 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The developmental origins of handedness remain elusive, though very early emergence suggests individual differences manifesting in utero could play an important role. Prenatal testosterone and Vitamin D exposure are considered, yet findings and interpretations remain equivocal. We examined n = 767 offspring from a population-based pregnancy cohort (The Raine Study) for whom early biological data and childhood/adolescent handedness data were available. We tested whether 18-week maternal circulatory Vitamin D (25[OH]D), and testosterone and estradiol from umbilical cord blood sampled at birth predicted variance in direction of hand preference (right/left), along with right- and left-hand speed, and the strength and direction of relative hand skill as measured by a finger-tapping task completed at 10 (Y10) and/or 16 (Y16) years. Although higher concentrations of Vitamin D predicted more leftward and less lateralized (regardless of direction) relative hand skill profiles, taken as a whole, statistically significant findings typically did not replicate across time-point (Y10/Y16) or sex (male/female) and were rarely detected across different (bivariate/multivariate) levels of analysis. Considering the number of statistical tests and generally inconsistent findings, our results suggest that perinatal testosterone and estradiol contribute minimally, if at all, to subsequent variance in handedness. Vitamin D, however, may be of interest in future studies.
Introduction
Consistent limb preferences have been reported in a range of non-human species (Ocklenburg, Isparta, Peterburs, & Papadatou-Pastou, Citation2019; Ströckens, Güntürkün, & Ocklenburg, Citation2013), but the distribution of handedness observed in Homo sapiens is striking. The vast majority of people show preference for the right hand for most tasks, with a recent meta-analysis (k = 262; n = 2,396,170) estimating the prevalence of left-handedness at 10.6% (Papadatou-Pastou et al., Citation2020). As the right hand is primarily controlled by the left motor cortex and the left hand by the right motor cortex, handedness can be considered the most salient and easily measurable estimate of cerebral lateralization (Beaton, Citation2003; Ocklenburg & Güntürkün, Citation2018). The phenomenon is therefore of interest to researchers working in various disciplines, and studies in this area are often motivated by the potential for advancing our understanding of developmental and psychiatric conditions associated with atypical patterns of cerebral asymmetry (Cuellar-Partida et al., Citation2021; Ocklenburg & Güntürkün, Citation2018; Ocklenburg, Berretz, Packheiser, & Friedrich, Citation2020).
Behavioural genetics studies suggest that approximately 25% of variance in handedness is explained by genetic factors, and that little, if any, is explained by shared environment (Medland, Duffy, Wright, Geffen, & Martin, Citation2006, Citation2009; Schmitz et al., Citation2021; Somers et al., Citation2015). Much of the residual variance likely reflects random developmental noise (McManus, Citation2021) and a certain amount of measurement error. However, as the direction of hand preference (i.e., right or left) is observable from infancy (Campbell, Marcinowski, & Michel, Citation2018; Michel & Harkins, Citation1986; Nelson, Campbell, & Michel, Citation2014), and perhaps even from the prenatal period (Hepper, Citation2013), it is plausible that individual differences in utero also play a role. Indeed there are reports that handedness is associated with birth weight, multiple births, maternal anxiety, birth order, birth stress, and the presence/absence of breastfeeding, as well as with the country, year, and season of birth (Beaton, Citation2003; de Kovel, Carrión-Castillo, & Francks, Citation2019). Together these data suggest that examining the environment present in utero may provide critical insight into the development of handedness.
There has been much interest in the association between handedness and season of birth, with results from this research potentially indicating a role for early Vitamin D exposure. However, findings from individual studies are inconsistent, (e.g., de Kovel et al., Citation2019; Leviton & Kilty, Citation1979; Martin & Jones, Citation1999; Nicholls, Citation1998; Stoyanov, Nikolova, & Pashalieva, Citation2011; Tran, Stieger, & Voracek, Citation2014) and some have observed no association between handedness and birth month (e.g., Cosenza & Mingoti, Citation1995; McManus, Citation1980; Milenković, Rock, Dragović, & Janca, Citation2008; Tonetti, Adan, Caci, Fabbri, & Natale, Citation2012). A meta-analysis by Jones and Martin (Citation2008) (k = 9; n = 39,379) reported a relatively high incidence of left-handedness in men born in the spring and early summer (March–July in the northern hemisphere; September–January in the southern hemisphere), though this effect could have been confounded by the small number of samples included as well as by the heterogeneous methods used for classifying handedness (see Beaton, Citation2008). More recently, de Kovel et al. (Citation2019) utilized a very large sample (n = 421,667) from the British Biobank and reported a slight excess of left-handedness in women born in summer; the peak for males was smaller and appeared in the autumn months. It is unclear why such effects occur, though seasonal variations in temperature and exposure to infectious agents (Leviton & Kilty, Citation1979), maternal anxiety (Jones & Martin, Citation2008), or Vitamin D could potentially act as mediators. Vitamin D is of interest here because relatively low concentrations in early life have been linked to autism (Kočovská, Fernell, Billstedt, Minnis, & Gillberg, Citation2012), language impairment (Whitehouse et al., Citation2012a) and schizophrenia (Cui, McGrath, Burne, & Eyles, Citation2021), all conditions that are associated with increased left-handedness (autism: Markou, Ahtam, & Papadatou-Pastou, Citation2017; language impairment: Abbondanza et al., Citation2022; schizophrenia: Hirnstein & Hugdahl, Citation2014). Additionally, Vitamin D levels correlate positively with testosterone (Wehr, Pilz, Boehm, März, & Obermayer-Pietsch, Citation2010), another possible mediator of the association between handedness and birth month (Geschwind & Galaburda, Citation1987; Nicholls, Citation1998), albeit the association is weak (D’Andrea et al., Citation2021).
The possibility that prenatal testosterone exposure plays a causal role in the development of handedness has been discussed in the scientific literature for nearly half a century, with several influential theories making competing predictions. The sexual differentiation hypothesis (Hines & Shipley, Citation1984; Levy & Gur, Citation1980) and the Geschwind-Behan-Galaburda (GBG) theory (Geschwind & Behan, Citation1982; Geschwind & Galaburda, Citation1985a, Citation1985b, Citation1985c, Citation1987) both posit that high levels of prenatal testosterone increase the likelihood of left-handedness. Predictions can also be derived from these theories that elevated prenatal testosterone will reduce the strength of lateralization, regardless of its direction (see Richards et al., Citation2021a). On the other hand, the callosal hypothesis (Witelson & Goldsmith, Citation1991; Witelson & Nowakowski, Citation1991) predicts that elevated prenatal testosterone increases the likelihood of right-handedness (at least in males). Lust et al. (Citation2011) extended this theory to predict that high levels of prenatal testosterone will result in relatively strong lateralization patterns (regardless of their direction), though the results obtained from their empirical study directly contradicted this idea (see also Richards et al., Citation2021a).
A range of methods has been applied to examine the effects of prenatal testosterone exposure on handedness. These include studying congenital adrenal hyperplasia (CAH; a suite of autosomal recessive conditions characterized by elevated prenatal androgen production), comparing same-sex (SS) and other-sex (OS) twins (the idea being that testosterone transferred via the amniotic fluid results in elevated exposure in females with a male as opposed to a female co-twin), retrospective examination of the effects of synthetic oestrogen administered during pregnancy (e.g., diethylstilbestrol [DES]), and correlating naturally varying testosterone levels assayed from amniotic fluid with subsequent handedness measures. A recent review of such research revealed a confusing literature replete with inconsistent findings and replication failures (Richards et al., Citation2021a), an observation that corroborates the non-significant effect size estimate determined by an earlier meta-analytic study (Pfannkuche, Bouma, & Groothuis, Citation2009). Similarly, a meta-analysis by Richards et al. (Citation2021b) of studies utilizing the second to fourth digit length ratio (2D:4D) as a proxy for prenatal testosterone exposure (see Manning, Citation2002; Manning, Scutt, Wilson, & Lewis-Jones, Citation1998) reported very small effect size estimates. Notably, the direction of correlation between left-handedness and right hand 2D:4D was negative whereas that between left-handedness and left hand 2D:4D was positive, diametrically opposite in terms of the implied effects of prenatal androgens.
Although the pattern of findings observed in the literature may suggest that prenatal testosterone is not a significant predictor of subsequent hand- edness outcomes, there are considerable validity issues regarding 2D:4D (e.g., Hickey et al., Citation2010; Hollier et al., Citation2015; Richards, Browne, & Constantinescu, Citation2021; Richards, Medland, & Beaton, Citation2021a) and studies using more direct methods have typically utilized sample sizes that lack the statistical power required to detect small or even medium sized effects. Furthermore, research relating to rare medical conditions (e.g., CAH), prenatal exposures (e.g., DES), complicated pregnancies (OS/SS twins) and samples that are not fully representative (e.g., children whose mothers underwent amniocentesis) may lack wider generalizability. One method that may be able to overcome these concerns, however, is to measure testosterone from umbilical cord blood sampled immediately after birth (Hollier, Keelan, Hickey, Maybery, & Whitehouse, Citation2014).
Tan and Tan (Citation2001) examined testosterone (both as total testosterone and as free testosterone, i.e., that which is unbound to sex hormone binding globulin [SHBG]) sampled from the umbilical artery in relation to neonatal grasp-reflex strength measured at 3–5 days post-partum in 116 full-term neonates (55 male, 61 female). Handedness (right or left) was defined in terms of which hand produced the stronger grasp-reflex. The study reported that right-handed males (n = 39) and right-handed females (n = 32) had higher concentrations of unbound testosterone than left-handed males (n = 16) and left-handed females (n = 29), respectively. Examination of the relative grasp-reflex strength revealed that stronger right-handedness increased with higher unbound testosterone levels; conversely, unbound testosterone levels correlated negatively with grasp-reflex strength for the left hand in males, and for both hands in females. This pattern of findings is not wholly consistent with any of the main theories linking testosterone with handedness (i.e., sexual differentiation, GBG, callosal), and indeed, no statistically significant associations were observed when examining total testosterone rather than free testosterone. It is also difficult to infer the meaning of these results. Considering that grasp-reflex strength is rarely examined in this literature, its relationship with other handedness measures is not well established, and handedness in general remains labile at such an early stage of infant development.
Although most research has focussed primarily on testosterone, other sex steroid hormones may play some role in the development of handedness. Three decades ago, Witelson (Citation1991, p. 144) noted
“It is not yet evident what the neuroanatomical substrate of handedness is in women and what role estrogen or other sex hormones may have in women in determining variations in structure related to handedness and other aspects of functional asymmetry”,
and this is still true now. However, there has been recent interest in the role that estradiol could play in neurodevelopment (Baron-Cohen et al., Citation2020; Schultheiss, Köllner, Busch, & Hofer, Citation2021; Tsompanidis et al., Citation2021), and recent studies have linked amniotic estradiol concentrations with aspects of functional asymmetry (Beking, Citation2018, Chapter 4) and handedness (Richards et al., Citation2021a). More specifically regarding handedness, high levels of estradiol in the amniotic fluid predicted relatively weak hand preference in females assessed at age 15 years with a modified Dutch language version (van Strien, Citation2002) of the Edinburgh Handedness Inventory (Oldfield, Citation1971). However, no such effect was observed for males, and estradiol did not correlate with relative hand skill as assessed via the Annett pegboard task (Annett, Citation1970).
Considering the inconsistent pattern of findings from studies examining early hormone exposure in relation to handedness, in the present investigation we used data from an Australian longitudinal pregnancy cohort study, The Raine Study, to test whether testosterone and estradiol measured in perinatal umbilical cord blood, as well as the level of Vitamin D present in the maternal circulation at 18-weeks’ gestation, were predictive of a range of handedness outcomes measured at 10- and/or 16-years of age. We pre-registered our analysis plan on the Open Science Framework (https://osf.io/rngq8) and hypothesised that perinatal testosterone, estradiol and Vitamin D would each be significant predictors of handedness. However, due to the equivocal pattern of findings in this literature, and due to the competing predictions posited by various theories, we did not specify directions of effect.
Method
Participants
Participants were part of The Raine Study (McKnight et al., Citation2012), which is an ongoing longitudinal, population-based examination of women and their offspring recruited from the public antenatal clinic at King Edward Memorial Hospital or surrounding private clinics in Perth, Western Australia. Between May 1989 and November 1991, 2,900 women were enrolled into the study if they were between 16 and 20 weeks pregnant with sufficient command of the English language to comprehend the study demands, a plan to deliver at King Edward Memorial Hospital, and an intention to continue residence in Western Australia (to enable future study of their child). By the end of the recruitment period, 2,868 live births (96%) were available for follow-up. Informed written consent was obtained from parents during initial recruitment and at each follow-up on behalf of their participating child who then provided their own consent after they turned 18 years of age. The study protocols were approved by the Human Research Ethics Committee at King Edward Memorial Hospital and/or Princess Margaret Hospital in Perth, Western Australia.
Measures
Maternal 25(OH)-Vitamin D
Venous blood was collected from 929 randomly selected women who were 18 weeks pregnant. Blood specimens were centrifuged, and serum was collected and stored at −80°C. In June 2011, serum 25(OH)-Vitamin D concentrations were measured using a commercial enzyme immunoassay kit (Scottsdale, AZ). Twenty-eight serum samples were also measured using isotope-dilution liquid chromatography tandem mass spectrometry by RMIT Drug Discovery Technologies (Melbourne, Australia). Levels of 25(OH)-Vitamin D measured using immunoassay strongly correlated with those of mass spectrometry, R2 = 0.87 (Whitehouse et al., Citation2012a) thus, confirming the low likelihood of molecules in the blood serum samples that may have interfered with the immunoassay of 25(OH)-Vitamin D.
Perinatal sex steroids
Mixed arterial and venous umbilical cord blood was obtained at the birth of n = 861 randomly selected deliveries. Blood samples were immediately centrifuged, plasma isolated, and then stored at −80°C. Detailed sequence analysis of DNA obtained from 10 mother–child pairs confirmed that the cord blood samples were not contaminated by maternal blood (Keelan et al., Citation2012). In January 2010, cord serum samples were thawed and aliquoted. Androgens including total testosterone (TT), Δ4-androstenedione (A4), and dehydroepiandrostenedione (see Keelan et al., Citation2012), and estrogens including estrone (E1), estradiol (E2), estriol (E3) and estetrol (E4) (see Hickey, Hart, & Keelan, Citation2014) were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) after solvent extraction. SHBG was assayed by ELISA using a commercial kit (IBL International, Hamburg, Germany) according to the manufacturer’s instructions. All samples were measured in duplicate by a single operator using assay kits from the same batch. Samples with an initial replicate coefficient of variability (CV) of >10% were reanalysed. The inter-assay imprecision (CV) was <4.5% (n = 25) and intra-assay CV was 5.2% (n = 861) (Keelan et al., Citation2012). In the current study we examined specifically free testosterone and total estradiol. Free testosterone was calculated for participants in The Raine Study (Hollier et al., Citation2015) as that un-sequestered by SHBG using the method described by Keelan et al. (Citation2012; see also Sartorius, Ly, Sikaris, McLachlan, & Handelsman, Citation2009).
Hand preference and relative hand skill
The McCarron Assessment of Neuromuscular Development (MAND; McCarron, Citation1997) is a standardized, quantitative, and reliable method of assessing motor proficiency designed for use with children between 3 and 16 years (Hands, Larkin, & Rose, Citation2013). A trained assessor administered the MAND to The Raine Study cohort at 10, 14, and 16 year follow-ups (Hands et al., Citation2013). Children’s hand preference was recorded via a single item measure, “Preferred Hand”, with response options being “Right” and “Left” (McCarron, Citation1997, p. 28). The assessment also involved 10 tasks examining children’s fine motor skills (beads in box, beads on rod, finger tapping, nut and bolt, and rod slide) and gross motor skills (hand strength, finger-nose-finger, jumping, heel-toe walk, and standing on one foot). We chose finger tapping from the 10 year (Y10) and 16 year (Y16) follow-ups as an index of relative hand skill because many previous studies in the laterality literature (e.g., Parker, Woodhead, Thompson, & Bishop, Citation2021; Peters & Durding, Citation1978; Tzourio Mazoyer et al., Citation2015) have utilized similar measures, whereas the other tasks that comprise the MAND have rarely (if at all) been examined in relation to handedness. We derived a laterality index (LI) using the following formula:
in which R and L are the number of finger taps made by the right and left index fingers, respectively, during a 10-second interval. The resulting values (signed LI) indicate both the direction and strength of relative hand skill; scores > 0 indicate faster right hand (relative to left hand) speed and scores further from 0 (either positive or negative) indicate stronger lateralization of relative hand skill. We also examined the unsigned (absolute) LI, which indicates the strength of relative hand skill regardless of its direction (higher scores indicating stronger lateralization). Additionally, right- and left-hand speed (measured by finger tapping scores for each hand) was also of interest (see Richards et al., Citation2021a).
Statistical analyses
In our pre-registered analysis plan (https://osf.io/rngq8) we hypothesised that handedness outcomes would be stable across the Y10 and Y16 follow-ups. We conducted a chi-square test to determine the level of association between self-reported hand preference (right or left), and used bootstrapped (10,000 resamples) Pearson’s correlations and paired-samples t tests to determine the level of association and difference for the continuous handedness outcomes (right-hand speed, left-hand speed, direction of relative hand skill, and strength of relative hand skill). We predicted that each of these measures would correlate positively across the time-points, that there would be no difference between them for direction or strength of relative hand skill, and that right- and left-hand speed would be faster at Y16 than Y10.
We transformed testosterone scores via natural logarithm (ln). Although bootstrapping does not assume a normal distribution of the error terms, there were some values that would be considered large outliers within the context of a normal distribution, which might still have exerted an undue influence (see for histograms of raw scores and log transformed scores). Although not pre-registered, we computed bootstrapped (10,000 samples) Pearson’s correlations, stratified by sex, between hormonal predictors (testosterone, estradiol, and Vitamin D) and handedness outcomes (hand preference [right or left], right-hand speed, left-hand speed, direction of relative hand skill, and strength of relative hand skill). (Note that when hand preference is the outcome, correlations are point-biserial.) Our reasoning for including these exploratory analyses is that they may be more directly comparable to findings from other studies that have not controlled for covariates statistically.
Figure 1. Histograms of the raw (a) and natural log transformed (b) scores for perinatal umbilical cord free testosterone.
We next conducted bootstrapped (10,000 samples) regression models with handedness variables as outcomes. Binary logistic regression was used when the outcome was dichotomous (hand preference: right or left) and linear regression was used when the outcome was continuous (right-hand speed, left-hand speed, direction of relative hand skill, strength of relative hand skill). These models each controlled for sociodemographic information recorded at 18 weeks’ pregnancy, antenatal factors recorded at 34 weeks’ pregnancy, and obstetric variables recorded at birth. We conducted hierarchical analyses with three steps (Step 1: enter covariates; Step 2: enter testosterone, testosterone × sex, estradiol, and estradiol × sex; Step 3: enter Vitamin D and Vitamin D × sex). The covariates included relate broadly to sociodemographic, obstetric, and antenatal variables. More specifically, these were delivery mode (presence or absence of labour), gestational age at delivery (number of days), maternal age at conception (years and months), birth weight (grams), maternal smoking during pregnancy (any vs. none), maternal alcohol consumption during pregnancy (any vs. none), maternal/paternal hand preference (one/both parent[s] vs. neither parent writes with left/either hand), family income ([1] less than AUD$7,000, [2] AUD$7,000-11,999, [3] AUD$12,000-23,999, [4] AUD$24,000-35,000, [5] more than AUD$36,000), parental education (average of maternal and paternal education: [0] none, [1] trade certificate or apprenticeship, [2] professional registration [non-degree], [3] college diploma or degree, [4] university degree), age at relevant assessment (years and months), sex (male or female), and Australian season of birth (spring [1; September, October, November], summer [2; December, January, February], autumn [3; March, April, May], or winter [4; June, July, August]). We also conducted exploratory multivariate analyses for Vitamin D. This was to increase statistical power, as the samples for which Vitamin D and testosterone and/or estradiol had been measured only overlapped partially. For these, Vitamin D and Vitamin D × sex were entered as predictors, along with the previously specified covariates, in a single-step simultaneous entry bootstrapped (10,000 samples) multiple regression model.
Statistical analyses were run using RStudio (Version 1.3.1073). All tests were two-tailed, and statistical significance was determined by p < 0.05 (non-bootstrapped analyses) or via bootstrapped 95% confidence intervals (CIs) that did not overlap with zero.
Results
Description of the sample and key variables
Of the original Raine Study cohort that was available for follow-up (N = 2,868), perinatal testosterone, estradiol, and Vitamin D data were measured for n = 827 (28.8%), n = 860 (30.0%), and n = 928 (32.4%), respectively. Both testosterone and estradiol were available for n = 827. However, of participants for whom Vitamin D had been assayed, testosterone and estradiol data were available for only n = 565 and n = 591, respectively. Handedness data (at least one outcome) were available for n = 1,644 (57.3%) at Y10 and n = 1,248 (43.5%) at Y16 (data from both follow-ups were available for n = 1,091).
Except where specified otherwise, we restricted our analyses to those participants for whom at least one perinatal hormone measure (testosterone, estradiol, or Vitamin D) and at least one handedness outcome (hand preference, right-hand speed, left-hand speed, direction of relative hand skill, strength of relative hand skill [from either or both of Y10 and Y16]) were available (n = 767). Descriptive statistics for this subsample, both as a whole and stratified by sex, are presented in . We did not compare this subsample with the wider cohort because those participants for whom testosterone and estradiol were assayed (Whitehouse et al., Citation2012b), as well as those for whom maternal Vitamin D samples were obtained (Whitehouse et al., Citation2012a), have already been shown to be fairly representative of the wider Raine Study cohort in terms of sociodemographic and obstetric characteristics.
Table 1. Descriptive statistics for hormonal, sociodemographic, obstetric, antenatal, and handedness variables.
Stability of handedness across childhood/adolescence
Of those who reported being right-handed at Y10, only 1 reported being left-handed at Y16; likewise, of those who reported being left-handed at Y10, only 3 reported being right-handed at Y16. Hand preference across these timepoints therefore correlated almost perfectly, χ2 (1, N = 464) = 424.875, p < 0.001, Cramér’s V = 0.957. Correlations across time-points were small-to-moderate for right-hand speed, r (453) 0.259 (BCa 95% CI: 0.178–0.339), and left-hand speed, r = (456) = 0.296 (BCa 95% CI: 0.207–0.379), but small and non-significant for relative hand skill direction, r = (450) = 0.071 (BCa 95% CI: −0.095–0.183), and relative hand skill strength, r (450) = 0.079 (BCa 95% CI: −0.019–0.248). Given that these latter two variables are expected to correlate when measured for the same participants at different time-points, we performed additional (not pre-registered) analyses to determine whether this was the case for the whole Raine Study cohort. For this analysis we did observe statistically significant, though very small, positive correlations for both direction, r (1061) = 0.088 (BCa 95% CI: 0.002–0.156), and strength of relative hand skill, r (1061) = 0.067 (BCa 95% CI: 0.008–0.151).
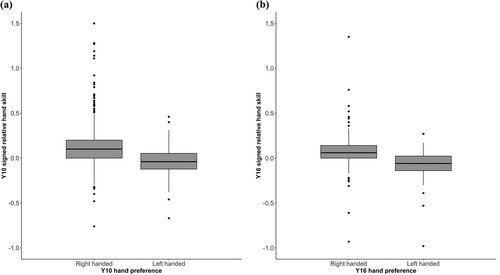
The number of taps made with the right hand at Y16 (M = 39.73, SD = 7.35) was significantly greater than that recorded at Y10 (M = 35.77, SD = 7.22), t(454) = −9.535, d = −0.544 (mean difference = −3.963, percentile 95% CI: −4.776 – −3.147), and a comparable effect was observed for the left hand (Y16: M = 37.72, SD = 6.97; Y10: M = 32.47, SD = 7.24), t(457) = −13.350, d = −0.740 (mean difference = −5.260, percentile 95% CI: −6.028 – −4.463). The mean (signed) relative hand skill LI at Y10 (M = 0.10, SD = 0.22) was significantly higher than at Y16 (M = 0.05, SD = 0.15), t(451) = 3.900, d = 0.250 (mean difference = 0.048, percentile 95% CI: 0.024–0.072), reflecting a larger difference at Y10 between the performance of the two hands in favour of the right hand. This was due to participants’ left-hand speed improving relatively more than their right-hand speed from Y10 to Y16. Likewise, the unsigned relative hand skill LI decreased from Y10 (M = 0.17, SD = 0.18) to Y16 (M = 0.11, SD = 0.11), t(451) = 5.670, d = 0.362 (mean difference = 0.055, percentile 95% CI: 0.036–0.074), suggesting that during adolescence participants became less lateralized overall. As would be expected, those reporting right hand preference had higher (more rightward) signed relative hand skill scores than did those reporting left hand preference ( for boxplots). This was the case at Y10 (right handers: n = 602, M = 0.12, SD = 0.22; left handers: n = 84, M = −0.05, SD = 0.17), t(123.05) = 8.074, d = 0.790 (mean difference = 0.167, percentile 95% CI: 0.127–0.208) and Y16 (right handers: n = 470, M = 0.07, SD = 0.15; left handers: n = 56, M = −0.08, SD = 0.19), t(63.651) = 5.906, d = 0.995 (mean difference = 0.153, percentile 95% CI: 0.106–0.205).
Perinatal testosterone, estradiol, and Vitamin D as predictors of handedness at Y10 and Y16
We first present exploratory (not pre-registered) bivariate analyses in which hormonal predictors are correlated with handedness outcomes (). These are included to ensure our findings are comparable with those of other studies that have not controlled for covariates. We next performed the multivariate analyses specified in our pre-registration () and followed these with sex-stratified models in cases where significant hormone × sex interaction effects were observed. Additionally, we report exploratory (not pre-registered) multiple regression models in which Vitamin D and Vitamin D × sex (but not testosterone, testosterone × sex, estradiol, or estradiol × sex) are included as predictors, along with the pre-specified covariates (). These were performed to explore whether effects of Vitamin D would be evident when examining a larger sample (i.e., we avoided listwise deletion of participants for whom Vitamin D was recorded but no data existed for testosterone and/or estradiol). For ease of comparison, we also summarise the findings from each stage of analysis qualitatively in .
Table 2. Bootstrapped (10,000 samples) Pearson’s correlations between hormonal predictors and handedness outcomes, stratified by sex.
Table 3. Bootstrapped (10,000 samples) multivariate regression models with hormones as predictors and handedness measures as outcomes.
Table 4. Bootstrapped (10,000 samples) multivariate regression models with Vitamin D and Vitamin D × sex as predictors and handedness measures as outcomes.
Table 5. Qualitative summary of results from bivariate and multivariate analyses of association between perinatal hormone concentrations and handedness outcomes measured at Y10 and Y16.
Before conducting the multivariate analyses, we imputed values for several covariates to maximize sample size. We imputed birth weight for n = 101 males and n = 117 females with the means (male M = 3233.50; female M = 3225.30) present for the subsample in which both handedness and hormone data were available. As relatively few mothers reported whether they smoked during pregnancy, we recoded missing values (n = 431) to indicate non-smoking. Likewise, missing values for alcohol consumption during pregnancy (n = 21) were replaced to indicate non-consumption. In cases for which parental handedness data were unavailable for both parents (n = 22), we replaced the values as indicating that both parents were right-handed. Note that when only one parent reported their handedness (n = 20, all mothers), cases were coded as neither parent being non-right-handed if the respondent was right-handed, and as either or both parent(s) being non-right-handed if the respondent was non-right-handed. As already described in the note beneath , parental education was calculated as the average of maternal and paternal educational level. If the father’s education was unknown but the mother’s education was known, the former was imputed with the latter (n = 94); likewise, if the mother’s educational level was not known but that of the father was known, the former was imputed with the latter (n = 1). In cases for which family income was not reported (n = 43), we replaced this with the modal value (≥ AUD$36,000).
As handedness outcomes (other than hand preference) were not strongly correlated across time-points, and because differences across time-points were observed (again, for outcomes other than hand preference), we correlated testosterone, estradiol, and Vitamin D with right-hand speed, left-hand speed, direction of relative hand skill, and strength of relative hand skill at both Y10 and Y16. For hand preference, we examined only Y10, as this yielded the larger sample size. Because we had specified our predictor variables a priori, and because the covariates in our models were used specifically to control for processes that may be associated with perinatal hormone concentrations and/or handedness, we report only the effect size estimates (β) and BCa 95% CIs for predictors and not covariates.
Hand preference
Hand preference (right or left) at Y10 was not associated with testosterone, estradiol, or Vitamin D in any of the bivariate or multivariate analyses, and in no case was a statistically significant hormone × sex interaction effect observed. For violin plots, see .
Signed relative hand skill
The bivariate analysis revealed no significant correlations between the hormonal predictors and signed relative hand skill in males. A significant negative correlation was detected between Vitamin D and signed relative hand skill in females at Y10, indicating higher levels of Vitamin D to be associated with a reduction in the extent of right hand advantage. Although this effect was not observed at the multivariate level at Y10, Vitamin D was again negatively correlated with signed relative hand skill at Y16 in the multivariate analysis. However, the robustness of this effect is unconvincing because it was not detected when the larger sample was analysed in which participants were included regardless of whether testosterone and/or estradiol data were available.
Unsigned relative hand skill
In the bivariate analysis, unsigned relative hand skill was positively correlated with estradiol in males at Y10, implying that higher estradiol levels may lead to greater disparity in skill between the hands. A significant estradiol × sex interaction was subsequently observed for Y10 unsigned relative hand skill in the multivariate analysis. Sex stratified models (males: n = 143, adjusted R2 = 0.048; females: n = 168, adjusted R2 = −0.034) revealed the same pattern of findings as the bivariate analysis: specifically, there was a positive correlation in males, β = 0.002 (BCa 95% CI: 0.0003–0.004) and no effect in females, β = 0.0001 (BCa 95% CI: −0.001–0.002). However, the robustness of this effect is questioned because it was detected at Step 3, but not at Step 2 when the sample size was larger. The bivariate analysis also revealed negative correlations between unsigned relative hand skill and Vitamin D in females at both Y10 and Y16. Although these effects were not observed in the pre-registered multivariate analyses (i.e., those which also included testosterone and estradiol as predictors), a negative correlation was again detected at Y16 (though as a main effect rather than an interaction effect) when the analysis was conducted on the larger sample that included participants for whom testosterone and/or estradiol data were unavailable (). However, the effect was not observed in the equivalent analysis at Y10.
Figure 4. Scatterplot showing a negative correlation between maternal gestational Vitamin D (25[OH]D) concentration and relative hand skill (unsigned finger tapping LI) measured at Y16.
Note. Scatterplot shows all available (unadjusted) data points (i.e., it includes those of participants who were deleted listwise in the multiple linear regression models); higher unsigned relative hand skill values indicate greater difference in speed between the right and left hands.
![Figure 4. Scatterplot showing a negative correlation between maternal gestational Vitamin D (25[OH]D) concentration and relative hand skill (unsigned finger tapping LI) measured at Y16.Note. Scatterplot shows all available (unadjusted) data points (i.e., it includes those of participants who were deleted listwise in the multiple linear regression models); higher unsigned relative hand skill values indicate greater difference in speed between the right and left hands.](/cms/asset/4a920dae-ecd9-458c-8fcb-8e0b675d9564/plat_a_2109656_f0004_oc.jpg)
Right-hand speed
Right-hand speed correlated negatively with testosterone in males at Y10 in the bivariate analysis, though no such effect was detected at the multivariate level. However, Y16 right-hand speed was negatively correlated with Vitamin D at the multivariate level, and this effect was qualified by a significant Vitamin D × sex interaction. Sex-stratified models (males: n = 109, adjusted R2 = 0.091; females: n = 131, adjusted R2 = 0.047) revealed a positive correlation in males, β = 0.069 (BCa 95% CI: 0.001–0.148) and a negative, but non-significant, correlation in females, β = −0.053 (BCa 95% CI: −0.107–0.021). Importantly, however, this effect was not detected in the larger analysis of Vitamin D conducted without exclusion of those participants for whom testosterone and/or estradiol data were unavailable.
Left-hand speed
Left-hand speed was positively correlated with Vitamin D in females at Y10 in the bivariate analysis, but this effect was not observed at the multivariate level. A significant testosterone × sex interaction was detected in relation to Y10 left-hand speed in the multivariate analysis. Sex-stratified models (males: n = 143, adjusted R2 = 0.056; females: n = 169, adjusted R2 = 0.007) revealed a positive correlation in males, β = 2.523 (BCa 95% CI: 0.068–5.424), and a non-significant negative correlation in females, β = −1.094 (BCa 95% CI: −2.789–1.001). However, this effect was not detected in the larger analysis that included participants for whom Vitamin D data were unavailable, and no hormonal effects were detected in relation to left hand speed at Y16.
Discussion
The current study aimed to examine whether perinatal concentrations of testosterone, estradiol, and Vitamin D are predictive of handedness outcomes measured during childhood/adolescence using a large dataset that includes perinatal (umbilical cord) sex steroid concentrations, second trimester maternal Vitamin D (25[OH]D) levels, and handedness outcomes measured at 10- (Y10) and/or 16-year (Y16) follow-up. Although some findings were statistically significant, most were not. Notably, of 54 bivariate correlations, only six were statistically significant.
Three effects could be considered to show a certain degree of consistency by having been replicated to some extent across different stages of analysis. First, a positive correlation was observed between umbilical cord estradiol and Y10 unsigned relative hand skill in males in both the bivariate analysis and at Step 3 of the hierarchical regression analysis. However, this effect was not detected at Step 2 of the hierarchical analysis. This is noteworthy because sample sizes available at Step 3 (n = 240-313) were considerably smaller than those of Step 2 (n = 360-466) due to listwise deletion of participants missing either Vitamin D or testosterone/estradiol data. Higher powered multivariate tests were therefore available for testosterone and estradiol (i.e., Step 2 of the hierarchical regression analysis) and Vitamin D (i.e., the exploratory analysis in which testosterone and estradiol were not included as predictors [n = 393-527]). For this reason, we are reluctant to consider significant effects at Step 3 of the hierarchical regression analysis as anything other than Type 1 errors unless they are accompanied by a comparable observation in the respective higher-powered analysis. A negative correlation between Vitamin D and signed relative hand skill (in females, but not males) was observed at Y10 in the bivariate analysis, and a similar negative correlation (in absence of a significant Vitamin D × sex interaction) was observed at Y16 at the multivariate level. However, neither of these effects directly replicated across other stages of analysis, and the multivariate effect (i.e., that observed at Y16) related to the restricted sample size available at Step 3 of the hierarchical regression analysis and was not observed in the larger analysis relating specifically to Vitamin D. The only other effect that may be considered to have replicated across different stages of analysis was a negative correlation between Vitamin D and unsigned relative hand skill. This effect was observed in females at both Y10 and Y16 in the bivariate analysis, and then again for the whole sample (i.e., in absence of a significant Vitamin D × sex interaction) in the higher powered of the multivariate level analyses at Y16 (but not Y10). Other than these exceptions, statistically significant effects did not follow any obvious pattern, as they were not replicated across sex (male or female) or time-point (Y10 or Y16). Additionally, all five statistically significant effects (including interactions) detected in the pre-registered analyses occurred at Step 3 of the hierarchical regression models. That is, they were detected in the lower-powered, but not in the higher-powered, multivariate analysis.
Our primary research aim was to test competing predictions from the sexual differentiation model (Hines & Shipley, Citation1984; Levy & Gur, Citation1980), GBG theory (Geschwind & Behan, Citation1982; Geschwind & Galaburda, Citation1985a, Citation1985b, Citation1985c, Citation1987) and callosal hypothesis (Witelson & Goldsmith, Citation1991; Witelson & Nowakowski, Citation1991) regarding associations between prenatal testosterone and handedness. Although no statistically significant hormonal differences were observed between right- and left-handed males and females, higher testosterone was associated with slower right hand speed (bivariate analysis) and faster left hand speed (Step 3, multivariate analysis) in males at Y10. However, neither effect was detected in the higher-powered (i.e., Step 2) level of the hierarchical multivariate regression analysis. Our pattern of findings also differs from that of Tan and Tan (Citation2001), who reported that unbound testosterone correlated negatively with grasp-reflex strength in males (left hand) and females (both hands). It also remains unclear whether, and, if so, how, the neonatal grasp reflex is related to child, adolescent, or adult patterns of handedness. This arguably makes it difficult to compare meaningfully the findings of Tan and Tan (Citation2001) with those of the present study, which did not utilize this measure.
Testosterone is aromatised to estradiol, hence umbilical cord estradiol concentrations may be associated with handedness. Also, prenatal (amniotic fluid) concentrations of estradiol have previously been associated with handedness (Richards et al., Citation2021a). A positive correlation between estradiol and unsigned relative hand skill was observed in males at Y10 follow-up. However, this finding is directly opposite to that of Richards et al. (Citation2021a) who reported that higher amniotic fluid estradiol concentrations in females (but not males) were associated with less lateralized hand preference profiles. Additionally, the effect observed in the current study was not observed in the more powerful (i.e., Step 2) level of the hierarchical regression model, and was not replicated at Y16, suggesting it may be a Type 1 error.
Vitamin D is thought to influence neurodevelopment but the hypothetical association between prenatal Vitamin D exposure and handedness has not previously been tested. Vitamin D may influence neurodevelopment, with recent research linking early life deficiency in this substance to autism (Kočovská et al., Citation2012), language impairment (Whitehouse et al., Citation2012a), and schizophrenia (Cui et al., Citation2021). Considering that Vitamin D is positively correlated with testosterone (D’Andrea et al., Citation2021) and exhibits similar seasonal fluctuations (Wehr et al., Citation2010), and that handedness has been widely (though often inconsistently) reported to correlate with month of birth (see de Kovel et al., Citation2019; Jones & Martin, Citation2008), we considered it plausible that prenatal Vitamin D exposure could play a role in the development of typical and/or atypical patterns of cerebral lateralization. As already noted, in females, Vitamin D was negatively correlated with signed relative hand skill at Y10; although this effect was not replicated in the multivariate analyses, a comparable negative correlation between signed relative hand skill at Y16 was observed (albeit this was in the whole sample and was not moderated by sex). Importantly, however, no significant association between Vitamin D and signed relative hand skill was detected at either Y10 or Y16 in our exploratory analyses of the larger sample in which participants without testosterone and/or estradiol were not subject to listwise deletion. These effects therefore do not appear to be robust. Vitamin D was also positively correlated with left-hand speed in females at Y10, although this effect was not detected in any subsequent multivariate level analysis. As already noted, Vitamin D was negatively correlated with unsigned relative hand skill in females at Y10 and Y16; although these effects were not observed in the pre-registered multivariate analyses, a significant negative correlation (albeit a main effect rather than an interaction effect) was observed in our higher-powered exploratory multivariate analysis at Y16. However, as with other cases, this effect may not be deemed wholly convincing because no comparable effect was observed in the equivalent multivariate analysis at Y10 for which a larger sample was available for analysis (Y10, n = 516; Y16, n = 393). Taken together, our findings indicate that there could be a link between 18-week maternal gestational Vitamin D concentrations and subsequent measures of handedness, although the effects observed here appear far from robust. Considering the preliminary nature of our investigation, however, we suggest that exploring associations between early Vitamin D concentrations and neurodevelopmental outcomes remains a promising area for future research.
An unexpected finding was that correlations for participants’ relative hand skill measured across timepoints were very low. More specifically, the correlations between Y10 and Y16 for both direction and strength of relative hand skill were negligible in size (Cohen, Citation1988) and only statistically significant when all participants with handedness data were considered rather than just the subsample for which hormonal measures had also been obtained. As hand preference (right or left) was essentially invariant, this suggests that finger tapping may be a noisy index of relative hand skill. This is consistent with the MAND’s manual, which notes that this is “the most difficult test for the evaluator to administer” (McCarron, Citation1997, p. 30) and suggests that assessors should practice on at least 10 people prior to administering the task in an actual testing situation. However, it should also be noted that this task relates to a repetitive movement and does not measure the ability to intrinsically control the fine muscles of the hand like other such tasks tend to do, and that correlations between different measures of relative hand skill are often fairly weak, even when measured at similar stages of development (Buenaventura Castillo, Lynch, & Paracchini, Citation2020). It would therefore be useful for future studies to thoroughly examine the test-retest reliability of finger-tapping as well as other measures of relative hand skill, such as those derived from the Annett pegboard task (Annett, Citation1970), and the Tapley and Bryden dot-filling task (Tapley & Bryden, Citation1985; see also McManus, Van Horn, & Bryden, Citation2016). We additionally noted that relative hand skill was less rightward and less lateralized at Y16 than Y10, indicating that participants made greater improvements in terms of left-hand speed (relative to right-hand speed) between these two follow-ups. This could be because most participants reported right hand preference, and dominant hand performance on such tasks may be near to one’s maximal ability, meaning there is more potential skill improvement to be gained via practice with the non-dominant (in most cases, left) hand (McManus et al., Citation2018).
Strengths and limitations
This study has several strengths. It employed a pre-registered analysis plan and utilized the largest dataset to date to measure the association between perinatal sex steroid concentrations and handedness outcomes. We also implemented bootstrapping procedures, which do not assume a normal distribution of the error terms (Diaconis & Efron, Citation1983). This may be advantageous when dealing with laterality indices (Wilke & Schmithorst, Citation2006) including those calculated for handedness (Richards et al., Citation2021a), which typically show marked deviations from the normal distribution as well as the presence of datapoints that would be considered outliers within the context of a normal distribution. However, our study also has some limitations. First, although The Raine Study cohort is large, data relating to umbilical cord hormones and maternal circulation were only collected for relatively small, and not completely overlapping, subsamples. Additionally, although these subsamples are generally representative of the overall Raine Study cohort, some differences have been noted. For example, females were proportionally over-represented in a previous analysis of maternal Vitamin D and offspring neurocognitive development (Whitehouse et al., Citation2012a), and sample attrition is known to be biased by socioeconomic status, with those from lower socioeconomic backgrounds less likely to participate in follow-up studies (Whitehouse et al., Citation2012b).
Our analyses were limited to the available handedness data. Although it is clearly a strength to have relative hand skill (both in terms of strength and direction) rather than simply hand preference, it should be noted that the MAND is primarily intended for use as “a standardized and quantitative method of assessing psychomotor skills” (McCarron, Citation1997, p. 25). Although a composite handedness score is posited in the manual for the MAND, we did not compute this because it combines several different, albeit related, phenomena. More specifically, this metric includes finger-tapping in addition to hand strength and a task relating to putting beads in a bag (McCarron, Citation1997, p. 28). We decided against conflating these various measures into a single variable (see Buenaventura Castillo et al., Citation2020), and, because we wanted to minimize the number of statistical tests required, chose to examine only the finger tapping task. Whilst finger-tapping measures have been widely used in handedness research, the technical difficulty in administering this test could potentially explain the relatively low test-retest correlations. Furthermore, low measurement reliability could have obscured associations between the hormonal measures and handedness outcomes. That said, it should be recalled that predictions from the sexual differentiation model, GBG theory, and callosal hypothesis are primarily related to direction of hand preference. Although Papadatou-Pastou, Martin, and Munafò (Citation2013) noted that self-reported hand preference measures (i.e., right or left) misclassify 13.5% of left-handers (but only 0.4% of right-handers) when compared with the results of handedness questionnaires, the complete lack of correlation between this more reliable/stable outcome and any of the hormonal predictors raises serious doubt regarding the idea that these variables are related in any meaningful way.
Conclusion
This was the largest study to prospectively examine perinatal (umbilical cord) concentrations of testosterone and estradiol in relation to handedness. Although higher estradiol was associated with stronger relative hand skill (irrespective of direction) in males at Y10, the effect was in the opposite direction to that reported by Richards et al. (Citation2021a) in regard to strength of hand preference in females. The effect was also not observed in the higher powered of the two multivariate analyses and did not replicate at Y16. Furthermore, testosterone was not consistently associated with any of the handedness outcomes at either time-point. Our findings therefore question the hypothesis that perinatal or prenatal sex steroid exposure is associated with handedness. The current study is also the first to examine the possible role of prenatal Vitamin D in the development of handedness. We observed that higher concentrations of Vitamin D (25[OH]D) in the mothers’ circulation predicted more leftward and less lateralized (regardless of direction) relative hand skill profiles in their children. However, although there was a certain degree of consistency across analyses for these findings, those statistically significant effects were still accompanied by some null results. Even so, when considering recent findings from research on early Vitamin D exposure and neurodevelopment, we suggest this area remains worthy of further investigation.
Author roles
GR, LL, DT, and AJOW obtained funding for the study. GR and DT wrote the manuscript. GR and ICM devised the statistical analysis plan, which was affirmed by the other co-authors prior to pre-registration. GR analysed the data. AAB, MH, MM, and ML revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Acknowledgements
We would like to acknowledge The Raine Study participants and their families for their ongoing participation in the study and The Raine Study team for study co-ordination and data collection. We also thank the NHMRC for their long-term contribution to funding the study over the last 30 years. The core management of The Raine Study is funded by The University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. The collection and analysis of prenatal hormone data were funded by The University of Western Australia, School of Women’s and Infants’ Health, King Edward Memorial Hospital, The University of Western Australia, Medical School, Royal Perth Hospital, and Telethon Kids Institute. The NHMRC project grant 1022134 funded the serum 25(OH)D assays that were conducted by RDDT in Melbourne, Victoria, Australia. The Raine Study Gen2-10 year follow-up was funded by the NHMRC and the Raine Medical Research Foundation. The Raine Study Gen2-22 year follow-up was funded by NHMRC project grants 1027449, 1044840 and 1021858. Funding was also generously provided by Safe Work Australia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this research are available from The Raine Study (https://rainestudy.org.au/information-for-researchers/). Restrictions apply to the availability of these data, which were used under license for this study. The R script that accompanies our analysis is available on the Open Science Framework (https://osf.io/yru2s/).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abbondanza, F., Dale, P. S., Wang, C. A., Hayiou-Thomas, M. E., Toseeb, U., Koomar, T. S., … Paracchini, S. (2022). Non-right handedness is associated with language and reading impairments. PsyArXiv, doi:10.31234/osf.io/74jsd
- Annett, M. (1970). The growth of manual preference and speed. British Journal of Psychology, 61(4), 545–558. doi:10.1111/j.2044-8295.1970.tb01274.x
- Baron-Cohen, S., Tsompanidis, A., Auyeung, B., Nørgaard-Pedersen, B., Hougaard, D. M., Abdallah, M. W., … Pohl, A. (2020). Foetal oestrogens and autism. Molecular Psychiatry, 25(11), 2970–2978. doi:10.1038/s41380-019-0454-9
- Beaton, A. A. (2003). The nature and determinants of handedness. In K. Hugdahl, & R. J. Davidson (Eds.), The asymmetrical brain (pp. 105–158). MIT Press. doi:10.7551/mitpress/1463.003.0006
- Beaton, A. A. (2008). Handedness assessment in studies of seasonal anisotropy. Cortex, 44(1), 97–98. doi:10.1016/j.cortex.2006.06.001
- Beking, T. (2018). Two sides to every story: Sex hormones, brain lateralization and gender development. University of Groningen.
- Buenaventura Castillo, C., Lynch, A. G., & Paracchini, S. (2020). Different laterality indexes are poorly correlated with one another but consistently show the tendency of males and females to be more left- and right-lateralized, respectively. Royal Society Open Science, 7(4), 191700. doi:10.1098/rsos.191700
- Campbell, J. M., Marcinowski, E. C., & Michel, G. F. (2018). The development of neuromotor skills and hand preference during infancy. Developmental Psychobiology, 60(2), 165–175. doi:10.1002/dev.21591
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Erlbaum Associates.
- Cosenza, R. M., & Mingoti, S. A. (1995). Season of birth and handedness revisited. Perceptual and Motor Skills, 81(2), 475–480. doi:10.2466/pms.1995.81.2.475
- Cuellar-Partida, G., Tung, J. Y., Eriksson, N., Albrecht, E., Aliev, F., Andreassen, O. A., … Medland, S. E. (2021). Genome-wide association study identifies 48 common genetic variants associated with handedness. Nature Human Behaviour, 5(1), 59–70. doi:10.1038/s41562-020-00956-y.
- Cui, X., McGrath, J. J., Burne, T. H. J., & Eyles, D. W. (2021). Vitamin D and schizophrenia: 20 years on. Molecular Psychiatry 26, 7, 2021, 2708–2720, doi:10.1038/s41380-021-01025-0
- D’Andrea, S., Martorella, A., Coccia, F., Castellini, C., Minaldi, E., Totaro, M., … Barbonetti, A. (2021). Relationship of vitamin D status with testosterone levels: A systematic review and meta-analysis. Endocrine, 72(1), 49–61. doi:10.1007/s12020-020-02482-3
- de Kovel, C. G. F., Carrión-Castillo, A., & Francks, C. (2019). A large-scale population study of early life factors influencing left-handedness. Scientific Reports, 9(1), 584. doi:10.1038/s41598-018-37423-8.
- Diaconis, P., & Efron, B. (1983). Computer-intensive methods in statistics. Scientific American, 248(5), 116–130. doi:10.1038/scientificamerican0583-116
- Geschwind, N., & Behan, P. (1982). Left-handedness: Association with immune disease, migraine, and developmental learning disorder. Proceedings of the National Academy of Sciences, 79(16), 5097–5100. doi:10.1073/pnas.79.16.5097
- Geschwind, N., & Galaburda, A. M. (1985a). Cerebral lateralization. Archives of Neurology, 42(5), 428–459. doi:10.1001/archneur.1985.04060050026008
- Geschwind, N., & Galaburda, A. M. (1985b). Cerebral lateralization. Archives of Neurology, 42(6), 521–552. doi:10.1001/archneur.1985.04060060019009
- Geschwind, N., & Galaburda, A. M. (1985c). Cerebral lateralization. Archives of Neurology, 42(7), 634–654. doi:10.1001/archneur.1985.04060070024012
- Geschwind, N., & Galaburda, A. M. (1987). Cerebral lateralization, biological mechanisms, associations, and pathology. MIT Press.
- Hands, B., Larkin, D., & Rose, E. (2013). Reprint of ‘The psychometric properties of the McCarron assessment of neuromuscular development as a longitudinal measure with Australian youth’. Human Movement Science, 32(5), 1163–1175. doi:10.1016/j.humov.2013.08.003
- Hepper, P. G. (2013). The developmental origins of laterality: Fetal handedness. Developmental Psychobiology, 55(6), 588–595. doi:10.1002/dev.21119
- Hickey, M., Doherty, D. A., Hart, R., Norman, R. J., Mattes, E., Atkinson, H. C., & Sloboda, D. M. (2010). Maternal and umbilical cord androgen concentrations do not predict digit ratio (2D:4D) in girls: A prospective cohort study. Psychoneuroendocrinology, 35(8), 1235–1244. doi:10.1016/j.psyneuen.2010.02.013
- Hickey, M., Hart, R., & Keelan, J. A. (2014). The relationship between umbilical cord estrogens and perinatal characteristics. Cancer Epidemiology, Biomarkers & Prevention, 23(6), 946–952. doi:10.1158/1055-9965.EPI-13-1321
- Hines, M., & Shipley, C. (1984). Prenatal exposure to diethylstilbestrol (DES) and the development of sexually dimorphic cognitive abilities and cerebral lateralization. Developmental Psychology, 20(1), 81–94. doi:10.1037/0012-1649.20.1.81
- Hirnstein, M., & Hugdahl, K. (2014). Excess of non-right-handedness in schizophrenia: Meta-analysis of gender effects and potential biases in handedness assessment. British Journal of Psychiatry, 205(4), 260–267. doi:10.1192/bjp.bp.113.137349
- Hollier, L. P., Keelan, J. A., Hickey, M., Maybery, M. T., & Whitehouse, A. J. O. (2014). Measurement of androgen and estrogen concentrations in cord blood: Accuracy, biological interpretation, and applications to understanding human behavioral development. Frontiers in Endocrinology, 5, 64. doi:10.3389/fendo.2014.00064
- Hollier, L. P., Keelan, J. A., Jamnadass, E. S. L., Maybery, M. T., Hickey, M., & Whitehouse, A. J. O. (2015). Adult digit ratio (2D:4D) is not related to umbilical cord androgen or estrogen concentrations, their ratios or net bioactivity. Early Human Development, 91(2), 111–117. doi:10.1016/j.earlhumdev.2014.12.011
- Jones, G. V., & Martin, M. (2008). Seasonal anisotropy in handedness. Cortex, 44(1), 8–12. doi:10.1016/j.cortex.2006.05.001
- Keelan, J. A., Mattes, E., Tan, H., Dinan, A., Newnham, J. P., Whitehouse, A. J. O., … Hickey, M. (2012). Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PLoS ONE, 7(8), e42827. doi:10.1371/journal.pone.0042827
- Kočovská, E., Fernell, E., Billstedt, E., Minnis, H., & Gillberg, C. (2012). Vitamin D and autism: Clinical review. Research in Developmental Disabilities, 33(5), 1541–1550. doi:10.1016/j.ridd.2012.02.015
- Leviton, A., & Kilty, T. (1979). Seasonal variation in the birth of left-handed schoolgirls. Archives of Neurology, 36(2), 115–116. doi:10.1001/archneur.1979.00500380085016
- Levy, J., & Gur, R. C. (1980). Individual differences in psychoneurological organization. In J. Herron (Ed.), Neuropsychology of left-handedness (pp. 199–210). Academic Press.
- Lust, J. M., Geuze, R. H., van de Beek, C., Cohen-Kettenis, P. T., Bouma, A., & Groothuis, T. G. G. (2011). Differential effects of prenatal testosterone on lateralization of handedness and language. Neuropsychology, 25(5), 581–589. doi:10.1037/a0023293
- Manning, J. T. (2002). Digit ratio: A pointer to fertility, behavior, and health. Rutgers University Press.
- Manning, J. T., Scutt, D., Wilson, J., & Lewis-Jones, D. I. (1998). The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Human Reproduction, 13(11), 3000–3004. doi:10.1093/humrep/13.11.3000
- Markou, P., Ahtam, B., & Papadatou-Pastou, M. (2017). Elevated levels of atypical handedness in autism: Meta-analyses. Neuropsychology Review, 27(3), 258–283. doi:10.1007/s11065-017-9354-4
- Martin, M., & Jones, G. V. (1999). Handedness and season of birth: A gender-invariant relation. Cortex, 35(1), 123–128. doi:10.1016/S0010-9452(08)70790-1
- McCarron, L. T. (1997). Mccarron assessment of neuromuscular development: Fine and gross motor abilities (revised). McCarron-Dial Systems.
- McKnight, C. M., Newnham, J. P., Stanley, F. J., Mountain, J. A., Landau, L. I., Beilin, L. J., … Mackey, D. A. (2012). Birth of a cohort — The first 20 years of the Raine study. Medical Journal of Australia, 197(11), 608–610. doi:10.5694/mja12.10698
- McManus, I. C. (1980). Season of birth of left-handers. Archives of Neurology, 37(1), 63–63. doi:10.1001/archneur.1980.00500500093020
- McManus, I. C. (2021). Is any but a tiny fraction of handedness variance likely to be due to the external environment? Laterality, 26(3), 310–314. doi:10.1080/1357650X.2021.1892126
- McManus, I. C., Buckens, G., Harris, N., Flint, A., Ng, H. L. A., & Vovou, F. (2018). Faking handedness: Individual differences in ability to fake handedness, social cognitions of the handedness of others, and a forensic application using Bayes’ theorem. Laterality: Asymmetries of Body, Brain and Cognition, 23(1), 67–100. doi:10.1080/1357650X.2017.1315430
- McManus, I. C., Van Horn, J. D., & Bryden, P. J. (2016). The Tapley and Bryden test of performance differences between the hands: The original data, newer data, and the relation to pegboard and other tasks. Laterality: Asymmetries of Body, Brain and Cognition, 21(4–6), 371–396. doi:10.1080/1357650X.2016.1141916
- Medland, S. E., Duffy, D. L., Wright, M. J., Geffen, G. M., Hay, D. A., Levy, F., … Boomsma, D. I. (2009). Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia, 47(2), 330–337. doi:10.1016/j.neuropsychologia.2008.09.005
- Medland, S. E., Duffy, D. L., Wright, M. J., Geffen, G. M., & Martin, N. G. (2006). Handedness in twins: Joint analysis of data from 35 samples. Twin Research and Human Genetics, 9(1), 46–53. doi:10.1375/twin.9.1.46
- Michel, G. F., & Harkins, D. A. (1986). Postural and lateral asymmetries in the ontogeny of handedness during infancy. Developmental Psychobiology, 19(3), 247–258. doi:10.1002/dev.420190310
- Milenković, S., Rock, D., Dragović, M., & Janca, A. (2008). Season of birth and handedness in serbian high school students. Annals of General Psychiatry, 7(1), 2. doi:10.1186/1744-859X-7-2
- Nelson, E. L., Campbell, J. M., & Michel, G. F. (2014). Early handedness in infancy predicts language ability in toddlers. Developmental Psychology, 50(3), 809–814. doi:10.1037/a0033803
- Nicholls, M. E. R. (1998). Seasonal trends in the birth of sinistrals. Laterality: Asymmetries of Body, Brain and Cognition, 3(3), 241–253. doi:10.1080/713754306
- Ocklenburg, S., Berretz, G., Packheiser, J., & Friedrich, P. (2020). Laterality 2020: Entering the next decade. Laterality 26, 265–297. doi:10.1080/1357650X.2020.1804396
- Ocklenburg, S., & Güntürkün, O. (2018). The lateralized brain: The neuroscience and evolution of hemispheric asymmetries. Academic Press.
- Ocklenburg, S., Isparta, S., Peterburs, J., & Papadatou-Pastou, M. (2019). Paw preferences in cats and dogs: Meta-analysis. Laterality: Asymmetries of Body, Brain and Cognition, 24(6), 647–677. doi:10.1080/1357650X.2019.1578228
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. doi:10.1016/0028-3932(71)90067-4
- Papadatou-Pastou, M., Martin, M., & Munafò, M. R. (2013). Measuring hand preference: A comparison among different response formats using a selected sample. Laterality: Asymmetries of Body, Brain and Cognition, 18(1), 68–107. doi:10.1080/1357650X.2011.628794
- Papadatou-Pastou, M., Ntolka, E., Schmitz, J., Martin, M., Munafò, M. R., Ocklenburg, S., & Paracchini, S. (2020). Human handedness: A meta-analysis. Psychological Bulletin, 146(6), 481–524. doi:10.1037/bul0000229
- Parker, A. J., Woodhead, Z. V. J., Thompson, P. A., & Bishop, D. V. M. (2021). Assessing the reliability of an online behavioural laterality battery: A pre-registered study. Laterality, 26(4), 359–397. doi:10.1080/1357650X.2020.1859526
- Peters, M., & Durding, B. M. (1978). Handedness measured by finger tapping: A continuous variable. Canadian Journal of Psychology/Revue Canadienne de Psychologie, 32(4), 257–261. doi:10.1037/h0081694
- Pfannkuche, K. A., Bouma, A., & Groothuis, T. G. G. (2009). Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1519), 929–942. doi:10.1098/rstb.2008.0282
- Richards, G., Beking, T., Kreukels, B. P. C., Geuze, R. H., Beaton, A. A., & Groothuis, T. (2021a). An examination of the influence of prenatal sex hormones on handedness: Literature review and amniotic fluid data. Hormones and Behavior, 129, 104929. doi:10.1016/j.yhbeh.2021.104929
- Richards, G., Browne, W. V., & Constantinescu, M. (2021). Digit ratio (2D:4D) and amniotic testosterone and estradiol: An attempted replication of Lutchmaya et al. (2004). Journal of Developmental Origins of Health and Disease, 12(6), 859–864. doi:10.1017/S2040174420001294
- Richards, G., Medland, S. E., & Beaton, A. A. (2021b). Digit ratio (2D:4D) and handedness: A meta-analysis of the available literature. Laterality, 26(4), 421–484. doi:10.1080/1357650X.2020.1862141
- Sartorius, G., Ly, L. P., Sikaris, K., McLachlan, R., & Handelsman, D. J. (2009). Predictive accuracy and sources of variability in calculated free testosterone estimates. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 46(2), 137–143. doi:10.1258/acb.2008.008171
- Schmitz, J., Zheng, M., Lui, K. F. H., McBride, C., Ho, C. S.-H., & Paracchini, S. (2022). Quantitative multidimensional phenotypes improve genetic analysis of laterality traits. Translational Psychiatry, 12(1), 68. doi:10.1038/s41398-022-01834-z
- Schultheiss, O. C., Köllner, M. G., Busch, H., & Hofer, J. (2021). Evidence for a robust, estradiol-associated sex difference in narrative-writing fluency. Neuropsychology, 35(3), 323–333. doi:10.1037/neu0000706
- Somers, M., Ophoff, R. A., Aukes, M. F., Cantor, R. M., Boks, M. P., Dauwan, M., … Sommer, I. E. (2015). Linkage analysis in a Dutch population isolate shows no major gene for left-handedness or atypical language lateralization. Journal of Neuroscience, 35(23), 8730–8736. doi:10.1523/JNEUROSCI.3287-14.2015
- Stoyanov, Z., Nikolova, P., & Pashalieva, I. (2011). Season of birth, Geschwind and Galaburda hypothesis, and handedness. Laterality: Asymmetries of Body, Brain and Cognition, 16(5), 607–619. doi:10.1080/1357650X.2010.506689
- Ströckens, F., Güntürkün, O., & Ocklenburg, S. (2013). Limb preferences in non-human vertebrates. Laterality: Asymmetries of Body, Brain and Cognition, 18(5), 536–575. doi:10.1080/1357650X.2012.723008
- Tan, U., & Tan, M. (2001). Testosterone and grasp-reflex differences in human neonates. Laterality: Asymmetries of Body, Brain and Cognition, 6(2), 181–192. doi:10.1080/713754405
- Tapley, S. M., & Bryden, M. P. (1985). A group test for the assessment of performance between the hands. Neuropsychologia, 23(2), 215–221. doi:10.1016/0028-3932(85)90105-8
- Tonetti, L., Adan, A., Caci, H., Fabbri, M., & Natale, V. (2012). Season of birth and handedness in young adults. Laterality: Asymmetries of Body, Brain and Cognition, 17(5), 597–601. doi:10.1080/1357650X.2011.599118
- Tran, U. S., Stieger, S., & Voracek, M. (2014). Latent variable analysis indicates that seasonal anisotropy accounts for the higher prevalence of left-handedness in men. Cortex, 57, 188–197. doi:10.1016/j.cortex.2014.04.011
- Tsompanidis, A., Aydin, E., Padaigaitė, E., Richards, G., Allison, C., Hackett, G., … Baron-Cohen, S. (2021). Maternal steroid levels and the autistic traits of the mother and infant. Molecular Autism, 12(1), 51. doi:10.1186/s13229-021-00453-7
- Tzourio Mazoyer, N., Petit, L., Zago, L., Crivello, F., Vinuesa, N., Joliot, M., … Mazoyer, B. (2015). Between-hand difference in ipsilateral deactivation is associated with hand lateralization: FMRI mapping of 284 volunteers balanced for handedness. Frontiers in Human Neuroscience, 9, 5. doi:10.3389/fnhum.2015.00005
- van Strien, J. W. (2002). Vragenlijst voor handvoorkeur. Nederlands Tijdschrift Voor de Psychologie En Haar Grensgebieden, 47(2), 88–92.
- Wehr, E., Pilz, S., Boehm, B. O., März, W., & Obermayer-Pietsch, B. (2010). Association of vitamin D status with serum androgen levels in men. Clinical Endocrinology, 73(2), 243–248. doi:10.1111/j.1365-2265.2009.03777.x
- Whitehouse, A. J. O., Holt, B. J., Serralha, M., Holt, P. G., Kusel, M. M. H., & Hart, P. H. (2012a). Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics, 129(3), 485–493. doi:10.1542/peds.2011-2644
- Whitehouse, A. J. O., Mattes, E., Maybery, M. T., Dissanayake, C., Sawyer, M., Jones, R. M., … Hickey, M. (2012b). Perinatal testosterone exposure and autistic-like traits in the general population: A longitudinal pregnancy-cohort study. Journal of Neurodevelopmental Disorders, 4(1), 25. doi:10.1186/1866-1955-4-25
- Wilke, M., & Schmithorst, V. J. (2006). A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage, 33(2), 522–530. doi:10.1016/j.neuroimage.2006.07.010
- Witelson, S. F. (1991). Neural sexual mosaicism: Sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology, 16(1–3), 131–153. doi:10.1016/0306-4530(91)90075-5
- Witelson, S. F., & Goldsmith, C. H. (1991). The relationship of hand preference to anatomy of the corpus callosum in men. Brain Research, 545(1–2), 175–182. doi:10.1016/0006-8993(91)91284-8
- Witelson, S. F., & Nowakowski, R. S. (1991). Left out axoms make men right: A hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia, 29(4), 327–333. doi:10.1016/0028-3932(91)90046-B

![Figure 3. Violin plots for free testosterone (a), total estradiol (b), and Vitamin D (25[OH]D) (c) stratified by Y10 hand preference (right or left).](/cms/asset/bc807e2d-d1e5-4d1e-ad7c-b8c800c65922/plat_a_2109656_f0003_oc.jpg)