Abstract
Background: Testosterone (TT) and dehydroepiandrosterone sulphate (DHEAS) are neurosteroids and their deficiencies constitute the hormone risk factors promoting the development of depression in elderly otherwise healthy men. We investigated the link between hypogonadism and depression in accordance with age and concomitant diseases in men with systolic HF using the novel scale previously dedicated for elderly population.
Methods: We analysed the prevalence of depression and severity of depressive symptoms in population of 226 men with systolic HF (40–80 years) compared to 379 healthy peers. The severity of depression was assessed using the Polish long version of Geriatric Depression Scale (GDS).
Results: In men aged 40–59 years the severity of depressive symptoms was greater in NYHA classes III–IV compared to NYHA classes I–II and reference group. In men aged 60–80 years depressive symptoms were more severe in NYHA class III-IV compared to controls (all p ≤ 0.001). In multivariate logistic regression model in men aged 40–59 years advanced NYHA class was associated with higher prevalence of mild depression (OR = 2.14, 95%CI: 1.07–4.29) and chronic obstructive pulmonary disease (COPD) with higher prevalence of severe depression (OR = 69.1, 95%CI: 2.11–2264.3). In men aged 60–80 years advanced NYHA class and TT deficiency were related to higher prevalence of mild depression (respectively: OR = 2.9, 95%CI: 1.3–6.4; OR = 3.6, 95%CI: 1.2–10.63).
Conclusion: TT deficiency, COPD and advanced NYHA class were associated with higher prevalence of depression in men with systolic HF.
Introduction
Depression is common in patients with heart failure (HF), with the prevalence ranging from 9% to 60% [Citation1]. The severity of depressive symptoms is particularly high in elderly men with advanced HF [Citation2,Citation3]. The presence of depressive symptoms has numerous unfavourable consequences for patients with HF, e.g. worsening their quality of life [Citation4–6] and deteriorating the subjective perception of exercise intolerance [Citation7], as well as increasing the risk of recurrent hospitalizations [Citation8,Citation9] and deaths [Citation8,10–12].
The pathogenesis of depression in the course of HF is multifactorial [Citation13,Citation14] and not completely understood [Citation14]. It has been established that endocrine abnormalities, such as deficiencies in testosterone (TT) [Citation15–19] and dehydroepiandrosterone sulphate (DHEAS) [Citation16,Citation20], may favour the development of depressive symptoms in otherwise healthy elderly men [Citation17] as well as those with chronic non-cardiovascular disorders [Citation21–23]. There are some premises that the aforementioned hormone derangements may be associated with more severe depressive symptoms in patients with HF [Citation24], and could have potential therapeutic implications. However, the available evidence regarding this issue is equivocal [Citation25–27].
The main aim of our study was to evaluate the prevalence of depression and androgen deficiencies in population of men with HF in accordance with age-matched groups and compared to healthy peers using the GDS scale. Moreover, we wanted to prove the hypothesis that androgen deficiencies are associated with depressive symptoms in population of men with HF. Finally, the additional analysis was performed to find the plausible links between the severity of depression symptoms and non-cardiovascular comorbidities in aforementioned population.
In assessment of severity of depressive symptoms, the Geriatric Depression Scale (GDS) was applied. According it, we have differentiated the severity of depression as a mild and severe disorder. The GDS scale, in our opinion, seems to be more suitable than other scales as it does not include any questions about somatic symptoms of depression which may be associated with ageing, HF or other chronic concomitant diseases. This is the first retrospective study which explores the direct links between depression and androgen deficiency using the GDS scale in population of men with HF.
Methods
Study population
Our study population consisted of men aged 40–80 years hospitalised or attended outpatient HF clinic (4th Military Hospital, Wroclaw, Poland). The inclusion criteria in the study were: (a) a documented history of chronic HF > 6 months, (b) left ventricular ejection fraction (LVEF) ≤ 45% as assessed by echocardiography, (c) clinical stability and unchanged chronic HF medications for ≥4 weeks preceding the study. The exclusion criteria were: (a) acute coronary syndrome or coronary revascularization within the 6 months preceding the study; (b) any acute/chronic illness that might influence hormonal metabolism; (c) any hormonal treatment either at the time of the study or in the past; (d) any history of psychiatric disorders and/or related therapy either at the time of the study or in the past [Citation28].
The reference population included healthy men aged 40–80 years living in the same area, who were examined in the year 2000 in the large Wroclaw Centre of Polish Preventive Medicine (DOLMED, Wroclaw, Poland). This population had no history of any physical and mental disorders [Citation28].
All participants signed informed consent. The study was approved by the local ethics committee and conducted in accordance with the Helsinki Declaration.
Assessment of depressive symptoms
Severity of depression was estimated based on Polish version of long form of Geriatric Depression Scale (GDS). It is a 30-item questionnaire in a simple yes/no response format [Citation29]. Following Brink et al. [Citation29] we considered a GDS ≥ 11 points as a diagnosis of mild depression and a GDS ≥ 21 points as a diagnosis of severe depression. This scale was applied in our study due to its simplicity with high sensitivity and specificity in confirmation of depression in elderly population (respectively: 82% and 78%) [Citation30]. Although it was previously designed for elderly people [Citation29], it turned out to be suitable also for a younger population, but older than 40 years old [Citation31].
Moreover, it was more precise than other scales as it did not include questions about somatic depressive symptoms (i.e. reduced living energy, sleep disorders or lack of sexual interest) which are common in a geriatric population [Citation29]. These symptoms are believed to be natural part of ageing and it is difficult to state if they have been caused by mental disorders or not.
Serum hormone levels and other laboratory measurements
The venous blood samples were collected in the morning after an overnight fast and a supine rest of at least 15 minutes. The samples were centrifuged and frozen at −70 °C. Serum level of TT and DHEAS was measured with immunoassays (Diagnostic Products Corp, San Francisco, CA) and expressed in nanogram per millilitre. The inter- and intra-assay variability coefficients were 12.0% and 6.8% for DHEAS and 9.8% and 7.4% for TT [Citation28].
DHEAS and TT deficiency were defined as a serum hormone level equal to or less than the 10th percentile calculated to equivalent age categories in the reference group of healthy men [Citation32,Citation33]. The cut off values for aged ≤ 45, 46–55, 56–65, ≥66 years were respectively: 3.2; 3.0; 2.7; 2.6 ng/ml for serum TT and 1411; 1048; 709; 310 ng/ml for serum DHEAS.
Additionally, we calculated the level of free testosterone (fTT) using validated equation of Vermeulen et al. [Citation34]. Deficiency of fTT was defined as serum level of fTT equal to or less than 10th percentile of fTT for the equivalent age-group of healthy men. The 10th percentiles of fTT for healthy men in the reference population aged ≤ 45, 46–55, 56–65, ≥66 years were respectively: 77, 64, 59, 52 pg/mL. In order to estimate the level of fTT, we also measured the serum level of sex hormone binding globulin (SHBG) that was expressed in nanomoles per litre. The interassay and intraassay variability coefficients were 5.2% and 3.0%, respectively [Citation28].
Plasma levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured with immunoassay based on electrochemiluminescence on the Elecsys 1010/2010 System (Roche Diagnostics GmbH, Mannheim, Germany). Normal value of plasma NT-proBNP in our country is less than 125 pg/mL. We used the Modification in Diet in Renal Disease Equation [Citation85] for calculation of estimated glomelurar filtration rate (eGFR; mL/min/1.73m2). Renal failure was defined as eGFR < 60 mL/min/1.73m2. Serum high-sensitive C-reactive protein (hsCRP, mg/L) was assessed using immunonephelometry (Dade Behring, Marburg GmbH, Germany).
Statistical analysis
Most analysed continuous variables had normal distribution, so we expressed them as a mean ± standard deviaton. The Kolmogorov–Smirnov test was used to determine if the variables had normal distribution. The intergroup differences were verified with the unpaired Student t-test and one-way ANOVA. NT-proBNP, DHEAS and high sensitive C-reactive protein (hsCRP) had skewed distribution so we presented them as a median with lower and upper quartiles. They were log transformed to normalise their distribution. The inter-group differences were tested using the t-test and one-way ANOVA for unpaired samples for normalised values. The categorical variables were expressed as numbers with percentages. The intergroup differences were tested using the χ2 test and one-way ANOVA.
The links between severity of depressive symptoms (no depression < 11 points, mild depression ≥11 points and severe depression ≥ 21 points in GDS scale) and sex steroids deficiency were assessed using univariate and multivariate logistic regression analyses separately in two groups of men with systolic HF: aged 40–59 and 60–80 years. In an univariate logistic regression model the potential determinants of severity of depressive symptoms were continuous variables such as: age, body mass index (BMI), heart rate (HR), systolic (SBP) and diastolic blood pressure (DBP), left ventricular ejection fraction (LVEF), eGFR, serum level of N-terminal-pro-B-type Natriuretic Peptid (NT-proBNP), serum sodium (Na), haemoglobin (Hb), white blood cells (WBC), platelets (PLT), total cholesterol (TC), hsCRP, DHEAS, TT and fTT, respectively; as well as dichotomised qualitative variables: ischaemic aetiology of heart failure, New York Heart Association Class (NYHA), treatment (use angiotensin-converting-enzyme inhibitor or angiotensin receptor blocker (ACEI/ARB), b-blockers, spironolactone, loop diuretics, thiazide diuretics, digoxin, statin, acetylsalicylic acid (ASA)) and comorbidities (previous myocardial infarction, hypertension, atrial fibrillation, diabetes, renal failure, previous stroke or transient ischaemic attack (TIA), peripheral arterial disease, chronic obstructive pulmonary disease (COPD), cancer). In a multivariate logistic regression model, we included variables that had been shown to be significant in univariate logistic regression models (p < 0.05). p < 0.05 was considered statistically significant. Statistical analyses were performed using the STATISTICA 9.1. data analysis software system (StatSoft, Inc, College Station, TX).
Results
The baseline characteristics of men with systolic HF and reference group
We examined 226 men aged 40–80 years with systolic HF (LVEF: 30 ± 8%, NYHA class I/II/III-IV: 16%/56%/28%, ischaemic aetiology: 69%). The detailed characteristic of the study population is presented in .
Table 1 Baseline characteristics of men with systolic HF depending on age.
In men aged 60–80 years we observed lower values of HR, eGFR, Hb, PLT, WBC, TC, serum DHEAS but higher values of serum Na than in men aged 40–59 years (all p < 0.05). Also, the severity of HF, prevalence of HF ischaemic aetiology and treatment with statin were more common in men aged 60–80 years than in men aged 40–59 years (all p < 0.05). Moreover, the prevalence of some comorbidities like previous myocardial infarction, hypertension, diabetes, renal failure and COPD was higher in elderly men as compared to men aged 40–59 years (p < 0.05). The reference group consisted of 292 men aged 40–59 years (median BMI 27.2 ± 3.6 kg/m2) and 87 men aged 60–80 years (median BMI 27.6 ± 3.0 kg/m2).
The severity of depressive symptoms in men with systolic HF and healthy men
The total GDS score was higher in men aged 60–80 years than in men aged 40–59 years (11 ± 6 versus 10 ± 6 points, p < 0.05). Both, in younger men (aged 40–59 years) and in older ones (60–80 years) the severity of depressive symptoms was higher in men with HF than in the reference group (respectively: 10 ± 6 versus 7 ± 4 points, p < 0.0001 and 11 ± 6 versus 10 ± 3 points, p = 0.02).
In younger group, the severity of depressive symptoms was higher in men with advanced HF (NYHA class III-IV) as compared to men in NYHA class I-II and the reference group. In men aged 60–80 years, depressive symptoms were more severe in men in NYHA class III-IV as compared to controls, but did not differ from those in NYHA class I-II (p = 0.2) ().
Figure 1. (A) The severity of depressive symptoms according to age and NYHA class. (B) The prevalence of depression according to age and NYHA class. (C) Serum testosterone level in men with systolic HF according to age and depression severity. (D) The prevalence of testosterone deficiency in men with systolic HF according to depression severity. (E) The prevalence of COPD and stroke/TIA in men with systolic heart failure according to age and depression severity. (F) The severity of depressive symptoms according to number of risk factors in men with systolic HF.
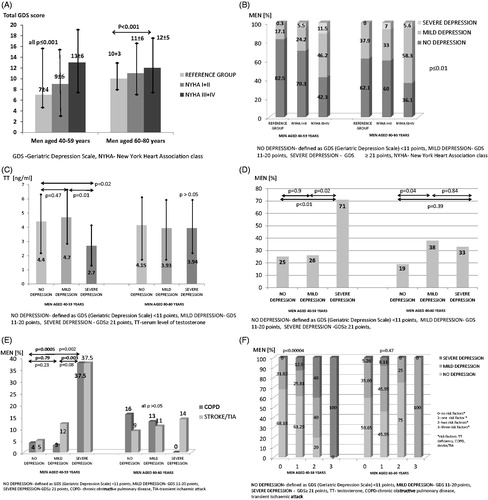
Prevalence of depression and severity of depressive symptoms in men with systolic HF and in healthy men
In men aged 40–59 years the prevalence of mild and severe depression was higher than in healthy peers at the same age (29% versus 17% and 7% versus 0.3%, all p < 0.007). In older men compared to the age-matched group, we only observed higher prevalence of severe depression (6% versus 0%, p = 0.02).
Prevalence of androgen deficiency in men with systolic HF and in healthy men
The prevalence of androgen deficiencies was higher in men with HF compared to healthy peers (TT: 28 versus 10% in men 40–59 years and 27 versus 10% in men aged 60–80 years, all p ≤ 0.004; DHEAS: 66 versus 10% and 54 versus 10%, p < 0.001, respectively).
Determinants of depression severity in younger men with systolic HF
In the univariate logistic regression analysis model, advanced NYHA class and lower serum haemoglobin level were related to higher prevalence of mild depression in younger men with systolic HF (all p < 0.05). TT deficiency, reduced level of TT, previous stroke or TIA, COPD and advanced NYHA class were associated with greater prevalence of severe depression (all p < 0.05) ().
Table 2. Risk factors of prevalence of mild and severe depression in younger and older men with HF.
In multivariate logistic regression model advanced NYHA class was associated with higher prevalence of mild depression (OR = 2.14, 95%CI: 1.07–4.29, χ2 = 4.748, p = 0.03). Furthermore, COPD was related to greater prevalence of severe depression (OR = 69.1, 95%CI: 2.11–2264.32, χ2 = 5.87 p = 0.02).
Determinants of depression severity in older men with systolic HF
In the univariate logistic regression analysis model, advanced NYHA class, lower serum Na and TT deficiency were linked to higher prevalence of mild depression (all p < 0.05) (). In multivariate logistic regression model advanced NYHA class and TT deficiency were associated with higher prevalence of mild depression (respectively: OR = 2.9, 95%CI: 1.3–6.4; χ2 = 7.14, p = 0.009; OR = 3.6, 95%CI: 1.2–10.63; χ2 = 5.37, p = 0.02).
Discussion
There are two major novel findings arising from our study. First of all, our data suggest that depression may be often associated with imbalance in sexual hormones, especially in relatively young subjects. Secondly, regardless of high NYHA class, the presence of some comorbidities like COPD, stroke, anaemia augmented the severity of depressive symptoms in the aforementioned population. Furthermore, we confirmed that depressive symptoms and deficiencies of androgens are common problems in men at different stages of heart failure as was published previously [Citation24].
Although the links between depression and androgen imbalance in general population have already been studied, they still remain ambiguous. In our paper we focused on HF population, where the aggravation of hormonal disturbances is high and may be associated with depressive symptoms independently to the severity of HF and concomitant diseases. Moreover, the role of androgen imbalance is stronger in younger compared to elderly men in the study population.
The prevalence of depression and androgen deficiency: the role of androgens in pathophysiology of depression
Depression is a common problem in HF population. According to our data, mild depressive disorders concerned almost one-third of patients with HF in younger age and about 40% men in older age, whereas severe depression was observed in about 7% men in both groups. These data were concordant with other studies where the prevalence of depression varied widely, ranging from 9% to 60% [Citation1]. Also, the prevalence of mild and severe depression in younger men with HF was greater compared to the reference group at the same age. In the elderly group we only observed higher prevalence of severe depression in men with HF compared to the healthy peers. This may be explained by the fact that in younger men serious chronic disease as HF changes their lives dramatically, causing physical disability, need for care and loss of job in quite short time. On the contrary, in the elderly ageing is a natural part of life, health worsening and reducing day activity progresses slowly so it is easier to get used to it and cope with it.
In our study the prevalence of androgen deficiency was high in men with HF and occurred more often than in healthy peers irrespective of age. The decline of androgen level is often observed in chronic diseases [Citation22,Citation35]. In HF patients there is an imbalance in anabolic and catabolic processes which emerge from the activation of neuroendocrine system and inflammation processes [Citation36]. It is plausible that lower level of DHEAS in HF patients may be caused by insulin resistance and hyperinsulinemia as insulin is an inhibitor of DHEAS secretion in healthy subjects [Citation37]. Therefore, the decline in DHEAS [Citation36] and TT [Citation38] is often observed in HF population, especially in advanced stages.
DHEA is mainly synthesised by adrenal glands in response to ACTH (adrenocorticotropic hormone) stimulation. Some data indicate that DHEA may be synthesised in brain as well [Citation39]. DHEA and its sulphated form DHEAS are precursors of all sex hormones. Moreover, acting as neurosteroids through binding to special membrane and nuclear cell receptors, they may directly affect metabolic processes in the human brain [Citation40]. Also DHEA modulates neurotransmission of important substances like serotonin, γ-amino butyric acid, glutamate and dopamine in the central nervous system [Citation41–43]. DHEAS is crucial in maintaining good memory and cognitive function [Citation40].
TT is in 95% produced by Leydig cell in testes under the stimulation of LH (Luteinizing Hormone). It is produced in small amounts by brain and adrenal cortex [Citation44,Citation45]. TT’s biologic effect is through binding to androgen receptors in the nucleus of the cells [Citation46]. It is essential in sexual function [Citation47], provides a good state of bones by stimulation of bone osteoblastic activity [Citation48], stimulates erythropoiesis [Citation49], body mass building [Citation50], ensures good memory and regulates cognitive processes [Citation51]. Beside these functions, TT stimulates dopamine [Citation52] and serotonin release [Citation53]- making mood improvement and enhancing greater activity. In brain TT may be intracellularly converted into oestradiol [Citation54] and more potent androgen dihydrotesterone which can bind to androgen receptors as well [Citation55,Citation56].
The plausible links between depression and androgens emerge from the imbalance in hypothalamus-pituitary–gonadal/adrenal axis and androgen ability to act on androgen receptors in brain directly. Long-term exposure to stress is associated with hypersecretion of corticotropin-releasing hormone (CRH) and cortisol [Citation57]. Similar phenomenon is observed in HF patients [Citation36]. Hypercortisolemia is associated with decline in production of DHEAS. It may be explained by the fact that there is a competition in their synthesis and release by the adrenal gland.
Furthermore, previous studies have shown the negative correlations between cortisol and testosterone. Research on animals and human models has confirmed that cortisol inhibits testicular Leidig function and causes decrease in the production of testosterone [Citation58,Citation59].
Undoubtedly, androgens play a significant role in neurotransmission in the central nervous system- that may explain their influence on cognitive function, activity and mood. The paraventricular nucleus (PVN) of the hypothalamus is directly involved into converging the neural and hormonal signals that control the production of CRH and that produce CRH itself. Moreover, other important neurotransmitters and neuromodules as dopamine, gonadotropin releasing hormone, vasopressin and oxytocin are overproduced in PVN in patients with depressive disturbances.
Dihydrotestosterone, metabolite of TT, a pure agonist of androgen receptor, can directly act on neurons of this regions and inhibits hypothalamus-pituitary-adrenal axis [Citation60]. Moreover, it can be converted into 3-beta-diol which can selectively bind to oestrogen receptors [Citation60]. Its special function is to inhibit the response to stress and some behaviour connected with stress like anxiety [Citation61,Citation62]. This function may play a significant role in the pathogenesis of depression.
Furthermore, in order to differentiate primary from secondary hypogonadism we measured the level of LH. The serum level of LH was 4.39 ± 2.95 IU/L in men aged 40–59 years and 4.98 ± 3.00 IU/L in men aged 60–80 years, respectively. There was no statistical difference between groups (p = 0.16).
Following Tajar et al. [Citation63] we divided our study population into two groups: patients with primary hypogonadism defined as TT level < 10.5 nmol/L and LH level > 9.4 U/L and secondary hypogonadism defined as TT < 10.5 nmol/L and LH ≤ 9.4 U/L. In our study, the majority of the patients presented lower level of TT and LH irrespective of age category (98% in men in 40–59 years and 95% men in 60–80 years, p > 0.5) what indicated the secondary character of hypogonadism which was often observed in chronic illnesses.
The severity of depressive symptoms compared to androgens imbalance and potential medical implications
In our paper depression was associated with androgen imbalance in both groups of men. In men aged 40–59 years lower TT level and TT deficiency were linked to higher prevalence of severe depression. In the elderly only a weak association between mild depression and TT deficiency was observed, but in multivariate model TT deficiency turned out to be a significant risk factor of development of mild depression. The inflammatory processes [Citation65], cachexia [Citation36] and activation of the neuroendocrine system [Citation36,Citation65,Citation66] are common in patients with systolic HF [Citation36]. These processes cause androgen disturbances which not only reduce exercise capacity [Citation68], worsen outcomes and quality of life [Citation24] but also aggravate depressive symptoms [Citation24]. Most published data show links between the reduced level of androgens and depressive symptoms in healthy men [Citation64,Citation69,Citation70] but there are only few studies concerning this problem in men with HF [Citation24,Citation67]. Some studies indicate that substitutional therapy with TT and DHEAS may improve mood and vital forces in men with depression [Citation25,Citation26,Citation71]. Stout demonstrated that testosterone therapy in hypogonadal men with HF reduced depressive symptoms assessed with Beck Depression Inventory [Citation72]. Similarly, Pugh published data where TT supplementation was related to improvement not only in exercise capacity and HF symptoms but also trend towards relieving depressive symptoms in men with HF [Citation27]. There are no data showing that TT replacement improves survival of patients with HF. Further studies are needed to establish the role of hormonal supplementation in depression's treatment in men with HF.
Impact of severity of heart disease and comorbidities on depressive symptoms
Moreover, our study suggests that advanced NYHA class is related to higher incidence of depression in both groups of men with systolic HF. Our results correspond with published data where advanced NYHA class was associated with higher prevalence of depression in patients with HF [Citation4,Citation73,Citation74].
In addition, it has been shown that some comorbidities like COPD and previous stroke or TIA are independent risk factors of depression in younger men with systolic HF. We have not observed such correlations in the older group. In our study they occurred quite often as they affected 9.7% of men with systolic HF. Our results are concordant with published data that COPD [Citation75,Citation76] and previous stroke or TIA [Citation77,Citation78] increase the risk of depression in population with and without HF.
Finally, we presented that lower serum Hb concentration corresponds to higher incidence of mild depression in men aged 40–59 years compared to healthy peers. It is well-known that anaemia is a risk factor of depressive symptoms in general population [Citation80], in patients after acute coronary syndrome [Citation81] and suffering from cancer [Citation82]. In OPTIMIZE study [Citation83] depression occurred more often in patients with anaemia compared to those with normal Hb level. In our study the reduction level of Hb by 1 g/dl was associated with increased risk of development of depression about 1.5 times. More studies are needed to establish the role of anaemia in the development of depression in HF population.
Study limitations
The limitation of our study is its observational character. We only underlined the problem of deficiency of androgens in HF population and its potential link with the depressive symptoms. Our data suggest that androgen deficiency and some comorbidities may be a risk factor of development of depression but further prospective studies are needed to confirm our observations.
Our study was not designed to clarify the underlying mechanism of androgen imbalance in HF. We only mentioned a complex pathophysiological role of androgens in provoking depression in the aforementioned population. Some information comes from experimental studies on animals and needs further research. It may have a significant role in the treatment of patients with depression in the future.
Conclusions
Summarizing, all patients with systolic HF should be screened for depressive symptoms. Mood disorders are common in the aforementioned population [Citation79], they reduce daily activity and increase mortality [Citation9,Citation84]. The psychiatric counseling some patients with systolic HF and depression should be taken into consideration in order to reduce symptoms of the disease and improve quality of life.
Moreover, searching for androgen deficiencies in HF population may be crucial in order to find population of higher risk of development of depression. There are studies showing that TT or DHEAS supplementation may improve mood and vital forces in men with depressive disorders [Citation25,Citation26,Citation71]. Accordingly, further research is necessary to establish the role of androgen substitutional therapy in alleviating depressive symptoms and improvement in survival of HF population.
Since concomitant diseases such as stroke/TIA or COPD may increase the risk of depression, prevention is very important. Treatment of atherosclerosis and arterial hypertension, ceasing smoking are only examples of methods how to reduce the risk of developing disorders mentioned above.
Declaration of interest
Authors have no conflicts of interest. This work was supported by the statutory grant for Department of Heart Diseases, Wroclaw Medical University, Poland [ST-436].
References
- Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48:1527–37
- Himelhoch S, Weller WE, Wu AW, et al. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care 2004;42:512–21
- Freedland KE, Carney RM, Rich MW, et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry 1991;24:59–71
- Gottlieb SS, Khatta M, Friedmann E, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol 2004;43:1542–9
- Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail 2007;13:643–8
- Carels RA. The association between disease severity, functional status, depression and daily quality of life in congestive heart failure patients. Qual Life Res 2004;13:63–72
- Skotzko CE, Krichten C, Zietowski G, et al. Depression is common and precludes accurate assessment of functional status in elderly patients with congestive heart failure. J Card Fail 2000;6:300–5
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001;161:1849–56
- Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med 2007;167:367–73
- Murberg TA, Bru E, Svebak S, et al. Depressed mood and subjective health symptoms as predictors of mortality in patients with congestive heart failure: a two-years follow-up study. Int J Psychiatry Med 1999;29:311–26
- Junger J, Schellberg D, Muller-Tasch T, et al. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail 2005;7:261–7
- Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Card Fail 2005;11:455–63
- Silver MA. Depression and heart failure: an overview of what we know and don't know. Cleve Clin J Med 2010;77:S7–S11
- Joynt KE, Whellan DJ, O’Connor CM. Why is depression bad for the failing heart? A review of the mechanistic relationship between depression and heart failure. J Card Fail 2004;10:258–71
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive Symptoms in older men. Clin Endocrinol (Oxf) 2010;72:232–40
- Morsink LF, Vogelzangs N, Nicklas BJ, et al. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology 2007;32:874–83
- Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162–7
- Bain J. Testosterone and the aging male: to treat or not to treat? Maturitas 2010;66:16–22
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Moriyama Y, Yasue H, Yoshimura M, et al. The plasma levels of dehydroepiandrosterone sulfate are decreased in patients with chronic heart failure in proportion to the severity. J Clin Endocrinol Metab 2000;85:1834–40
- Amini Lari M, Parsa N, Marzban M, et al. Depression, testosterone concentration, sexual dysfunction and methadone use among men with hypogonadism and HIV infection. AIDS Behav 2012;16:2236–43
- Debigaré R, Marquis K, Côté CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 2003;124:83–9
- Halabi S, Collins EG, Thorevska N, et al. Relationship between depressive symptoms and hypogonadism in men with COPD. COPD 2011;8:346–53
- Jankowska EA, Drohomirecka A, Ponikowska B, et al. Deficiencies in circulating testosterone and dehydroepiandrosterone sulphate, and depression in men with systolic chronic heart failure. Eur J Heart Fail 2010;12:966–73
- Pope HG, Jr Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105–11
- Khera M, Bhattacharya RK, Blick G, et al. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012;15:14–21
- Pugh PJ, Jones RD, West JN, et al. Testosterone treatment for men with chronic heart failure. Heart 2004;90:446–7
- Jankowska EA, Biel B, Majda J, et al. Anabolic Deficiency in men with chronic heart failure prevalence and detrimental impact on survival. Circulation 2006;114:1829–37
- Brink TL, Yesavage JA, Lum O, et al. Screening tests for geriatric depression. Clinical Gerontologist 1982;1:37–44
- Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry 2010;18:1066–77
- Rule BG, Harvey HZ, Dobbs AR. Reliability of GDS in adult population. Clinical Gerontologist 1989;9:37–43
- Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2005;62:64–73
- Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med 2002;40:1151–60
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am 2007;36:333–48
- Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997;96:526–34
- Nestler JE. Regulation of human dehydroepiandrosterone metabolism by insulin. Ann NY Acad Sci 1995;774:73–81
- Tappler B, Katz M. Pituitary-gonadal dysfunction in low-output cardiac failure. Clin. Endocrinol (Oxf) 1979;10:219–26
- Maninger N, Wolkowitz OM, Reus VI, et al. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA Sulfate (DHEAS). Front Neuroendocrinol 2009;30:65–91
- Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab 2002;13:288–94
- Kimonides VG, Khatibi NH, Svendsen CN, et al. Dehydroepiandrosterone (DHEA) and DHEA(S) ulfate (DHEAS) protecthippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA 1998;95:1852–7
- Robichaud M, Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol 2004;182:11–21
- Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone(DHEA)—a precursor steroid or an active hormone in human physiology. J Sex Med 2011;8:2960–82
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol 2001;46:1–32
- Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci 2008;65:777–97
- Wierman ME. Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ 2007;31:26–33
- Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35
- Tivesten A, Moverare-Skrtic S, Chagin A, et al. Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. J Bone Miner Res 2004;19:1833–9
- Ferruci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 2006;166:1380–8
- Baker JR, Bemben MG, Anderson MA, Bemben DA. Effects of age on testosterone responses to resistance exercise and musculoskeletal variables in men. J Strength Cond Res 2006;20:874–81
- Azad N, Pitales S, Barres WE, et al. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab 2003;88:3064–8
- Alderson LM, Baum MJ. Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain Res 1981;218:189–206
- Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol 2005;17:179–85
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology 1985;117:2471–7
- Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology 1977;101:841–8
- Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Rev 2001;37:25–37
- Rubin RT, Poland RE, Lesser IM, et al. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry 1987;44:328–36
- Bambino TH, Hsueh AJ. Direct inhibitory effect of glucocorticoids upon testicular lutenizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology 1981;108:2142–8
- Cumming DC, Quigley ME, Yen SC. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab 1983;57:671–3
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alphaandrostan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 2006;26:1448–56
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine 2005;28:235–42
- Krezel W, Dupont S, Krust A, et al. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci USA 2005;98:12278–82
- Tajar A, Forti G, O'Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810–18
- McIntyre RS, Mancini D, Eisfeld BS, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology 2006;31:1029–35
- Sharma R, Coats AJ, Anker SD. The role of inflammatory mediators in chronic heart failure: cytokines, nitric oxide, and endothelin-1. Int J Cardiol 2000;72:175–86
- Sharma R, Anker SD. Immune and neurohormonal pathways in chronic heart failure. Congest Heart Fail 2002;8:23–8
- Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J 2006;27:57–64
- Jankowska EA, Filippatos G, Ponikowska B, et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Cardiac Fail 2009;15:442–50
- Seidman SN, Araujo AB, Roose SP, et al. Low testosterone levels in elderly men with dysthymic disorder. Am J Psychiatry 2002;159:456–9
- Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 1996;81:3578–83
- Schmidt PJ, Daly RC, Bloch M, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry 2005;62:154–62
- Stout M, Tew GA, Doll H, et al. Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J 2012;164:893–901
- Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol 2001;38:199–205
- Freedland KE, Rich MW, Skala JA, et al. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003;65:119–28
- van Manen JG, Bindels PJE, Dekker FW, et al. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002;57:412–16
- Schneider C, Jick SS, Bothner U, Meier CR. COPD and the risk of depression. Chest 2010 Feb;137:341–7
- Stewart R, Prince M, Mann A, et al. Stroke, vascular risk factors and depression: cross-sectional study in a UK Caribbean-born population. Br J Psychiatry 2001;178:23–8
- Hackett ML, Yapa C, Parag V, Craig S. Frequency of depression after stroke a systematic review of observational studies by Anderson. Stroke 2005;36:1330–40
- Lesman-Leegte I, Jaarsman T, Sanderman R, et al. Depressive symptoms are prominent among elderly hospitalised heart failure patients. Eur J Heart Fail 2006;8:634–40
- Onder G, Penninx BW, Cesari M, et al. Anemia is associated with depression in older adults: results from the InCHIANTI study. J Gerontol a Biol Sci Med Sci 2005;60:1168–72
- Steptoe A, Wikman A, Molloy GJ, Kaski JC. Anaemia and the development of depressive symptoms following acute coronary syndrome: longitudinal clinical observational study. BMJ Open 2012;2:e000551–3
- Skarstein J, Bjelland I, Dahl AA, et al. Is there an association between haemoglobin, depression, and anxiety in cancer patients? J Psychosom Res 2005;58:477–83
- Albert NM, Fonarow GC, Abraham WT, et al. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. Am J Med 2009;122:366–73
- Jiang W, Kuchibhatla M, Cuffe MS, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation 2004;110:3452–6
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Internal Med 1999;130:461–70
