ABSTRACT
Introduction
Interleukin-17A (IL-17A) is a well-established pro-inflammatory cytokine, which plays a pivotal role in immune and autoimmune diseases including psoriasis, asthma, psoriatic arthritis, and rheumatoid arthritis. Three currently approved monoclonal antibodies (mAbs) are in clinical practice for the treatment of multiple immune diseases. However, the disadvantages of the mAbs, such as non-oral administration, poor tissue penetration, lacking blood-brain barrier penetration, often long half-life times, narrow its application. Thus, intensive research is performed to discover potent small molecules, peptides, and macrocycles targeting the IL-17A/IL-17 RA protein–protein interaction (PPI) to modulate immune responses as an attractive approach for immunotherapy.
Areas covered
Small molecules, macrocycles, and peptides targeting IL-17A/IL-17RA PPI from 2013 to 2021.
Expert opinion
The rapid increase in the identification of small-molecule inhibitors of IL-17 should translate into a supplement of current biotherapeutics with mAbs. Potential advantages of small molecules over mAbs show room for clinical treatment improvement and new indication areas . An increasing number of patents and articles are recently published on small-molecule immunomodulators (SMIMs). Two compounds from Lilly and Leo Pharma are currently investigated in early clinical trials, followed by a Dice molecule. The outcome of these trials will influence future development of IL-17 inhibitors for treatment of inflammation-related diseases.
1. Introduction
The family of interleukin-17 (IL-17) cytokines, comprising IL-17A through IL-17 F, promotes the maintenance of both adaptive and innate immunity. The released cytokines act through their membrane-bound IL-17 receptor (IL-17R), a family of five receptors (IL-17RA through IL-17RE), and activating the IL-17 signal pathway [Citation1,Citation2]. Dysregulation expression of IL-17 may contribute to inflammatory and autoimmune diseases such as psoriasis, psoriatic arthritis, rheumatoid arthritis, and multiple sclerosis () [Citation3]. As such, they are highly interesting new therapeutic targets for inflammatory diseases [Citation4].
Figure 1. Effects of IL-17 under different disease conditions and IL-17 receptor mediated signaling.
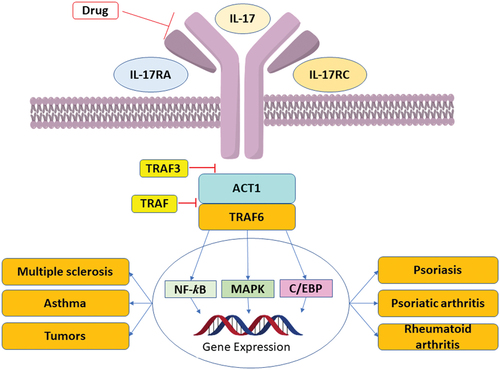
Interleukin-17A (IL-17A) is the best investigated IL17 family member. It is well established as a pro-inflammatory cytokine, which plays a pivotal role in immune and autoimmune related diseases including psoriasis [Citation5], asthma [Citation6], psoriatic arthritis [Citation7], and rheumatoid arthritis [Citation8]. IL-17A forms homodimers or heterodimers with IL-17A or IL-17F and is a major cytokine mainly secreted from Th17 cells. It signals through its membrane-bound receptors, IL-17RA and IL-17RC, and modulates IL-17A signaling pathway and triggers multiple inflammatory and immune responses. Thus, IL-17A has emerged as a major topic of interest for treating inflammatory-associated diseases.
Antagonizing IL-17A/IL-17RA protein–protein interaction (PPI) was hypothesized to reduce overexaggerated inflammation in autoimmune diseases. There are several ways to block IL-17A signaling by targeting IL-17A proteins or receptors. Clinically, three monoclonal antibodies (mAbs) are already approved for different immunological diseases, secukinumab and ixekizumab target IL-17A while brodalumab targets IL-17RA () [Citation9]. Numerous clinical trials of anti-IL-17A and IL-17RA antibodies are currently in progress. IL-17A/IL-17RA directed biotherapeutics have achieved impressive successes in durable clinical treatment
Table 1. IL17 directed mAbs on the market and small molecules in clinical trials.
of psoriasis, asthma, psoriatic arthritis, and rheumatoid arthritis. However, IL17A has also major implications in central nervous system neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and glaucoma [Citation10,Citation11]. However, mAbs have limited applications due to non-oral administration, poor tissue penetration, lacking blood-brain barrier penetration, often long half-life times, high cost-of-good, and most importantly being applicable only to extracellular targets [Citation12]. For these reasons, the development of small-molecule modulators targeting IL-17A/IL-17RA is an emerging area, which potentially could considerably widening current indication areas. Small molecules, generally have a good tissue penetration with potentially higher efficacy and a tunable half-life time, and are orally bioavailable facilitating patient treatment. Herein we summarized patents and literature of non-mAb IL-17A inhibitors from 2013 to 2021.
2. Structural information
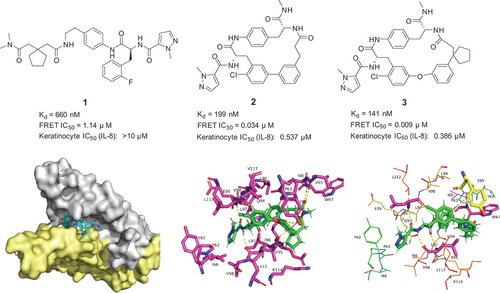
The co-crystal structure of interleukin 17A in complex with IL-17RA receptor has been determined for the first time by Pfizer scientists Liu and colleagues in 2013 [Citation13]. The mechanism of IL-17 receptors recognition is detailed in the paper. This complex shows the large buried surface area of IL-17A – IL-17RA of ~2000 Å2. The overall interface between the two proteins is large, flat, and featureless, without deep binding pockets, resulting in numerous additive but weak polar and hydrophobic interactions and making the interface likely a challenging target for small molecules. Subsequently, Alfonso and his colleagues at Eli Lilly showed that the β-hairpin pocket is the binding site for Ensemble macrocycles through a combination of computational methods and hydrogen/deuterium exchange mass spectrometry (HDX-MS) experiments [Citation14]. Based on further understanding of these studies, Pfizer scientists Liu and his colleagues firstly disclosed the co-crystal structures of interleukin 17A in complex with a small molecule and macrocycles in 2016 [Citation15]. Inspired by the previous findings that an anti-IL-17A Fab stabilized the complex of the cytokine with a high-affinity peptide antagonist (HAP) [Citation16], and based on disclosed inhibitors [Citation16–18] exemplified by compound 1, the first co-crystal structure of compound 1 with IL17A was achieved and shows that the binding site is a U shape pocket. Subsequently, Pfizer researchers designed the potent macrocycles 2 and 3 and elucidated their high-resolution co-crystal structure with IL-17A as shown in . For example, macrocycle 3 shows multiple interactions with its dimeric receptor, including hydrogen bonds (L97 and W67), hydrophobic interactions (main pocket: Y62, P63, W67, L97, and L112; subpocket: R114, E95, and L97) and stacking interactions (Y52 and L97) of the aromatic components of the macrocycle (). Notably, the atoms of the spirocyclopentyl moiety are important activity elements in all active macrocycle series, which can be rationalized by the shape and electrostatic complementarity with the Lys114, Leu97 pocket. Simultaneously in surface plasmon resonance (SPR) and fluorescence resonance energy transfer (FRET) experiments, compound 2 and 3 not only showed desirable activities (SPR Kd < 200 nM; FRET IC50 < 35 nM) but also showed significant specificity for IL-17 F or IL-17RA, showing no measurable binding to IL-17 F or IL-17RA at concentrations up to 13.3 μM. Moreover, these compounds potently modulated IL-17A-stimulated production of the pro-inflammatory cytokine IL-8 (IC50 < 540 nM) in the psoriasis-relevant keratinocyte cellular assay. Furthermore, compound 3 did not inhibit the baseline IL-8 production of keratinocytes stimulated by TNF-α alone.
3. Patent review
3.1. Ensemble therapeutics corporation
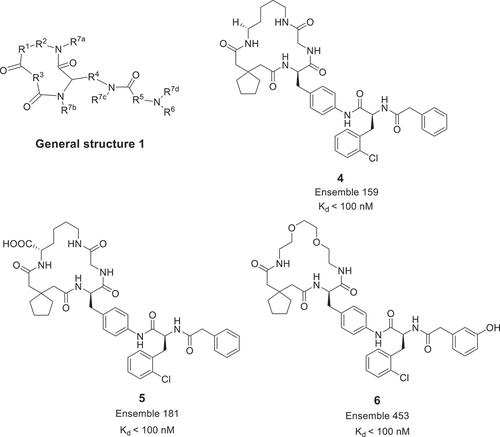
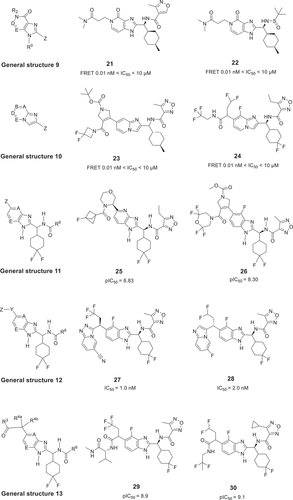
Two patents to describe chemical matter inhibiting IL-17 has been disclosed by Ensemble Therapeutics Corporation in 2013 and 2015. Using DNA-encoded library (DEL) synthesis technology, a plentiful macrocycle library was developed for screening IL-17 inhibitors. Most macrocycles with general structure 1 () are claimed to have excellent binding to IL-17A [Citation17,Citation18]. These macrocycles mostly use –CH2-(1,4-phenylene)– as a linker to connect the large cyclic amide ring and the aromatic motif. For example, compound 4 and its derivatives 5 and 6 were measured through enzyme-linked immunosorbent assay (ELISA), SPR assay, HT-
29 cell-based functional assay and a rheumatoid arthritis synovial fibroblast (RASF) cell assay. (). Notably, macrocycle 4, 5, and 6 were shown to bind to IL-17A with very good binding affinities Kd < 100 nM. Furthermore, the best compounds of these macrocycles have IC50 values of less than 1.0 μM in the HT-29 cell assay.
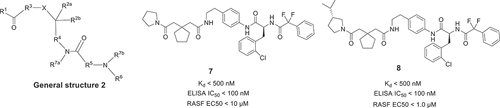
Workers from Ensemble Therapeutics Corporation also claimed linear peptides with general structure 2 () as modulator of IL-17/IL-17RA pathway in 2014 [Citation19]. The inhibitory activity of exemplary compounds 7 and 8 was assessed by SPR and ELISA, and the Kd and the IC50 values of them were below 500 nM and below 100 nM, respectively. Interestingly, in the RASF cell assay, compound 8 showed significant improvement with EC50 < 1.0 μΜ.
3.2. UCB Biopharma Sprl
Based on the better understanding of intracellular signaling pathways and new hit finding technologies, especially the co-crystal structures of IL-17A and small molecules published by Pfizer scientists, the discovery of modulators of IL-17A is recently making major progress.
In the last 4 years, scientists at UCB Biopharma Sprl company have published 13 patent applications describing small-molecule IL-17A inhibitors. These compounds are claimed to be useful in modulating the human IL-17A activity and also be potentially used in combination with conventional biological therapies to treat and/or prevent various human ailments, such as inflammatory and autoimmune disorders.
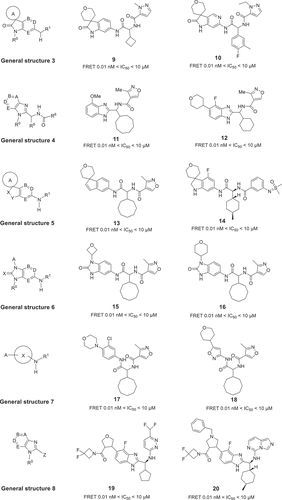
The first patent, disclosed in 2018, describes a series of spirocyclic 2-oxoindoline derivatives with general structure 3 [Citation20] that inhibit the IL-17/IL-17RA protein–protein interaction (PPI). Most spirocyclic oxoindolines were claimed, with a cycloalkyl ring A likely pointed toward the hydrophobic pocket, fused aromatic ring acting as the proposed binder and the 6-substituted amide group likely directed toward the inside pocket forming hydrogen bonds. Many of these compounds, tested by FRET assay and HDF (human dermal fibroblast) cell-line assay, show IC50 values in the range of 0.01 nM–10 μM (). Subsequently, the second patent was reported by UCB Biopharma Sprl company chemists in 2019, including a series of substituted fused imidazole derivatives with general structure 4 () [Citation21]. An imidazole rings was used as an amide bond mimic, fused with the phenyl ring. Furthermore, the proposed substituents at R5 for these compounds are likely conformation-preserving alkyl rings. The activities were measured by FRET and HDF, and exhibit IC50 values in the range of about 0.01 nM to about 10 μM, such as 11 and 12.
Then, another six patents were disclosed in 2020 by UCB Biopharma Sprl researchers. Some of these compounds have very similar core scaffolds and also showed potent disruption between the IL-17A and soluble IL-17RA. General structures 5 [Citation22] and 6 [Citation23] are complementary to general structure 3, as shown in . The spirocyclic indolines were modified to obtain the general structure 5 of the spirocyclic indane analogs, and some representative examples such as compounds 13 and 14 showed desirable activities in the range of 0.01 nM to 10 μM as tested by FRET and HDF assays. Another general structure 6 of benzimidazol-2-one derivatives is formed by modifying general structure 3 by replacing the carbon in the fused 5-membered ring with nitrogen. This change not only expands the diversity of patents but also preserves the activity of compounds that bind to IL-17, such as example compounds 15 and 16. The functionalized amine derivatives with general structure 7 [Citation24], as shown in , were designed as the promising antagonists. These amines with large alkyl rings inhibited the IL-17 and IL-17RA PPI with IC50 values between 0.01 nM and 10 μM (example 18 and 19). A series of substituted fused bicyclic imidazole derivatives with general structure 8 [Citation25] as shown in , were modified from general structure 4. Chemists payed more attention to the substitution of imidazole fused rings. For example, a substituted aliphatic ring was introduced as a substituent at the 5-position of benzimidazole. These compounds also modulated the IL-17/IL-17RA PPI with remarkable activities, for example compound 19 and 20 with IC50 values in the range of 0.1 nM–10 μM. Another series of fused bicyclic imidazole derivatives with general structure 9 () were also disclosed with another patent in 2020, including 4 H-imidazo[4,5-c]pyridin-4-one derivatives and analogues [Citation26]. According to the FRET and HDF measurements, these novel inhibitors also were potent modulating the human IL-17A activity, for example compounds 21 and 22 gave IC50 values in the range of 0.1 nM–10 μM. The imidazopyridine derivatives with general structure 10 () were the last patent disclosed by UCB Biopharma Sprl chemists in 2020 [Citation27]. This imidazopyridine scaffold is completely different from previously published fused imidazole cores. Importantly, it is not only expanding the molecular diversity but also keeps the activity competitive, for instance, the example compounds 23 and 24 with IC50 values from 0.1 nM to 10 μM.
With more detailed study of IL-17A, another four patents were disclosed by the UCB Biopharma Sprl scientists in 2021. All compounds of these patents are difluorocyclohexyl derivatives with general structure 11 [Citation28,Citation29], 12 [Citation30], 13 [Citation31] as shown in . These substituted difluorocyclohexyl compounds, derived from fused imidazole modulators, potently inhibit the ability of IL-17A to bind IL-17RA, as assessed by FRET and HDF assays. For example, these heterocyclic compounds with general structure 11 exhibit a pIC50 (pIC50 equals -log10[IC50], in which IC50 is expressed as a molar concentration, higher pIC50 figure means more active compound) value of 5.0 or more, such as the pIC50 values of the example 25 and 26 are 8.83 and 8.30, respectively. Based on a difluorocyclohexyl core as a hinge-binding motif, compounds with general structure 12, using the imidazole, triazole, tetrazole, and triazol[4,3-a]pyridine as functional groups, effectively inhibited the interaction of IL-17A and IL-17RA. For example, compounds 27 and 28 have IC50 values 1.0 nM and 2.0 nM, respectively. Interestingly, the best compounds with general structure 13 exhibit higher pIC50 than the compounds with the general structure 11, such as compounds 29 and 30 have pIC50 values of 8.9 and 9.1, respectively.
3.3. Hitgen Inc
Over the last 3 years, the Chinese biotech company Hitgen Inc. has released many details of its research progress in discovering IL-17A modulators, especially in 2021, disclosing 10 patents. All of these reported inhibitors focus on the treatment of IL-17A mediated disease, including inflammation, autoimmune diseases, infectious diseases, cancer, and precancerous syndrome.
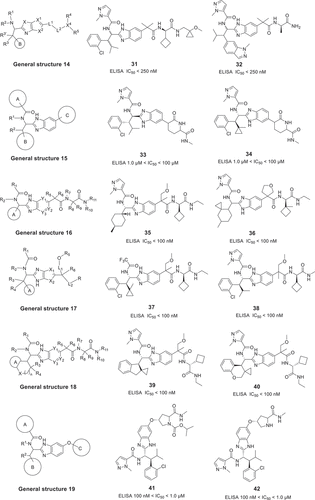
In these disclosed patents, most scaffolds appear to have a common imidazole core fused with a 5-substituted 6-membered ring. There are two types of core scaffolds in these published patents. All of these modulators were measured by ELISA assays and exhibited promising activity. Most modulators of the general structures 14 [Citation32], 15 [Citation33], 16 [Citation34], 17 [Citation35], 18 [Citation36], 19 [Citation37], 20 [Citation38], 21 [Citation39] and 22 [Citation40] were claimed with the imidazole core fused to a 6-membered ring as shown in .
In 2019, Hitgen Inc chemists disclosed the imidazole derivatives with general structure 14 that inhibit IL-17A/IL-17RA PPI with exemplary promising IC50 values less than 250 nM. Subsequently, a class of ((methyl)pyrazolecarboxamido)methylbenzimidazoles with general structure 15 was disclosed by Hitgen Inc in 2021. These compounds (examples 33, 34) are characterized with the IC50 values in the range of 1.0–100 μM as immunomodulators, which are useful for the inhibiting IL-17A. A series of (benzimidazolyl)(cyclobutylmethyl)acetamides with general structure 16 was reported by researchers from Hitgen Inc as shown in . These compounds are more potent IL-17A/IL-17RA inhibitors (compounds 35, 36 IC50 < 100 nM) compared to their previously reported inhibitors. The general structure 17 for one series of these compounds was also disclosed by Hitgen Inc as immunomodulator and used for treating IL-17A-mediated diseases. Some representative examples with IC50 values of less than 100 nM are shown in . Another class of ((methyl)pyrazolecarboxamido)methylbenzimidazoles with general structure 18 was disclosed by Hitgen Inc binding to IL-17A as promising inhibitors. Potentially to lock in the bound conformation, a cyclization strategy between the ring A and the R3 was used among compounds of general structure 17 to form the derivatives of general structure 18 as shown in . The best compounds with novel structure also have IC50 values below 100 nM for targeting IL-17A (example compounds 39 and 40). Chemists at Hitgen Inc. reported a class of (benzimidazole)oxypyrrolidines with general structure 19 in 2021 as the modulators for IL-17A related-disease. The claimed compounds 41 and 42 of general structures 19 showed activity (IC50) in the range of 0.01–1.0 μM. In order to lock the sidechain conformation, a ring is introduced at the carbon between the phenyl ring and the amide to form the derivatives of general structures 20 as shown in . Compounds with general structures 21 and 22, directed used hetero ring to lock the conformation, are also potent inhibitors for IL-17A/IL-17RA PPI, exemplary promising compounds 45 and 47 exhibit IC50 values in the range of 0.01–1.0 μM.
Hitgen Inc disclosed their latest patent with the general structure 23 () in 2021 [Citation41]. Guided by the ring-opening strategy, a para-substituted aromatic ring scaffold acts as a linker to replace the imidazole ring. However, these compounds have only moderate activity in binding to IL-17A. For example, compounds 49 and 50 have IC50 values in the range of 1.0 μM to 100 μM.
3.4. Dice Alpha, Inc
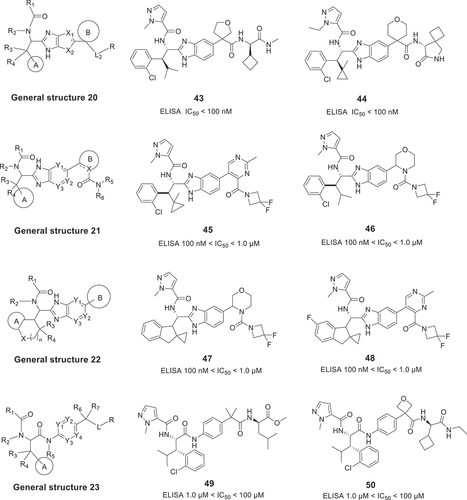
Dice Alpha, Inc. has disclosed two carboxamide derivative patents for targeting the IL-17A/IL-17RA PPI. The general structures 24 [Citation42] and 25 [Citation43] are shown in , and the inhibition activity of these compounds was measured by IL-17A/A or IL-17A/F HEK-blue cell assay. The first patent, which disclosed in 2020, contains 419 compounds with the general structure 24 and many of these analogs are structurally related to general structure 23 derivatives. The promising compounds gave the IC50 values less than 10 μM, such as, compounds 50 and 51. A second application claims compounds with the general structure 25 and exemplifies 810 IL-17A inhibitors in 2021. These compounds are complementary to the first application with more detailed research, and the best example compounds have IC50 values less than 0.3 μM. Based on their research, Dice Alpha, Inc has already started a Phase I clinical trial in 2022 (link).
3.5. Eli Lilly and Company
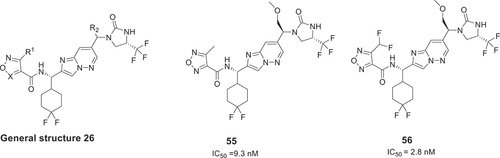
Researchers from Eli Lilly and Company described a series of potent imidazo[1,2-b]pyridazine derivatives with general structure 26 () in 2020 [Citation44]. Compounds in this patent are claimed to treat certain symptoms of psoriasis, rheumatoid arthritis, or multiple sclerosis. These compounds utilize a difluorocyclohexyl-imidazopyridazinyl-imidazolidinone core and are very similar to compound 24 () reported by UCB Biopharma Sprl. This application only exemplifies 28 modulators and the inhibition activity of these compounds was measured by AlphaLISA assay as well as cell-based human IL-17A neutralization assay. The best compounds illustrate promising IC50 values in neutralizing human IL-17A-mediated signaling
in HT-29 cells, for example, compounds 55 and 56 are 9.3 nM and 2.8 nM, respectively. Importantly, Eli Lilly initiated a Phase I clinical trial (link) of compound 55 to study IL-17A-related diseases in 2021. But unfortunately, the clinical trial was suspended due to safety concerns.
3.6. Leo Pharma A/S
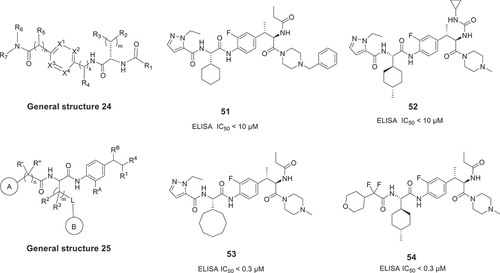
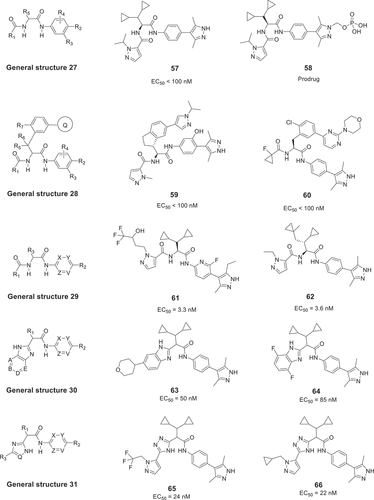
In 2020 and 2021, Leo Pharma A/S filed 5 patents for treating or ameliorating a variety of diseases, which involve up- or de-regulation of IL-17A, such as psoriasis, ankylosing spondylitis, and psoriatic arthritis. The disclosed general structures 27 [Citation45], 28 [Citation46], 29 [Citation47], 30 [Citation48], and 31 [Citation49] are shown in . The inhibition activity of these compounds was measured by IL-8 release assay in human epithelial keratinocytes adult, and some compounds had good EC50 values and good oral efficacy.
The amino-acid anilides and derivatives with general structures 27 [Citation45] and 28 [Citation46], which were published in 2020, are shown in . Between these two disclosed patents, the general structure 28 uses bulky aromatic moiety at the position of the group R5. Among these compounds, the most promising compounds have EC50 values in a range of less than 100 nM, such as 57, 58, 59, and 60. Notably, the compound 57 showed a promising oral bioavailability in mouse and dog pharmacokinetic studies with 26% and 23% when dosed orally at 50 and 1 mg/kg, respectively. In addition, compound 58, as a prodrug of compound 57, showed a significantly higher pharmacokinetic profile than compound 57 in animal studies [Citation45]. Subsequently, in order to expand the diversity of compounds, Leo Pharma A/S researchers disclosed a patent with general structure 29 in 2021, as shown in [Citation47]. Substituting the benzene ring nucleus with a pyridine, pyrimidine, or pyridazine ring, these new compounds are not only structurally novel but also exhibit strong activities, such as compound 61 with an EC50 value of 3.3 nM. Finally, applying bioisosteric replacements, 1 H-imidazolyl, 1 H-1,2,4-triazolyl scaffolds with potentially better properties can replace the amide group. For example, the general structure 30 is claimed to use an aromatic 6-membered ring fused with imidazole to replace the amide bond () [Citation48]. These compounds exhibit good activity, such as compounds 63 and 64 with EC50 values of 50 nM and 85 nM, respectively. In contrast, using 1 H-imidazolyl or 1 H-1,2,4-triazolyl scaffolds as linkers in the general structure 31 (), the activity of the most promising compounds increased slightly, e.g. compounds 65 and 66 showed EC50 values of 24 nM and 22 nM, respectively. Compound 58, as a prodrug, has entered a phase I clinical trial (link) for treatment of IL-17A-related diseases in 2021 [Citation49].
4. Expert opinion
Currently, biotherapeutics of antibody therapy are leading the way in the treatment of IL-17-related diseases, three FDA approved monoclonal antibodies (mAbs) (secukinumab, ixekizumab, and brodalumab) have been made significant advances in the field of immunology therapy. However, these antibodies have a number of disadvantages as well, such as non-oral applications, poor tissue penetration, and long half-life times. In contrast, small-molecule modulators are highly suitable for oral administration as well as flexible treatment regimen to overcome drawbacks of mAbs for patient treating. Moreover, the small-molecule compounds may be safer due to the faster withdrawal rate of the drug should adverse events occur, and they also facilitate topical therapy. Hence, from a small-molecule perspective, IL-17A is of significant interest since it is pro-inflammatory cytokine playing a key role in pathogenic autoimmunity.
Although the co-crystal structure of IL-17A and IL-17RA were reported in 2013, targeting large featureless protein surfaces with small molecules remains a challenge for effective drug discovery. Fortunately, scientists at Pfizer reported co-crystal structures of small molecules in complex with IL-17A in 2016, and structure-based IL-17A/IL-17RA PPI drug discovery has burst out recently. Especially recently years, several companies have reported dozens of patents for small-molecule modulators for the treatment of IL-17A-related diseases. For example, UCB Biopharma Sprl company reported the spirocyclic and imidazole derivatives as the novel modulators of IL-17 in 13 patents. Hitgen Inc. company also disclosed a series of imidazole derivatives in 11 patents for the IL-17A treatment. Eli Lilly and LEO Pharma have initiated phase I clinical trials for treating IL-17A related disease, and DICE Molecules is also submitted an Investigational New Drug (IND) application.
Currently, patent protected small molecules antagonizing IL17a receptor interaction PPI are rather similar scaffolds, all build on a central bis amide motif or a bioisostere thereof. It can be speculated that all disclosed scaffolds have a similar binding mode to the interface of the IL-17a dimer involving four hydrogen bonds of the bisamide to the backbone of Leu97A and Leu97B. It remains to be seen if other fundamentally different classes of small molecules able to potently antagonize the IL17a receptor interaction PPI can be discovered.
Current discovery efforts should translate into an increase in the number of IL-17A inhibitors entering the clinic. This will lead to further insight into efficacy of IL-17A inhibitors as standalone and in combination with antibody therapies.
Article highlights
IL-17/IL-17RA is a hot target for human immune therapy.
A number of small-molecule IL-17/IL-17RA inhibitors have been identified.
High-resolution cocrystals have been disclosed.
A consensus approach to overcoming the shortcomings of mAbs.
With these IL-17/IL-17RA inhibitors in phase I clinical trials, the outcome of these trials will likely influence future drug development in the immunotherapy of disease treatment.
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer has consulted for Eli Lilly and Aclaris Therapeutics on some aspects of IL-17 signaling in the last 12 months. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567.
- Li X, Bechara R, Zhao J, et al. IL-17 receptor–based signaling and implications for disease. Nat Immunol. 2019;20(12):1594–1602.
- Jin W, Dong C . IL-17 cytokines in immunity and inflammation. Emerging Microbes and Infections. 2013; 2(1): e60.
- McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–902.
- Tollenaere MAX, Hebsgaard J, Ewald DA, et al. Signalling of multiple interleukin (IL)-17 family cytokines via IL-17 receptor A drives psoriasis-related inflammatory pathways. Br J Dermatol. 2021;185(3):585–594.
- Ostling J, Geest M, Schofield JPR, et al. IL-17–high asthma with features of a psoriasis immunophenotype. Journal of Allergy and Clinical Immunology. 2019;144(5):1198–1213.
- Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390.
- Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune- mediated diseases psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–142.
- Thomas LW, Lee EB, Wu -J-J. Systematic review of anti-drug antibodies of IL-17 inhibitors for psoriasis. J Dermatolog Treat. 2019;30(2):110–116.
- Fu J, Huang Y, Bao T, et al. The role of Th17 cells/IL‑17A in AD, PD, ALS and the strategic therapy targeting on IL17A. J Neuroinflammation. 2022;19(1):98–110.
- Chen J, Liu X, Zhong Y. Interleukin-17A: the key cytokine in neurodegenerative diseases. Front Aging Neurosci. 2020;12:566922.
- Wang W-J, Groves MR, Dömling A. Artificial macrocycles as IL-17A/IL-17RA Antagonists. Med Chem Commun. 2018;9(1):22–26.
- Liu S-P, Song X, Chrunyk BA. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nat Commun. 2013;4(1):1888–1897.
- Espada A, Broughton H, Jones S. A binding site on IL-17A for inhibitory macrocycles revealed by hydrogen/deuterium exchange mass spectrometry. J Med Chem. 2016;59(5):2255–2260.
- Liu S-P, Dakin LA, Xing L, et al., Binding site elucidation and structure guided design of macrocyclic IL-17A antagonists. Sci Rep. 2016. 6(1): 30859.
- Liu S-P, Desharnais J, Sahasrabudhe PV, et al. Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide.Scientific Reports. 2016;6:26071.
- Ensemble Therapeutics Corporation. Macrocyclic compounds for modulating IL-17. WO2013116682A1. 2013.
- Ensemble Therapeutics Corporation. Macrocyclic compounds for modulating IL-17. US20150005319A1. 2015.
- Ensemble Therapeutics Corporation. Compounds for modulating IL-17. WO2014066726A2. 2014.
- Ucb Biopharma Sprl. Spirocyclic indolines as IL-17 modulators. WO2018229079A1. 2018.
- Ucb Biopharma Sprl. Fused imidazole derivatives as IL-17 modulators. WO2019138017A1. 2019.
- Ucb Biopharma Sprl. Spirocyclic indane analogues as IL-17 modulators. WO2020011731A1. 2020.
- Ucb Biopharma Sprl. Benzimidazolone derivatives, and analogues thereof, as IL-17 modulators. WO2020120140A1. 2020.
- Ucb Biopharma Sprl. Benzimidazolone derivatives, and analogues thereof, as IL-17 modulators. WO2020120141A1. 2020.
- Ucb Biopharma Sprl. Fused imidazole derivatives as IL-17 modulators. WO2020260425A1. 2020.
- Ucb Biopharma Sprl. Fused imidazole derivatives as IL-17 modulators. WO2020260426A1. 2020.
- Ucb Biopharma Sprl. Imidazopyridine derivatives as IL-17 modulators. WO2020261141A1. 2020.
- Ucb Biopharma Sprl. Difluorocyclohexyl derivatives as IL-17 modulators. WO2021170627A1. 2021.
- Ucb Biopharma Sprl. Difluorocyclohexyl derivatives as IL-17 modulators. WO2021170631A1. 2021.
- Ucb Biopharma Sprl. Difluorocyclohexyl derivatives as IL-17 modulators. WO2021204800A1. 2021.
- Ucb Biopharma Sprl. Difluorocyclohexyl derivatives as IL-17 modulators. WO2021204801A1. 2021.
- Hitgen Inc. Preparation of immunomodulator for treating IL-17A mediated diseases. WO2019223718A1. 2019.
- Hitgen Inc. Enantioselective prepn. of ((methyl)pyrazolecarboxamido)methyl benzimidazoles as IL-17 immunomodulators. CN112341435A. 2021.
- Hitgen Inc. Preparation of (benzimidazolyl)(cyclobutylmethyl)acetamides as IL-17 immunomodulators. CN112341439A. 2021.
- Hitgen Inc. Preparation of imidazole derivatives as immunomodulators and used for treating IL-17A-mediated diseases. CN112341440A. 2021.
- Hitgen Inc. Enantioselective preparation of ((methyl)pyrazolecarboxamido)methyl benzimidazoles as IL-17 immunomodulators. CN112341441A.
- Hitgen Inc. Enantioselective preparation of (benzimidazolyl)oxypyrrolidine carboxylates as IL-17 immunomodulators. CN112341442A. 2021.
- Hitgen Inc. Preparation of benzimidazole derivatives as immune regulators. CN112341446A. 2021.
- Hitgen Inc. Preparation of benzimidazole derivatives as immune regulators. CN112341450A. 2021.
- Hitgen Inc. Compound as IL-17A inhibitor and use in the preparation of drugs for treating IL-17A-mediated diseases thereof. CN112341451A. 2021.
- Hitgen Inc. Immunomodulator, and application thereof in treating IL-17A mediated disease. CN112341519A. 2021.
- Dice Alpha Inc. IL-17 Ligands And Uses Thereof. US2020247785A1. 2020.
- Dice Alpha Inc. IL-17A modulators and uses thereof. WO2021055376A1. 2021.
- Eli Lilly And Company. Imidazo[1,2-b]pyridazine IL-17A inhibitors. WO2020146194A1. 2020.
- Leo Pharma A/S. Amino-acid anilides as small molecule modulators of IL-17. WO2020127685A1. 2020.
- Leo Pharma A/S. Amino-acid anilides as small molecule modulators of IL-17. WO2020182666A1. 2020.
- Leo Pharma A/S. Small molecule modulators of IL-17. WO2021250194A1. 2021.
- Leo Pharma A/S. Small molecule modulators of IL-17. WO2021255086A1. 2021.
- Leo Pharma A/S. Small molecule modulators of IL-17. WO2021255174A1. 2021.