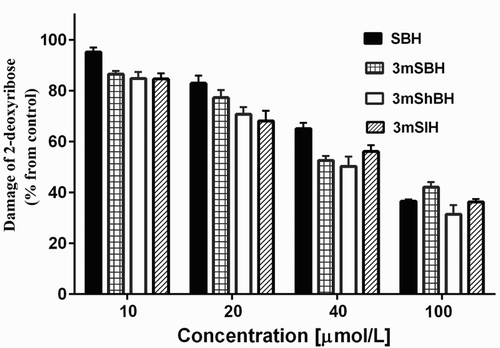
The results for C50 obtained via the chemiluminescent iron-induced hydroxyl radical formation and from the spectrophotometric AOA evaluation in the model system with UV irradiation for the methoxy derivatives were very similar (). Comparing these data with the one of the active iron chelators SBH [Citation31] in the same model system, it was suggested that the three methoxy derivatives work as antioxidant using similar mechanism of action in the compared model systems different from the Fe-chelation.
Figure 4. Comparison between the C50 values obtained from the chemiluminescent system of Fe-induced hydroxyl radical formation and from the spectrophotometric assay of UV-induced 2-deoxyribose degradation. Values of C50 were calculated from data depicted in and 3 representing the effect of the concentration of the hydrazone derivatives (solutions and experimental conditions are as described in the figure 1 and 3 legends).
The studies in the UV-model system not only verified the protection effect of the investigated compounds on the process of OH● induced damage of the deoxyribose molecule but also excluded the possibility of quenching effect-induced diminishment of the chemiluminescent response in the system of iron-dependent hydroxyl radical formation. The similar C50 values for both systems () suggested that chemiluminescent methods are appropriate for determining the scavenging properties of the methoxy derivatives and will provide adequate experimental results concerning their effectiveness against ROS.
As can be seen on , all derivatives have excellent scavenging effect against the superoxide radical and the hypochlorous anion (C50 varies from 1.44 to 3.67 µmol/L). There are two possible basic mechanisms defining the potential deactivating effect of the studied compounds against ROS – hydrogen atom transfer associated with bond dissociation and electron transfer correlated with ionization potential. Difficulties in the analysis of the experimental data are associated with the fact that the intermediates obtained can interact with each other and/or participate in secondary free radical reactions. The performed studies in this area indicate that it is more likely to observe mix mechanism of action: either by sequential proton loss and followed electron transfer or by electron transfer and subsequent proton transfer [Citation32,Citation33]. According to the knowledge on the electrochemical reduction of hydrazones under aqueous conditions, the reaction consists of two-electron, two-proton transfer which converts the C=N-NH to CH-NH-NH group [Citation34–36]. Our data clearly indicated different effect on the luminol-dependent CL in a concentration-dependent manner. The obtained C50 values for the compounds were similar but statistically different. However, despite the fact that the OH● is the most potent oxidizing agent among the studied ROS, the influence of the derivatives on the chemiluminescent luminosity in the model system of Fe-induced hydroxyl radical formation was moderate. The observed effect was more than ten times lower (the probe exhibited the highest C50 values) compared to the one obtained in the two other model systems. A possible explanation of the results obtained is that due to its extremely reactivity OH● is lacking specificity and could be involved in the competition reaction of oxidative attack on any of the presented molecules in the model system (EDTA, luminol, ascorbate).
The results indicated that 3mShBH, 3-methoxysalicylaldehyde-4-hydroxybenzoylhydrazone and 3-methoxysalicylaldehyde isonicotinoylhydrazone displayed changeable radical scavenge activity depending on the mechanism of ROS generation. Despite the differences in the demonstrated antioxidant properties between the compounds, they exhibited similar behaviour in the selected in vitro chemiluminescent systems. In all the assays, 3mShBH seemed to be the most potent compound, indicating the beneficial effect of the presence of a hydroxyl group in the molecular skeleton of the investigated properties.
The principal object of the investigation in the ABTS model systems investigation was to explore the stability of the antioxidant effect of the derivatives under study for a long period of time. A further task was to provide a stabilization method allowing for storage for considerable period, without decomposition and activity reduction. The investigated derivatives were encapsulated in polysaccharide particles consisting of chitosan and alginate. Hydrazone-loaded particles were characterized by an average diameter of 350 nm. The antioxidant activity of non-encapsulated and encapsulated into the particles’ derivatives was measured every ten days during a 30-day investigation period. The experimental data revealed differences in AOA of the 3-methoxy-bearing hydrazone derivatives in liquid formulation during the storage period (). Less stable was 3mSBH whose total antioxidant capacity was diminished by two and a half times on the tenth day of the storage period and more than three times until the end of the investigation. For the same period, the antioxidant activity of 3mShBH and 3mSIH solutions was reduced by 30.1% and 22.6%, respectively. These data suggested that the incorporation of the hydroxyl group or the substitution by a nitrogen atom in the benzene ring of 3mSBH significantly increased the investigated properties – stability of the 3-methoxy derivatives.
Figure 5. Kinetic changes of the AOA of free hydrazones and hydrazones encapsulated into chitosan-alginate particles against the stable ABTS radical. The antioxidant activity was determined during 30-day investigation period. Data are presented as mean ± SD (n = 3). The empty chitosan-alginate particles did not exhibit any antioxidant activity (0.98 ± 0.06%).
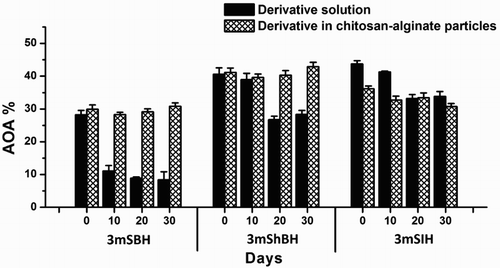
The encapsulation of the hydrazone derivatives in chitosan–alginate particles definitely stabilized their antioxidant activity. This effect was most noticeable for the 3mSBH-NP and 3mShBH-NP samples since no statistically significant variation in the AOA was observed during the whole storage period. At the end of the investigated period, the AOA of the stock solution of 3mSBH was three and a half times lower than that of the encapsulated 3mSBH. Only the AOA of the 3mSIH-NP diminished during the explored period by 14.85%.
In vitro cytotoxicity
In order to evaluate the growth inhibitory effects of the hydrazone derivatives, we have used human hepatocellular carcinoma cell line – HEP-G2, human embryotic kidney cell line HEK-293 and human neuroblastoma cells – SH-SY-5Y. After 72 hours of incubation with the studied 3-methoxy derivatives, the cell viability was determined by the MTT assay. The cell viability of all compounds was decreased in a concentration-dependent manner ().
Figure 6. Concentration – response curves of the investigated 3-methoxy derivatives against HEP-G2 (hepatocellular carcinoma), HEK-293 (human embryotic kidney) and SH-SY-5Y (human neuroblastoma) cell lines as assessed by the MTT-dye reduction assay after 72 h exposure. Each data point represents the arithmetic mean ± sd of eight separate experiments.
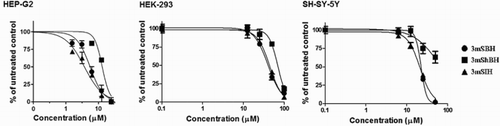
The incubation of Hep-G2 cell with micromolar concentration of the studied derivatives has shown significant difference in viability compared with that of the untreated control sample. The in vitro concentration-dependent effect is presented in . Cell viability was almost zero after 72 hours of incubation of the human hepatoblastoma cells with the 3-methoxy derivatives at concentration 25 μM. The parent (3mSBH) and the pyridine bearing derivative (3mSIH) exhibited higher cell growth inhibitory effect compared to the hydroxyl bearing derivative (3mShBH). The IC50 value of 3mShBH was more than two fold higher as compared to that of 3mSBH and more than 4 times higher than that of 3mSIH.
SH-SY-5Y neuroblastoma cell lines are differentiated and have neuronal characteristics i.e. dopamine and norepinephrine synthesis, expression of dopamine transporters. The evaluation of the chemosensitivity of SH-SY-5Y cells to the hydrazones derivatives was tested in the concentration range between 0.1 and 50 µM. The 3-methoxy derivative with no subsequent structural modifications (3mSBH) and the pyridyl analog (3mSIH) demonstrated similar patterns of activity. At the maximal investigated concentration (50 μM), these compounds have caused drastic decrease in the cell viability by more than 95%. The IC50 values derived from the concentration––response curve are 22.2 µM for 3mSBH and 21.42 µM for 3 m SIH. The data for the hydroxyl group bearing derivative (3mShBH) suggest relatively less-prominent effect. At the highest tested concentration, cell viability was 63% and the compound failed to induce 50% cell viability. We have performed additional experiments investigating the cytoprotective effects of 3mShBH against NMDA-induced excitotoxicity in SH-SY-5Y human neuronal cells (). The results obtained reveal dose-dependent manner of the observed cytoprotective effect.
Table 1. Cytoprotective effects of the tested compounds’ formulation against NMDA-induced excitotoxicity in SH-SY5Y human neuronal cells, as assessed by the MTT-dye reduction assay after 72 h incubation.
The investigations with the non-malignant HEK-293 cell line revealed less-prominent cytotoxicity and the concentration range of the measurements was increased to 100 μM. The observed IC50 values were in the range 39.9–69.69 μmol/L. Again 3mSIH exerted the most pronounced cytotoxic effect followed by 3mSBH.
The comparison of the obtained IC50 results reveals that the studied 3-methoxy compounds have the capacity to inhibit the growth of malignant and normal cell lines (). All the derivatives were more potent on malignant cells and expressed less cell toxicity on the non-malignant HEK-293. In all cell systems, the structural modifications afforded similar patterns of cytotoxicity. Their activity decreases in the following order – 3mSIH>3mSBH>3mShBH.
Table 2. Cytotoxic activity of the tested hydrazones on HEP-G2 (hepatocellular carcinoma cell line), HEK-293 (human embryotic kidney cell line) and SH-SY-5Y (human neuroblastoma cells).
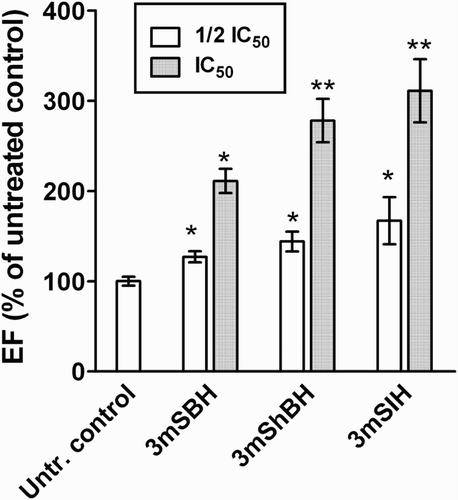
Moreover, the ability of the tested agents to induce apoptosis was evaluated using a commercial ELISA-kit for quantification of histone-associated mono- and oligonucleosomal DNA-fragments, as an established biomarker of cellular apoptosis. The results from the apoptosis-induction assay are depicted in . Evident from the data, the 24 h treatment of HEP-G2 cells is associated with a statistically significant increase in the level of the apoptotic DNA fragmentation in a concentration-dependent manner. These findings imply that the growth inhibitory effects of the presented compounds are, at least partly, mediated by the recruitment of the apoptotic cell signalling cascades and induction of programmed cell death.
Figure 7. Apoptotic DNA fragmentation after 24 h exposure of HEP-G2 cells to the tested compounds, as assessed by ‘Cell death detection ELISA™’ kit. The tests were run in quadruplicate and presented as percentage of the untreated control. The asterisks indicate statistically significant differences vs. the untreated control at p ≤ 0.05 (*) or p ≤ 0.01 taken as an indicator of significance level.
Conclusion
The present study demonstrated a well-pronounced in vitro antioxidant activity of 3-methoxy derivatives of SBH in scavenging different forms of reactive species – hypochlorous anion, hydroxyl radical, superoxide anion radical and ABTS radical. The investigations performed in different model systems suggested that the studied compounds acted by a mechanism of direct antioxidant activity and radical scavenging. The structural modifications in the initial compound associated with incorporation of a hydroxyl group or substitution with hetero atom in the hydrazide part enhanced the scavenging capacity.
Overall, the effect of the hydroxyl-bearing derivative exceeded the one observed for the SBH structural derivative containing a hetero atom. Thus, the designed original methoxy hydrazones can be used in the development of new class of antioxidant protectors with potential application in oxidative stress pathologies. The encapsulation of hydrazones into polysaccharide particles based on chitosan and sodium alginate could be a useful approach leading to the preservation of their antioxidant activity. The incorporation of hydroxyl group in the hydrazide part of the molecule (3 mShBH) relates to better antioxidant effect in most of the studied systems and is associated with higher IC50 values in all cytotoxicity experiments. This compound proved to exert cytoprotective effect against NMDA-induced excitotoxicity in SH-SY5Y human neuronal cells.
The compounds exerted antiproliferative effects in human tumour cell lines, which are at least partly mediated by the induction of programmed cell death through apoptosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nadya Hristova-Avakumova http://orcid.org/0000-0001-7763-9791
Vera Hadjimitova http://orcid.org/0000-0001-7735-5406
Notes on contributors
Nadia Hristova-Avakumova is an assistant professor in the Department of Medical Physics and Biophysics in the Faculty of Medicine at Medical University of Sofia. She graduated with a master’s degree in molecular biology with specialization in biophysics. Her scientific interests are related to free radical processes, oxidative stress and evaluation of the antioxidant properties of pharmaceutical products, new designed compounds and natural products.
Krassimira Yoncheva is a lecturer in the Department of Pharmaceutical Technology and Biopharmaceutics in the Faculty of Pharmacy at Medical University of Sofia. The research interests of Prof. Yoncheva are associated with the development and characterization of biodegradable polymeric micro- and nanoparticles as drug delivery systems. The surface modification of the particles, e.g. pegylation or coating with bioadhesive polymers, is in the focus of her research.
Boryana Nikolova-Mladenova works in the Department of Chemistry in the Faculty of Pharmacy at Medical University of Sofia. The scientific and research work of Nikolova-Mladenova is focused on several areas of modern pharmaceutical science and practice, such as design and synthesis of new bioactive hydrazones and their metal complexes with the metals Ga, Cu, Zn, Fe, Co and Ni; study of the structure and stability of the new ligands and complexes; pharmacological investigation of the compounds; research of the structure–activity relation.
Trayko Traykov graduated physical chemistry at Medical University of Sofia ‘St Kliment Ohridski’. He has been working at Medical University of Sofia since 1982. He is the author or co-author of 95 publications, 4 textbooks and 7 laboratory manuals. He is delivering lectures on Medical Biophysics and several elective courses for students at Medical University of Sofia. He is a member of the scientific direction in the university ‘Free radical processes in biological systems’.
Georgi Momekov is a lecturer of Pharmacology and pharmacotherapy at the Faculty of Pharmacy, Medical University of Sofia, Bulgaria. He has graduated Pharmacy and has acquired a PhD degree in Pharmacology from the same university in 1998 and 2007, respectively. His academic career began in 1999 as an assistant professor; he became associate and full professor in 2011 and 2014, respectively. His research interests include experimental anticancer chemotherapy, ethnopharmacology and phytopharmacology.
Vera Hadjimitova is an associate professor in the Department of Medical Physics and Biophysics at Medical University of Sofia. She is delivering lectures on Medical Physics and Biophysics of Bulgarian and foreign students studying Medicine, Dental Medicine and Pharmacy. Dr Hadjimitova is a co-lecture in elective courses: ‘Oxidative stress and free radical processes’ and ‘Physics methods for diagnostics and therapy’. She holds a PhD in the field of free radical processes and oxidative stress which is her main field of scientific interest. Her current research interests include in the improvement and modernization of Medical Physics and Biophysics educations. Dr Hadjimitova is a member of the Board of Union of the physicists in Bulgaria.
Additional information
Funding
References
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96.
- Júnior WB, Alexandre-Moreira MS, Alves MA, et al. Analgesic and anti-inflammatory activities of salicylaldehyde 2-chlorobenzoyl hydrazone (H(2)LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H(2)LASSBio-1064) and their zinc(II) complexes. Molecules. 2011;16(8):6902–6915. doi: 10.3390/molecules16086902
- El-Sayed MAA, Adbel-Aziz NI, Abdel-Aziz AAM, et al. Design, synthesis and biological evaluation of substituted hydrazones and pyraloze derivatives as selective COX-2 inhibitors: Molecular docking study. Bioorg Med Chem. 2011;19(11):3416–3424. doi: 10.1016/j.bmc.2011.04.027
- Kumar P, Narasimhan B. Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium. Mini Rev Med Chem. 2013;13(7):971–987. doi: 10.2174/1389557511313070003
- Metwally KA, Abdel-Aziz LM, Lashine ESM, et al. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: synthesis and preliminary evaluation as antimicrobial agents. Bioorg Med Chem. 2006;14(24):8675–8682. doi: 10.1016/j.bmc.2006.08.022
- Oviddiu O, Ndongo J, Moldovan C, et al. Synthesis and antimicrobial activity of some new 2-hydrazone-thiazoline-4-ones. Farmacia. 2012;60(6):785–797.
- Silva AG, Zapata-Sudo G, Kummerle AE, et al. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem. 2005;13(10):3431–3437. doi: 10.1016/j.bmc.2005.03.003
- Jois HS, Kalluraya B, Vishwanath T. Synthesis, spectroscopic properties and antioxidant activity of bis-hydrazones and schiff's bases derived from terephthalic dihydrazide. J Fluoresc. 2015;25(3):481–488. doi: 10.1007/s10895-015-1558-5
- Nikolaevskii A, Kniga O, Khizhan E, et al. Antioxidant activity of hydrazones with sterically hindered phenol fragments. Russ J Phys Chem A. 2012;86(12):1816–1820. doi: 10.1134/S0036024412120205
- Belkheiri N, Bouguerne B, Bedos-Belval F, et al. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur J Med Chem. 2010;45(7):3019–3026. doi: 10.1016/j.ejmech.2010.03.031
- Nikolova-Mladenova B, Halachev N, Iankova R, et al. Synthesis, characterization and cytotoxic activity of new salicylaldehyde benzoylhydrazone derivatives as potential anti-proliferative agents. Arzneimittel For/Drug Res. 2011;61(12):714–718.
- Liu Z, Jiao Y, Wang Y, et al. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001
- Porfire AS, Zabaleta V, Gamazo C, et al. Influence of dextran on the bioadhesive properties of poly(anhydride) nanoparticles. Int J Pharm. 2010;390(1):37–44. doi: 10.1016/j.ijpharm.2009.08.017
- Kumar MN, Muzzarelli RA, Muzzarelli C, et al. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017–6084. doi: 10.1021/cr030441b
- Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56(3):290–299. doi: 10.1016/j.yrtph.2009.09.015
- Hadjimitova V, Traykov T, Mileva M, et al. Effect of some psychotropic drugs on luminol-dependent chemiluminescence induced by O2.-,OH., HOCL. Z Naturforsch C. 2002;57c:1066–1071.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3
- Traykov T, Hadjimitova V, Goliysky P, et al. Effect of phenothiazines on activated macrophage-induced luminol-dependent chemiluminescence. Gen Physiol Biophys. 1997;16(1):3–14.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4
- Van Dyke K, Van Dyke C, Woodfork K, editors. Luminescence biotechnology: instruments and applications. Boca Raton: CRC Press; 2002.
- Roda A, Guardigli M, Pasini P. Bioluminescence and chemiluminescence in drug screening. Anal Bioanal Chem. 2003;377(5):826–833. doi: 10.1007/s00216-003-2096-6
- Campbell AK. Chemiluminescence: principles and applications in biology and medicine. Cambridge: VCH; 1988. chap 5.
- Schwamberger G, Flesh I, Ferber E. Tumorocidal effector molecules of murine macrophages. Pathobiology. 1991;59(4):248–253. doi: 10.1159/000163656
- Sies H, de Groot H. Role of reactive oxygen species in cell toxicity. Toxicol Lett. 1992;64–65:547–551.
- Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12(4):274–277. doi: 10.1016/S0899-9007(96)00000-8
- Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22(4–5):189–216. doi: 10.1016/S0098-2997(01)00010-3
- Forman HJ, Torres M. Signaling by the respiratory burst in macrophages. IUBMN Life. 2001;51(6):365–371. doi: 10.1080/152165401753366122
- Avakumova N, Hadjimitova V, Traykov T. Inhibition of oxygen free radicals induced luminol-dependent chemiluminescence by 4-methoxy derivatives of salicylaldehyde benzoyl hydrazine. Luminescence. 2014;29(S1):61–62.
- Leopoldini M, Russo N, Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. doi: 10.1016/j.foodchem.2010.08.012
- Wright JS, Johnson ER, DiLabio GA. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc. 2001;123(6):1173–1183. doi: 10.1021/ja002455u
- Demirel Özel A, Durmuş Z, Yılmaz İ, et al. Electroreduction of some substituted hydrazones on platinum electrode in dimethyl formamide. Acta Chim Slov. 2009;56:797–806.
- Bakalbassis EG, Lithoxoidou AT, Vafiadis AP. Theoretical insights, in the liquid phase, into the antioxidant mechanism-related parameters in the 2- monosubstituted phenols. J Phys Chem A. 2006;110:11151–11159. doi: 10.1021/jp061718p
- Kareem HS, Ariffin A, Nordin N, et al. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur J Med Chem. 2015;103:497–505. doi: 10.1016/j.ejmech.2015.09.016
