Plain Language Summary
What is this summary about?
This plain language summary describes the results of the phase 2 study called PAISLEY which tested deucravacitinib, a new medicine under investigation before approval, in people living with lupus. In this trial, researchers wanted to find out if deucravacitinib would be safe and reduce the symptoms and disease activity in people living with lupus. PAISLEY looked at the type of lupus known as systemic lupus erythematosus, shortened to SLE.
What happened in the study?
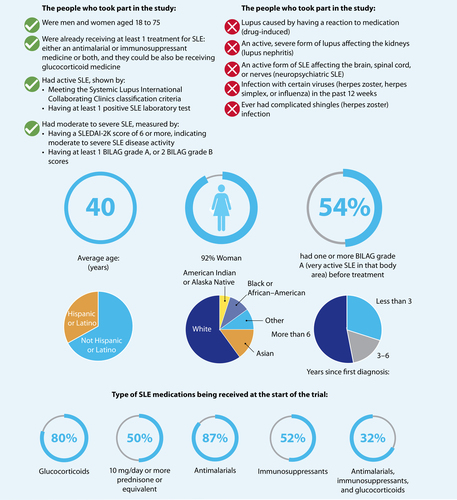
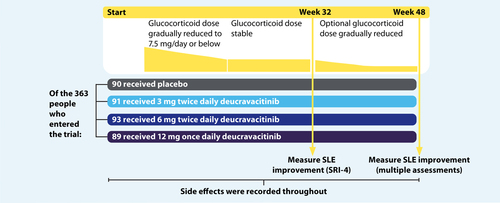
The study included 363 people from 17 countries who had SLE and were between 18 and 75 years of age. The participants were divided into 4 groups at random. One group was given placebo (a fake or dummy pill that contains no medicine) and the other 3 groups took deucravacitinib, a pill taken by mouth. Each of the groups taking deucravacitinib took a different dose, either 3 milligrams (mg) twice daily, 6 mg twice daily, or 12 mg once daily. After 32 and 48 weeks, researchers measured the number of people in each group who had improvements in their SLE symptoms and disease activity, as measured by different tests. They also looked at any side effects people experienced, which may or may not have been caused by the medicine.
What do the results mean?
After 32 weeks of treatment, SLE symptoms and disease activity improved in more people in each of the deucravacitinib dose groups compared with the people taking placebo (the dummy pill). After 48 weeks of treatment, SLE symptoms and disease activity were still improved in more people taking deucravacitinib compared with people taking placebo, and this was measured in several different ways. The best results were seen in people taking deucravacitinib 3 mg twice daily. The number of serious side effects was similar for people taking deucravacitinib and those taking placebo. The most common side effects that were seen in people taking deucravacitinib were infections such as sore throat, cough, or bronchitis (upper respiratory tract), infltion in the nose (nasopharyngitis), headaches, and urinary tract infections. More people taking deucravacitinib than placebo had acne, rash, and cold sores (oral herpes). These were not serious and did not have any long-term effects on patient health or lead to patients stopping treatment.
How to say (double click sound icon to play sound)…
Systemic lupus erythematosus: SIS-teh-MIC LOO-puhs Eh-RE-the-ma-TOE-sus
Deucravacitinib: doo-KRAV-a-sih-ti-nib
Enzyme: EN-zime
Interferon: in-tur-FER-on
Placebo: pluh-SEE-boh
Tyrosine kinase: TY-ruh-seen KY-nays
TYK2: TIK-tu
Who should read this article?
This summary may be useful for people who want to learn about the PAISLEY study, including people with SLE, their families, caregivers, and healthcare providers. Please look at the final Glossary section for more information on some of the scientific words (highlighted in green) used in this article.
Where can I find the original article on which this summary is based?
The original article, called “Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial” was published in February 2023 in the scientific journal, Arthritis & Rheumatology, volume 75, issue 2, page 242. You can read it for free at: https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42391
Who sponsored the study?
The study was sponsored by Bristol Myers Squibb, who would like to thank the people living with SLE who volunteered to take part in this study and their families, as well as PAISLEY investigators and study teams.
What is systemic lupus erythematousus?
There are 4 main types of lupus. Systemic lupus erythematosus (shortened to SLE, often known simply as “lupus”) is a long- term condition that can affect the whole body. It occurs when a person’s immune system (the body’s natural defense system) gets out of balance and causes damage to the body’s own healthy tissues by causing inflammation. This is known as an autoimmune condition. In people living with SLE, this can damage different parts of the body. People living with SLE may therefore experience a wide range of symptoms, including skin rashes, fevers, swelling and aches in the joints and muscles, as well as harmful inflammation in internal organs. SLE affects more women than men. African, Asian, Native American, and people of mixed race are also more likely to have SLE, which can be more severe, than people of European descent, but SLE does occur in all populations. The symptoms of SLE often come and go in waves, called “flares” or “flare-ups”, when symptoms worsen acutely. There is no cure for SLE, but symptoms can be controlled with treatment. Even with current treatments, there is a risk of severe damage to the organs, especially kidneys, and a greater chance of dying at a younger age. Several treatments are available to help people living with SLE, including antimalarial medicines, steroids (glucocorticoids), immunosuppressants, and biologics.
Antimalarial medicines were developed to treat and prevent malaria, but they also help with SLE
Glucocorticoids are a type of hormone called a steroid that is made naturally at low amounts in the body. Manufactured steroids are an effective medication to reduce the body’s immune responses and resulting inflammation
Immunosuppressants are medicines that stop or reduce different parts of the immune response and inflammation in the body
Biologics are newer medicines, generally designed to look and work similar to molecules made by living organisms, that decrease inflammation by targeting specific pathways (a series of natural chemical reactions) in the body’s immune system
However, many of these treatments are “borrowed” from other conditions. This means they have not been approved specifically to treat people living with SLE, and often means that they have not been fully studied in SLE. This is why researchers are looking for new treatments.
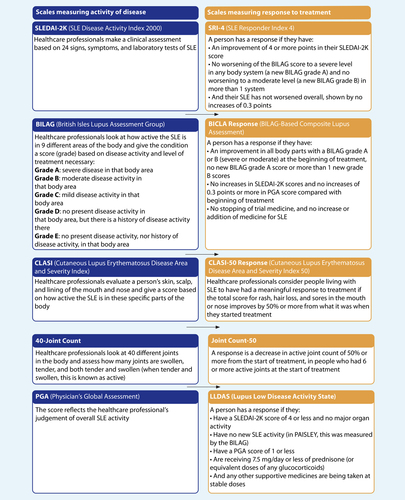
Healthcare professionals use several different tools to measure how severely SLE affects a person living with the condition, and if their symptoms change over time. Several measuring tools were used in the PAISLEY study. They have all been validated
(confirmed by healthcare professionals). The short names of the tools used in PAISLEY were: SRI-4, BICLA, LLDAS, CLASI-50, and the Joint Count-50. Of these tools, the SRI-4 outcome after 32 weeks of treatment was assessed as the main outcome measure of the study, referred to as the primary endpoint.
Before the people in the PAISLEY study started receiving treatment, their SLE symptoms were measured using the activity scales to give researchers a baseline of where each patient was starting. Following treatment, people on study completed the response scales so that researchers could measure how their SLE symptoms and disease activity had changed after receiving the treatment. In the language of clinical research, a change for the better is called a “response”. Each tool is measured through different tests and values by the healthcare professionals. For example, if a person scored a 20 out of 40 on the 40-Joint Count activity scale prior to receiving treatment and, following treatment, scored a 5 out of 40 on the Joint Count-50 response scale, their joint-related SLE symptoms had improved (responded to treatment) in a measurable way, as they had shown a greater than 50% decrease in joint disease. Further details of these tools are shown in the Supplement.
Healthcare professionals also use blood tests to measure how active the SLE is. These tests can include:
Levels of antibodies made by the immune system that target DNA released from cells in the patient’s body, which is not broken down (double-stranded DNA). These antibodies often occur in people living with SLE and are associated with more severe disease.
Levels of two immune system particles called complement 3 and 4, which are involved in natural inflammation and immune responses. Low complement 3 and 4 levels are often seen in people living with SLE because when the immune system is very active, they get used up.
A specific pattern of immune activation called the interferon signature. This is increased in patients living with SLE when immune components called interferons stimulate several immune system processes.
What is deucravacitinib?
Deucravacitinib is a pill taken by mouth, with or without food. It is approved for clinical use in multiple countries for treating certain people with the autoimmune condition called plaque psoriasis.
Based on the ways it works, researchers believe it may be a useful treatment also for people living with SLE. Deucravacitinib works by blocking a signaling protein inside the body’s cells called tyrosine kinase 2 (shortened to TYK2). This protein operates as an enzyme, which helps speed up key interactions or changes between other proteins.
TYK2 is found in most cells in the body but has an essential role in the body’s immune response. TYK2 translates the body’s response to an immune activation or infection, relayed by proteins released in the blood called cytokines, and sends the message into cells to activate parts of the immune system, especially those parts driven by interferons and some other immune components.
This activity would normally be helpful to fight infections. In SLE, there is an unusually high TYK2 activity, which contributes to the symptoms of the condition. Blocking the TYK2 enzyme with deucravacitinib may reduce SLE symptoms.
Where is the PAISLEY study in the drug development timeline?
Phase 2 studies usually include hundreds of patients and look at how safe a treatment is and how well it works at multiple doses in a particular condition. For a medicine to become approved for use outside of clinical studies, it must usually go through larger studies (known as phase 3 trials). PAISLEY was a phase 2 study that tested different doses of deucravacitinib in people living with SLE, to decide on the best dose to be used in the final phase 3 trial
What did the PAISLEY study look at?
The main aim of the study was to compare how many people in each group had an improvement in their SLE after taking deucravacitinib or placebo for 32 weeks, as shown by an SRI-4 response. Researchers also wanted to find out how many people in each group had an improvement in their SLE after 48 weeks, as shown by several different measures: an SRI-4 response, a BICLA response, an LLDAS response, a CLASI-50 response, and the Joint Count-50. Also, blood tests looking at the levels of components of the immune system, complement 3 and 4, levels of antibodies targeting double-stranded DNA, and the interferon signature were done over the course of treatment.
The researchers also wanted to know what side effects may be seen with deucravacitinib in people living with SLE, so they compared the side effects seen in the people taking the placebo with those seen in the people taking deucravacitinib.
How was the study carried out?
People taking part in the study were randomly divided into 4 groups, meaning that the treatment they received was decided by chance. PAISLEY was a “double-blind” study, so neither the healthcare professionals nor the patients knew if they were taking deucravacitinib or placebo, and the researchers only found out once the trial was finished.
What were the main results of the study?
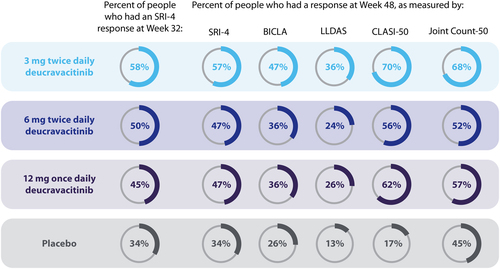
More people who were taking any dose of deucravacitinib had an improvement in SLE (measured by SRI-4 response) after 32 weeks of treatment compared with people taking placebo.
After 48 weeks, more people taking deucravacitinib had an improvement in SLE, when assessed by the SRI-4, BICLA, LLDAS, CLASI-50, and Joint Count-50 response measures compared with people taking placebo.
The details of exactly what it means to have a response according to the different measures are described in the Supplemental picture.
Although treatment with all 3 deucravacitinib doses resulted in more people being responders than placebo at Week 32 and Week 48, people taking the 3-mg twice daily dose showed the greatest improvements compared with placebo.
Blood tests showed that:
Over 48 weeks of treatment, the levels of antibodies targeting double-stranded DNA decreased in the blood of people taking each of the deucravacitinib doses but did not change in the people taking placebo. This suggests that treatment with deucravacitinib helped reduce the immune activation targeting the patient’s own DNA and tissues.
In the patients who had low levels of complement 3 and 4 at the start of treatment, indicating immune activation, these levels increased over the 48 weeks in people taking deucravacitinib but not placebo, suggesting that deucravacitinib helped complement levels stay within a healthy range.
From 4 to 44 weeks of treatment, the interferon signature was also reduced in people taking each of the deucravacitinib doses, but not in those taking placebo, suggesting that deucravacitinib helped reduce immune activation.
What were the side effects?
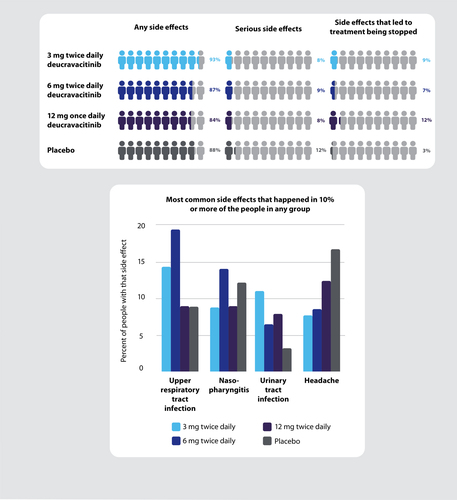
The most common side effects seen in people who took deucravacitinib were infections such as sore throat, cough, or bronchitis (upper respiratory tract), inflammation in the nose (nasopharyngitis), headaches, and urinary tract infections. Most side effects were mild, and the healthcare professionals treating the patients for those side effects thought that most were not related to the study treatment. Low frequency of acne, rash, and cold sores (oral herpes), mostly mild, were seen in people taking deucravacitinib that were higher than seen with placebo. Eighteen of 273 people (7%) taking deucravacitinib had acne compared with 4 of 90 (4%) taking placebo. Twelve of 273 people (4%) taking deucravacitinib had rash, and 13 of 273 (5%) had cold sores, whereas none of the 90 people receiving placebo had rash or cold sores. Most side effects seen in people who took deucravacitinib were considered by healthcare professionals to not be directly linked to receiving deucravacitinib treatment, meaning that they might have occurred to various reasons independent of deucravacitinib.
No one died during the study. The number of serious side effects, which are any medical event that results in death, having to go to or stay in hospital, or a persistent or significant incapacity, was low and similar in each of the 4 groups including the placebo group.
What do the results of the PAISLEY study mean?
In this study, more people living with SLE who took deucravacitinib had a clinically meaningful reduction in SLE symptoms and disease activity than those who took placebo. The side effects in people taking deucravacitinib were generally mild, and the amount of side effects was similar between people taking deucravacitinib and people taking placebo. Overall, these results suggest that deucravacitinib could become a new treatment for SLE in the future. There are larger phase 3 studies being carried out with a greater number of people living with SLE to further understand the benefits of deucravacitinib.
Where can I find more information on the study?
The original article, called “Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial”, was published in Arthritis & Rheumatology (2023), volume 75, issue 2, pages 242-252. You can read it for free at https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42391.
You can find more information on the PAISLEY study (NCT03252587) on the official ClinicalTrials.gov website: https://www.clinicaltrials.gov/study/NCT03252587.
PLSP disclosure
This plain language summary represents the opinion of the authors. This plain language summary has been developed to accompany the original article and is not intended for any other use.
Financial and competeing interests disclosure
For a full list of declarations, including author disclosure statements, please see the original article. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
The summary was prepared by Christine Ingleby, DPhil, CMPP, of Envision Pharma, Inc, and funded by Bristol Myers Squibb.
Glossary
Autoimmune condition: a condition in which the body’s internal defense mechanism, the immune system, becomes imbalanced, potentially leading to damage to the body’s own healthy tissues.
Antibodies: a natural part of the body’s defense system, different antibodies specifically react against many different targets
– usually foreign invaders such as bacteria or viruses, but antibodies sometimes react with the body’s own healthy tissues leading to production of, for example, antibodies targeting double-stranded DNA.
Complement: a family of proteins made by the body that plays an important role in the immune system.
DNA: the hereditary material found in every cell of the body; it holds the genetic code and is sometimes called the building block of life. All proteins made in the body use DNA as a blueprint.
Glucocorticoid: a type of steroid that is made naturally by the body and can also be manufactured and used as a medicine to reduce the body’s immune response.
Immunosuppressant: a type of medicine that stops or decreases immune responses in the body
Inflammation: a natural part of the body’s immune system that recognizes and removes harmful or foreign invaders such as bacteria or viruses. It causes heat, pain, swelling, and redness locally and may have other impacts depending on the affected area.
Interferon: a key part of the body’s defense system, interferon is one of the first steps in immune activation when there is a foreign invader such as a virus.
Protein: a type of molecule made by cells that do many different jobs in the body and are the foundation of every cell and bodily process.
Systemic lupus erythematosus: a chronic autoimmune condition that affects various organs and may cause a wide range of symptoms, including skin rashes, fevers, and swelling and aches in the joints and muscles.