Abstract
Introduction
In light of potential negative health effects of cobalt exposure, a characterization of inflammatory mechanisms in exposed individuals is warranted. The current study investigated cobalt exposure in the Swedish hard metal industry and its relationship to inflammatory markers, including NLRP3 inflammasome activation and white blood cell (WBC) counts.
Materials and methods
Inhalable cobalt and dust exposures, and systemic cobalt levels, were determined for 72 workers in the hard metal industry and linear regression models were applied to correlate exposure to markers of inflammasome activation and WBC counts.
Results
Mean exposures to inhalable dust (0.11 mg/m3) and cobalt (0.0034 mg/m3) were below the Swedish occupational exposure limits, and these low exposures did not correlate with any investigated outcomes. Instead, cobalt blood levels significantly correlated with a ca 10% decrease in IL-18 plasma levels per 10 nM cobalt increase. Furthermore, pre-shift cobalt blood and/or urine levels significantly correlated with some WBC measures, including decreased neutrophil-to-lymphocyte ratio, increased lymphocyte-to-monocyte ratio, and lymphocyte counts.
Conclusion
The low inhalable particle exposures had no impact on WBC counts and inflammasome activation. Instead, systemic cobalt levels, which also include skin exposure, demonstrated possible suppressive effects on inflammatory responses in cobalt-exposed individuals in the hard metal industry.
Introduction
Cobalt is used in a variety of applications, including the production of hard metals and in lithium-ion batteries. With increasing and innovative use of cobalt globally, new occupations at risk of exposure are introduced, and further understanding of the biological effects of cobalt is needed to ensure its safe use and handling, as exposure has been found to cause adverse health effects (Leyssens et al. Citation2017). Occupational exposure to cobalt per se has been associated with adverse health effects that include allergic dermatitis, asthma, hard metal lung disease, and possible effects on myocardial tissue (Linna et al. Citation2004, Leyssens et al. Citation2017). Cobalt metal with tungsten carbide is furthermore classified as probably carcinogenic by the IARC (IARC Citation2006). Therefore, monitoring levels of cobalt and risk reduction of exposure is well established within the hard metal industry, where cobalt is used as a binder together with tungsten carbide to produce the hard metal used mainly for cutting tools.
Particle exposure can generate inflammatory responses locally in the lungs, contributing to development of lung diseases, including pneumoconiosis and lung cancer (Valavanidis et al. Citation2013, Perlman and Maier Citation2019), but also systemic inflammatory responses (Rückerl et al. Citation2014, Pope et al. Citation2016, Guan et al. Citation2019). The systemic inflammatory reactions are likely contributing to the strong relationship between particle exposure and cardiovascular diseases (CVD) (Cohen et al., Citation2017, Liu et al. Citation2017) due to the central role of inflammation in the pathogenesis of CVD (Golia et al. Citation2014). Although the mechanism of particle-induced inflammation varies, some particles, including cobalt alloys, silica, and monosodium urate crystals, have been shown to activate a specific inflammatory complex named the NLR family pyrin domain containing 3 (NLRP3) inflammasome (Martinon et al. Citation2006, Cassel et al. Citation2008, Caicedo et al. Citation2013). When activated, the enzymatic activity of the NLRP3 inflammasome is mediated by caspase-1 allowing it to cleave the proinflammatory cytokines pro-interleukin (IL)-1β and pro-IL-18 into their biologically active forms (Kelley et al. Citation2019). The NLRP3 inflammasome is activated not only by particles, but by various chemicals, including cobalt and other metal ions (Ferko and Catelas Citation2018, Klasson et al. Citation2021).
Inflammatory responses mediated by certain inflammatory stimuli, e.g., bacterial or viral infections, will increase haematopoiesis of certain leukocyte populations, resulting in increased white blood cell (WBC) counts or changes in the ratios of lymphocyte populations, including the neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR). Although cobalt has been shown to affect haematopoiesis, it is predominantly shown for erythrocytes (Ebert and Jelkmann Citation2014), while there are conflicting results regarding cobalt exposure and its effect on leucocyte counts (Hart et al. Citation2009, Penny et al. Citation2013, Honkasaari et al. Citation2020).
There is a lack of knowledge regarding the current exposure levels and occupational exposure limits to dust and cobalt in the hard metal industry and the effects in systemic inflammatory responses. We have previously reported a detailed description of particle exposures per se in the hard metal industry and their relationship to general markers of inflammation (Andersson et al. Citation2021). In the present study, effects of dust and cobalt exposures in the hard metal industry is studied, focussing on inflammatory mechanisms of relevance to cobalt exposure in particular, including activation of the NLRP3 inflammasome and effects on WBC counts.
Materials and methods
Study group
Descriptive statistics of the study population can be seen in . A more detailed description of the study group has been reported previously, including job titles and self-reported health status such as diseases and respiratory symptoms (Andersson et al. Citation2020). In short, the study was performed on 72 individuals working at two Swedish hard metal industries. A majority worked at the pressing and powder departments but also forming (prototype), laboratory, and maintenance departments were included. The produced hard metal products were based on tungsten carbide with cobalt used as a binder.
Table 1. Descriptive statistics of the 72 hard metal industry workers included in the study.
Study design
The study was performed between March 2017 and October 2018 at two different hard metal industries during six different sampling occasions. Air sampling of inhalable dust was performed during the second or third day following a work-free weekend. On the same day, blood and urine samples were collected in the morning before work, and in the afternoon following an eight-hour work shift. Samples were analyzed for cobalt levels in blood and urine, plasma inflammatory markers, blood counts, and ex vivo stimulation of whole blood. Analysis of WBC counts and ex vivo-experiments were performed on-site, and for circulating markers of inflammasome activation, EDTA-plasma was frozen immediately on dry ice and stored at −70 °C until analysis. Blood samples for cobalt analysis were collected in sodium heparin-tubes. Both blood and urine samples for cobalt analysis were stored refrigerated until analysis.
NLRP3 associated cytokine measurements and white blood cell counts
In the pre- and post-shift plasma samples, concentrations of IL-18 and IL-1Ra were measured in duplicates in EDTA-plasma on the QuickPlex SQ120 instrument (Mesoscale diagnostics, Rockville, MD) using the U-plex assay according to manufacturer’s instructions. All samples were within quantifiable levels with a CV within all duplicates <20%. IL-1β was measured in EDTA-plasma in singlets with the Simoa bead technology (Quanterix, Billerica, MA, USA) performed at the company’s facility. All but two samples were above the limit of detection (LOD, 0.016 pg/mL) and 60% were above LOD but below the lower limit of quantification (LLOQ). For the samples below LLOQ, the concentrations given from the standard curve regression calculation were used. For the samples below LOD, half the concentration of LOD was used.
White blood cell (WBC) counts were performed on freshly isolated blood using the Hemocue WBC Diff System (Hemocue, Ängelholm, Sweden). The WBC counts included total WBC, 5-part differential counts (neutrophils, lymphocytes, monocytes, eosinophils, basophils) and the neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR). An elevated NLR or reduced LMR have been proposed as markers of systemic inflammation (Afari and Bhat Citation2016, Huang et al. Citation2018).
Ex vivo NLRP3 inflammasome activation
Before and after a work shift, freshly drawn whole blood from the study participants was mixed with an equal amount of RPMI supplemented with 10% foetal calf serum (FCS) and 25 mM HEPES (all from Invitrogen, Carlsbad, CA, USA) and were incubated either with or without 1 ng/mL lipopolysaccharide (LPS-B5 ultrapure, Invivogen, San Diego, CA, USA) for three hours at 37 °C. For analysis of cytokine release, the cells were incubated an additional 45 min with or without 250 µM adenosine triphosphate (ATP, Sigma Aldrich, St. Louis, MO), generating in total four different experimental conditions for each study participant: control, only LPS, only ATP, and combined LPS + ATP. At the end of the incubation time, the samples were centrifuged for five min at 1,000 × g to collect supernatants which were stored at −70 °C until analysis of IL-1β, IL-18 and IL-1Ra concentrations using the U-plex assay (mesoscale) according to manufacturer’s instructions, measured in singlets. For ex vivo caspase-1 enzymatic activity measurements, CD14-APC antibody (IM2580, Beckman Coulter, Brea, CA, USA) and FAM-WEHD-FMK Caspase-1 probe (Vergent Biosciences, Minneapolis, MN, USA) were added during the incubation step with ATP for 45 min. The reaction was stopped by adding 1-step fix-lyse solution (eBioscience, San Diego, CA, USA). Following lysis, the samples were washed two times with PBS prior to analysis on an Accuri C6 flow cytometer (BD, Franklin Lake, NJ, USA). Caspase-1 activity was detected by the FAM-WEHD probe in monocytes separated in the data by their CD14 expression. Enzymatic activation was calculated as the percent change in FAM-WEHD signal compared to the unstimulated control sample for each individual and time-point.
Aerosol measurements and cobalt analysis
Aerosol measurement of inhalable dust was performed by personal sampling as described previously (Andersson et al. Citation2020). In short, personal aerosol measurements were performed during the second or third day after a work-free weekend, on the same day as the blood and urine samples were collected. The personal dust sampling was carried out as eight-hour full-shift samples according to standards for measurement of inhalable dust using GSP-samplers (GSA Messgerätebau GmbH, Ratingen, Germany) with a 37 mm filter (3 µm pore-size) connected to a sampling pump (SKC Aircheck XR5000, Eighty Four PA, or GSA SG 5100, GSA Messgerätebau GmbH) operating at an air flow of 3.5 L/min. To determine cobalt levels in the dust, the dust filters were dissolved with acid (concentrated nitric acid with 10% hydrogen peroxide) and analyzed by inductively coupled plasma mass spectrometry (ICP-MS).
Stationary measurements of inhalable dust from different departments were performed as described previously (Andersson et al. Citation2020) to determine background air concentrations. The background air concentrations were used to calculate exposures for the individuals working with respirators.
The cobalt concentration in blood and urine was analyzed by ICP-MS. The urine concentrations were normalized to the urine specific gravity in the linear regression analyses.
Exposure measures
Exposure to inhalable dust and inhalable cobalt is presented as eight-hour time-weighted averages (TWA, mg/m3). In total, 26 individuals worked with respirators some part of the day. For these individuals, the exposure measures were adjusted to account for this. It was assumed that the workers were null exposed while wearing a respirator mask, and the remainder of the time, either stationary measurement or personal sampling were used to calculate exposure. Exposure measures also included pre- and post-shift levels of cobalt concentration in blood and urine.
The morning levels of NLRP3 associated markers and cell counts were used to model effects of chronic exposures, considering the measured inhalable levels of dust and cobalt as average daily exposures for the participants. The morning levels of inflammatory markers and cell counts were also analyzed in relation to the morning levels of cobalt in blood and urine. In contrast, the afternoon levels of NLRP3 associated markers and cell counts were used to model acute effects of the daily exposures to inhalable dust or cobalt. The afternoon values were also analyzed for the afternoon levels of cobalt in blood or urine.
Statistical analysis
Multiple linear regression models were used to determine correlations between NLRP3 associated markers or WBC counts and the included exposure measures: inhalable dust, inhalable cobalt, cobalt blood concentration, and cobalt urine concentration. Continuous variables were used for all exposure measures. The multiple linear regression models included sex (male or female), age (>45; <45 years), body mass index (BMI) (dichotomized by median) and smoking (non-smoker, ex-smoker, smoker) as covariates. For the analytes IL-1β, IL-18 and IL-1Ra, log-normal distribution was used in the regression analysis, and the anti-log values presented in the graphs.
All 72 participants were included in the analyses for the inhalable exposure measures, except for one afternoon sample in which no blood sample was available. For the analyses using cobalt levels in blood or urine as independent variables, one outlier with very high cobalt levels was excluded. The statistics were performed using SPSS 22.0, and the graphs shown in this article were made using GraphPad Prism 5.03.
Ethics
Written informed consent was obtained for all study participants. The study was approved by the Regional Ethical Review Board, Uppsala, Sweden (dnr. 2017/050).
Results
Exposure levels to inhalable dust and cobalt and systemic cobalt concentrations in blood and urine
An extensive description of the workers’ dust exposures has been published previously, including exposures to different particle size fractions, particle surface area, and particle numbers (Andersson et al. Citation2020). The exposure levels for the dust exposure measures used in the present study are shown in . In general, dust exposures were low, with a mean exposure of 0.11 mg/m3 inhalable dust (range 0.039–0.36 mg/m3) and a mean inhalable cobalt exposure of 0.0034 mg/m3, with the highest exposures observed in the powder departments. When accounting for respirator use, the average inhalable cobalt exposures were reduced by 50% to 0.0017 mg/m3, and the average inhalable dust exposures reduced by 28% to 0.079 mg/m3. Cobalt concentrations in blood and urine, for the morning and afternoon sampling, are shown in . The average cobalt concentration in blood in the morning was 6 nM, ranging from 3.8-12 nM. The cobalt blood concentration increased by 9% on average in the afternoon, to 7 nM (p < 0.0001, Wilcoxon signed-rank test). The average morning cobalt concentration in urine was 33.9 nM (range 3.9–250 nM) and increased by 23% on average in the afternoon, reaching 44 nM (p = 0.0041, Wilcoxon signed-rank test).
Table 2. Dust and cobalt exposures, and concentration of biological markers for the pre- (AM) and post-shift (PM) sampling of the 72 hard metal industry workers.
IL-18 is associated with cobalt blood concentrations
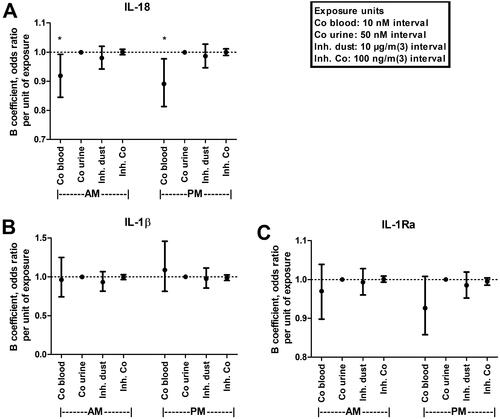
Descriptive statistics on the plasma concentrations of NLRP3 associated cytokines IL-1β, IL-18, and IL-1Ra, are shown in . To evaluate the effects of exposure on NLRP3 associated cytokines, multiple linear regression analyses of IL-1β, IL-18, and IL-1Ra levels, and dust exposures and cobalt levels, respectively, in blood and urine was performed (). Both morning and afternoon levels of cobalt in blood significantly correlated with decreased IL-18 levels, with 8% (B = 0.919, 95% confidence interval (CI)=0.845–0.992, p = 0.038) and 11% decrease (B = 0.891, 95% CI = 0.813–0.977, p = 0.015) in IL-18 per 10 nM increase in cobalt blood concentration, for the morning and afternoon levels, respectively. No significant associations were found for IL-1β or IL-1Ra in any of the studied exposure measures (inhalable dust, inhalable cobalt, cobalt in blood, and cobalt in urine) or time point (morning and afternoon). For the included covariates in the regression analyses, BMI was significantly correlated with IL-1Ra, and IL-18 with smoking status (current smoker) in all regression models (results not shown).
Figure 1. Multiple linear regression models of IL-18 (A), IL-1β (B), and IL-1Ra (C) cytokine plasma levels sampled in the morning (AM), or afternoon (PM) correlated to indicated cobalt or dust exposures. Points and error bars indicate anti-logged beta coefficient value (odds ratio) and 95% confidence interval, respectively. *Indicate significant correlation (p < 0.05). Dotted line marks odds ratio of 1 (no correlation). Co: cobalt; Inh: inhalable.
NLRP3 inflammasome activation does not correlate with cobalt exposure
To study if cobalt dust exposures affect the NLRP3 inflammasome, whole blood from the workers were stimulated ex vivo with the inflammasome triggers LPS and/or ATP to determine if cobalt exposures affect the level of inflammasome activation in terms of caspase-1 enzymatic activity and the release of NLRP3-associated analytes (IL-1β, IL-18, and IL-1Ra).
The increase in cytokine levels after ex vivo NLRP3 activation is shown in and multiple linear regression models of the ex vivo stimulated cytokine release and exposure to dust or cobalt in blood and urine, respectively, is shown in . LPS treatment of the blood cells resulted in release of all three measured cytokines, i.e. IL-1Ra as well as the NLRP3-regulated cytokines IL-18 and IL-1β. As expected, adding ATP after LPS stimulation enhanced the release of IL-18 and IL-1β, but not IL-1Ra. ATP alone did not stimulate release of the measured cytokines. Furthermore, LPS treatment resulted in significantly higher IL-18, IL-1β, and IL-1Ra release in the post-shift compared to pre-shift samples, and the same results were seen for IL-18 and IL-1Ra when also adding ATP (paired t-test, results not shown).
Figure 2. Multiple linear regression models of dust or cobalt exposure and changes in NLRP3 associated inflammatory responses to ex vivo stimulation of whole blood with LPS (1 ng/mL) with or without ATP (250 µM) sampled in the morning (AM) or afternoon (PM). NLRP3 inflammasome associated responses measured include release of indicated cytokines (IL-1β, IL-18 and IL-1Ra) (A and B) or caspase-1 enzymatic activation in monocytes (C and D). Points and error bars indicate beta coefficient value and 95% confidence interval, respectively. ATP: adenosine triphosphate; Co: cobalt; Inh: inhalable; LPS: lipopolysaccharide.
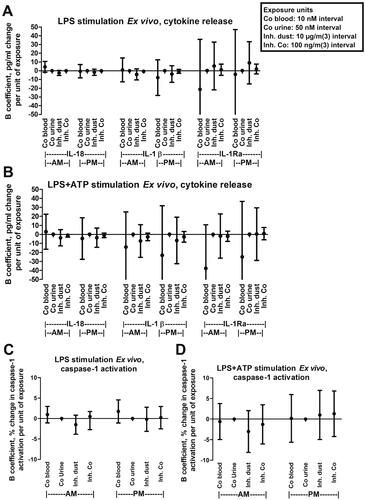
Table 3. Ex vivo stimulation of whole blood for pre- (AM) and post-shift (PM) sampling of the 72 hard metal industry workers.
No significant correlation was found for ex vivo stimulated cytokine release and exposure to dust or cobalt levels in blood or urine ()). Furthermore, using the same NLRP3 activators (LPS and/or ATP), ex vivo caspase-1 enzymatic activity in monocytes from the participants blood was measured and correlated to dust exposures or cobalt levels in blood and urine, respectively. Treatment with LPS increased monocyte caspase-1 activity by approximately 50% on average (), and LPS + ATP increased it on average by approximately 140%. Caspase-1 activation for pre- and post-shift sampling did not differ significantly using a paired t-test (results not shown). In accordance with the ex vivo cytokine release, no significant correlation between exposure measures and caspase-1 activation was found ().
Cobalt levels correlate with the lymphocyte-to-monocyte and the neutrophil-to-lymphocyte ratios
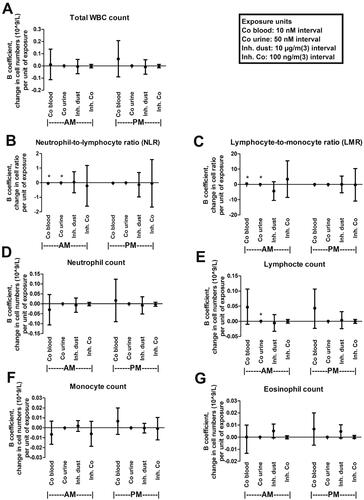
Descriptive statistics for the cell counts are presented in , and results of the multiple linear regression analyses of cell counts, including the cell ratios of NLR and LMR in relation to dust exposures and cobalt levels in blood and urine, respectively, are presented in . When the morning samples were investigated, NLR showed a negative correlation with cobalt levels in blood and urine, whereas LMR had a positive correlation. For NLR, a 0.043 decrease was found for each 10 nM increase in blood cobalt concentration (B= −0.043, 95% CI= −0.080 to −0.007, p = 0.021). For urine, each 50 nM increase in urine cobalt concentration correlated with a 0.0002 decrease in NLR (B= −0.0002, 95% CI = 0.0004 to −0.0000 p = 0.046). For LMR, each 10 nM increase in cobalt blood concentration correlated with a 0.397 increase in LMR (B = 0.397, 95% CI = 0.080 − 0.713, p = 0.015) and each 50 nM cobalt increase in urine correlated with a 0.003 increase in LMR (B = 0.003, 95% CI = 0.001 − 0.004, p = 0.004). In addition, the morning cobalt concentration in urine correlated positively with the total lymphocyte count. Each 50 nM increase in cobalt urine concentration correlated with a 0.00038 × 109 cells/L increase in blood lymphocytes (B 0.00038, 95% CI = 0.000051 − 0.00070, p = 0.024). No significant correlation was found between any exposure measure and the other cell counts measured, including total WBC, neutrophils, monocytes, eosinophils, or basophils.
Figure 3. Multiple linear regression of NLR, LMR (B and C) or indicated cell counts (A,D–G) sampled in the morning (AM) or afternoon (PM) and exposure to dust or cobalt levels in blood or urine. Points and error bars indicate beta coefficient value and 95% confidence interval, respectively. *Indicate significant correlation (p < 0.05). Co: cobalt; Inh: inhalable; WBC: white blood cell.
Discussion
The current study investigated inflammatory markers and their association to dust and cobalt exposure for 72 participants working in the Swedish hard metal industry. The inflammatory markers included NLRP3 inflammasome-associated cytokine levels (IL-1β, IL-18, and IL-1Ra) and enzyme activity (caspase-1), WBC counts, NLR, and LMR. The exposure measures included inhalable dust and cobalt exposures, as well as systemic cobalt exposure measured as cobalt levels in blood and urine. Inhalable cobalt exposures may have different biological effects compared to effects of cobalt levels of blood and urine, for two main reasons. Firstly, inhalable cobalt primarily exposes the lungs, compared to cobalt in blood and urine, which are measures of systemic cobalt exposure. Secondly, inhalable cobalt is in the form of particulate matter, whereas cobalt in blood and urine is a measure of cobalt ions, which could have different biological effects. In general, the inhalable dust and cobalt levels were low at the two industries. The mean 8-h TWA inhalable dust exposure was 0.11 mg/m3 and the mean cobalt exposure was 0.0034 mg/m3, without adjusting for respirator use. Considering that these exposures are around 50 times and five times below the current Swedish occupational exposure limits for inhalable dust and cobalt, respectively, the results of the study should be interpreted with these low exposures in mind.
In vitro studies have shown that cobalt alloy particles (WC-Co and Co-Cr-Mo) as well as soluble cobalt ions can induce the NLRP3 inflammasome and release of IL-1β (Caicedo et al. Citation2010, Oblak et al. Citation2015, Armstead and Li Citation2016, Klasson et al. Citation2021). In the present study, no significant relationships between inhalable particle exposure – either for the inhalable dust or inhalable cobalt exposure measures – and inflammasome activity were found either in plasma cytokine levels or by ex vivo experiments on whole blood. This might be explained by the fact that exposure levels to inhalable dust and to cobalt particles in this study are below the threshold limit that might be required to affect the NLRP3 inflammasome. In a similar study performed in an iron foundry environment, we found a correlation between dust exposure and caspase-1 enzymatic activity (Hedbrant et al. Citation2020). The difference in dust levels and dust composition may explain these differences, as the iron foundry environment had a 30-times-higher average exposure to dust, which also contained quartz, another known activator of the NLRP3 inflammasome.
The inhalable dust and the inhalable cobalt exposure levels did not correlate with any of the studied WBC counts nor with cell ratios. In a previous study that included 82 cobalt-exposed workers at a cobalt refinery, an increase in total WBC counts compared to the control group was found (Swennen et al. Citation1993); however, in that study, considerably higher cobalt exposure was reported compared to in our study, with a median cobalt air exposure of 0.01 mg/m3 and about 25% of exposures above 0.5 mg/m3, which could explain the difference in results.
In addition to inhalable particulate exposure, systemic cobalt exposure measures, including cobalt concentrations in blood and urine, were investigated. We have previously shown that both skin uptake and inhalable exposure to cobalt contribute to the total systemic cobalt exposure within the hard metal industry, as there are significant correlations between both exposures and cobalt levels in blood (Klasson et al. Citation2017, Wahlqvist et al. Citation2020). Thus, cobalt in blood and urine can be a useful measure of the total systemic exposure to which blood cells and endothelia, among other cells, are exposed. In general, cobalt levels in urine can be used as an indicator of acute exposure. Cobalt exposure will generate a rapid increase in cobalt concentration in urine that peaks some hours after exposure, and the elevated cobalt levels will rapidly decrease within the following 24 hours (Krug et al. Citation2014). In contrast, cobalt in blood is eliminated slowly, with an estimated half-life of 12 days, and can thus be used as an indicator of average chronic exposure levels (Princivalle et al. Citation2017). Cobalt exposure in the hard metal industry is well established as a source of elevated cobalt levels in blood and urine, but other sources cannot be excluded, including dietary intake.
The average cobalt blood concentration was around 7 nM, ranging from 4 to 14 nM, or 0.2–0.8 µg/L, and corresponding values for cobalt in urine was around 40 nM on average, ranging from 3 to 250 nM or 0.2 to 15 µg/L. Sweden lacks thresholds on cobalt levels in biological fluids; however, the American Conference of Governmental Industrial Hygienists has established recommended reference values of 1 and 15 µg/L for cobalt in blood and urine, respectively (ACGIH Citation2016). In this regard, all measurements in this study were at or below the suggested limits for cobalt in these body fluids. Regarding the NLPR3 inflammasome-associated markers, IL-18 was found to significantly correlate with cobalt concentration in blood, revealing an inverse relationship. Similar results were observed for morning as well as afternoon samples, giving an estimated decrease of about 10% in IL-18 plasma concentration per 10 nM increase in blood cobalt levels. These results, across pre- and post-work shift sampling, are reasonable given that both IL-18 and blood cobalt levels were similar across both time points. The immune suppressive role of cobalt ions on cytokine release are in line with a number of in vitro studies showing immune suppression by cobalt ions in biologically relevant nM/low µM range, i.e. inhibition of IL-2, IL-6, and interferon gamma release from PBMCs (Wang et al. Citation1996a), and inhibition of transforming growth factor B release from monocytes (Wang et al. Citation1996b). On the other hand, at higher doses, i.e. 100 µM and above, studies demonstrate induced inflammatory responses, e.g. increased release of IL-8 and CXCL-10 from monocytes (Lawrence et al. Citation2014), and activation of the TLR4 receptor (Oblak et al. Citation2015). The high doses of cobalt mentioned above could also induce hypoxic reactions of the cells, as these doses are commonly used to mimic hypoxia in cell culture under normoxic conditions (Muñoz-Sánchez and Chánez-Cárdenas Citation2019). Thus, cobalt may have different immune regulatory mechanisms depending on high vs low cobalt concentrations; however, more insight is needed into cobalt levels and their regulation on immune functions, especially in vivo. Previous studies on cobalt ions and IL-18 are scarce. There is one study of a reconstructed epidermis model demonstrating increased IL-18 release upon cobalt ion exposure (Gibbs et al. Citation2018), but to our knowledge, there are no studies on systemic cobalt ion exposure and IL-18.
For the white blood counts, a significant correlation for both NLR and LMR and cobalt concentrations, in blood and urine sampled pre-shift, was found. The NLR ratio was reduced, whereas the LMR increased in relation to elevated cobalt concentrations in blood and urine. The NLR ratio was developed due to an increase in neutrophils, usually observed during increased systemic inflammatory conditions, while lymphocyte counts tend to stay somewhat unaffected, giving rise to a higher NLR ratio. Elevated NLR is generally associated with worse outcomes when used as a prognostic tool for e.g. cancer and CVD (Angkananard et al. Citation2018, Cupp et al. Citation2020). Similarly, a lower LMR ratio is generally associated with worse outcomes for a number of diseases, including e.g. cancers and coronary artery disease (Chan et al. Citation2017, Gong et al. Citation2018). A reduced NLR, together with increased LMR, as observed by increasing cobalt blood and urine levels in this study, would therefore generally be considered as a reduced systemic inflammatory state; however, in this study the lymphocyte counts were positively correlated with cobalt concentration in urine, and the same trend was observed for cobalt in blood, while neutrophils and monocytes were not significantly correlated. These changes are opposite to the changes generally expected when using the ratios for inflammatory assessment and, therefore, any possible health implications of these findings would need further studies.
Cobalt levels in blood and urine were not significantly associated with WBC counts or any individual cell population except for lymphocyte counts and cobalt urine concentration. A study on 1,900 metal-on-metal (MoM) hip replacement patients found no correlation for cobalt blood levels and white WBC counts or specific WBC populations (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) (Honkasaari et al. Citation2020). However, two other studies of MoM arthroplasty patients have found correlation between cobalt blood levels and lymphocyte subsets, although in contrast to our study, a reduction in specific T-cell subsets, primarily CD8+ T-cells with increased cobalt blood levels were observed (Hart et al. Citation2009, Penny et al. Citation2013).
We have previously published results of markers of inflammation and exposures to dust or cobalt from this cohort (Andersson et al. Citation2021). In line with the results of the current study, indicating no or even dampened inflammatory responses to exposure, previous studies have found no correlation for IL-6, IL-8, tumour necrosis factor, C-reactive protein, or Serum amyloid A and exposures to dust or cobalt. However, some particulate matter exposure measures were associated with an increase in the lung specific marker CC16. This could indicate that the inhalable dust exposures are below the limit required to generate a systemic inflammatory response, but still enough to have some local effects in the lungs.
A potential limitation of the study is the lack of a completely unexposed control group. Instead, in order to reveal potential health effects of occupational cobalt exposure, this study was designed to correlate levels of exposure to inflammatory responses, rather than comparing the exposed group to a control group.
In conclusion, no correlation between inhalable dust or inhalable cobalt levels and inflammasome-associated outcomes or cell counts was found. Cobalt levels in blood and urine, which also take skin exposure into account, correlated with reduced IL-18 plasma concentration and increased NLR as well as decreased LMR. The significant findings were primarily observed in the pre-shift samples, indicating that the effects more likely arise from chronic long-term exposure rather than acute exposure. Any health implications of the results would need further studies.
Author contributions
Alexander Hedbrant: conceptualization, writing – original draft, methodology, investigation, visualization, project administration, funding acquisition. Daniel Eklund: methodology, investigation, writing – review and editing. Lena Andersson: methodology, investigation, project administration, writing – review and editing, funding acquisition. Ing-Liss Bryngelsson: methodology, formal analysis, writing – review and editing. Alexander Persson: conceptualization, writing – review and editing, funding acquisition. Håkan Westberg: conceptualization, methodology, writing – review and editing, funding acquisition. Eva Särndahl: supervision, conceptualization, writing – review and editing, funding acquisition.
Acknowledgements
We would like to acknowledge the support from the representatives of participating companies and we would like to thank the employees, who provided assistance throughout the study.
We would like to thank Annette Ericsson, Ina Lindell, Anders Johansson, Jessica Westerlund, Louise Fornander and Lennart Andersson at the Department of Occupational and Environmental Medicine, Örebro, for their contribution to air and blood sampling, and Kristine Midtbö at Inflammatory Response and Infection Susceptibility Centre (iRiSC), Örebro University for valuable support with blood sampling, storage, and analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s). The participating companies and the funders had no role in the study design, data collection, and analysis, preparation of the manuscript, or decision to publish.
Data availability statement
Data cannot be shared publicly due to legal restrictions imposed by Swedish Law regarding identifiable data. Data access requests can be directed to Etikprövningsmyndigheten i Uppsala (Swedish Ethical Review Authority in Uppsala): https://etikprovningsmyndigheten.se or [email protected]. Previously reported inhalable dust and cobalt exposure measurement data were used to support this study (Andersson et al. Citation2020, Andersson et al. Citation2021). This prior study is cited at relevant places within the text.
Additional information
Funding
References
- ACGIH, 2016. American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 114.
- Afari, M.E., and Bhat, T., 2016. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert review of cardiovascular therapy, 14 (5), 573–577.
- Andersson, L., et al., 2020. Respiratory health and inflammatory markers-exposure to cobalt in the Swedish hard metal industry. Journal of occupational and environmental medicine, 62 (10), 820–829.
- Andersson, L., et al., 2021. Inflammatory and coagulatory markers and exposure to different size fractions of particle mass, number and surface area air concentrations in the Swedish hard metal industry, in particular to cobalt. Biomarkers, 26 (6), 538–557.
- Angkananard, T., et al., 2018. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. BioMed research international, 2018, 2703518–2703518.
- Armstead, A.L., and Li, B., 2016. In vitro inflammatory effects of hard metal (WC-Co) nanoparticle exposure. International journal of nanomedicine, 11, 6195–6206.
- Caicedo, M.S., et al., 2010. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. Journal of biomedical materials research. Part A, 93 (4), 1312–1321.
- Caicedo, M.S., et al., 2013. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. Journal of orthopaedic research, 31 (10), 1633–1642.
- Cassel, S.L., et al., 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proceedings of the National Academy of Sciences of the United States of America, 105 (26), 9035–9040.
- Chan, J.C.Y., et al., 2017. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resecs colorectal cancer. Annals of surgery, 265 (3), 539–546.
- Cohen, A.J., et al., 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The lancet, 389 (10082), 1907–1918.
- Cupp, M.A., et al., 2020. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC medicine, 18 (1), 360–360.
- Ebert, B., and Jelkmann, W., 2014. Intolerability of cobalt salt as erythropoietic agent. Drug testing and analysis, 6 (3), 185–189.
- Ferko, M.A., and Catelas, I., 2018. Effects of metal ions on caspase-1 activation and interleukin-1β release in murine bone marrow-derived macrophages. PLOS one, 13 (8), e0199936.
- Gibbs, S., et al., 2018. Assessment of metal sensitizer potency with the reconstructed human epidermis IL-18 assay. Toxicology, 393, 62–72.
- Golia, E., et al., 2014. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Current atherosclerosis reports, 16 (9), 435.
- Gong, S., et al., 2018. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine, 97 (43), e12813-e12813.
- Guan, L., et al., 2019. PM(2.5) exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environmental toxicology., 34 (4), 530–538.
- Hart, A.J., et al., 2009. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J bone joint surg Br, 91-B (6), 835–842.
- Hedbrant, A., et al., 2020. Quartz dust exposure affects NLRP3 inflammasome activation and plasma levels of IL-18 and IL-1Ra in iron foundry workers. Mediators of inflammation, 2020, 1–10.
- Honkasaari, N., et al., 2020. No association between blood count levels and whole-blood cobalt and chromium levels in 1,900 patients with metal-on-metal hip arthroplasty. Acta orthopaedica, 91 (6), 711–716.
- Huang, Y., et al., 2018. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. International immunopharmacology., 57, 43–46.
- IARC, 2006. IARC Monographs on the evaluation of carcinogenic risks to humans. International agency for research on cancer, 86, 35–133.
- Kelley, N., et al., 2019. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. International journal of molecular sciences, 20 (13), 3328.
- Klasson, M., et al., 2017. Biological monitoring of dermal and air exposure to cobalt at a Swedish hard metal production plant: does dermal exposure contribute to uptake? Contact dermatitis, 77 (4), 201–207.
- Klasson, M., et al., 2021. Dermal exposure to cobalt studied in vitro in keratinocytes - effects of cobalt exposure on inflammasome activated cytokines, and mRNA response. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 26 (8), 637–674.
- Krug, O., et al., 2014. Quantifying cobalt in doping control urine samples-a pilot study. Drug testing and analysis, 6 (11–12), 1186–1190.
- Lawrence, H., et al., 2014. The immunobiology of cobalt: demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip. The bone & joint journal, 96-b (9), 1172–1177.
- Leyssens, L., et al., 2017. Cobalt toxicity in humans-a review of the potential sources and systemic health effects. Toxicology, 387, 43–56.
- Linna, A., et al., 2004. Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart. Occupational and environmental medicine, 61 (11), 877–885.
- Liu, Y., et al., 2017. Total and cause-specific mortality risk associated with low-level exposure to crystalline silica: a 44-year cohort study from China. American journal of epidemiology, 186 (4), 481–490.
- Martinon, F., et al., 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440 (7081), 237–241.
- Muñoz-Sánchez, J., and Chánez-Cárdenas, M.E., 2019. The use of cobalt chloride as a chemical hypoxia model. Journal of applied toxicology, 39 (4), 556–570.
- Oblak, A., Pohar, J., and Jerala, R., 2015. MD-2 determinants of nickel and cobalt-mediated activation of human TLR4. PLOS one, 10 (3), e0120583.
- Penny, J., et al., 2013. Metal ion levels and lymphocyte counts: ASR hip resurfacing prosthesis vs. standard THA: 2-year results from a randomized study. Acta orthopaedica, 84 (2), 130–137.
- Perlman, D.M., and Maier, L.A., 2019. Occupational Lung Disease. The medical clinics of North America, 103 (3), 535–548.
- Pope, C.A., 3rd., et al., 2016. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circulation research, 119 (11), 1204–1214.
- Princivalle, A., et al., 2017. Biological monitoring of cobalt in hard metal factory workers. International archives of occupational and environmental health, 90 (2), 243–254.
- Rückerl, R., et al., 2014. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environment international, 70, 32–49.
- Swennen, B., et al., 1993. Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. British journal of industrial medicine, 50 (9), 835–842.
- Valavanidis, A., et al., 2013. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. International journal of environmental research and public health, 10 (9), 3886–3907.
- Wahlqvist, F., et al., 2020. Dermal and inhalable cobalt exposure—uptake of cobalt for workers at Swedish hard metal plants. PLOS one, 15 (8), e0237100.
- Wang, J.Y., et al., 1996a. Inhibition of T and B cell proliferation by titanium, cobalt, and chromium: role of IL-2 and IL-6. Journal of biomedical materials research, 32 (4), 655–661.
- Wang, J.Y., et al., 1996b. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials, 17 (23), 2233–2240.
