 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: The prevalence of chronic kidney disease (CKD) in the elderly is high. Serum cystatin C is an accurate marker of kidney function and it also has prognostic utility in CKD patients. The aim of our study was to determine the prediction of serum cystatin C and other markers of kidney function on long-term survival in elderly CKD patients.
Methods: Fifty eight adult Caucasian patients, older than 65 years, without known malignancy, thyroid disease and/or not on steroid therapy were enrolled in the study. In each patient, 51CrEDTA clearance, serum creatinine, serum cystatin C, and estimated glomerular filtration rate using different equations were determined on the same day and patients were then followed for 11 years or until their death.
Results: The means are as follows: 51CrEDTA clearance 53.3 ± 17.4 ml/min/1.73 m2, serum creatinine 1.62 ± 0.5 mg/dl, serum cystatin C 1.79 ± 0.5 mg/l, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation 40.1 ± 14 ml/min/1.73 m2, Berlin Initiative Study 2 (BIS2) equation 38.9 ± 10.7 ml/min/1.73 m2, full age spectrum (FAS) creatinine equation 43.8 ± 13.8 ml/min/1.73 m2, FAS cystatin C equation 40.1 ± 11.7 ml/min/1.73 m2. In the follow up period, 47 (81%) patients died. Cox regression analysis showed different hazard ratios (HRs) for death: for 51CrEDTA clearance HR 1.022 (95% CI 1.004–1.042; p = .015), serum creatinine HR 1.013 (95% CI 1.006–1.019; p = .001), serum cystatin C HR 2.028 (95% CI 1.267–3.241; p = .003), CKD-EPI creatinine equation HR 1.048 (95% CI 1.019–1.076; p = .001), BIS2 equation HR 1.055 (95% CI 1.021–1.088; p = .001), FAS creatinine equation HR 1.046 (95% CI 1.017–1.074; p = .001), FAS cystatin C equation HR 1.039 (95% CI 1.010–1.071; p = .009).
Conclusions: Our results showed the highest HR for serum cystatin C among kidney function markers for prediction of outcome in elderly CKD patients.
Introduction
Chronic kidney disease (CKD) is a worldwide health problem and its prevalence is rising, particularly in the elderly, reflecting mainly the worldwide increased life expectancy of the population [Citation1–4]. Estimation of glomerular filtration rate (GFR) is essential for the evaluation of patients with CKD and allows the detection of early impairment of kidney function, the prevention of further deterioration and complications, the correction of the dosage of drugs cleared by the kidney to avoid potential drug toxicity and facilitate management of CKD patients. All these facts are particularly important to be considered in the elderly [Citation3,Citation4]. Today, GFR is usually estimated through the use of equations that include a serum concentration of an endogenous filtration marker like serum creatinine and/or serum cystatin C [Citation3–6]. Both serum creatinine and serum cystatin C are affected by factors other than GFR. Serum cystatin C is considered to be less biased by age and weight, however, it has been demonstrated that serum cystatin C levels are variously affected by dysthyroidisms (hypothyroidism or hyperthyroidism), corticosteroid therapy, chronic inflammation, and malignancy [Citation3,Citation7,Citation8].
In previously published studies, an independent, graded association was observed between renal dysfunction estimated by GFR and the risk of hospitalization, cardiovascular events and death in a large, community-based population [Citation8–11]. The studies have shown that renal impairment is an independent risk factor for cardiovascular disease and all-cause mortality [Citation10,Citation11]. Furthermore, among the known markers to estimate kidney function, serum cystatin C may have prognostic importance as a predictor of adverse outcomes independent of renal function. The studies have reported that compared to estimated GFR by serum creatinine concentrations, estimated GFR with serum cystatin C demonstrated a stronger association with hard outcomes, such as cardiovascular events, cardiovascular mortality, and all-cause mortality [Citation12–14].
The aim of our study was to determine the prediction of serum cystatin C, measured GFR (51CrEDTA clearance) and recently described serum creatinine and/or serum cystatin C based GFR equations (Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation, Berlin Initiative Study 2 (BIS2) equation, full age spectrum (FAS) equations) on long-term survival in a well-defined population of elderly CKD patients.
Materials and methods
We included 58 adult Caucasian patients older than 65 years (29 women, 29 men; mean age 73 years; range from 65 to 85 years). All patients were referred for measuring 51CrEDTA clearance by nephrologists, diabetologists, cardiologists, or general internists because of suspected or established renal dysfunction. 51CrEDTA was injected intravenously; blood samples were obtained 120, 180, and 240 min after the injection. GFR was measured from 51CrEDTA clearance according to the Committee on renal clearance recommendations [Citation15]. 51CrEDTA clearance was calculated in millilitre per minute per 1.73 m2. Before 51CrEDTA was injected, blood was withdrawn for measuring serum creatinine and serum cystatin C. Serum creatinine was measured by using the kinetic method according to the Jaffé method without deproteinisation (isotope dilution mass spectrometry traceable method; Roche Diagnostics, Mannheim, Germany). This is a compensated method based on manufacturer instructions and was described previously [Citation16]. Serum cystatin C was measured by the particle-enhanced immunonephelometric method (Dade Behring, Marburg, Germany). The values were recalculated to the certified reference standard, using multiplication factor, according to the manufacture’s specifications.
The GFR was calculated according to different equations: CKD-EPI creatinine equation (1), BIS2 equation (2) and FAS creatinine equation (3) and FAS cystatin C equation (4) [Citation17–20].
1. GRF calculated according to the CKD-EPI creatinine equation:
The variable a takes on the following values on the basis of race and sex:
women = 144; men = 141
The variable b takes on the following values on the basis of sex:
women = 0.7; men = 0.9
The variable c takes on the following values on the basis of sex and serum creatinine measurement:
women: serum creatinine ≤0.7 mg/dl = –0.329; serum creatinine >0.7 mg/dl = –1.209
men: serum creatinine ≤0.7 mg/dl = –0.411; serum creatinine >0.7 mg/dl = –1.209
2. GFR calculated according to BIS2 equation:
3. GFR calculated according to FAS creatinine equation:
for adult Caucasians, the value of Q is constant and equals 0.7 mg/dl for women and 0.9 mg/dl for men.
4. GFR calculated according to FAS cystatin C equation:
for all ages ≤70 years Q is 0.82 mg/l and 0.95 mg/l for older ages.
In each patient, 51CrEDTA clearance, serum creatinine, serum cystatin C, and estimated GFR using different equations were determined on the same day and patients were then followed for 11 years or until their death.
For statistical analysis, SPSS for Windows (version 17.0, SPSS, Chicago, IL) was used. The mean value, range, and SD were calculated. The percentages of survival of enrolled patients according to gender were defined. Cox’s regression model was used to assess the influence of serum creatinine, serum cystatin C, 51CrEDTA clearance, and GFR calculated from before mentioned equations on all-cause mortality in our patients. Cox’s model was presented with beta coefficients, hazard ratios (HRs) along with their 95% confidence intervals (CIs) and p values, which described the associations between different markers of kidney function and long-term all-cause mortality in elderly CKD patients. Originally, the statistical analysis of HRs for death showed that every increase in measured (51CrEDTA clearance) and estimated GFR (equations) resulting in a decrease of chance to die (presented with HRs values below 1). It is expected that a decrease in measured and estimated GFR with equations would be associated with death, therefore inverse HRs for measured and estimated GFR were calculated and presented in our study.
The study protocol was in conformity with ethical guidelines and informed consent was obtained from every patient.
Results
Baseline characteristics of our patients are presented in . The means are as follows: 51CrEDTA clearance 53.3 ± 17.4 ml/min/1.73 m2, serum creatinine 1.62 ± 0.5 mg/dl, serum cystatin C 1.79 ± 0.5 mg/l, CKD-EPI creatinine equation 40.1 ± 14 ml/min/1.73 m2, BIS2 equation 38.9 ± 10.7 ml/min/1.73 m2, FAS creatinine equation 43.8 ± 13.8 ml/min/1.73 m2, FAS cystatin C equation 40.1 ± 11.7 ml/min/1.73 m2. In the follow up period of 11 years, 47 (81%) of our elderly CKD patients (23 women and 24 men) died. Baseline characteristics of our patients who died and those who survived are presented in . Cox’s regression analysis showed different HRs for death: for 51CrEDTA clearance HR 1.022 (95% CI 1.004–1.042; p = .015), serum creatinine HR 1.013 (95% CI 1.006–1.019; p = .001), serum cystatin C HR 2.028 (95% CI 1.267–3.241; p = .003), CKD-EPI creatinine equation HR 1.048 (95% CI 1.019–1.076; p = .001), BIS2 equation HR 1.055 (95% CI 1.021–1.088; p = .001), FAS creatinine equation HR 1.046 (95% CI 1.017–1.074; p = .001), and FAS cystatin C equation HR 1.039 (95% CI 1.010–1.071; p = .009) (, ).
Figure 1. Hazard ratios for death for different markers of kidney function in elderly CKD patients. CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; BIS: Berlin Initiative Study; FAS: full age spectrum.
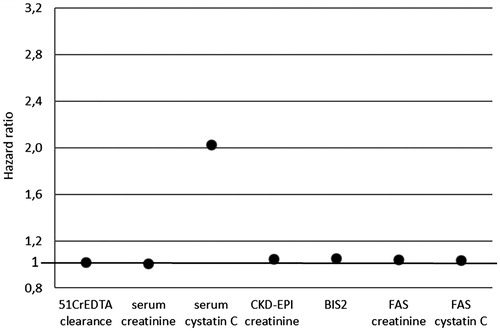
Table 1. Baseline characteristics of our patients.
Table 2. Baseline characteristics of patients who died and those who survived.
Table 3. Cox regression analysis for risk of death based on different markers of kidney function in elderly CKD patients.
Discussion
Over the past decade, serum cystatin C has been often suggested as a marker of kidney function. Strong evidence has shown that serum cystatin C alone or in combination with serum creatinine may improve classification of GFR to identify CKD in certain clinical populations. Serum cystatin C equations based on the serum cystatin C reference standard are considered state-of-the-art to estimate kidney function and the latest CKD guidelines included several suggestions and recommendations that relate to serum cystatin C [Citation1,Citation3,Citation21,Citation22]. The results of previous studies have also suggested that serum cystatin C might be more than just a GFR marker, as it may provide complementary information to established risk determinants, especially for high-risk populations. Additionally, studies have reported that serum cystatin C may be a useful prognostic indicator of cardiovascular disease [Citation9–13]. In studies, where new and more precise equations (based on serum creatinine and serum cystatin C or both) were used, differences between GFR estimation markers in prediction of mortality in different patient populations were observed [Citation21–24]. Estimated GFR based on serum creatinine appeared to have J-shaped association with the risk of death. On the contrary, estimated GFR based on serum cystatin C showed a linear association with mortality. GFR estimated from both serum creatinine and serum cystatin C was significantly more predictive of outcomes than estimated GFR by serum creatinine, but significantly less predictive of outcomes than estimated GFR by serum cystatin C alone [Citation21–24]. The reason for these differences was not completely clear, but can be partly explained by the non-GFR determinants of serum cystatin C [Citation25]. However, Shlipak et al. warned us about those conclusions. They believed that this difference in prediction was almost certainly due to the characteristics of the populations studied. GFR measurement studies consistently recruit relatively healthy persons who are more likely to have predictable muscle mass, the CKD population in GFR studies is much younger and has fewer comorbid diseases than the overall kidney disease population, and participants without CKD tend to be either middle-aged volunteers or prospective kidney donors. In contrast, prognosis studies have included a higher proportion of elderly persons and a greater prevalence of chronic diseases that make serum creatinine level less reliable than serum cystatin C level. The alternative viewpoint to explain these findings is that serum cystatin C has a direct link to adverse outcomes that is independent of its role as a GFR marker, such as inflammation or adiposity [Citation21]. Furthermore, in advanced CKD, in which serum cystatin C and creatinine levels are highest, estimated GFR based on serum creatinine and/or serum cystatin C concentrations, and measured GFR all have very similar associations with mortality [Citation26].
Despite of conclusions reached in before mentioned studies and discussions regarding the prediction of serum cystatin C for outcome in elderly CKD patients, we analyzed our data of elderly CKD patients. Our study examined prognosis of patients on the basis of different kidney function markers (serum cystatin C, serum creatinine, measured GFR with 51CrEDTA clearance, estimated GFR with different serum creatinine and/or serum cystatin C based GFR equations, some of them recently described) in a well-defined population of Caucasian elderly CKD patients.
The results of our study showed different HRs for death for studied kidney function markers. All markers showed a positive prediction for death in the observed period; however, serum cystatin C had an importantly higher HR (2.0). The HR of other kidney function markers, including the gold standard – 51CrEDTA clearance, serum creatinine, serum creatinine based equations, serum cystatin C based equation and combined serum creatinine–cystatin C equations were much lover.
The strength of our study is a well-defined homogeneous population of elderly patients with mild to moderate CKD, with excluded present malignancy, use of steroids and dysthyroidisms. To define the possible influence of adiposity on serum cystatin C in the studied population, we made a sub-analysis of our results excluding four obese patients (the results of sub-analysis are not presented in the manuscript). However, the results did not change importantly and our conclusions remained. Unfortunately, we were less capable to evaluate the inflammatory status of our patients which may also affect serum cystatin C levels to exclude chronic inflammation as condition. Another strength is that we examined a variety of clinically useful methods for GFR estimation in a non-advanced or preterminal population of elderly CKD patients, including the gold standard for GFR measurement and standardized measurements of serum cystatin C and creatinine. Our patients were followed for a long time. An important limitation of our study is a small number of enrolled elderly CKD patients. Furthermore, we need to be aware that the widely used CKD-EPI equation was developed in a non-elderly CKD population. Moreover, due to the difference in scale of the biomarkers, compared to estimated GFR, a direct comparison of HRs between biomarkers and GFR equations remains the subjects of discussion.
Conclusions
Our results suggest that serum cystatin C adds more value than other kidney function markers (including the gold standard) in predicting death in elderly patients with mild to moderate CKD. Although some studies argued that serum cystatin C is unlikely to have substantial associations with adverse outcomes outside its role as a marker of GFR, serum cystatin C could be a useful predictor of all-cause mortality in a well-defined population, like elderly CKD patients.
Ethical approval
The Slovenian National Medical Ethics Committee approved the protocols in this study (approval no. 29/02/08).
Informed consent
The informed consent was obtained from every patient.
Acknowledgements
The results presented in this paper have been accepted for poster presentation at the American Society of Nephrology Kidney week 2017 Annual Meeting, October 31–November 5 in New Orleans, LA.
Disclosure statement
The authors have declared that no conflict of interest exists.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.
- Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;2010:195–133.
- Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–114.
- Farrington K, Covic A, Aucella F, et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant. 2017;32:9–16.
- Hojs R, Bevc S, Ekart R, et al. Kidney function estimating equations in patients with chronic kidney disease. Int J Clin Pract. 2011;65:458–464.
- Bevc S, Hojs N, Hojs R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients – comparison of three novel sophisticated equations and simple cystatin c equation. Ther Apher Dial. 2017;21:126–132.
- Chew JS, Saleem M, Florkowski CM, et al. Cystatin C—a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008;29:47–62.
- Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169.
- Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcome and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372.
- Ix JH, Chertow GM, Shlipak MG, et al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539.
- Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246.
- Hojs Fabjan T, Penko M, Hojs R. Newer glomerular filtration rate estimating equations for the full age spectrum based on serum creatinine and cystatin C in predicting mortality in patients with ischemic stroke. Eur J Intern Med. 2018;52:67–72.
- Blaufox MD, Aurell M, Bubeck B, et al. Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med. 1996;37:1883–1890.
- Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffe creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–55.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration. Ann Intern Med. 2009;150:604–612.
- Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481.
- Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31:798–806.
- Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant. 2017;32:497–507.
- Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62:595–603.
- Bevc S, Ekart R, Hojs R. Cystatin C – a marker of kidney function and predictor of cardiovascular disease and mortality. Acta Med Biotechn. 2014;7:9–15.
- Astor BC, Shaikh S, Chaudhry M. Associations of endogenous markers of kidney function with outcomes: more and less than glomerular filtration rate. Curr Opin Nephrol Hypertens. 2013;22:331–335.
- Hojs Fabjan T, Penko M, Hojs R. Cystatin C, creatinine, estimated glomerular filtration, and long-term mortality in stroke patients. Ren Fail. 2014;36:81–86.
- Astor BC, Levey AS, Stevens LA, et al. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222.
- Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23:351–359.
