ABSTRACT
Objective: The purpose of this study was to investigate the effects of chromium picolinate (CrPic) supplementation associated with aerobic exercise using measures of oxidative stress in rats exposed to air pollution.
Methods: Sixty-one male Wistar rats were divided into eight groups: residual oil fly ash (ROFA) exposure and sedentary (ROFA-SED); ROFA exposure, sedentary and supplemented (ROFA-SED-CrPic); ROFA exposure and trained (ROFA-AT); ROFA exposure, supplemented and trained (ROFA-AT-CrPic); sedentary (Sal-SED); sedentary and supplemented (Sal-SED-CrPic); trained (Sal-AT); and supplemented and trained (Sal-AT-CrPic). Rats exposed to ROFA (air pollution) received 50 µg of ROFA daily via intranasal instillation. Supplemented rats received CrPic (1 mg/kg/day) daily by oral gavage. Exercise training was performed on a rat treadmill (5×/week). Oxidative parameters were evaluated at the end of protocols.
Results: Trained groups demonstrated lower gain of body mass (P < .001) and increased exercise tolerance (P < .0001). In the gastrocnemius, trained groups demonstrated increased SOD activity (P < .0001) and decrease levels of TBARS (P = .0014), although CAT activity did not differ among groups (P = .4487).
Conclusion: Air pollution exposure did not lead to alterations in oxidative markers in lungs and heart, and exercise training was responsible for decreasing oxidative stress of the gastrocnemius.
1. Introduction
Air pollution is currently classified as a leading environmental cause of cancer and ranked as one of the top 10 causes of disability [Citation1–3]. Furthermore, polluted air is related to premature mortality, estimated to cause a global mortality burden of more than 3 million premature deaths/year. The projections of emission scenarios indicate that these values could double by 2050, if any measure of air quality control could be made [Citation4,Citation5]. There is strong evidence that pollutants present in air are responsible for the detrimental effects of air pollution, triggering oxidative stress and systemic inflammation [Citation3,Citation6,Citation7]. These effects contribute to the pathological mechanism, increasing the susceptibility of the population to developing chronic diseases [Citation6,Citation7].
Physical inactivity is one of the most significant public health problems of the twenty-first century and is the fourth leading cause of death worldwide [Citation8,Citation9], whereas physical activity demonstrates well-established health benefits [Citation10–12]. Exercise training induces the formation of reactive oxygen species (ROS), which act as important mediators of physiologic signaling and cellular adaptations, modulation of muscle contraction, regulation of antioxidant protection and repair of oxidative damage [Citation13,Citation14]. The majority of studies investigating air pollution and exercise, however, have demonstrated controversial findings [Citation13–16].
Chromium picolinate (CrPic) is a trivalent chromium complex largely used to control glucose levels and improve insulin sensitivity. Furthermore, CrPic supplementation has exhibited antioxidant activity in animals with established oxidative stress, being responsible for increasing glutathione (GSH) levels, catalase (CAT) and superoxide dismutase (SOD) activity, in addition to decreasing lipid peroxidation [Citation17–22]. CrPic antioxidant activity has not been investigated in association with air pollution exposure, however. Considering the antioxidant properties of this supplement, it could possibly decrease the damaging effects of air pollution on health, attenuating the action of oxidative molecules and, subsequently, the damage they induce [Citation23,Citation24].
The aim of the present study was to examine the effects of CrPic supplementation associated with aerobic exercise during subchronic air pollution exposure in measures of oxidative stress. In addition, we investigated the adaptation of aerobic exercise in groups exposed to air pollution and supplemented with CrPic.
We tested the hypothesis that air pollution exposure may increase body mass and decrease exercise tolerance, as well as inducing oxidative damage in the lungs, heart and gastrocnemius, whereas supplementation of CrPic and aerobic training protocols may lead to beneficial effects on those variables.
2. Materials and methods
2.1. Animals
This study was performed using 61 male Wistar rats (45 days old) obtained from the Animal Breeding Unit of the Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA). The animals were housed under standard conditions (food and water ad libitum, temperature between 22 and 24°C, light–dark cycle of 12 h). The handling of the animals obeyed resolutions of the National Council on Animal Experimentation and all procedures were in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the National Institute of Health (NIH-USA). This study was approved by CEUA/UFCSPA, under the protocol number 159/15.
2.2. Experimental design
Sixty-one male Wistar rats were divided into eight experimental groups: residual oil fly ash (ROFA) exposure and sedentary (ROFA-SED, n = 8); ROFA exposure, sedentary and supplemented (ROFA-SED-CrPic, n = 6); ROFA exposure and trained (ROFA-AT, n = 8); ROFA exposure, supplemented and trained (ROFA-AT-CrPic, n = 7); sedentary (Sal-SED, n = 8); sedentary and supplemented (Sal-SED-CrPic, n = 8); trained (Sal-AT, n = 8); supplemented and trained (Sal-AT-CrPic, n = 8). Intranasal instillation of ROFA and CrPic supplementation protocols were performed daily for 90 days, and the training protocol was performed for the same period of time (5×/week).
2.3. Intranasal instillation of ROFA
Animals exposed to air pollution received 50 µg of ROFA via intranasal instillation daily for 90 days. ROFA was applied as a recognized form of particulate matter. The dose used represents a concentration of 29 µg/m³, which is the value found in a polluted city [Citation25]. ROFA particles were collected from an electrostatic precipitator installed in one of the chimneys of a large steel plant in São Paulo, Brazil. Characterization of ROFA is included in . A suspension of 50 µg of ROFA was prepared in 10 µl of sterile saline solution. When rats were 60 and 90 days old, ROFA suspensions of 50 µg were prepared in 20 and 30 µl sterile saline solutions, respectively. The volume was adjusted to ensure that the suspension would reach the lungs, considering that as the rats’ respiratory systems developed, a greater volume would be necessary [Citation26,Citation27]. Control groups underwent the same instillation protocol, but received only saline.
Table 1. Characterization of metals in residual oil fly ash.
2.4. Chromium picolinate supplementation
Supplemented groups received 1 mg/kg of CrPic in 1 ml sterile saline solution (presentation form: powder, with purity of ≥ 98%, Pharma Nostra®, Brazil) by oral gavage daily for 90 days. Animals were weighed every 15 days to allow for dose adjustment [Citation19]. Control groups underwent the same supplementation protocol, but received only saline.
2.5. Exercise tolerance test
All rats underwent an exercise tolerance test to measure their maximal running capacity before and after the period of experiments. First, animals were subjected to an adaptation period of five days and run for 10 min/day [Citation28]. The test consisted of running on an electric treadmill with an inclination of 15°, starting with a speed of 5 m/min and increasing by an increment of 5 m/min every 3 min until exhaustion. Exhaustion was established as the time at which the animal was unable to run for at least 15 s, even while receiving an electrical stimulus (1.5 μA) [Citation29].
2.6. Training protocol
The animals in trained groups underwent aerobic exercise training, which was performed on a motorized treadmill five days/week with a moderate intensity of 70% for 90 days. The running time started at 20 min on the first week and was extended by 10 min/week until 50 min/day was reached (all rats were running) [Citation29,Citation30].
2.7. Tissue collection
After 90 days of experimental protocols, animals were anesthetized via exposure to isoflurane in oxygen (induction 5%, 2 L/min) for 5 min in an induction chamber and then euthanized via the exsanguination method. Lungs, heart and gastrocnemius were dissected and stored in −80°C for subsequent analyses of oxidative stress.
2.8. Oxidative stress analysis
2.8.1. Tissue preparation
To prepare tissues, lungs, heart and gastrocnemius were defrosted, weighed in an analytical balance and homogenized in KPi buffer (KCl 1.15%, pH 7.4) containing protein inhibitors. Homogenization was performed in a tissue homogenizer (CT-136.1, Cientec®), after which samples were centrifuged and supernatants were stored at −80°C until oxidative stress analyses were conducted.
2.8.2. Protein concentration
Protein concentration of the tissues homogenates was measured via Bradford’s method [Citation31] using bovine serum albumin as a standard. The sample absorbance was determined at 595 nm, using a Lambda 35 spectrophotometer (Perkin-Elmer of Brazil, SP, Brazil).
2.8.3. Superoxide dismutase activity
SOD activity was determined based on the inhibition of pyrogallol auto-oxidation by the enzyme, following the method described by Marklund and Marklund [Citation32]. Sample absorbances were determined using a Lambda 35 spectrophotometer (Perkin-Elmer of Brazil, SP, Brazil), at 420 nm after 60 and 120 s. The results were expressed as USOD/mg of total protein.
2.8.4. CAT activity
CAT activity was determined based on the decomposition of hydrogen peroxide at 25°C, following the method described by Aebi [Citation33]. Sample absorbances were determined using a Lambda 35 spectrophotometer (Perkin-Elmer of Brazil, SP, Brazil), at 240 nm for 120 s. The results were expressed in nmol/mg of total protein.
2.8.5. Thiobarbituric acid-reactive substances
To determine lipid peroxidation, thiobarbituric acid-reactive substances (TBARS) levels were measured according to the technique described by Esterbauer and Cheeseman [Citation34]. Sample absorbances were determined at 535 nm using a Lambda 35 spectrophotometer (Perkin-Elmer of Brazil, SP, Brazil). TBARS concentration was expressed in nmol/mg of total proteins. To calculate TBARS levels, a standard curve generated based on known concentrations of 100 nmol/ml 1,1,3,3-tetrametoxypropane in 1% H2SO4 solution was utilized.
2.9. Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analyses were begun using the Kolmogorov–Smirnov test to evaluate normality of all variables. Two-way repeated measures analysis of variance (ANOVA) was then performed, followed by Tukey’s post hoc test to compare body mass variables between treatment and control groups. Other variables were analyzed using one-way ANOVA, followed by Tukey’s post hoc test to compare between treatment and control groups. For statistical analysis and graphics creation, SigmaPlot version 12.0 for Windows (Systat Software, Inc.) and GraphPad Prism version 6.0 for Windows (Prism 6; GraphPad Software, Inc.) were used. A P < .05 was considered statistically significant.
3. Results
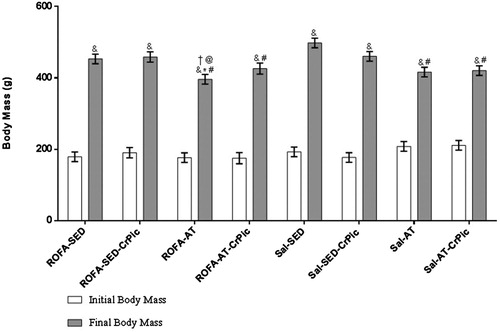
Our study started with 64 animals; however, during the study, there were three losses (one from the ROFA-AT-CrPic group and two from the ROFA-SED-CrPic group) due to causes not related to the experiments (data not shown). Initial body mass did not differ among groups (P > .05) and at the end of the study, all groups showed a mean increase in body mass of 236% (P < .001). Furthermore, when final body mass was compared among groups, the trained groups demonstrated lower gain of body mass in comparison to the Sal-SED group (P < .05; ).
Figure 1. Body mass before and after 12 weeks of chromium picolinate (CrPic) supplementation and aerobic exercise intervention in rats exposed to residual oil fly ash (ROFA). Values presented as mean ± SD. Statistical analysis: two-way repeated measures analysis of variance (ANOVA) followed by Tukey’s post hoc test. ROFA-SED, ROFA exposure and sedentary (n = 8); ROFA-SED-CrPic, ROFA exposure, sedentary and supplemented (n = 6); ROFA-AT, ROFA exposure and trained (n = 8); ROFA-AT-CrPic, ROFA exposure, supplemented and trained (n = 7); Sal-SED, sedentary (n = 8); Sal-SED-CrPic, sedentary and supplemented (n = 8); Sal-AT, trained (n = 8); Sal-AT-CrPic, supplemented and trained (n = 8). Symbols represent comparisons among groups based on the post hoc analysis: &P < .05 vs. Initial Body Mass; *P < .05 vs. ROFA-SED; @P < .05 vs. ROFA-SED-CrPic; #P < .05 vs. Sal-SED; †P < .05 vs. Sal-SED-CrPic.
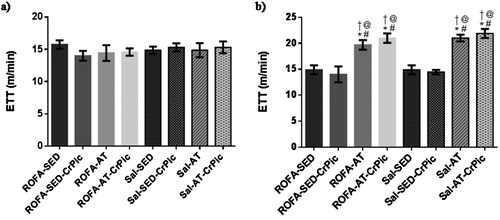
Exercise tolerance tests performed at the beginning of the study demonstrated no difference among groups (P = .8879). In the final exercise tolerance test, however, an increase in exercise tolerance was observed in the trained groups when compared to the sedentary groups (P < .0001; ).
Figure 2. Exercise tolerance test before and after 12 weeks of chromium picolinate (CrPic) supplementation and aerobic exercise intervention in rats exposed to residual oil fly ash (ROFA). (a) Initial exercise tolerance test; (b) final time of exercise tolerance test. Values presented as mean ± SD. Statistical analysis: one-way ANOVA followed by Tukey’s post hoc test. ROFA-SED, ROFA exposure and sedentary (n = 8); ROFA-SED-CrPic, ROFA exposure, sedentary and supplemented (n = 6); ROFA-AT, ROFA exposure and trained (n = 8); ROFA-AT-CrPic, ROFA exposure, supplemented and trained (n = 7); Sal-SED, sedentary (n = 8); Sal-SED-CrPic, sedentary and supplemented (n = 8); Sal-AT, trained (n = 8); Sal-AT-CrPic, supplemented and trained (n = 8). Symbols represent comparisons among groups based on the post hoc analysis: *P < .05 vs. ROFA-SED; @ P < .05 vs. ROFA-SED-CrPic; # P < .05 vs. Sal-SED; †P < .05 vs. Sal-SED-CrPic.
Activity levels of SOD and CAT in lung tissue did not differ among groups (P = .2756 and P = .1198, respectively), nor did TBARS levels differ among groups (P = .7189; ). Similarly, in heart tissue, no differences were observed among groups in relation to SOD (P = .0763) and CAT (P = .4999) activity levels, or TBARS levels (P = .8656; ).
Figure 3. Oxidative stress in lung tissue after 12 weeks of chromium picolinate (CrPic) supplementation and aerobic exercise intervention in rats exposed to residual oil fly ash (ROFA). Analyses of (a) superoxide dismutase (SOD) activity in lung tissue; (b) catalase (CAT) activity in lung tissue and (c) thiobarbituric acid-reactive substance (TBARS) levels in lung tissue. Values presented as mean ± SD. Statistical analysis: one-way ANOVA followed by Tukey’s post hoc test. ROFA-SED, ROFA exposure and sedentary (n = 8); ROFA-SED-CrPic, ROFA exposure, sedentary and supplemented (n = 6); ROFA-AT, ROFA exposure and trained (n = 8); ROFA-AT-CrPic, ROFA exposure, supplemented and trained (n = 7); Sal-SED, sedentary (n = 8); Sal-SED-CrPic, sedentary and supplemented (n = 8); Sal-AT, trained (n = 8); Sal-AT-CrPic, supplemented and trained (n = 8).
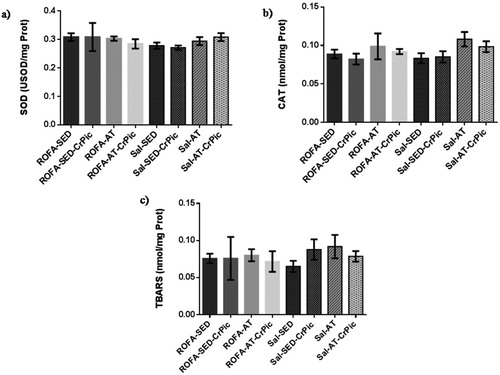
Figure 4. Oxidative stress in heart tissue after 12 weeks of chromium picolinate (CrPic) supplementation and aerobic exercise intervention in rats exposed to residual fly oil ash (ROFA). Analyses of (a) superoxide dismutase (SOD) activity in heart tissue; (b) catalase (CAT) activity in heart tissue and (c) thiobarbituric acid-reactive substance (TBARS) levels in heart tissue. Values presented as mean ± SD. Statistical analysis: one-way ANOVA followed by Tukey’s post hoc test. ROFA-SED, ROFA exposure and sedentary (n = 8); ROFA-SED-CrPic, ROFA exposure, sedentary and supplemented (n = 6); ROFA-AT, ROFA exposure and trained (n = 8); ROFA-AT-CrPic, ROFA exposure, supplemented and trained (n = 7); Sal-SED, sedentary (n = 8); Sal-SED-CrPic, sedentary and supplemented (n = 8); Sal-AT, trained (n = 8); Sal-AT-CrPic, supplemented and trained (n = 8).
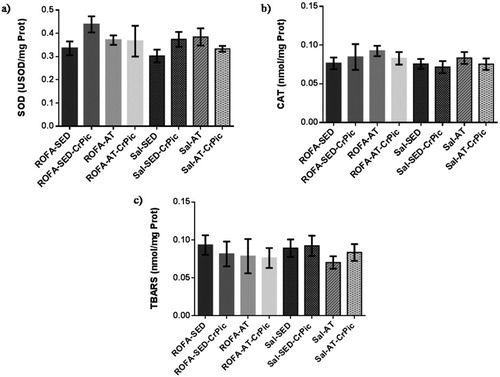
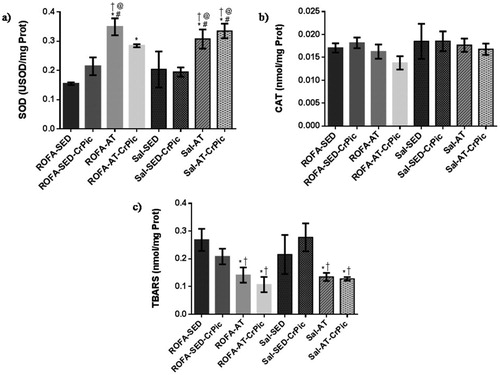
In the gastrocnemius, SOD activity was higher in the groups that underwent aerobic training (ROFA-AT, Sal-AT and Sal-AT-CrPic) than in all sedentary groups (ROFA-SED, ROFA-SED-CrPic, Sal-SED and Sal-SED-CrPic; P < .0001; ). No differences in CAT activity were observed among groups (P = .4487), but levels of TBARS were lower in trained groups when compared to the ROFA + SED and Sal-SED-CrPic groups (P = .0014).
Figure 5. Oxidative stress in gastrocnemius muscle after 12 weeks of chromium picolinate (CrPic) supplementation and aerobic exercise intervention in rats exposed to residual oil fly ash (ROFA). Analyses of (a) superoxide dismutase (SOD) activity in gastrocnemius tissue; (b) catalase (CAT) activity in gastrocnemius tissue and (c) thiobarbituric acid-reactive substance (TBARS) levels in gastrocnemius tissue. Values presented as mean ± SD. Statistical analysis: one-way ANOVA followed by Tukey’s post hoc test. ROFA-SED, ROFA exposure and sedentary (n = 8); ROFA-SED-CrPic, ROFA exposure, sedentary and supplemented (n = 6); ROFA-AT, ROFA exposure and trained (n = 8); ROFA-AT-CrPic, ROFA exposure, supplemented and trained (n = 7); Sal-SED, sedentary (n = 8); Sal-SED-CrPic, sedentary and supplemented (n = 8); Sal-AT, trained (n = 8); Sal-AT-CrPic, supplemented and trained (n = 8). Symbols represent comparisons among groups based on the post hoc analysis: *P < .05 vs. ROFA-SED; @P < .05 vs. ROFA-SED-CrPic; #P < .05 vs. Sal-SED; †P < .05 vs. Sal-SED-CrPic.
4. Discussion
The main findings of the present study were an improvement in exercise tolerance and a reduction in oxidative stress in the gastrocnemius muscle in rats that underwent aerobic training. These findings were evidenced by an increase in both exercise tolerance and SOD activity, and a decrease in TBARS levels in the gastrocnemius muscle. Oxidative stress parameters evaluated in lung and heart tissues, however, did not differ among groups.
Regarding body mass, all groups exhibited increases in body mass during the study, which was expected due to the growth process. Conversely, aerobic exercise could attenuate mass gain, likely due to increased energy expenditure, resulting in a negative energy balance and, consequently, a reduction of mass gain. Such an outcome was observed in an experimental study by Cigarroa et al. [Citation35], which demonstrated that a treadmill intervention could counterbalance the mass gain of animals fed a cafeteria diet.
Our findings suggest that aerobic training leads to exercise tolerance, evidenced by an increased maximal velocity, as demonstrated in experimental studies that use aerobic training [Citation28,Citation29]. The repetitive muscle contraction during exercise training can lead to a variety of responses that increase aerobic metabolism capacity and exercise tolerance [Citation36–38].
In the present study, no differences in SOD and CAT activity, or TBARS levels, in the lungs and heart were observed among groups. Consistent with our results, a study reporting exposure to 50 and 250 µg of ROFA for 90 days also observed no change in oxidative stress markers, such as SOD, CAT and TBARS [Citation39]. Interestingly, acute inhalation of concentrated ambient particles and ROFA has been found to lead to potential oxidant injuries, followed by an up-regulation of antioxidant defenses. After a 24 h inhalation period, reversibility of oxidative stress occurs, suggesting that oxidants mediated by pollution exposure may trigger adaptive responses in the lungs and heart [Citation40–43]. Moreover, our study was performed using healthy animals. In contrast, studies using different experimental models of diseases have demonstrated that an underlying condition represents the greatest risk after ROFA exposure, as once oxidants and inflammatory migration occur at sites where there is a pre-established inflammation; this is not observed in healthy animals [Citation44–46].
Regarding evaluations of oxidative stress in the gastrocnemius, we found increased SOD activity together with decreased TBARS levels in trained groups, suggesting a positive effect of aerobic exercise on oxidative stress in the gastrocnemius muscle. Supporting our results, muscle activity during exercise increases ROS formation and simultaneously promotes an increase in the antioxidant defense system, as well as improving resistance to oxidative stress [Citation38,Citation47]. Furthermore, higher levels of SOD activity in skeletal muscle are related to intensity and duration of exercise, as evidenced by studies reporting that higher intensities and longer durations of exercise were associated with increased SOD activity [Citation47].
We found no significant difference in CAT activity among groups, indicating no alteration in levels of this enzyme in skeletal muscle in response to exercise. Likely, CAT activity did not exhibit changes due to the action of other antioxidant mechanisms in skeletal muscle that neutralize hydrogen peroxide, such as glutathione peroxidase (GPx) and peroxiredoxins. Endurance exercise promotes an increase of 20–177% in GPx activity and peroxiredoxins are constitutively secreted from the skeletal muscle, becoming more abundant than CAT and GPx [Citation47–49].
Notably, the reduced TBARS levels observed in trained groups in our study could be associated with the increased SOD activity, which may prevent against lipid peroxidation, considering that ROS generation induced by exercise is a stimulus for activating the expression of antioxidant enzymes [Citation6,Citation50]. Naturally, a decrease in TBARS levels would be a consequence of the SOD activity [Citation38,Citation47].
In relation to the lack of effect of supplementation, CrPic can play an antioxidant effect when there is an oxidative disruption, because CrPic supplementation could preserve the antioxidant status when there are a depletion of antioxidant enzymes and an increase in oxidative stress [Citation51–53]. In the present study, positive action of CrPic was not observed, likely because there was no depletion of antioxidant enzymes or increased oxidative stress in our sample. Other studies investigating the effects of antioxidant supplementation have reported that supplementation has led to beneficial effects and reduced oxidative stress only in individuals with low baseline antioxidant profiles [Citation43,Citation51,Citation54,Citation55].
In conclusion, this study showed that in a healthy sample, subchronic ROFA exposure did not lead to alterations in oxidative markers. Furthermore, exercise training could decrease body mass gain and increase exercise tolerance, as well as increasing SOD activity and decreasing lipid peroxidation of skeletal muscles, such as the gastrocnemius.
Acknowledgements
We are thankful to Camila Scheid, M.Sc., Caroline Gamalho da Silveira, Eloísa Basso and M.Sc. Roseana Boek Carvalho for their support during the development of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Bruna Marmett holds a bachelor’s degree in Nutrition and a master’s degree in Health Sciences from the Federal University of Health Sciences of Porto Alegre (UFCSPA). Currently, she is a doctoral student in Health Sciences (UFCSPA), research line Pharmacology and Toxicology, participating in research at the Atmospheric Pollution Laboratory in the areas of atmospheric pollution, supplementation, physical exercise, oxidative stress and DNA damage.
Ramiro Barcos Nunes is professor in the Department of Education, Research and Extension (DEPEX) at the Federal Institute Sul-Rio-Grandense (IFSUL) and contributing professor at UFCSPA. He graduated in Physical Education from the Federal University of Rio Grande do Sul (UFRGS), with master’s and doctorate degrees in Health Sciences from UFCSPA.
Kellen Sábio de Souza is a student of Physiotherapy at UFCSPA.
Pedro Dal Lago is professor and research coordinator of the UFCSPA. He is a member of the Advisory Committee on Health Sciences at FAPERGS, a consultant to FWO, Belgium.
Cláudia Ramos Rhoden is professor and coordinator of the Atmospheric Pollution Laboratory at UFCSPA. She holds a bachelor degree in Pharmacy from the Universidade Federal do Rio Grande do Sul (UFRGS), a Master in Pharmacology from UFCSPA, a Ph.D. in Biological Sciences (Physiology) from the UFRGS and a postdoctorate in the Physiology Program at Harvard University. She is also an associate researcher at the Department of Pathology, Faculty of Medicine, University of São Paulo.
ORCID
Bruna Marmett http://orcid.org/0000-0002-2982-0323
Pedro Dal Lago http://orcid.org/0000-0001-9907-7689
Cláudia Ramos Rhoden http://orcid.org/0000-0002-3099-9375
Additional information
Funding
References
- IARC (International Agency for Research on Cancer). IARC: Outdoor air pollution a leading environmental cause of cancer deaths. IARC Scientific Publications. World Health Organization. 2013; 161.
- WHO. Air quality guidelines: Global update 2005. World Health Organization. 2006.
- Aguilera I, Dratva J, Caviezel S, et al. Particulate matter and subclinical atherosclerosis: associations between different particle sizes and sources with carotid intima-media thickness in the SAPALDIA study. Environ Health Perspect. 2016;124:1700–1706. doi: 10.1289/EHP161
- Silva RA, Adelman Z, Fry MM, et al. The impact of individual anthropogenic emissions sectors on the global burden of human mortality due to ambient air pollution. Environ Health Perspect. 2016;124:1776–1784. doi: 10.1289/EHP177
- Lelieveld J, Evans JS, Fnais M, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371
- Fraunberger EA, Scola G, Laliberté VL, et al. Redox modulations, antioxidants, and neuropsychiatric disorders. Oxid Med Cell Longev. 2016. doi:10.1155/2016/4729192.
- Pope CA, Bhatnagar A, McCracken J, et al. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;11:1204–1214. doi: 10.1161/CIRCRESAHA.116.309279
- Blair SN. Physical inactivity: The biggest public health problem of the 21st century. Br J Sports Med. 2009;43:1–2.
- Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380:294–305. doi: 10.1016/S0140-6736(12)60898-8
- Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5
- Lauzé M, Daneault JF, Duval C. The effects of physical activity in Parkinson’s disease: A review. J Parkinson’s Dis. 2016;6:685–698. doi: 10.3233/JPD-160790
- Stubbs B, Koyanagi A, Hallgren M, et al. Physical activity and anxiety: A perspective from the world health survey. J Affect Disord. 2016;208:545–552. doi: 10.1016/j.jad.2016.10.028
- Giles LV, Koehle MS. The health effects of exercising in air pollution. Sports Med. 2014;44:223–249. doi: 10.1007/s40279-013-0108-z
- Matt F, Cole-Hunter T, Donaire-Gonzalez D, et al. Acute respiratory response to traffic-related air pollution during physical activity performance. Environ Int. 2016;97:45–55. doi: 10.1016/j.envint.2016.10.011
- Powers SK, Duarte J, Kavazis NA, et al. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526
- Buresh R, Berg K. A tutorial on oxidative stress and redox signaling with application to exercise and sedentariness. Sports Med Open. 2015;1:3–12. doi: 10.1186/s40798-014-0003-7
- Al-Rasheed NM, Attia HA, Mohamed RA, et al. Preventive effects of selenium yeast, chromium picolinate, zinc sulfate and their combination on oxidative stress, inflammation, impaired angiogenesis and atherogenesis in myocardial infarction in rats. Journal of Pharmacy and Pharmaceutical Sciences. 2013;16:848–867. doi: 10.18433/J34C7N
- Sundaram B, Singhal K, Sandhir R. Anti-atherogenic effect of chromium picolinate in streptozotocin-induced experimental diabetes. J Diabetes. 2013a;5:43–50. doi: 10.1111/j.1753-0407.2012.00211.x
- Sundaram B, Aggarwal A, Sandhir R. Chromium picolinate attenuates hyperglycemia-induced oxidative stress in streptozotocin-induced diabetic rats. J Trace Elem Med Biol. 2013b;27:117–121. doi: 10.1016/j.jtemb.2012.09.002
- Tezuka M, Ishii S, Okada S. Chromium (III) decreases carbon tetrachloride-originated trichloromethyl radical in mice. J Inorg Biochem. 1991;44:261–265. doi: 10.1016/0162-0134(91)84031-4
- Refaie FM, Esmat AY, Mohamed AF, et al. Effect of chromium supplementation on the diabetes induced-oxidative stress in liver and brain of adult rats. Biometals. 2009;22:1075–1087. doi: 10.1007/s10534-009-9258-8
- Zhou J, Xu H, Huang K. Organoselenium small molecules and chromium (III) complexes for intervention in chronic low-grade inflammation and type 2 diabetes. Curr Top Med Chem. 2016;16:823–834. doi: 10.2174/1568026615666150827094815
- Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochem Biophys Acta. 2016;1860:2891–2898. doi: 10.1016/j.bbagen.2016.05.014
- Andersen ZJ, de Nazelle A, Mendez MA, et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: The Danish diet, cancer, and health cohort. Environ Health Perspect. 2015;123:557–563. doi: 10.1289/ehp.1408698
- Medeiros N, Rivero D, Kasahara DI, et al. Acute pulmonary and hematological effects of two types of particle surrogates are influenced by their elemental composition. Environ Research. 2004;95:62–70. doi: 10.1016/j.envres.2003.07.007
- OECD. Guideline for the testing of chemicals. Original Test Guideline No 413, Environment Directorate, OECD. 2009.
- Southam DS, Dolovich M, O’Byrne PM, et al. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:833–839. doi: 10.1152/ajplung.00173.2001
- Marton O, Koltai E, Takeda M, et al. The rate of training response to aerobic exercise affects brain function of rats. Neurochem Int. 2016;99:16–23. doi: 10.1016/j.neuint.2016.05.012
- Ferreira JCB, Rolim NPL, Bartholomeu JB, et al. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol. 2007;34:760–765. doi: 10.1111/j.1440-1681.2007.04635.x
- Nunes RB, Alves JP, Kessler LP, et al. Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clinics (Sao Paulo). 2013;68:876–882. doi: 10.6061/clinics/2013(06)24
- Schleicher E, Wieland OH. Evaluation of Bradford method for protein determination in body-fluids. J Clin Chem Clin Biochem. 1978;16:533–534.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
- Aebi H. Catalase invitro. Methods Enzymol 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H
- Cigarroa I, Lalanza JF, Caimari A, et al. Treadmill intervention attenuates the cafeteria diet-induced impairment of stress-coping strategies in young adult female rats. PLoS One. 2016. doi: 10.1371/journal.pone.0153687
- Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol-London. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327
- Radak Z, Zhao Z, Koltai E, et al. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498
- Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5:356–377. doi: 10.3390/biom5020356
- Damiani RM, Piva MO, Petry MR, et al. Is cardiac tissue more susceptible than lung to oxidative effects induced by chronic nasotropic instillation of residual oil fly ash (ROFA)? Toxicol Mech Methods. 2012;22:533–539. doi: 10.3109/15376516.2012.692109
- Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749
- Carvalho GM, Nagato LK, Fagundes SS, et al. Time course of pulmonary burden in mice exposed to residual oil fly ash. Front Physiol. 2014. doi:10.3389/fphys.2014.00366.
- Magnani ND, Marchini T, Tasat DR, et al. Lung oxidative metabolism after exposure to ambient particles. Biochem Biophys Res Commun. 2011;412:667–672. doi: 10.1016/j.bbrc.2011.08.021
- Marchini T, Magnani ND, Paz ML, et al. Time course of systemic oxidative stress and inflammatory response induced by an acute exposure to residual oil fly ash. Toxicol Appl Pharmacol. 2014;274:274–282. doi: 10.1016/j.taap.2013.11.013
- Farraj AK, Haykal-Coates N, Winsett DW, et al. Increased non-conducted p-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environ Health Perspect. 2009;117:709–715. doi: 10.1289/ehp.0800129
- Marchini T, Wolf D, Michel NA, et al. Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages. Basic Res Cardiol. 2016. doi:10.1007/s00395-016-0562-5.
- Meng X, Zhang Y, Yang KQ, et al. Potential harmful effects of PM2.5 on occurrence and progression of acute coronary syndrome: epidemiology, mechanisms, and prevention measures. Int J Environ Res Public Health. 2016. doi:10.3390/ijerph13080748.
- Powers SK, Knuak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594:5081–5092. doi: 10.1113/JP270646
- Boccatonda A, Tripaldi R, Davì G, et al. Oxidative stress modulation through habitual physical activity. Curr Pharm Des. 2016;22:3648–3680. doi: 10.2174/1381612822666160413123806
- Perkins A, Nelson KJ, Parsonage D, et al. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004
- Marmett B, Nunes RB. Effects of chromium picolinate supplementation on control of metabolic variables: A systematic review. J Food Nutr Research. 2016;4:633–639.
- Cefalu WT, Rood J, Pinsonat P, et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;59:755–762. doi: 10.1016/j.metabol.2009.09.023
- Wang ZQ, Qin J, Martin J, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56:1652–1655. doi: 10.1016/j.metabol.2007.07.007
- Paschalis V, Theodorou AA, Kyparos A, et al. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur J Nutr. 2016;55:45–53. doi: 10.1007/s00394-014-0821-x
- Paschalis V, Theodorou AA, Margaritelis NV, et al. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic Biol Med. 2018;115:288–297. doi: 10.1016/j.freeradbiomed.2017.12.007
