ABSTRACT
Emodin is a naturally occurring anthraquinone derivative with a wide range of pharmacological activities, including neuroprotective and anti-inflammatory activities. We aim to assess the anticancer activity of emodin against hepatocellular carcinoma (HCC) in rat models using the proliferation, invasion, and angiogenesis biomarkers. After induction of HCC, assessment of the liver impairment and the histopathology of liver sections were investigated. Hepatic expression of both mRNA and protein of the oxidative stress biomarkers, HO-1, Nrf2; the mitogenic activation biomarkers, ERK5, PKCδ; the tissue destruction biomarker, ADAMTS4; the tissue homeostasis biomarker, aggregan; the cellular fibrinolytic biomarker, MMP3; and of the cellular angiogenesis biomarker, VEGF were measured. Emodin increased the survival percentage and reduced the number of hepatic nodules compared to the HCC group. Besides, emodin reduced the elevated expression of both mRNA and proteins of all PKC, ERK5, ADAMTS4, MMP3, and VEGF compared with the HCC group. On the other hand, emodin increased the expression of mRNA and proteins of Nrf2, HO-1, and aggrecan compared with the HCC group. Therefore, emodin is a promising anticancer agent against HCC preventing the cancer prognosis and infiltration. It works through many mechanisms of action, such as blocking oxidative stress, proliferation, invasion, and angiogenesis.
1. Introduction
The global concern of hepatic cancer is increasing annually [Citation1]. According to the World Health Organization (WHO), by 2030, the annual global death from hepatocellular carcinoma (HCC) will exceed one million individuals. HCC can explicate about 90% of the total primary liver cancer cases, with approximately 800,000 new cases identified each year worldwide [Citation2]. Due to the late stage upon diagnosis, resistance to existing therapy, and a high likelihood of recurrence, the prognosis for liver cancer patients is bleak. The five-year total survival rate for liver cancer patients is 18%, and the recurrence rate is more than 50% five years after surgery [Citation3]. Even though therapeutic targets for HCC have gotten a lot of global attention, there are just a few effective treatment options for HCC patients [Citation4]. Furthermore, because of the strong angio-invasive capability of the tumor, the therapeutic alternatives for HCC patients are limited [Citation5].
ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family members are diverse metalloendopeptidase enzymes that have diverse roles in tissue morphogenesis and pathophysiological remodeling, inflammation, and vascular biology [Citation6]. Recently, this family has been implicated in the pathophysiology of a growing number of vascular diseases, such as coronary artery disease and coagulation disorders [Citation7]. It has been sub-grouped into 19 members according to their substrates [Citation8]. ADAMTS4 represents the basic organizational structure of all of these family members [Citation6,Citation7] ADAMTS4 can cleave hyaluronan-binding chondroitin sulfate proteoglycan (CSPG) extracellular proteins, including aggrecan, versican, brevican and neurocan [Citation9]. It has also been labeled ‘angio-inhibitory’ based on the original identification of ADAMTS4 as an anti-angiogenic factor. Many studies reported that inhibition of ADAMTS4 may be a good molecular target for the treatment of arthritis [Citation10]. It has been reported that its level is highly regulated in some types of cancers, especially in the tumor microenvironment [Citation11]. So, it could be a promising molecular target for the treatment of HCC.
Emodin is a chemical derivative of the anthraquinone family. It is an active constituent extensively present in a vast collection of roots and bark of plants, Molds, and lichens [Citation12]. Extensive research has been performed regarding the neuroprotective functions of emodin, such as antidiuretic, antibacterial, antiulcer, anti-inflammatory, anticancer, and antinociceptive. an antimalarial and antiallergic agent, most related to its molecular structure, antioxidant properties, and inhibitory effect on many cells signaling pathways [Citation13]. It has been confirmed that emodin inhibits processes of neoplasia at the stages of proliferation, invasion and angiogenesis [Citation14]. Still, there is a poor investigation of the effect of emodin against the PKCδ/ADAMST4 signaling pathway. We aim in this research to uncover the regulatory effect of emodin on the ADAMST4 through PKCδ and to show the output effect of such regulatory action on the prognosis and infiltration of the thioacetamide-induced HCC rat model. Besides, we investigated oxidative stress, tissue homeostasis, mitogenic activation, tissue destruction, and cellular fibrinolytic and cellular angiogenesis biomarkers.
2. Materials and methods
2.1. Drugs and chemicals
Emodine (CAS Number 518-82-1) was purchased from Aladdin Reagent Co., Ltd (Shanghai, China). It is dissolved in distilled water. thioacetamide with 99% purity and CA were purchased from (Tocris, Bristol, UK). It is dissolved in distilled water.
2.2. Experimental design
The research involved forty Sprague – Dawley rats, consisting of male and female rats weighing 180 and 200 g. These rats were acquired from the college's animal facility without undergoing any prior procedures. They were kept at a consistent temperature, exposed to a 12-hour light–dark cycle, and given five days to acclimate before the start of the study. The Research Ethics Committee of the Faculty of Pharmacy at Delta University of Science and Technology approved the animal protocol under the reference number FPDU8/2023. Throughout the experiment, the rats had unrestricted access to food and water. They were divided into four groups, each housing ten rats, with five rats in each cage. All treatments given to the rats were administered by the same research team member, following the same sequence and schedule in the morning ().
Control group: Rats were given normal saline daily for sixteen weeks.
Treated control group: Rats were given 40 mg/kg of emodin orally in normal saline once daily for sixteen weeks.
HCC group: Rats were given 200 mg/kg of thioacetamide in normal saline via intraperitoneal injection twice weekly for sixteen weeks [Citation15–18].
HCC and emodin group: Rats were given 200 mg/kg of thioacetamide intraperitoneally in normal saline twice weekly, along with 40 mg/kg of emodin orally in normal saline daily for sixteen weeks.
Previously, emodin was utilized solely for the treatment of HCC cell lines, and no prior research had demonstrated its efficacy in treating HCC induced in rats. Consequently, initial studies investigated four distinct concentrations of emodin: 30, 40, 50, and 60 mg/kg. Following the findings, the dosage of 40 mg/kg was chosen as it was determined to be the minimal concentration that yielded therapeutic benefits.
2.3. Sample collection
Thiopental sodium (40 mg/kg, i.p.) was used to anesthetize rats. Serum specimens were centrifuged at 3000 rpm for 5 min after being collected from the retro-orbital plexus. Samples were stored at – 80 ⁰C. A portion of the hepatic tissues with tumor was separated in a fresh condition and sliced, and a 10% (w/v) buffered formalin solution was used for tissue fixation, followed by investigating the morphological features. Another portion of the hepatic tumor tissues was homogenized in a 10-fold volume sodium-potassium phosphate buffer of pH 7.4 and stored at – 80 ⁰C.
2.4. Liver gross appearance and evaluation of the number of hepatic nodules
The macroscopic features of the harvested liver, including color, size, texture, and presence of nodules, were carefully recorded. Abnormal nodules were identified by their subtle grayish-white hue, distinguishing them from the normal ones. The entire liver tissues were excised, preserved, and then sliced into 2mm-thick sections. Nodules in the liver measuring 3 mm or more were counted by two separate investigators.
2.5. Histopathological examination and immunohistochemistry
Hepatic slices that are kept in 10% formalin were processed by routine histopathological methods and embedded in paraffin blocks, cut at 5-micrometer thickness. Sections were stained with hematoxylin/eosin. Sections were anonymously coded and examined in a masked manner using a digital camera-aided computer system (Nikon Corporation). For immunohistochemistry, five-micrometer-thick paraffin sections were incubated with monoclonal anti-ADAMTS-4 (Sigma Aldrich Chemicals Co., St. Louis, MO, US). Sections were counterstained with hematoxylin. The degree of intensity of the color of immunohistochemical staining was recorded using a score of 0 for no positive cells in the high-power field, a score of 1 for infrequent positive cells, a score of 2 for a moderate number of positive cells, and a score of 3 for widespread staining. A pathologist blinded to the treatment groups performed all readings [Citation19–21].
2.6. Evaluation of oxidative stress and antioxidant activities
The levels of malondialdehyde (MDA) and reduced glutathione in hepatic tissue were quantified using commercially available kits from BioDiagnostic Co. in Giza, Egypt, and a BioTek spectrophotometer in Highland, VT, U.S.A.
2.7. Enzyme-linked immunosorbent assays (ELISA) determination
α-fetoprotein (AFP), Nrf2, HO-1 (USCN Business Co., Ltd.), PKC, and ERK5 (MyBioSource, San Diego, CA, USA) protein concentrations were analyzed using the accessible ELISA kits in the market.
2.8. Western blotting
The concentrations of the expressed proteins of ADAMTS-4, MMP3, VEGF, and aggregan in hepatic samples were analyzed and calculated as previously mentioned [Citation22]. The total protein extracted was quantified using a protein assay commercially available from Bio-Rad Laboratories, Inc. Subsequently, the entire protein extract was separated by SDS-PAGE at 20 μg per lane. Primary antibodies (Sigma-Aldrich, Merck KGaA) (1:500) were incubated overnight with the membranes a 4˚C. 1:2000 β-actin antibody (Sigma-Aldrich; Merck KGaA) reprobed the membrane at room temperature in PBST reagent with 5% non-fat milk. Following primary incubation, membranes were incubated with HRP-conjugated sheep anti-rabbit secondary antibodies (1:5000). Protein bands were visualized using enhanced chemiluminescence. These data are expressed as the relative optical density using ImageJ software.
2.9. Quantitative real-time polymerase chain reaction (RT–PCR)
The procedure was conducted as described previously by our group [Citation23–25]. Briefly, total RNA was extracted using an RNeasy Mini kit (Qiagen, USA), and the concentration was evaluated with a Maxima® SYBR Green/Fluorescein Master Mix (Fermentas, USA). Then, 1 µg RNA was reverse transcribed into cDNA by a QuantiTect® Reverse Transcription Kit (Qiagen, USA). The expression levels of Nrf2, HO-1, ERK5, PKCδ, ADAMTS-4, aggrecans, MMP3, and VEGF mRNA in rat hepatic lysates were measured with Maxima® SYBR Green/Fluorescein qPCR Master Mix and Rotor-Gene Q (Qiagen, USA). Finally, for housekeeping and internal referencing, rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used. The gene-specific PCR primers used are summarized in . The results of the RT–PCR analysis were expressed as Cycle threshold (Ct) values. The PCR data sheet contains the Ct values of the gene of interest relative to the reference housekeeping gene (GAPDH). A control sample was employed to assess the gene expression of a particular gene. The Relative Quantification (RQ) of each target gene was calculated and standardized to the housekeeping gene using the delta-delta Ct (ΔΔCt) method. The RQ of each gene was determined using the formula 2-ΔΔCt.
Table 1. Primer Sequences for real-time PCR assay.
2.10. Statistical analysis
Mean ± SEM was used to show the values. Kolmogorov – Smirnov test was used to test the normality of the sample distribution and multiple comparisons. The Kaplan-Meier method was used to examine the significant difference in the survival percentage of the group of rat models. One-way ANOVA followed by the Bonferroni post hoc test was used to test the significant difference among groups. SPSS version 22 (IBM Corp.) was used for the statistical analysis, and a statistically significant difference was considered whenever p < 0.05.
3. Results
3.1. Effect of emodin on antioxidant biomarkers; Nrf2 and HO-1
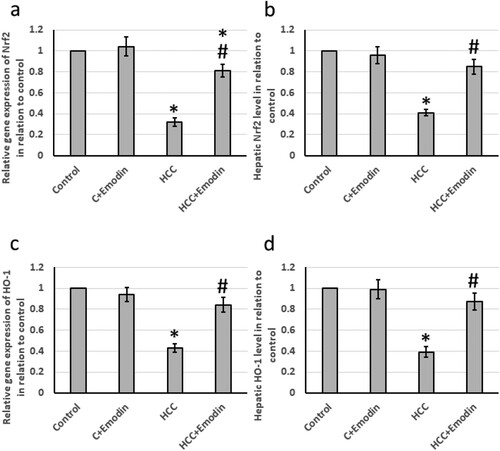
The HCC group showed significant downregulation of the mRNA and protein levels of both Nrf2 and HO-1. Treatment of rats with emodin significantly increased the gene and protein expression of Nrf2 and HO-1 in the HCC group without affecting the control group ().
Figure 2. Effect of oral administration of emodin (40 mg/kg, P.O) on both mRNA and protein levels of the antioxidant biomarkers; Nrf2 and HO-1. (a) The relative expression of the hepatic Nrf2 mRNA, (b) the relative hepatic NRF2 protein levels, (c) the relative expression of the hepatic HO-1 mRNA and (d) the relative hepatic HO-1 protein. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. C, control; HCC, hepatocellular carcinoma; HO-1; Heme oxigenase variant 1; Nrf2, nuclear factor erythroid 2-related factor 2.
3.2. Effect of emodin on the oxidative stress
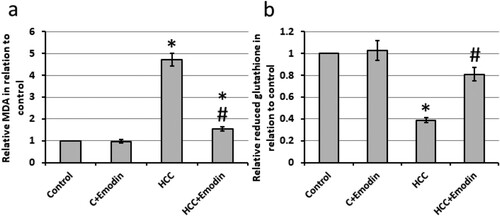
HCC rats exhibited a 4.71-fold increase in hepatic levels of MDA and a 61% reduction in the hepatic concentration of reduced glutathione compared to the control rats. Treatment with emodin reversed these effects in HCC rats without impacting the control rats (see ).
Figure 3. Effect of oral administration of emodin (40 mg/kg, P.O) on oxidative status of the hepatic cells. (a) The relative hepatic tissues of MDA. (b) The relative hepatic tissues of reduced glutathione. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. C, control; HCC, hepatocellular carcinoma; MDA; malondialdehyde.
3.3. Antitumor effects of emodin on rats
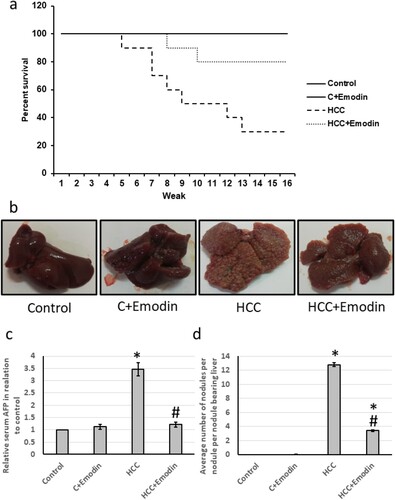
The survival percent of control rats was normally over 16 weeks with no extension for using emodin in the treated control group. The HCC group had a reduced survival percentage of about 70.63%. The HCC group showed a reduced percent survival to 4.7 weeks (about 29.37% of the survival rate of the control group). Emodin increased the percent survival percent to 8 weeks about a 1.7-fold increase of the survival rate of (the HCC group). Therefore, emodin extended the survival percentage of the HCC group by about 45%. In addition, the HCC group showed an average relative serum AFP level of about 3.5 times that of the control group. Meanwhile, emodin succeeded in significantly decreasing the upregulated serum AFP (about 71% reduction), restoring it to its normal level in the control group. HCC showed a significant increase in the number of hepatic nodules compared with the control group (about 12.4 times). The emodin-treated HCC group showed an average of about three nodules (about 23% of that number in the HCC group). So, emodin significantly reduced (about 77%) the hepatic nodules than the HCC group ().
Figure 4. Effect of oral administration of emodin (40 mg/kg, P.O) on the percent survival and hepatic nodules. (a) The percent of the survival of the four groups of the experiment over sixteen weeks. (b) Representative images of livers separated from different treated groups. (c) Relative serum cancer market AFP in tested groups. (d) The average number of hepatic nodules in groups. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. C, control; HCC, hepatocellular carcinoma; AFP, alpha Fetoprotein.
3.4. Effect of emodin on the histology of the liver tissue
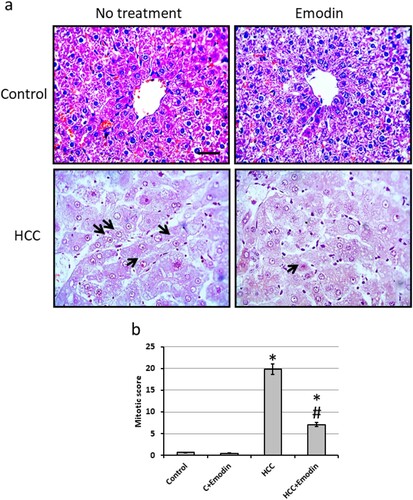
Microscopic pictures of liver sections from the control group stained with H&E show normally arranged hepatic cords around central veins. The liver section from the HCC bearing group shows well-differentiated HCC. Cells of HCC are polygonal with distinct cell membranes, an eosinophilic granular cytoplasm, and prominent nucleoli (black arrows). Sections from the HCC group treated with emodin showed improvement in the structure of hepatocytes. In addition, investigation of the mitotic score revealed a significant reduction in HCC rats treated with emodin as compared with the HCC group without affecting the control group ().
Figure 5. Effect of oral administration of emodin (40 mg/kg, P.O) on liver sections stained with Haematoxylin/eosin. (a) Representatives of microscopic pictures of liver sections stained with haematoxylin/eosin showing normally arranged hepatic cords around central veins. Liver section from HCC group show well-differentiated HCC. Cells of HCC are polygonal with distinct cell membranes, an eosinophilic granular cytoplasm and prominent nucleoli (black arrows). Sections from HCC group orally treated with 40 mg/kg emodin show improvement of the structure of hepatocytes. Scale bare represented 50 µm. (b) Mitotic score calculated using high field powers in 10 different areas of each rat. The results of the mitotic figure are presented as the mean ± SE. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. C, control; HCC, hepatocellular carcinoma.
3.5. Effect of emodin on both mRNA and protein levels of mitogenic activation biomarkers; PKCδ and ERK5
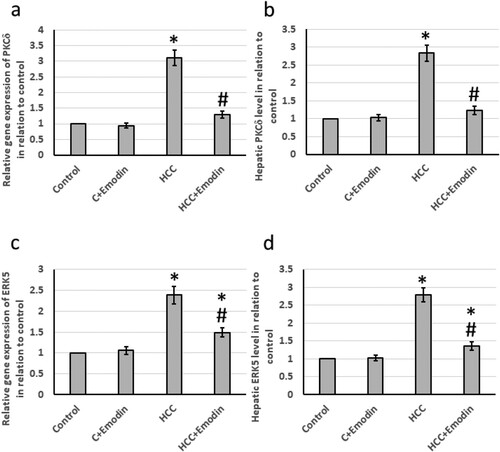
The HCC group showed significantly up-regulated both mRNA and protein levels of the hepatic PKCδ (3 and 2.75 times respectively) relative to the control group. Emodin succeeded in reducing the up-regulated levels for both the mRNA levels of PKCδ and its protein level (about 59.68% and 57.14%, respectively) relative to those in the HCC group. Moreover, the HCC group showed significant upregulated both mRNA and protein levels of the hepatic ERK5 (2.4 and 2.8 times, respectively) relative to the control group. Emodin reduced its elevated mRNA and protein levels (about 40% and 50% respectively) relative to those in the HCC group ().
Figure 6. Effect of oral administration of emodin (40 mg/kg, P.O) on both mRNA and protein levels of the mitogenic activation biomarkers; PKCδ and ERK5. (a) The relative expression of the hepatic PKCδ mRNA, (b) the relative hepatic PKCδ protein, (c) the relative expression of the hepatic ERK5 mRNA and (d) the relative hepatic ERK5 protein. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. C, control; ERK5, extracellular-signal-regulated kinase 5; HCC, hepatocellular carcinoma; PKCδ; Protein kinase C delta type.
3.6. Effect of emodin on the immunohistochemistry of ADAMTS4
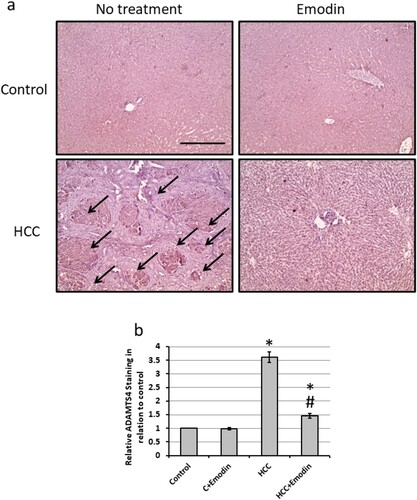
Immunohistochemistry of liver tissues showed a significantly higher level of ADAMTS4 in the HCC group than in the control group. Meanwhile, the emodin-treated HCC group was relatively similar to the control group ().
Figure 7. Effect of oral administration of emodin (40 mg/kg, P.O) on liver sections stained with anti-ADAMTS4 antibodies. (a) Representative images of hepatic tissues stained with anti-ADAMTS-4 antibodies in different treated groups showing increased staining in the HCC group, which was reduced by treatment with emodin. (b) The positive staining score was determined through immunohistochemistry in ten different fields. Black arrows indicated the areas of positive immune staining. Scale bar represents 100 μm.
3.7. Effect of emodin on the expression of tissue destruction biomarker
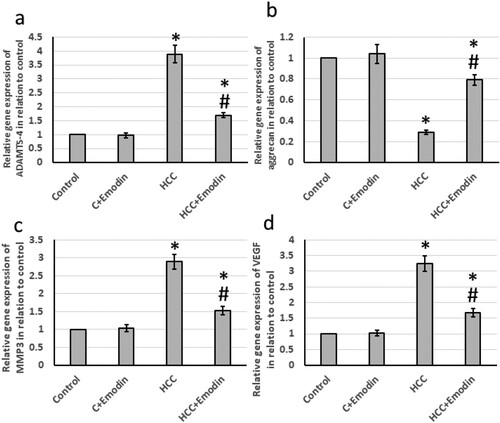
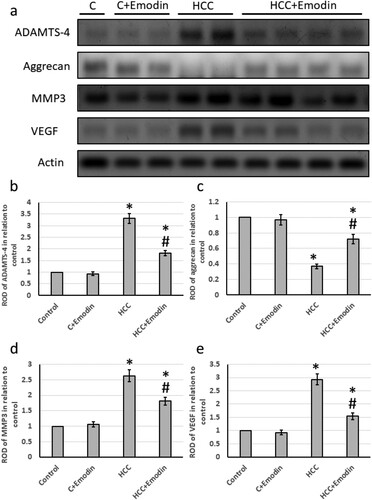
The HCC group showed significantly elevated levels of gene expression of ADAMTS4 (3.3 times), MMP3 (2.6 times), and VEGF (2.94 times) associated with a 62% reduction in the expression of aggrecan. Treatment of HCC with emodin reversed all these effects in HCC rats without affecting the control rats (). In addition, the HCC group exhibited significantly higher levels of protein expression of hepatic ADAMTS4 (3.9 times), MMP3 (2.8 times), and VEGF (3.3 times), along with a 70% decrease in the protein expression of aggrecans. Treatment of HCC with emodin reversed all these effects in HCC rats without affecting the control rats (see ).
Figure 8. Effect of oral administration of emodin (40 mg/kg, P.O) on mRNA expression of hepatic ADAMTS4, aggrecans, MMP3 and VEGF. (a) The relative expression of the hepatic ADAMTS-4 mRNA and (b) the relative expression of the hepatic aggrecans mRNA. (c) the relative expression of the hepatic MMP3 mRNA. (d) the relative expression of the hepatic VEGF mRNA. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. ADAMTS4, A disintegrin and metalloproteinase with thrombospondin motifs 4; C, control; HCC, hepatocellular carcinoma; MMP3, matrix metallopeptidase 3; VEGF, vascular endothelial growth factor.
4. Discussion
Anthraquinones are structural analogs of some natural compounds that have anticancer activity. Emodin possesses three fused rings with a structural composition and 3D configuration, allowing a double-directional oxidation–reduction reaction. Emodin was found to increase the mitochondrial membrane potential, up-regulated the NQO1 and HO-1 expression levels, and activate the Nrf2 pathway, which is the main role of anti-oxidative stress and an anti-apoptotic agent. It also has been reported that emodin decreased the phosphorylation process of Smad3 and NF-κB and reduced levels of some inflammatory factors [Citation26]. It has been confirmed that emodin inhibits processes of neoplasia at the stages of proliferation, invasion, and angiogenesis. For example, emodin inhibits cyclin D1/E, MAPK and MMP2-9 responsible for cancer angiogenesis and metastasis [Citation27]. In other studies, emodin reduced the viability and the motility of hepatic cancer cells in a time-dependent and dose-dependent manner [Citation28]. The molecular mechanism includes blocking the cell cycle evolution in the S – or G2/M phases through decreasing the expression of Bcl-2, cyclin-A, and CDK2 and through increasing of the expression of p53, p21, Bax, cyclin E, cleaved caspase-3, 8 and 9, and cleaved PARP [Citation29] Our findings confirmed the biological activity of emodin as an anticancer in vivo, however, through alteration of other subcellular biomarkers such as PKCδ, ADMAST4, and ERK5. These factors affect the tissue destruction and cellular invasion of cancer cells. Moreover, our histopathological investigations also showed a reduction in the number of hepatic nodules and the tumor marker AFP.
Figure 9. Effect of oral administration of emodin (40 mg/kg, P.O) on protein expression of hepatic ADAMTS4, aggrecans, MMP3 and VEGF. (a) Representative images of Western blot analysis of hepatic ADAMTS4, aggrecans MMP3 and VEGV. (b) ROD of Western blot analysis of ADAMTS4 in relation to control. (c) ROD of Western blot analysis of aggrecans in relation to control. (d) ROD of Western blot analysis of MMP3 in relation to control. (e) ROD of Western blot analysis of VEGF in relation to control. * Significant difference as compared with the control groups at p < 0.05. # Significant difference as compared with the HCC group at p < 0.05. ADAMTS4, A disintegrin and metalloproteinase with thrombospondin motifs 4; C, control; HCC, hepatocellular carcinoma; MMP3, matrix metallopeptidase 3; VEGF, vascular endothelial growth factor.
Oxidative stress plays a significant role in the development and progression of HCC by impacting cell growth, cell death, and the cell cycle. In addition, oxidative stress as indicated by the elevation in MDA and reduction in reduced glutathione can be harmful to various cells in the body, leading to the advancement of chronic liver disease [Citation30]. Interestingly, oxidative stress induces autophagy and activates stress response molecules such as Nrf2. The buildup of cytoplasmic Nrf2 prompts nuclear translocation and enhances the production of antioxidant and detoxification agents, enabling cells to mount a protective response against oxidative stress. Conversely, the deactivation of Nrf2 has been linked to the progression of HCC, including its metastasis [Citation31]. Furthermore, blocking Nrf2 has been demonstrated to significantly enhance the suppression of HCC induced by erastin and sorafenib [Citation32]. One of the downstream targets of Nrf2 is HO-1, an antioxidant protein. The overexpression of HO-1 has been implicated in the development and advancement of certain cancers, and the activation of HO-1 is believed to confer cellular protection [Citation33]. However, we found that treatment of HCC rats with emodin increased the expression of Nrf2 and HO-1, associated with elevation in the hepatic MDA levels and reduction in the hepatic reduced glutathione levels. Emodin was reported previously to activate Nrf2 and HO-1 in intestinal mucosal layer damage [Citation34], acetaminophen-induced hepatotoxicity [Citation34], and LPS-induced neuroinflammation [Citation35]. However, this is the first study to illustrate the ability of emodin to increase the expression of Nrf2 and HO-1 in HCC.
ADAMSTs are a subfamily of proteins called A disintegrin and metalloproteinases, which are involved in numerous subcellular pathways responsible for many events related to cell adhesion, cellular migration, cellular signaling, membrane protein shedding, and proteolysis [Citation36]. They have been highly expressed in tumor cells. Generally, it has been proved that they have critical rules in cellular growth and integrin function [Citation37]. Owing to their ability to degrade aggrecan, ADAMTS4, and ADAMTS5 subfamily members can be named aggrecanase-1 and aggrecanase-2, respectively [Citation38]. They participate in the deterioration of articular cartilage and joint degeneration [Citation39]. Therefore, they have to be tightly controlled at different levels. On the gene expression level, ADAMTS4 can be regulated by the transcription factors foxm1, TNF-α, TGF-β, and reactive oxygen species [Citation40]. They are also controlled through the presence of deactivators such as TIMP-3 [Citation41] or through coupling with fibronectin [Citation42]. We found that emodin can reduce the ADAMTS4 on both the mRNA and protein expressions in HCC rat without affecting the control group.
MMPs are proteolytic enzymes which are playing a crucial role in the modification of the micro-environment of the tissues for cancer prognosis such as the decomposition of the extracellular matrix between cells [Citation43]. Thus, up-regulation of the MMPs has been reported to be related to cancer. MMP3 has been proven to play an important role in tumor growth and dysregulated angiogenesis [Citation44]. MMP3 releases soluble E-cadherin which interferes with the cell-to-cell interaction system. Besides, the membrane type 1-MMP is able to transform pro-αv, pro-α3, and pro-α5 integrins into active forms that play an active role with αvβ3 and α2β1 integrins to mediate the signaling and migration of some cancers such as in breast carcinoma [Citation45]. We found that emodin can reduce MPP3 on both the mRNA and protein expression in HCC rats without affecting the control rats.
Protein kinase C (PKC) is a family of enzymes that are responsible for the phosphorylation of serine and threonine residues [Citation46]. They are also part of myriads of cell functions, including mitogenic signaling, cytoskeleton rearrangement, glucose metabolism, differentiation, and regulation of cell survival and apoptosis [Citation47]. Most of the previous cellular functions are part of the etiology and the prognosis of human diseases. There are at least eleven structurally and functionally related to the PKC isozymes which are closely like them, however, they are more or less different in their tissue distribution, subcellular localization, and substrate specificity [Citation48]. Among these proteins, PKCδ contains a different C-terminal kinase domain and N-terminal regulatory domains than other subfamily members [Citation49]. The functional C1A and C1B domains of PKCδ bind to the phosphatidyl derivative of serine amino acid and a C2 domain, which binds anionic lipids and cannot bind to Calcium ion [Citation50]. Up-regulation of PKCδ is directly associated with inflammation and cancer. It is worth mentioning that it is the rate-limiting step in the production of the matrix degrades [Citation51]. In our research, we report that emodin successfully reduced the up-regulated levels of mRNA and protein of PKCδ in the HCC rat model.
Tumor angiogenesis, the process of new blood vessel formation that supplies tumors with nutrients and oxygen, is primarily driven by VEGF. While VEGF is mainly produced in endothelial cells, it is commonly observed to be excessively expressed in various tumor types [Citation52]. MMP-9 is responsible for releasing a significant amount of stored VEGF, thereby initiating the development of new blood vessels. VEGF acts as a potent stimulant for the in vivo proliferation of tumor cells and is considered essential for promoting the growth and multiplication of endothelial cells in the majority of metastasis patients [Citation53]. Therefore, VEGF plays a critical role in tumor angiogenesis, and its overexpression is frequently associated with tumor progression and metastasis. We found that HCC increased the expression of HCC and that emodin significantly reduced the expression of VEGF in HCC rats, without affecting the control rats. Emodin was not reported previously to reduce the expression of VEGF in HCC.
5. Conclusion
Our findings illustrated that emodin downregulated PKCδ, ERK5, ADAMTS-4, MMP3, and VGEF associated with overexpression of Nrf2, HO-1, and aggrecan in rat models of HCC. This indicates that emodin can control HCC metastasis and proliferation. Histopathological pictures for the liver sections emphasized the promising usage of emodin to reduce mitotic score and fibrotic area, leading to amelioration of HCC. Emodin reduced the hepatic nodules by 75% and improved the structure of the hepatocytes compared to the HCC group. It adds the promising activity of using emodin in one of the highest-killing cancers through different mechanisms, such as enhancing antioxidant activity and blocking proliferation, invasion, and angiogenesis pathways.
Authors’ contributions
HMH, AH, OB and MMHA were responsible for performing the biochemical analysis. AA and RA were responsible for performing the animal experiments. OB and MMHA performed the pathological and immunohistochemistry analysis. HMH, AH and OB performed the statistical analysis. MMHA came up with the concept for the study and supervised the work. HMH, AH, AA and RA helped develop and design the present study. All authors contributed to the writing of the manuscript and approved the final version.
Availability of data
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical approval
The Research Ethics Committee of the Faculty of Pharmacy at Delta University for Science and Technology approved all animal procedures under reference number FPDU8/2023.
Suppl_2.pptx
Download MS Power Point (418.8 KB)Suppl 1.tif
Download TIFF Image (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol. 2021 Jul;12(Suppl 2):S361–S373.
- Elewa MA, Al-Gayyar MM, Schaalan MF, et al. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin Exp Metastasis. 2015 Jun;32(5):479–493. doi:10.1007/s10585-015-9721-6
- Sas Z, Cendrowicz E, Weinhauser I, et al. Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for New treatment options. Int J Mol Sci. 2022 Mar 29;23(7).
- Manzar GS, De BS, Abana CO, et al. Outcomes and toxicities of modern combined modality therapy with atezolizumab plus bevacizumab and radiation therapy for hepatocellular carcinoma. Cancers (Basel). 2022 Apr 9;14(8).
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019 Apr 15;144(8):1941–1953. doi:10.1002/ijc.31937
- Kelwick R, Desanlis I, Wheeler GN, et al. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015 May 30;16:113, doi:10.1186/s13059-015-0676-3
- Novak R, Hrkac S, Salai G, et al. The role of ADAMTS-4 in atherosclerosis and vessel wall abnormalities. J Vasc Res. 2022;59(2):69–77. doi:10.1159/000521498
- Rienks M, Barallobre-Barreiro J, Mayr M. The emerging role of the ADAMTS family in vascular diseases. Circ Res. 2018 Dec 7;123(12):1279–1281. doi:10.1161/CIRCRESAHA.118.313737
- Stanton H, Melrose J, Little CB, et al. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta. 2011 Dec;1812(12):1616–1629. doi:10.1016/j.bbadis.2011.08.009
- Kuznik-Trocha K, Winsz-Szczotka K, Lachor-Motyka I, et al. The effects of TNF-alpha inhibition on the metabolism of cartilage: relationship between KS, HA, HAPLN1 and ADAMTS4, ADAMTS5, TOS and TGF-beta1 Plasma Concentrations in Patients with Juvenile Idiopathic Arthritis. J Clin Med. 2022 Apr 4;11(7).
- Fontanil T, Mohamedi Y, Cobo T, et al. Novel associations within the tumor microenvironment: fibulins meet ADAMTSs. Front Oncol. 2019;9:796, doi:10.3389/fonc.2019.00796
- Dong X, Zeng Y, Liu Y, et al. Aloe-emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytother Res. 2020 Feb;34(2):270–281. doi:10.1002/ptr.6532
- Mitra S, Anjum J, Muni M, et al. Exploring the journey of emodin as a potential neuroprotective agent: novel therapeutic insights with molecular mechanism of action. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2022 May;149:112877, doi:10.1016/j.biopha.2022.112877
- Stompor-Goracy M. The health benefits of emodin, a natural anthraquinone derived from rhubarb-A summary update. Int J Mol Sci. 2021 Sep 1;22(17).
- Alshehri SA, Almarwani WA, Albalawi AZ, et al. Role of arctiin in fibrosis and apoptosis in experimentally induced hepatocellular carcinoma in rats. Cureus. 2024 Jan;16(1):e51997.
- Albalawi AZ, Alatawi AS, Al-Atwi SM, et al. Echinacoside ameliorates hepatic fibrosis and tumor invasion in rats with thioacetamide-induced hepatocellular carcinoma. Biomol Biomed. 2024 Mar 10.
- Elewa MAF, Eldehna WM, Hamdan AME, et al. WRH-2412 alleviates the progression of hepatocellular carcinoma through regulation of TGF-β/β-catenin/α-SMA pathway. J Enzyme Inhib Med Chem. 2023 Dec;38(1):2185761, doi:10.1080/14756366.2023.2185761
- El-Far YM, Khodir AE, Noor AO, et al. Selective cytotoxic activity and protective effects of sodium ascorbate against hepatocellular carcinoma through its effect on oxidative stress and apoptosis in vivo and in vitro. Redox Report: Communications in Free Radical Research. 2020 Dec;25(1):17–25.
- Bayomi HS, Elsherbiny NM, El-Gayar AM, et al. Evaluation of renal protective effects of inhibiting TGF-β type I receptor in a cisplatin-induced nephrotoxicity model. Eur Cytokine Netw. 2013 Nov 1;24(4):139–147. doi:10.1684/ecn.2014.0344
- Albalawi GA, Albalawi MZ, Alsubaie KT, et al. Curative effects of crocin in ulcerative colitis via modulating apoptosis and inflammation. Int Immunopharmacol. 2023 May;118:110138, doi:10.1016/j.intimp.2023.110138
- Elsherbiny NM, Al-Gayyar MM, Abd El Galil KH. Nephroprotective role of dipyridamole in diabetic nephropathy: effect on inflammation and apoptosis. Life Sci 2015 Dec 15;143:8–17. doi:10.1016/j.lfs.2015.10.026
- El-Far YM, Khodir AE, Emarah ZA, et al. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Report: Communications in Free Radical Research. 2022 Dec;27(1):9–20.
- Alattar A, Alshaman R, Al-Gayyar MMH. Therapeutic effects of sulforaphane in ulcerative colitis: effect on antioxidant activity, mitochondrial biogenesis and DNA polymerization. Redox Report: Communications in Free Radical Research. 2022 Dec;27(1):128–138.
- Bagalagel A, Diri R, Noor A, et al. Curative effects of fucoidan on acetic acid induced ulcerative colitis in rats via modulating aryl hydrocarbon receptor and phosphodiesterase-4. BMC Complement Med Ther. 2022 Jul 23;22(1):196, doi:10.1186/s12906-022-03680-4
- Alharbi KM, Alshehri SA, Almarwani WA, et al. Effects of cycloastragenol on Alzheimer's disease in rats by reducing oxidative stress, inflammation, and apoptosis. Curr Alzheimer Res. 2024 May 17.
- Pang X, Shao L, Nie X, et al. Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int Immunopharmacol. 2021 Feb;91:107277, doi:10.1016/j.intimp.2020.107277
- Wang Z, Chen H, Chen J, et al. Emodin sensitizes human pancreatic cancer cells to EGFR inhibitor through suppressing Stat3 signaling pathway. Cancer Manag Res. 2019;11:8463–8473. doi:10.2147/CMAR.S221877
- Gu J, Cui CF, Yang L, et al. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Oncol Res 2019 Feb 5;27(2):193–202. doi:10.3727/096504018X15150662230295
- Dong X, Ni B, Fu J, et al. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspasedependent pathway. Oncol Rep. 2018 Oct;40(4):1985–1993.
- Darweish MM, Abbas A, Ebrahim MA, et al. Chemopreventive and hepatoprotective effects of Epigallocatechin-gallate against hepatocellular carcinoma: role of heparan sulfate proteoglycans pathway. J Pharm Pharmacol. 2014 Jul;66(7):1032–1045. doi:10.1111/jphp.12229
- Kawaguchi T, Nakano D, Koga H, et al. Effects of a DPP4 inhibitor on progression of NASH-related HCC and the p62/ Keap1/Nrf2-pentose phosphate pathway in a mouse model. Liver Cancer. 2019 Oct;8(5):359–372. doi:10.1159/000491763
- Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016 Jan;63(1):173–184. doi:10.1002/hep.28251
- Yeh CN, Wu RC, Cheng CT, et al. HO-1 is a favorable prognostic factor for HBV-HCC patients who underwent hepatectomy. Cancer Manag Res. 2018;10:6049–6059. doi:10.2147/CMAR.S186931
- Shang L, Liu Y, Li J, et al. Emodin protects sepsis associated damage to the intestinal mucosal barrier through the VDR/ Nrf2 /HO-1 pathway. Front Pharmacol. 2021;12:724511, doi:10.3389/fphar.2021.724511
- Park SY, Jin ML, Ko MJ, et al. Anti-neuroinflammatory effect of emodin in LPS-stimulated microglia: involvement of AMPK/Nrf2 activation. Neurochem Res. 2016 Nov;41(11):2981–2992. doi:10.1007/s11064-016-2018-6
- Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007 May;98(5):621–628. doi:10.1111/j.1349-7006.2007.00434.x
- Lukaszewicz-Zajac M, Paczek S, Mroczko B. A disintegrin and metalloproteinase (ADAM) family-novel biomarkers of selected gastrointestinal (GI) Malignancies? Cancers (Basel). 2022 May 6;14(9).
- Xiong X, Liu L, Xu F, et al. Feprazone ameliorates TNF-α-induced loss of aggrecan via inhibition of the SOX-4/ADAMTS-5 signaling pathway. ACS Omega. 2021 Mar 23;6(11):7638–7645. doi:10.1021/acsomega.0c06212
- Santamaria S. ADAMTS-5: a difficult teenager turning 20. Int J Exp Pathol. 2020 Feb;101(1-2):4–20. doi:10.1111/iep.12344
- Kim IM, Ackerson T, Ramakrishna S, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006 Feb 15;66(4):2153–2161. doi:10.1158/0008-5472.CAN-05-3003
- Casagrande V, Iuliani G, Menini S, et al. Restoration of renal TIMP3 levels via genetics and pharmacological approach prevents experimental diabetic nephropathy. Clin Transl Med. 2021 Feb;11(2):e305, doi:10.1002/ctm2.305
- Hashimoto G, Shimoda M, Okada Y. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J Biol Chem. 2004 Jul 30;279(31):32483–32491. doi:10.1074/jbc.M314216200
- Michalczyk K, Cymbaluk-Ploska A. Metalloproteinases in Endometrial Cancer-Are They Worth Measuring? Int J Mol Sci. 2021 Nov 19;22(22).
- Roy R, Morad G, Jedinak A, et al. Metalloproteinases and their roles in human cancer. Anat Rec (Hoboken). 2020 Jun;303(6):1557–1572. doi:10.1002/ar.24188
- Roy R, Yang J, Moses MA. Matrix metalloproteinases As novel biomarker s and potential therapeutic targets in human cancer. J Clin Oncol. 2009 Nov 1;27(31):5287–5297. doi:10.1200/JCO.2009.23.5556
- Umachandran S, Mohamed W, Jayaraman M, et al. A PKC that controls polyphosphate levels, pinocytosis and exocytosis, regulates stationary phase onset in Dictyostelium. J Cell Sci. 2022 May 1;135(9).
- Tyagi K, Roy A. Evaluating the current status of protein kinase C (PKC)-protein kinase D (PKD) signalling axis as a novel therapeutic target in ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021 Jan;1875(1):188496, doi:10.1016/j.bbcan.2020.188496
- Cohen P. Protein kinases - the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002 Apr;1(4):309–315. doi:10.1038/nrd773
- Parihar SP, Ozturk M, Marakalala MJ, et al. Protein kinase C-delta (PKCδ), a marker of inflammation and tuberculosis disease progression in humans, is important for optimal macrophage killing effector functions and survival in mice. Mucosal Immunol. 2018 Mar;11(2):496–511. doi:10.1038/mi.2017.68
- Wang J, Sun L, Nie Y, et al. Protein kinase C delta (PKCdelta) attenuates bleomycin induced pulmonary fibrosis via inhibiting NF-kappaB signaling pathway. Front Physiol. 2020;11:367.
- Ellman MB, Kim JS, An HS, et al. The pathophysiologic role of the protein kinase Cδ pathway in the intervertebral discs of rabbits and mice: in vitro, ex vivo, and in vivo studies. Arthritis Rheum. 2012 Jun;64(6):1950–1959. doi:10.1002/art.34337
- Teleanu RI, Chircov C, Grumezescu AM, et al. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2019 Dec 29;9(1).
- Alfair BM, Jabarti AA, Albalawi SS, et al. Arctiin inhibits inflammation, fibrosis, and tumor cell migration in rats With ehrlich solid carcinoma. Cureus. 2023 Sep;15(9):e44987.