Abstract
Aim
Myocardial performance index (MPI) is an easy-to-apply and non-invasive method that shows both systolic and diastolic functions of the heart. In this study, it was aimed to evaluate the relationship between erectile dysfunction (ED) and MPI. Methods: The study included 45 male patients admitted to the urology outpatient clinic for ED and 48 healthy male volunteers. Echocardiographic evaluation of all participants was performed. Isovolumetric contraction time (IVCT), isovolumetric relaxation time (IVRT) and ejection time (ET) were measured. MPI was calculated using the IVCT + IVRT/ET formula. Results: The average age of the study population was 50 ± 5.3. Early diastolic mitral inflow (E)/late diastolic mitral inflow (A) ratio was significantly lower in the ED group (p ≤ 0.05). In the TDI evaluation between the groups, while early diastolic mitral annular velocity (Em) was significantly higher in the ED group, there was no significant difference in late diastolic mitral annular velocity (Am) and systolic peak velocities (Sm) (p < 0.01 and p = 0.417 and p = 0.092, respectively). While IVRT was significantly lower in the ED group (p < 0.05), there was no significant difference in IVCT and ET (p = 311 and p = 0.261, respectively). MPI was statistically significantly higher in the ED group (p < 0.05). Conclusion: ED has been found to affect MPI. This parameter, which is easily and non-invasively measured, can be used to predict the risk of CVDs in ED.
Introduction
Consistent or repetitive inability to achieve and/or maintain adequate penile erection for sexual satisfaction is defined as erectile dysfunction (ED) [Citation1]. It is a condition that affects more than 50 percent of men between the ages of 40 and 70 [Citation2]. The relationship between ED and cardiovascular diseases (CVD) is well defined and many studies have been done to support this [Citation3,Citation4]. Hypertension, diabetes mellitus, smoking, hyperlipidemia, and sedentary lifestyle are the main risk factors for CVD and ED [Citation5]. The pathophysiological fundamental for both is atherosclerosis and endothelial dysfunction [Citation6]. ED has been shown to occur on average 3–5 years before cardiovascular events and therefore can be a powerful predictive factor for CVDs [Citation7]. By early detection of ED, the risk of future cardiovascular events can be reduced and there may be an opportunity to intervene to restrain the progression of ED severity. Nitric oxide (NO) is a signaling molecule released from endothelial cells and has an important role in the mechanism of erection, and endothelial cell dysfunction causes a decrease in NO production. Endothelium-dependent vasodilation and structural vascular disorders due to NO path disruption have been demonstrated in both ED and CAD [Citation8].
Many questionnaire forms have been developed in which sexual interest, performance and satisfaction are questioned and evaluated by the scoring system. The most common among these is the International Index of Erectile Function (IIEF) questionnaire [Citation9].
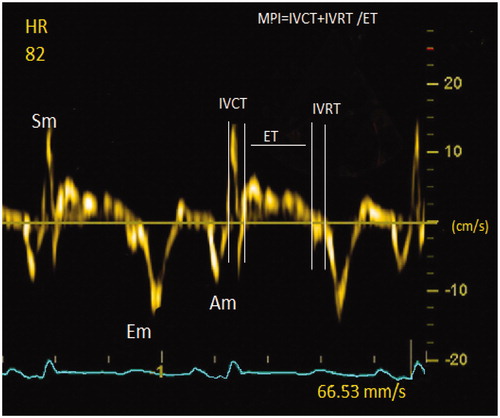
In order for the heart to work as an effective pump, it must not only eject but also fill the left ventricle with blood. This is known as the diastolic function [Citation10]. Myocardial performance index (MPI) is an echocardiographic measurement and is used to evaluate both systolic and diastolic functions of the left ventricle (LV). It is an index obtained from mitral and aortic flows that are not affected by preload, ventricular structure and heart rate and can be easily measured from Doppler traces. It is measured by the following formula; Isovolumetric relaxation time (IVRT) + Isovolumetric contraction time (IVCT)/ejection time (ET). It is a widely accepted parameter because variability among observers is very low and can be easily measured. In a study on patients with heart failure (HF), this index was found to be a strong predictor of cardiovascular mortality in patients with systolic HF [Citation11]. MPI can be a valuable tool to assess possible systolic and diastolic heart failure in the future. In our study, we aimed to determine whether MPI will be a predictive parameter for possible CVD development in ED patients and to provide an important source for future studies.
Materials and methods
Study population and study design
The study was designed prospectively and cross-sectionally and was approved by the local ethics committee (2020/016). Forty-five male patients aged 18–65 years who were diagnosed with ED in the urology polyclinic between 1 February and 30 March 2020 were included in the study. For the control group, 48 age-matched healthy volunteers were recruited. A complete medical history was taken from the participants, and a physical examination was performed and the findings were recorded. Waist circumference, height and weight of all participants were measured, body mass indexes (BMI) were calculated using the formula BMI = weight (kg)/[height (meter)]2. After the first application and after 12 h of fasting, venous blood samples were taken and centrifuged at 3000 rpm for biochemical analysis. Complete blood counts were measured for all participants. During biochemical analysis: fasting blood glucose, sodium, potassium, creatinine, blood urea nitrogen, uric acid, calcium, total protein, albumin, hepatic enzymes, thyroid-stimulating hormone, C-reactive protein (CRP), and lipid parameters were measured.
Exclusion criteria:
Patients diagnosed with hypertension or using antihypertensive drugs
Detected coronary artery disease and peripheral artery disease
Systolic heart failure
Moderate or severe valvular heart disease
Primary and secondary pulmonary hypertension or chronic pulmonary disease
Chronic kidney disease (GFR <60 ml/min)
Diabetes mellitus (Type I or II)
Thyroid dysfunction
Obesity (BMI > 30 kg/m2)
Patients who have undergone major pelvic surgery
Cigarette smokers and alcohol consumers
Patients with active infections
Anemia
Patients who have previously received surgical or medical treatment for erectile dysfunction
Patients without previous sexual intercourse and therefore erectile functions cannot be evaluated.
Evaluation of erectile function
The degree of ED was determined by filling the International Erectile Function Index-5 (IIEF-5) form. The minimum score was 5 and the maximum score was 25. According to this form, IIEF-5 scores were calculated. Scores between 5 and 9 were severe ED, 10–15 medium ED, and scores 16–21 light ED. Values 22 and above were evaluated as normal erectile function [Citation10]. Patients were divided into two groups according to this scoring system. The first group is the erectile dysfunction group with mild ED, moderate ED and severe ED. The second group was the healthy non-ED control group.
Echocardiographic evaluation
Echocardiographic examinations were performed at the echocardiography laboratory with the Epiq 7 device (Philips, Amsterdam, Netherlands) in the left lateral decubitus position as recommended by the American Echocardiographic Association [Citation12]. With parasternal long-axis imaging, the diameter of the left atrium and the aortic diameter were measured. M-mode images; thickness of left ventricular walls, left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD) were recorded. Left ventricular ejection fraction was measured with the modified Simpson method. Early diastolic mitral inflow (E), late diastolic mitral inflow (A) waves were measured by pulse wave (PW) Doppler from apical four-chamber windows. E/A ratios of the patients were calculated using these values. Mitral annular velocities were obtained by tissue doppler imaging (TDI) using the pulsed-wave mode. Early diastolic mitral annular (Em), late diastolic (Am), systolic peak velocities (Sm) were measured at the annulus level of the left ventricular lateral edges. All measurements were made in five consecutive cardiac cycles and the means of these were used. As shown in , Doppler time intervals were measured from the time intervals of mitral inflow and LV outflow velocities. MPI was calculated with the formula IVRT + IVCT/ET. Echocardiographic measurements of 20 patients included in the study were repeated 15 days later to determine the interobserver variability. The repeatability of the measurements was statistically significant (intraclass correlation coefficient of 0.923; p < 0.001).
Statistical analysis
Statistical analyzes were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed as mean ± standard deviation, while categorical variables are shown as frequency and percentage (%). The distribution of the data was evaluated by the Kolmogorov–Smirnov test. A comparison between groups was made using the Mann–Whitney U- or t-test according to the distribution of data. Correlation analysis was performed with Pearson or Spearman correlation test Chi-square test was used to evaluate the differences of categorical variables between groups. In the statistical analysis, p-value <0.05 was considered significant.
Results
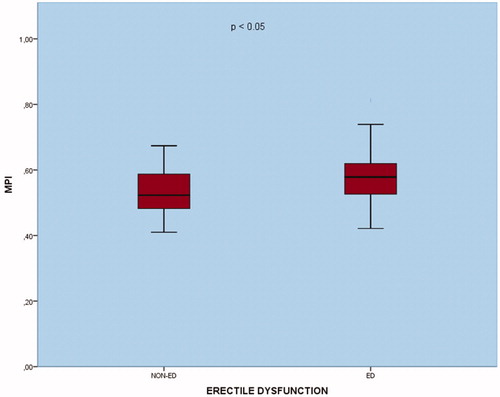
The mean age in the study population was 50 ± 5.3. There was no statistical difference between the patient and control groups in the age groups (p = 0.712). In the ED group, the weight, BMI and waist circumference were higher than the control group, but this difference was not statistically significant (p = 0.356, p = 0.135 and p = 0.363, respectively). As expected, the IIEF 5 score was lower in the ED group than in the control group (p < 0.01). Demographic and anthropometric measurements of the study groups are shown in . In conventional echocardiographic evaluation, aortic diameter, interventricular septum (IVS), posterior wall (PW) thickness and LVEF measurements were similar in the groups. LVESD and LVEDD values were higher in the ED group but did not have statistical significance (p = 0.316 and p = 0.487, respectively). There was no statistical difference between the groups in the measurements of mitral E wave and A waves (p = 0.221 and p = 0.057, respectively). E/A ratio was significantly lower in the ED group (p < 0.05). In TDI evaluation, while Em was significantly higher in ED, there was no significant difference in Am value and Sm value (p < 0.01 and p = 0.417 and p = 0.092, respectively). IVRT was significantly lower in the ED group (p < 0.05), there was no significant difference in IVCT and ET (p = 0.311 and p = 0.261, respectively). MPI was statistically significantly larger in the ED group (p < 0.05) (). shows the echocardiographic measurements.
Table 1. Demographic and anthropometric measurements.
Table 2. Echocardiographic measurements.
Discussion
Our study showed that MPI, which is an indicator of LV dysfunction, is associated with ED. We also observed that IVRT, which is one of the parameters in the measurement of MPI, is prolonged in the ED group. IVRT, a phase of diastolic functions, is a highly energy-dependent, very active period. Sufficient adenosine triphosphate (ATP) cannot be produced in the cell due to ischemia, and lactic acid accumulation occurs, which prolongs the contraction members’ separation time. This occurs not only concerning ischemia but also in cases where LV’s functions are impaired and cause indirect ischemia.
ED prevalence is gradually increasing with the aging caused by the natural process and the emergence of chronic diseases such as diabetes mellitus, hypertension and vascular diseases. Especially its association with CVD is frequent. Similar pathophysiological mechanisms and similar risk factors explain the association between ED and CVD. Endothelial dysfunction is at the beginning of these mechanisms [Citation13]. A positive correlation between the severity of ED in men with a high atherogenic index has recently been demonstrated [Citation14]. The IIEF-5 test is a well-established method to evaluate ED [Citation9].
ED symptoms begin 3–5 years before CVD appears. Cardiovascular and all-cause mortality is higher in ED patients than in the general population [Citation15]. In the meta-analysis, which included 25 suitable studies involving 154,794 individuals, it was observed that ED patients had a significantly increased risk of CVD by 43% compared to men without ED [Citation16]. Besides, studies have shown that ED may be the first clinical sign and predictor of cardiovascular disorders [Citation17].
ED has a considerable pathophysiological relationship with CVD risk factors. Cyclic guanosine monophosphate (cGMP) increases by taking NO, which provides penile erection, into smooth muscle tissue. The same physiological mechanism has a role in the dilatation of coronary arteries, pulmonary arteries and systemic arteries [Citation18]. cGMP activates protein kinase G (PKG), which acts as a brake in the signal pathways of cardiomyocyte stiffness and hypertrophy. Stiffer and larger cardiomyocytes contribute to active relaxation and passive stiffness. Thus, the endothelium-derived NO directly regulates the cardiac diastolic functions [Citation19]. In addition to these and similar effects, NO also has antiatherogenic effects, so NO reduction is prominent in the early stages of atherosclerosis. Therefore, ED is considered an important marker with an increased risk of CVD. In a study investigating cardiac risk factors in ED patients, CVD risk factors were common even in mild ED patients [Citation20]. Another study in 395 men with hypogonadism showed an increased risk of CVD with loss of libido and ED severity [Citation21]. It has also been shown that in men with hypogonadism who were given long-term testosterone treatment, the IIEF score improved significantly, improved quality of life and significant improvement in all cardiometabolic risk factors. It has been stated that it may be an additional precaution in the secondary prevention of cardiovascular events in men with hypogonadism with a history of CVD [Citation22–24].
In a previous study (E/Em) ratio and IVRT were shown to be increased with ED severity [Citation8]. The findings of the study supported ours. But in our study, factors that play a role in CVD and its etiology were disabled. The purpose of doing this was to see the effects of ED on LV functions in plain form. Another difference of our study was that it was measured in IVCT, ET and MPI, which are other objective indicators of diastolic functions. From this aspect, we think that our study may have superiority. In the studies carried out, an increase in atherosclerotic heart diseases, peripheral arteries and especially CIMT has been observed in patients with ED [Citation25]. It is known that atherosclerosis is an important risk factor in the onset of diastolic dysfunctions.
A study by Karagöz et al., in middle-aged men with ED, stated that even though there is no coronary artery disease, the left ventricular global longitudinal and circumferential strain values increase with ED severity, ED severity is associated with left ventricular systolic and diastolic dysfunction [Citation3]. In this study, while there was a history of drug use that could make ED in patients (ACEI, ARB, diuretics, etc.), drug use was excluded in our study. Similarly, in another study conducted in patients without apparent CAD showed that endothelial functions and left ventricular diastolic functions such as mitral E/A ratio, mitral E/Em ratio, and IVRT were impaired in patients with ED [Citation26]. In another recent study, the negative collation was detected between the left ventricular diastolic dysfunction stage and IIEF 5 score [Citation27].
In our study, the measurement of these parameters was similar. What made our study different was that it showed diastolic dysfunctions by measuring MPI. This was the first study in literature. In our study, the advantage of MPI measured by tissue doppler imaging method in showing diastolic parameters was demonstrated in previous studies [Citation28]. It has also been shown that MPI can be used in patients with poor echocardiographic image quality. Another advantage of MPI is that it is less affected by heart rate. Heart rates at 50–120 beats/min do not require correction [Citation29].
Phosphodiesterase-5 (PDE-5) inhibitors are the drugs used as the first choice in the treatment of ED. The mechanism of action is to prevent the inactivation of cGMP in the way of conversion to GMP. Some authors have shown cardioprotective effects through regulation of endothelial dysfunction with continuous use of PDE 5 inhibitors, and it has also been shown that there may be positive hemodynamic effects in patients with HF [Citation30]. A recent study showed that the E′/A′ ratio as an indicator of diastolic functions was positively correlated with IIEF-5 scores before treatment and negatively correlated with the beneficial effect of vardenafil therapy [Citation31]. However, these studies are short-lived.
The limitations of our study can be considered as the relatively low number of patients and being single-centered. Another limitation may be asymptomatic and silent CAD, which has not been detected in patients, although there are CAD exclusion criteria in the study group. Indeed, sometimes the stress test and coronary computed tomography may not detect CAD.
As a result, the relationship between CVD and ED has been known for a long time. Although ED patients are expected to develop cardiac diseases, including diastolic dysfunction in the coming years, there are scarce parameters to measure this developmental process. We have shown that MPI can be a useful method to objective this relationship and identify high-risk patients.
Author contributions
IA and MC and conceived the study design. IA, MC and AHY were involved in data collection. IA and MC performed the statistical analysis. IA, MC and AHY interpreted data and prepared the manuscript draft. All authors critically reviewed the final version of the manuscript. All authors approved the final version of the manuscript.
Acknowledgments
We thank the urologist Ali Haydar Yilmaz, who contributed to data collection and interpretation in our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13(2):135–143.
- Miner M, Kim ED. Cardiovascular disease and male sexual dysfunction. Asian J Androl. 2015;17(1):3–4.
- Karagöz A, Günaydin ZY, Bektaş O, et al. Subclinical left ventricular deterioration in patients with erectile dysfunction. Acta Cardiol. 2016;71(5):557–563.
- Chew KK, Bremner A, Stuckey B, et al. Sex life after 65: how does erectile dysfunction affect ageing and elderly men? Aging Male. 2009;12(2–3):41–46.
- Kałka D, Zdrojowy R, Womperski K, et al. Should information about sexual health be included in education directed toward men with cardiovascular diseases? Aging Male. 2018;21(4):243–250.
- Leoni LA, Fukushima AR, Rocha LY, et al. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male. 2014;17(3):125–130.
- Diaconu CC, Manea M, Marcu DR, et al. The erectile dysfunction as a marker of cardiovascular disease: a review. Acta Cardiol. [cited 2019 Apr 6]; [7 p.]. DOI:10.1080/00015385.2019.1590498
- El-Sakka AI, Morsy AM, Fagih BI. Severity of erectile dysfunction could predict left ventricular diastolic dysfunction in patients without overt cardiac complaint. J Sex Med. 2011;8(9):2590–2597.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830.
- Yu HP, Jen HL, Yin WH, et al. Circulating adiponectin levels following treatment can predict late clinical outcomes in chronic heart failure. Acta Cardiol Sin. 2017;33(2):139–149.
- Olson JM, Samad BA, Alam M. Myocardial performance index determined by tissue doppler imaging in patients with systolic heart failure predicts poor long-term prognosis: an observational cohort study. J Card Fail. 2016;22(8):611–617.
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2(5):358–367.
- Chaudhary RK, Shamsi BH, Chen HM, et al. Risk factors for erectile dysfunction in patients with cardiovascular disease. J Int Med Res. 2016;44(3):718–727.
- Culha MG, Canat L, Degirmentepe RB, et al. The correlation between atherogenic indexes and erectile dysfunction. Aging Male. [cited 2020 Apr 8]; [5 p.]. DOI:10.1080/13685538.2020.1749996
- Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, et al. The triad: erectile dysfunction-endothelial dysfunction-cardiovascular disease. Curr Pharm Des. 2008;14(35):3700–3714.
- Zhao B, Hong Z, Wei Y, et al. Erectile dysfunction predicts cardiovascular events as an independent risk factor: a systematic review and meta-analysis. J Sex Med. 2019;16(7):1005–1017.
- Araujo AB, Travison TG, Ganz P, et al. Erectile dysfunction and mortality. J Sex Med. 2009;6(9):2445–2454.
- Naylor AM. Endogenous neurotransmitters mediating penile erection. Br J Urol. 1998;81(3):424–431.
- Segers VFM, Brutsaert DL, De Keulenaer GW. Cardiac remodeling: endothelial cells have more to say than just NO. Front Physiol. 2018;9:382
- Cho SY, Son H, Kim SW, et al. Should men with mild erectile dysfunction be closely evaluated for cardiovascular diseases in the Korean population?. Aging Male. 2014;17(2):81–86.
- Ho CH, Wu CC, Chen KC, et al. Erectile dysfunction, loss of libido and low sexual frequency increase the risk of cardiovascular disease in men with low testosterone. Aging Male. 2016;19(2):96–101.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199(1):257–265.
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–261.
- Traish AM, Haider A, Haider KS, et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22(5):414–433.
- Elkamshoushi AAM, Hassan EM, El Abd AM, et al. Serum endocan as a predictive biomarker of cardiovascular risk in erectile dysfunction patients. Andrologia. 2018;50(10):e13113.
- Uslu N, Eren M, Gorgulu S, et al. Left ventricular diastolic function and endothelial function in patients with erectile dysfunction. Am J Cardiol. 2006;97(12):1785–1788.
- Ceyhun G, Erbay G. A rare cause of erectile dysfunction: left ventricular diastolic dysfunction. Minerva Cardioangiol. [cited 2020 Mar 11]. DOI:10.23736/S0026-4725.20.05149-X
- Ozdemir K, Balci S, Duzenli MA, et al. Effect of preload and heart rate on the doppler and tissue doppler-derived myocardial performance index. Clin Cardiol. 2007;30(7):342–348.
- Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10(2):169–178.
- Pofi R, Gianfrilli D, Badagliacca R, et al. Everything you ever wanted to know about phosphodiesterase 5 inhibitors and the heart (but never dared ask): how do they work? J Endocrinol Invest. 2016;39(2):131–142.
- Zengin K, Ede H, Tanik S, et al. Cardiac factors affecting the success of vardenafil in erectile dysfunction. Turk J Med Sci. 2015;45(4):751–757.